Abstract
Background
A common measure of rehabilitation effectiveness post-stroke is self-selected walking speed, yet individuals may achieve the same speed using different coordination strategies. Asymmetry in the propulsion generated by each leg can provide insight into paretic leg coordination due to its relatively strong correlation with hemiparetic severity. Subjects walking at the same speed can exhibit different propulsion asymmetry, with some subjects relying more on the paretic leg and others on the nonparetic leg. The goal of this study was to assess whether analyzing propulsion asymmetry can help distinguish between improved paretic leg coordination versus nonparetic leg compensation.
Methods
Three-dimensional forward dynamics simulations were developed for two post-stroke hemiparetic subjects walking at identical speeds before/after rehabilitation with opposite changes in propulsion asymmetry. Changes in the individual muscle contributions to forward propulsion were examined.
Findings
The major source of increased forward propulsion in both subjects was from the ankle plantarflexors. How they were utilized differed and appears related to changes in propulsion asymmetry. Subject A increased propulsion generated from the paretic plantarflexors, while Subject B increased propulsion generated from the nonparetic plantarflexors. Each subject’s strategy to increase speed also included differences in other muscle groups (e.g. hamstrings) that did not appear related to propulsion asymmetry.
Interpretation
The results of this study highlight how speed cannot be used to elucidate underlying muscle coordination changes following rehabilitation. In contrast, propulsion asymmetry appears to provide insight into changes in plantarflexor output affecting propulsion generation and may be useful in monitoring rehabilitation outcomes.
Keywords: Forward dynamics simulations, Gait, Post-stroke hemiparesis, Rehabilitation
1. Introduction
Stroke is a leading cause of long-term disability in the United States (Roger et al., 2012) that often leaves survivors with various levels of hemiparesis. Due to altered muscle coordination (e.g., Den Otter et al., 2007; Turns et al., 2007), post-stroke hemiparetic subjects typically walk at slow walking speeds with asymmetrical gait patterns (e.g., Olney and Richards, 1996). Previous studies of healthy walking have found that proper muscle coordination is critical for successfully generating the necessary biomechanical functions to walk (Neptune et al., 2001; Neptune et al., 2004). Thus, to improve walking performance, post-stroke rehabilitation programs should focus on improving muscle coordination. However, the effects of stroke are heterogeneous and specific muscle coordination impairments vary within the post-stroke hemiparetic population (e.g., De Quervain et al., 1996; Knutsson and Richards, 1979; Shiavi et al., 1987).
A common measure of rehabilitation effectiveness is self-selected walking speed as it is closely related to quality of life (Perry et al., 1995; Schmid et al., 2007). Several recent modeling studies have investigated muscle coordination in hemiparetic subjects walking at different speeds (Hall et al., 2011; Peterson et al., 2010) and found that limited community walkers (self-selected walking speed between 0.4 and ≥ 0.8 m/s) have reduced paretic leg plantarflexor contributions to forward propulsion, while community walkers (speed 0.8 m/s) have decreased paretic leg hip flexor contributions to swing initiation (Peterson et al., 2010). In addition, compared to limited community walkers, community walkers have increased paretic leg contributions to both forward propulsion and swing initiation (Hall et al., 2011). However, subjects may achieve the same walking speed using different strategies, with increases in walking speed achieved through different mechanisms (e.g., output from the paretic leg or increased reliance on the nonparetic leg). Therefore, speed alone does not provide adequate information regarding underlying muscle coordination impairments.
An important factor in producing walking speed is generating sufficient propulsion, which acts to propel the body center-of-mass (COM) forward. In non-impaired walking, the plantarflexors are critical for generating propulsion (Liu et al., 2008; Neptune et al., 2001) and in the post-stroke hemiparetic population the paretic leg plantarflexors are often impaired (e.g., Lamontagne et al., 2007; Turns et al., 2007), resulting in altered propulsion generation. The asymmetry in propulsion generated between the paretic and nonparetic legs (percent of paretic propulsion, PP, paretic / paretic + nonparetic propulsion) is considered to be representative of the coordinated output of the paretic leg due to its relatively strong correlation with hemiparetic severity (Bowden et al., 2006). While PP was found to also have a weaker relationship to walking speed (r = 0.55), subjects walking at the same speed exhibited a variety of asymmetry values, with some subjects relying more on the paretic leg and others on the nonparetic leg to generate the same speed. This variability is likely due to the use of different underlying coordination strategies. Therefore, analyzing PP throughout rehabilitation may provide information that distinguishes improved paretic leg coordination versus compensation by the non-paretic leg (i.e., increased output from the paretic (nonparetic) leg would increase (decrease) PP).
If PP is a good indicator of changes in underlying muscle coordination post-stroke, we believe that it may also prove to be useful in developing and monitoring rehabilitation progress. Therefore, the goal of this study was to assess the potential of PP to provide information regarding underlying changes in muscle coordination and function as a result of rehabilitation. Specifically, forward dynamic simulations were performed for two post-stroke hemiparetic subjects who walked at identical speeds pre-rehabilitation, had identical speed increases following rehabilitation, but had approximately equal but opposite changes in their PP values (i.e., from asymmetric to symmetric versus from symmetric to asymmetric). Since the plantarflexors are the primary contributors to forward propulsion (Liu et al., 2006; Liu et al., 2008; Neptune et al., 2001), we hypothesized that changes in propulsion asymmetry following rehabilitation would be associated with changes in plantarflexor function.
2. Methods
2.1 Subjects
The subjects analyzed were a subset a larger study of chronic post-stroke hemiparetic subjects at the VA Brain Rehabilitation Research Center at the University of Florida (Bowden et al., 2012). Each subject signed an institutionally approved informed consent and protocol. Two subjects were chosen who walked at the same speed pre-rehabilitation with identical post-rehabilitation speed increases, but had opposite changes in their values of PP (increased versus decreased; Table 1).
Table 1.
Subject demographics and clinical measures of function and performance. (BBS = Berg Balance Scale, DGI = Dynamic Gait Index, FM- L = Fugl-Meyer Lower Extremity Score, PP = Percent Paretic Propulsion)
| Subject | Age | Sex | Affected Side | Time since stroke (months) | BBS | DGI | FM-L | Speed (m/s) | PP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||
| A | 68 | M | L | 17 | 46 | 52 | 15 | 14 | 23 | 22 | 0.8 | 1.1 | 0.43 | 0.51 |
| B | 56 | M | L | 12 | 50 | 52 | 17 | 16 | 24 | 29 | 0.8 | 1.1 | 0.50 | 0.40 |
2.2 Rehabilitation
Each subject completed a 12-week rehabilitation training program that consisted of therapy three times a week with 20 minutes of actual stepping using a body weight supported treadmill modality (Hesse et al., 1995; Plummer et al., 2007; Visintin et al., 1998) followed by 20 minutes of immediate translation of skills acquired during walking on the treadmill to overground walking (as described in Bowden et al., 2012; Duncan et al., 2007). Training began with 40% of body weight support (BWS) and progressed as tolerated across sessions to no BWS when possible. Training took place between 2.0 and 3.0 mph with manual assistance provided by physical therapists at the hip and/or lower legs to approximate normal trunk, pelvis and lower-limb limb kinematics as well as the spatial-temporal patterns of walking (Bowden et al., 2012; Duncan et al., 2007).
2.3 Experimental data
Each subject walked for 30s on a split-belt instrumented treadmill (Techmachine) at their fastest comfortable speed pre- and post-rehabilitation (Table 1). A safety harness mounted to the laboratory ceiling was worn across the shoulders to protect the subject in case of a loss of balance. One or more practice trials were performed to ensure subjects were comfortable with the setup. Subjects walked approximately 10s prior to data collection to ensure a steady-state walking pattern had been reached. A modified Helen-Hayes reflective marker set was recorded using a 12-camera motion analysis system (Vicon Motion Systems) to capture bilateral 3D kinematics at 100 Hz. A 16-channel electromyography (EMG) system (Konigsburg Instruments) was used to record bilaterally at 2000 Hz from the medial gastrocnemius, soleus, tibialis anterior, rectus femoris, vastus lateralis, biceps femoris long head, semimebranosus, and gluteus medius. Bilateral 3D ground reaction forces (GRFs) were collected at 2000Hz.
Visual 3D (C-Motion) was used to process all data. Marker and GRF data were low-pass filtered using a 4th-order Butterworth filter with cutoff frequencies of 6 Hz and 20 Hz, respectively. EMG was high-pass filtered with a cutoff frequency of 40 Hz, de-meaned and low-pass filtered with a cutoff frequency of 10 Hz. PP was calculated as the propulsion generated by the paretic leg divided by the total propulsion generated by both legs, where propulsion was calculated as the time integral of the positive anterior-posterior (AP) GRFs generated by the leg. The individual gait cycle for each subject with the minimum difference in kinematics and GRFs compared to that subject’s average data was then used as the simulation tracking data.
2.4 Musculoskeletal model
A previously described 3D musculoskeletal model (Allen and Neptune, 2012) with 23 degrees-of-freedom was developed using SIMM (Musculographics, Inc.) and included rigid segments representing the trunk, pelvis and two legs (thigh, shank, talus, calcaneus and toes). The pelvis had six degrees-of-freedom (3 translations, 3 rotations) with the trunk and hip joints modeled using spherical joints. The knee, ankle, subtalar and metatarsophalangeal joints were modeled as single degree-of-freedom revolute joints. Foot-ground contact forces were modeled with 31 independent visco-elastic elements attached to each foot (Neptune et al., 2000). Passive torques representing forces applied by ligaments, passive tissue and joint structures were applied at each joint (Anderson, 1999). The dynamical equations-of-motion were generated using SD/FAST (PTC).
The model was driven by 38 Hill-type musculotendon actuators per leg that were combined into 34 muscle groups based on anatomical classification (Table 2), with muscles within each group receiving the same excitation pattern. Each group was excited by either one or two Gaussian excitation patterns with timing constrained based on the recorded EMG. For those muscles in which EMG were not recorded, broad timing constraints were based on published EMG data (Perry, 1967; Sutherland, 2001). A first-order differential equation was used to represent activation dynamics (Raasch et al., 1997), with activation and deactivation time constants derived from Winters and Stark (1988).
Table 2.
The 38 muscles on each leg were combined into 34 groups, with muscles within each group receiving the same excitation, and then into 18 groups when analyzing muscle function. Excitation patterns for each group consisted of one or two blocks (paretic, nonparetic when two values listed).
| Muscle name | Analysis Group | Excitation group | # Excitation Blocks | |
|---|---|---|---|---|
| Subject 1 | Subject 2 | |||
| Iliacus | IL | IL | 1 | 1 |
| Psoas | IL | IL | 1 | 1 |
|
| ||||
| Adductor Longus | AL | AL | 1 | 1 |
| Adductor Brevus | AL | AB | 1 | 1 |
| Pectineus | AL | PECT | 1 | 1 |
|
| ||||
| Quadratis Femoris | QF | QF | 1 | 1 |
|
| ||||
| Adductor Magnus 1 | AM | AM1 | 1 | 1 |
| Adductor Magnus 2 | AM | AM2 | 1 | 1 |
| Adductor Magnus 3 | AM | AM3 | 1 | 1 |
|
| ||||
| Sartorius | SAR | SAR | 1 | 1 |
|
| ||||
| Rectus Femoris | RF | RF | 2 | 2 |
|
| ||||
| Vastus Medialis | VAS | MVAS | 1 | 1 |
| Vastus Lateralis | VAS | LVAS | 1 | 1 |
| Vastus Intermedialis | VAS | LVAS | 1 | 1 |
|
| ||||
| Gluteus Medius 1 | GMED | GMED1 | 2 | 2,1 |
| Gluteus Medius 2 | GMED | GMED2 | 2 | 2,1 |
| Gluteus Medius 3 | GMED | GMED3 | 2 | 2,1 |
|
| ||||
| Gluteus Minimus 1 | GMIN | GMIN1 | 2 | 2,1 |
| Gluteus Minimus 2 | GMIN | GMIN2 | 2 | 2,1 |
| Gluteus Minimus 3 | GMIN | GMIN3 | 2 | 2,1 |
|
| ||||
| Piriformis | PIRI | PIRI | 2 | 2,1 |
|
| ||||
| Gemellus | GEM | GEM | 2 | 2,1 |
|
| ||||
| Tensor Fascia Lata | TFL | TFL | 1 | 1 |
|
| ||||
| Gluteus Maximus 1 | GMAX | GMAX1 | 1 | 1 |
| Gluteus Maximus 2 | GMAX | GMAX2 | 1 | 1 |
| Gluteus Maximus 3 | GMAX | GMAX3 | 1 | 1 |
|
| ||||
| Semitendinosus | HAM | MH | 1,2 | 1,2 |
| Semimembranosus | HAM | MH | 1,2 | 1,2 |
| Gracilis | HAM | GRAC | 1,2 | 1,2 |
| Biceps Femoris Long Head | HAM | BFLH | 1,2 | 1 |
|
| ||||
| Biceps Femoris Short Head | BFSH | BFSH | 1 | 1 |
|
| ||||
| Medial Gastrocnemius | GAS | MGAS | 1 | 1 |
| Lateral Gastrocnemius | GAS | LGAS | 1 | 1 |
|
| ||||
| Soleus | SOL | SOL | 1 | 1 |
| Tibialis Posterior | SOL | TP | 1 | 1 |
| Flexor Digitorum Longus | SOL | FDL | 1 | 1 |
|
| ||||
| Tibialis Anterior | TA | TA | 1 | 1 |
| Extensor Digitorum Longus | TA | TA | 1 | 1 |
2.5 Dynamic optimization
Simulations were generated from paretic mid-stance to non-paretic toe-off in order to capture both the paretic and nonparetic propulsive phases of gait. A simulated annealing algorithm (Goffe et al., 1994) fine-tuned the muscle excitation patterns and initial joint velocities such that the difference between the simulated and experimentally measured walking data was minimized. Quantities included in the cost function were differences in 3D pelvis translations, trunk, pelvis, hip, knee and ankle joint angles and GRFs.
2.6 Analysis of muscle function
A previously described ground reaction force decomposition (Neptune et al., 2001) was performed to quantify individual muscle contributions to forward propulsion (through the GRF). The contribution of each muscle to forward propulsion (braking) was calculated as the time integral of the positive (negative) GRF in the AP direction resulting from that muscle’s activity during the paretic and nonparetic propulsive phases, where a propulsive phase consists of late single leg stance and the double support immediately preceding swing. Muscles on each leg were combined into 18 groups based on similar anatomical and functional classification for the simulation analyses (Table 2).
3. Results
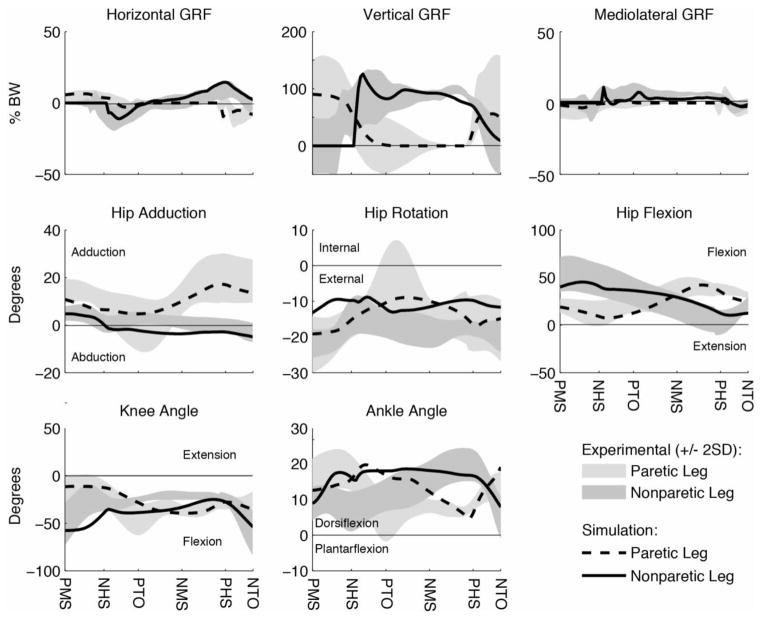
Each simulation emulated well the experimental tracking data with the average kinematic and GRF deviation generally within two standard deviations (2SD) of the experimental data (Fig. 1): 4.32° (experimental 2SD = 5.92°) and 4.83% body weight (BW, experimental 2SD = 13.44% BW) for Subject A pre-rehabilitation; 4.49° (experimental 2SD = 6.78°) and 5.86% BW (experimental 2SD = 11.70% BW) for Subject A post-rehabilitation; 4.00° (experimental 2SD = 4.82°) and 4.17% BW (experimental 2SD = 6.48% BW) for Subject B pre-rehabilitation; and 4.01° (experimental 2SD = 3.74°) and 4.54% BW (experimental 2SD = 5.02% BW) for Subject B post-rehabilitation.
Figure 1.
Example comparison plot of simulated and experimental data (see supplementary data for all comparison plots). Simulations were generated from paretic mid-stance to nonparetic toe-off. The paretic (nonparetic) propulsive phase was defined as paretic (nonparetic) mid-stance to paretic (nonparetic) toe-off. Abbreviations: PMS = paretic mid-stance; NHS = Nonparetic heel-strike; PTO = paretic toe-off; NMS = nonparetic mid-stance; PHS = paretic heel-strike; NTO = nonparetic toe-off.
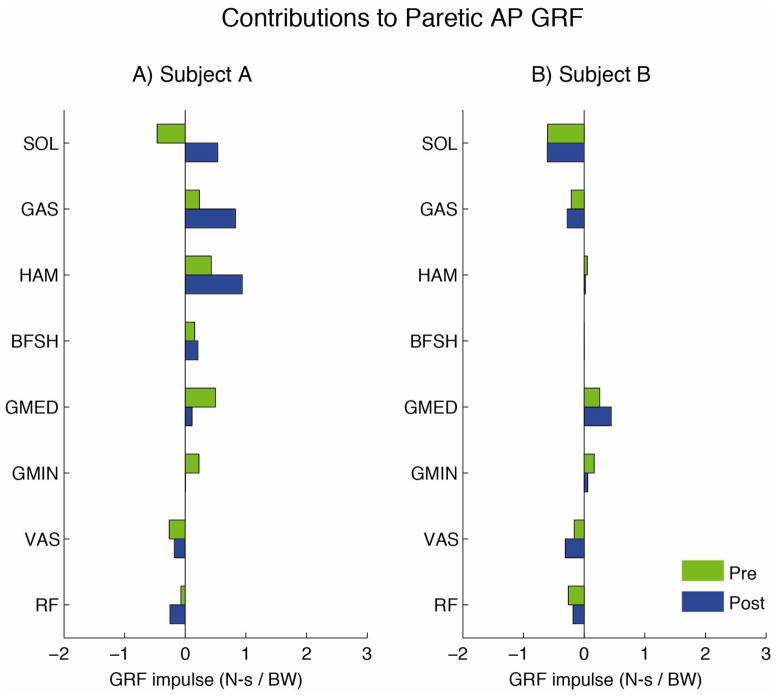
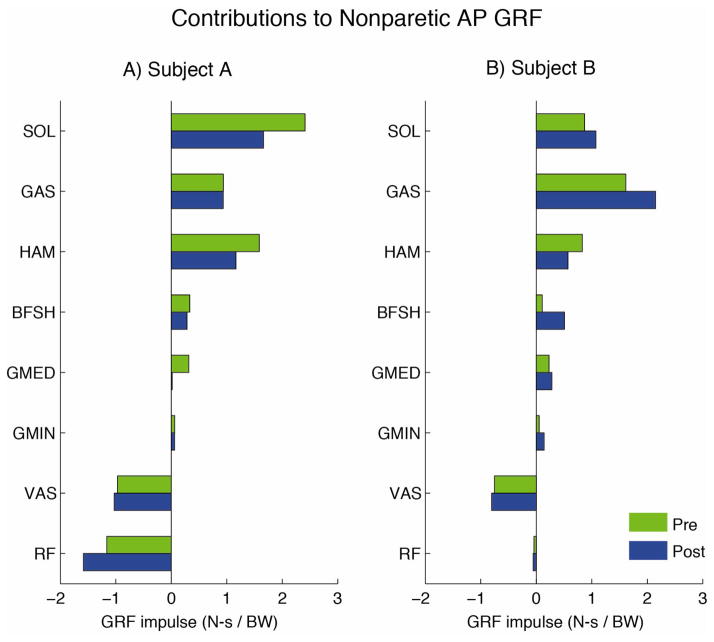
As expected, the major contributors to the AP GRFs during the paretic (nonparetic) propulsive phase were the paretic (nonparetic) leg muscles. Contributions from the muscles of the nonparetic (paretic) leg to the paretic (nonparetic) GRF during the paretic (nonparetic) propulsion phase were minimal and are not presented.
3.1 Subject A (PP increased with rehabilitation)
The major contributors to forward propulsion during the paretic propulsive phase before rehabilitation were the paretic plantarflexors (GAS), hamstrings (HAM and BFSH) and hip extensors/abductors (GMED and GMIN, Fig. 2A). Both GAS and HAM had increased contributions to the AP GRFs after rehabilitation, while GMED and GMIN had decreased contributions. The major sources of braking during the paretic propulsive phases before rehabilitation were the paretic uniarticular plantarflexors (SOL), and the uniarticular (VAS) and biarticular (RF) knee extensors (Fig. 2A). After rehabilitation, when PP increased, SOL no longer generated braking and was instead a major source of forward propulsion. The biarticular RF had increased contributions to braking after rehabilitation while the uniarticular VAS had similar contributions before and after rehabilitation.
Figure 2.
Contributions of muscles on the paretic leg to the anterior-posterior ground reaction forces during the paretic propulsive phase for A) Subject A and B) Subject B.
The nonparetic plantarflexors (SOL and GAS), hamstrings (HAM and BFSH) and hip extensors/abductors (GMED) were the major sources of forward propulsion during the nonparetic propulsive phase before rehabilitation (Fig. 3A). After rehabilitation, the nonparetic plantarflexors either had a decreased contribution (SOL) or remained unchanged (GAS). Both HAM and GMED had decreased contributions after rehabilitation. The major source of braking during the nonparetic propulsive phase before rehabilitation was the knee extensors (Fig. 3A). The contribution from the biarticular RF increased while the contribution from the uniarticular VAS remained the same after rehabilitation.
Figure 3.
Contributions of muscles on the nonparetic leg to the anterior-posterior ground reaction forces during the nonparetic propulsive phase for A) Subject A and B) Subject B.
3.2 Subject B (PP decreased with rehabilitation)
The major contributors to forward propulsion during the paretic propulsive phase before rehabilitation were the paretic hip abductors/extensors (GMED and GMIN, Fig. 2B). GMED had an increased contribution and GMIN a decreased contribution after rehabilitation. The plantarflexor (SOL and GAS) and knee extensor (VAS and RF) were both major contributors to braking before rehabilitation and had only minor changes after rehabilitation when PP worsened (Fig. 2B).
The nonparetic plantarflexors (SOL and GAS), hamstrings (HAM and BFSH), and hip abductors/extensors (GMED and GMIN) all contributed to forward propulsion during the nonparetic propulsive phase before rehabilitation (Fig. 3B). All muscle groups had an increased contribution to forward propulsion after rehabilitation except for HAM, which had a small decrease. The major source of braking during the nonparetic propulsive phase was the nonparetic uniarticular VAS, which had a similar contribution before and after rehabilitation (Fig. 3B).
4. Discussion
While increased walking speed is a primary goal post-stroke (Bohannon et al., 1988), speed can be increased using different strategies (Olney et al., 1998), and therefore walking speed alone cannot be used to direct rehabilitation towards improving specific gait deficits. In order to assess how walking speed was improved and to evaluate the effectiveness of rehabilitation efforts in improving coordination, additional measures are needed to identify the underlying coordination mechanisms. Therefore, the purpose of this study was to assess the potential of PP, a measure of asymmetry in the generation of forward propulsion between legs, to identify underlying changes in muscle coordination following rehabilitation. Specifically, two subjects were analyzed using musculoskeletal simulations that walked at the same speed pre-rehabilitation and had identical speed increases following rehabilitation yet exhibited opposite changes in PP. The results show that the increases in speed were achieved through different coordination mechanisms of which some appear strongly related to the changes in PP.
A major source of increased forward propulsion in both subjects was the plantarflexors, which is consistent with previous studies showing the plantarflexors to be a primary contributor to forward propulsion (i.e., Liu et al., 2008; McGowan et al., 2008; Neptune et al., 2001) and a mechanism for attaining higher walking speeds in both non-impaired (Liu et al., 2008; Neptune et al., 2008) and post-stroke hemiparetic walking (Hall et al., 2011; Olney et al., 1994; Parvataneni et al., 2007). However, the extent to which the plantarflexors were utilized to increase forward propulsion differed between subjects despite their identical increase in walking speed. Subject A increased the propulsion generated from the paretic leg plantarflexors (SOL and GAS, Fig. 3A), while Subject B increased the propulsion generated from the nonparetic leg plantarflexors (Fig. 3B). In agreement with our hypothesis, these opposite changes in plantarflexor output between legs were consistent with the opposite changes in PP that accompanied the speed increases following rehabilitation in each subject (Table 1); an increase in PP (i.e., more reliance on the paretic leg) was accompanied with increased output from the paretic leg plantarflexors (Subject A) whereas a decrease in PP (i.e., increased reliance on the nonparetic leg) was accompanied with an increased output from the nonparetic leg plantarflexors (Subject B). This suggests that changes in PP may be useful in monitoring the effectiveness of rehabilitation to improve muscle coordination by providing information regarding changes in plantarflexor coordination and function.
Interestingly, the paretic leg soleus contributed to braking instead of forward propulsion in both subjects before rehabilitation, but only Subject A had an improvement in muscle coordination after rehabilitation such that both plantarflexors contributed to forward propulsion. While the soleus is a primary generator of forward propulsion during non-impaired walking (Liu et al., 2008; Neptune et al., 2004), the fact that it can contribute to braking in individuals with post-stroke hemiparesis may be a common impairment mechanism. For example, a similar result was seen in a recent simulation study that examined the function of the ankle plantarflexors in several individuals with post-stroke hemiparesis before and after rehabilitation (Knarr et al., 2013). Knarr et al. found that in almost all individuals the paretic leg plantarflexors generated braking during pre-swing by acting to decelerate the center-of-mass before rehabilitation and in only a few individuals did they switch to generating forward propulsion following rehabilitation. The fact that only some individuals improve paretic plantarflexor contributions to forward propulsion highlights how a particular rehabilitation intervention does not necessarily induce the same improvement in coordination mechanisms in all subjects.
Thus, while PP appears to be a promising measure to determine whether a subject is relying more on the paretic or nonparetic leg plantarflexors to increase speed throughout rehabilitation, an interesting question remains as to what predisposes an individual to use one mechanism over another. Many studies have reported an increase in metabolic cost in hemiparetic populations compared to healthy walkers (Bard, 1963; Detrembleur et al., 2003; Zamparo et al., 1995). One reason individuals may utilize different strategies is to minimize this cost, which likely depends on an individual’s specific impairments and their remaining neuromuscular resources. In addition, rehabilitation may not be able to influence all impairments (e.g. contractures, disrupted neural pathways) that affect the resources on which an individual can draw when improving walking through rehabilitation. Because the effects of stroke are highly variable, the differences in functional resources available and the types of impairments present may dictate the strategy used to increase speed throughout rehabilitation and measures other than PP may be necessary to identify these types of impairments.
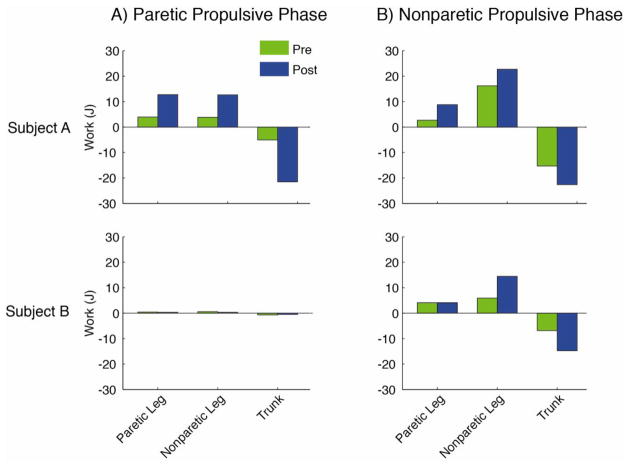
In addition to using different strategies at the level of the ankle plantarflexors, each subject’s strategy to increase speed included differences in other muscle groups, which do not appear related to PP. For example, while the increase in the paretic leg hamstrings contribution to forward propulsion in Subject A is consistent with this subject’s increased reliance on the paretic leg after rehabilitation, Subject B did not utilize the paretic hamstrings for propulsion generation (Fig. 2). Further, while only Subject A relied on the paretic leg hamstrings to generate forward propulsion, both subjects utilized the nonparetic leg hamstrings for forward propulsion generation (Fig. 3). This suggests that PP may not be useful to elucidate changes in hamstrings function throughout rehabilitation. In non-impaired walking the hamstrings are typically active only into early stance, and therefore do not generate propulsion during late stance (Neptune et al., 2004). Prolonged activity of both the paretic (Den Otter et al., 2007; Knutsson and Richards, 1979) and nonparetic (Raja et al., 2012) hamstrings later into stance has previously been documented post-stroke. This prolonged duration may represent a compensatory mechanism to overcome plantarflexor weakness that does not appear related to PP. A post-hoc segmental power analysis (Neptune et al., 2004) revealed that hamstrings absorb energy from the trunk and deliver energy to the legs (Fig. 4). Energy delivered to the leg prior to swing is critical for initiating leg swing and increasing energy delivered to the leg prior to swing can improve swing time and/or step length such that walking speed is increased. Therefore, measures more focused on leg swing, such as the asymmetry in swing time and/or step length, may be more appropriate for identifying reliance on compensatory mechanisms related to leg swing.
Figure 4.
Energy delivered to the paretic leg, nonparetic leg and trunk from the A) paretic hamstrings (HAM and BFSH) during the paretic propulsive phase and B) nonparetic hamstrings (HAM and BFSH) during the nonparetic propulsive phase.
A potential limitation of this study is that that model parameters were based on quantities found in healthy individuals while muscle weakness, spasticity, and joint stiffness, common among post-stroke hemiparetic individuals (Olney and Richards, 1996), were not included. For example, stroke survivors often have shorter optimal fiber length and increased muscle stiffness (Gao et al., 2009) that alters the intrinsic force-length relationship. However, the optimization scales the excitation magnitudes such that the total force generated by each muscle remains unaffected, thus compensating for any differences in muscle parameters. In addition, to counteract the problem of muscle redundancy in solving for the optimal coordination pattern, we constrained the muscle excitation timing in the optimization to closely match measured EMG. Further, the results of this study are specific to subjects with coordination similar to the two subjects studied, and due to the heterogeneity of the post-stroke hemiparetic population, may not generalize to other subjects with similar changes in PP but different coordination mechanisms. However, due to the importance of the plantarflexors in generating forward propulsion, we expect that the relationship between PP and plantarflexor output would be reasonably consistent across the post-stroke population. Finally, calculating PP requires expensive force-plates and technical staff that are unlikely to be available in most clinical settings. Translating these findings to the clinic may require the use of less-expensive devices that can be implemented by the clinicians, such as accelerometers that can approximate whole-body COM, and further research is needed to determine if such devices can serve as a proxy for PP. Nevertheless, we believe that PP is a useful measure to assess in research labs for novel rehabilitation programs that are designed to improve plantarflexor recruitment and function.
5. Conclusions
Speed alone cannot elucidate underlying muscle coordination changes following rehabilitation and the percent of paretic propulsion may provide some of this missing information. Specifically, percent of paretic propulsion can provide insight into changes in plantarflexor output affecting propulsion generation. However, other coordination changes cannot be explained by this measure, such as compensatory mechanisms that utilize the hip muscles to alter leg swing control. Future work should examine whether different measures of asymmetry can be used to identify changes in coordination related to leg swing control, such as asymmetry in step lengths or swing times.
Supplementary Material
Highlights.
We analyzed propulsion asymmetry in post-stroke subjects pre/post rehabilitation.
Modeling and simulation was used to identify muscle contributions to propulsion.
Propulsion asymmetry appears to provide insight into changes in plantarflexor output.
Propulsion asymmetry may be useful in monitoring rehabilitation outcomes.
Walking speed cannot be used to elucidate underlying muscle coordination changes.
Acknowledgments
The authors would like to thank Dr. Mark Bowden and the intervention research team for their contributions to the data collection and processing. This project was supported by NIH grant R01 NS55380, the Rehabilitation Research & Development Service of the VA, and the NSF Graduate Research Fellowship Program.
Footnotes
The comments are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NICHD, VA or NSF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JL, Neptune RR. Three-dimensional modular control of human walking. J Biomech. 2012;45(12):2157–63. doi: 10.1016/j.jbiomech.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson FC. Doctoral Dissertation. The University of Texas; Austin: 1999. A dynamic optimization solution for a complete cycle of normal gait. [Google Scholar]
- Bard G. Energy expenditure of hemiplegic subjects during walking. Arch Phys Med Rehabil. 1963;44:368–70. [PubMed] [Google Scholar]
- Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. International Journal of Rehabilitation Research. 1988;11(2):181–184. [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37(3):872–6. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor Rehabilitation of Individuals with Chronic Stroke: Difference between Responders and Non-Responders. Arch Phys Med Rehabil. 2012 doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78(10):1506–14. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2007;25(3):342–52. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait & Posture. 2003;18(2):47–55. doi: 10.1016/s0966-6362(02)00193-5. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007:7–39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009;90(5):819–26. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Goffe WL, Ferrier GD, Rogers J. Global optimization of statistical functions with simulated annealing. J Econometrics. 1994;60(1–2):65–99. [Google Scholar]
- Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech. 2011;26(5):509–15. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse S, Bertelt C, Jahnke MT, Schaffrin A, Baake P, Malezic M, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26(6):976–81. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- Knarr BA, Kesar TM, Reisman DS, Binder-Macleod SA, Higginson JS. Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors. J Neuroeng Rehabil. 2013:10–12. doi: 10.1186/1743-0003-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102(2):405–30. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- Lamontagne A, Stephenson JL, Fung J. Physiological evaluation of gait disturbances post stroke. Clin Neurophysiol. 2007;118(4):717–29. doi: 10.1016/j.clinph.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. J Biomech. 2006;39(14):2623–30. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. J Biomech. 2008;41(15):3243–52. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Kram R. Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J Appl Physiol. 2008;105(2):486–94. doi: 10.1152/japplphysiol.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture. 2008;28(1):135–43. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Wright IC, Van Den Bogert AJ. A Method for Numerical Simulation of Single Limb Ground Contact Events: Application to Heel-Toe Running. Comput Methods Biomech Biomed Engin. 2000;3(4):321–334. doi: 10.1080/10255840008915275. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19(2):194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74(9):872–85. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Multivariate examination of data from gait analysis of persons with stroke. Phys Ther. 1998;78(8):814–28. doi: 10.1093/ptj/78.8.814. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture. 1996;4(2):136–148. [Google Scholar]
- Parvataneni K, Olney SJ, Brouwer B. Changes in muscle group work associated with changes in gait speed of persons with stroke. Clin Biomech (Bristol, Avon) 2007;22(7):813–20. doi: 10.1016/j.clinbiomech.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Perry J. The mechanics of walking. A clinical interpretation. Phys Ther. 1967;47(9):778–801. doi: 10.1093/ptj/47.9.778. [DOI] [PubMed] [Google Scholar]
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43(12):2348–55. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer P, Behrman AL, Duncan PW, Spigel P, Saracino D, Martin J, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21(2):137–51. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum-speed pedaling. J Biomech. 1997;30(6):595–602. doi: 10.1016/s0021-9290(96)00188-1. [DOI] [PubMed] [Google Scholar]
- Raja B, Neptune RR, Kautz SA. Coordination of the non-paretic leg during hemiparetic gait: Expected and novel compensatory patterns. Clin Biomech (Bristol, Avon) 2012;27(10):1023–30. doi: 10.1016/j.clinbiomech.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2012 update: A report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- Shiavi R, Bugle HJ, Limbird T. Electromyographic gait assessment, Part 2: Preliminary assessment of hemiparetic synergy patterns. J Rehabil Res Dev. 1987;24(2):24–30. [PubMed] [Google Scholar]
- Sutherland DH. The evolution of clinical gait analysis part l: kinesiological EMG. Gait Posture. 2001;14(1):61–70. doi: 10.1016/s0966-6362(01)00100-x. [DOI] [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88(9):1127–35. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122–8. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- Winters JM, Stark L. Estimated mechanical properties of synergistic muscles involved in movements of a variety of human joints. J Biomech. 1988;21(12):1027–41. doi: 10.1016/0021-9290(88)90249-7. [DOI] [PubMed] [Google Scholar]
- Zamparo P, Francescato MP, De Luca G, Lovati L, di Prampero PE. The energy cost of level walking in patients with hemiplegia. Scand J Med Sci Sports. 1995;5(6):348–52. doi: 10.1111/j.1600-0838.1995.tb00057.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.