Abstract
The amygdala is a key brain region with efferent and afferent neural connections that involve complex behaviors such as pain, reward, fear and anxiety. This study evaluated resting state functional connectivity of the amygdala with cortical and subcortical regions in a group of chronic pain patients (pediatric complex regional pain syndrome) with age-gender matched controls before and after intensive physical-biobehavioral pain treatment. Our main findings include (1) enhanced functional connectivity from the amygdala to multiple cortical, subcortical, and cerebellar regions in patients compared to controls, with differences predominantly in the left amygdala in the pre-treated condition (disease state); (2) dampened hyperconnectivity from the left amygdala to the motor cortex, parietal lobe, and cingulate cortex after intensive pain rehabilitation treatment within patients with nominal differences observed among healthy controls from Time 1 to Time 2 (treatment effects); (3) functional connectivity to several regions key to fear circuitry (prefrontal cortex, bilateral middle temporal lobe, bilateral cingulate, hippocampus) correlated with higher pain-related fear scores and (4) decreases in pain-related fear associated with decreased connectivity between the amygdala and the motor and somatosensory cortex, cingulate, and frontal areas. Our data suggest that there are rapid changes in amygdala connectivity following an aggressive treatment program in children with chronic pain and intrinsic amygdala functional connectivity activity serving as a potential indicator of treatment response.
Keywords: Fear, Children, Chronic Pain, Treatment Response, fMRI, Brain, Neuropathic
Introduction
Chronic pain is a complex experience that involves several neural networks tied to sensation, motor activity, cognition, and emotion. Brain regions often associated with the pain experience include the primary and secondary somatosensory cortex (S1 and S2), spinal cord, thalamus, insula, anterior cingulate cortex, prefrontal cortex [3,60,78], midbrain areas including the periaqueductal gray [44] and cerebellum [52], and subcortical structures including the hippocampus, basal ganglia, and amygdala [9,48,65,72]. There is a growing interest in the cognitive and emotional aspects of pain [10] with the amygdala emerging as a key region of interest [72].
A role for the amygdala in pain processing has been derived from a number of preclinical and clinical investigations. Preclinical data has provided a number of insights into the role of the amygdala in pain including: (i) a relay station for afferent nociceptive information from the parabrachial nucleus [6,20]; (ii) negative affective states [33,54]; (iii) increased excitability and plasticity in the amygdala in chronic pain [27]; and (iv) lesions inhibiting the development of chronic pain [41]. Clinical data on the amygdala’s role in pain have been derived mainly from neuroimaging. While a number of human studies have implicated the amygdala in acute pain [5,8,58,59] and emotional disorders, such as anxiety [18] and depression [69], alterations in amygdala connectivity in chronic pain has been of recent scientific interest [72]. Alterations in amygdala connectivity have been identified among adult migraine [23], adult fibromyalgia [31], and adult irritable bowel syndrome [37] patients. Pain-induced amygdala hyperconnectivity has also been found in pediatric patients with complex regional pain syndrome (CRPS) that persists after symptom resolution [42]. These findings consistently suggest alterations in emotional-arousal circuitry among patients with chronic pain syndromes.
The aim of the present study was to characterize and compare functional connectivity of the amygdala with the rest of the brain in pediatric CPRS and healthy controls. Our group has previously reported that pediatric CRPS is highly responsive to intensive physical-biobehavioral treatments [45]. Based on this finding and recent reports of resting state network connectivity changes that occur with chronification of pain [26] and treatment [53] functional connectivity changes in the amygdala before and after intensive physical-biobehavioral treatment compared with age- and gender-matched healthy controls was examined. Using a relatively rapid treatment paradigm to examine the role of the amygdala in CRPS, we hypothesized to find (i) significant differences in functional connectivity in CRPS vs. Controls (Disease Effect); and (ii) a normalization in functional connectivity in CRPS patients following their treatment (Treatment Effect). In addition, given that the amygdala is involved in fear [39,56], we hypothesized that the psychological measure of fear avoidance in pain [73] correlates with the synchronicity of activity across typical fear networks (amygdala-insula connection, amygdala-anterior cingulate connection [19].
Methods and Materials
Subjects
Twelve CRPS patients between the ages of 10–18 years with unilateral CRPS of the lower extremity were recruited from an intensive interdisciplinary pediatric pain rehabilitation program (see Table 1). Twelve healthy control controls were individually age- and gender matched. Selection Criteria: (i) for patients, diagnosis of CRPS based on the Budapest Criteria [24] as determined by an experienced pain physician on the basis of neurological examination and comprehensive record reviews; (ii) no other neurological, severe medical or psychiatric problems (e.g., active suicidality); (iii) absence of magnetic implants of any type; (iv) no current pregnancy; (v) no history of claustrophobia; (vi) weight <285 lbs. (the limit of the MRI table); (vii) right handed.
Table 1.
Demographic and clinical characteristics of the CRPS patients.
| Patient | Gender | Age | Etiology | Pain location | Pain Duration (mo) | Medication |
|---|---|---|---|---|---|---|
| 1 | F | 17 | Knee injury | R knee | 2.5 | AC |
| 2 | F | 10 | Twisted ankle | R foot | 18.7 | AD |
| 3 | M | 11 | Foot injury | L foot | 8 | AC, AD |
| 4 | F | 15 | Crush injury foot | L ankle | 5 | AD |
| 5 | M | 15 | No known injury | R knee | 12 | AC, AD |
| 6 | F | 11 | Knee injury | L knee | 6.5 | AC, AD |
| 7 | F | 16 | Fractured distal fibula | L ankle | 48 | AD |
| 8 | F | 17 | Foot and knee injury | L ankle | 19 | AD |
| 9 | F | 14 | Sprained ankle | L foot | 2.5 | AC |
| 10 | M | 13 | Post surgery | L ankle | 6 | AC |
| 11 | F | 17 | Twisted ankle | R foot | 85 | AD |
| 12 | F | 13 | Ankle sprain | L foot | 13 | AC |
|
| ||||||
| Mean (SEM) | 14.1 (.72) | 18.9 (7.0) | ||||
R=right; L=left. AC, anti-convulsivants; AD, anti-depressants.
Procedure
The study was approved by the Boston Children’s Hospital institutional Review Board (IRB). Informed parental consent and participant’s assent were obtained at study enrollment. Participants attended two study sessions, at admission and at discharge from the Pediatric Pain Rehabilitation Center (PPRC) for patients and at a matched time interval for controls. No new medications were prescribed during treatment (i.e., each patient remained on the same pharmacological treatment as when they entered into the program). During each study session, participants underwent a focused neurological exam and MRI scanning.
PPRC Interdisciplinary Treatment
Patients referred to the PPRC from the multidisciplinary outpatient pain clinic have persistent pain with significant impairment of mobility and daily function. Admission was based on 1) problem duration or acuity and 2) inability to progress in outpatient treatment. PPRC enrollment was dependent upon 1) family willingness to enroll in the intensive program and 2) insurance provider coverage. Patients with active suicidality or current eating disorder were not eligible. The rehabilitation program entails intensive daily physical, occupational, and psychological therapies eight hours a day, five days per week for a typical length of stay of three weeks (for details see [45]). Patients received 3–4 hours of physical and occupational therapy sessions each day. Psychological treatment entails daily individual and group-based cognitive behavioral therapy and families are actively incorporated into the program, with family therapy and parent education provided. Psychological therapy targets include: 1) teaching a self-management approach to pain, 2) addressing negative thinking and fears about pain, 3) engaging in valued activities and relationships in the presence of pain, and 4) reducing parental attention and protective responses to pain [45]. A physician and nurse evaluate patients daily to ensure continued appropriateness of treatment (e.g., continued medical stability) and to address acute and/or ongoing medical issues.
Pain and Psychological Assessment
CRPS patients reported average pain ratings at admission and at discharge. On an 11-point numerical rating scale, scores between 0–3 were considered mild, 4–6 moderate, and > 7 severe [80]. CRPS patients also completed a battery of psychological measures at admission and discharge from the program, which included the Fear of Pain Questionnaire ((FOPQ); [73]). For the FOPQ, scores of > 40 are considered elevated and scores of > 51 clinically significant.
MRI acquisition and analysis
Data acquisition
Participants were scanned on a 3T Tim Trio (Siemens Medical, Erlangen, Germany) scanner using a 12-channel head coil. Anatomical: A 3D T1-weighted anatomical scan was acquired using a magnetization prepared rapid gradient echo (MPRAGE) sequence (128 sagittal slices; field of view = 256 × 256 mm; TR = 2100 ms; TE = 2.74 ms; TI = 1,100 ms; 1.33 × 1 × 1 mm voxels). Functional: A resting-state functional (f)MRI scan was acquired using a T2*-weighted echo-planar pulse imaging (EPI) sequence (41 slices; TR = 2.5 ms; TE = 30 ms; 64 × 64 matrix; 3 × 3 × 3 mm voxels). During the restingstate fMRI acquisition period, subjects were asked to remain awake with their eyes open and observed a blank screen. All scans were examined separately for excessive motion (> 3mm).
Structural and functional MRI analysis
All preprocessing, first-level and second-level group analyses were performed using FMRIB Software Library (FSL) http://www.fmrib.ax.ac.uk/fsl.
Preprocessing steps
For each subject, the following preprocessing steps were taken: (i) MPRAGE and EPI images were skull-stripped using the brain extraction tool (BET) [75]; (ii) functional images were B0 unwarped using FSL FUGUE; (iii) motion correction using FMRIB’s Linear Motion Correction (MCFLIRT ; (iv) spatial smoothing at 5 mm full-width at half maximum (FWHM); (v) affine registration of the resting state fMRI dataset to the Montreal Neurological Institute (MNI)-152 2mm template brain using FMRIB’s Linear Image Registration Tool [28,29]; and (vi) highpass temporal filtering (0.01 Hz). Low pass filtering was not included as patients with chronic pain have been observed to have oscillations beyond 0.1 Hz [49,55].
Amygdala time courses
Using the Juelich probabilistic brain atlas [2] and a probability threshold of 50%, masks for the left and right amygdala as separate regions of interest (ROI) were defined (see Figure 1S) as has been done in prior functional connectivity analyses of the amygdala [63]. The ROIs were converted from standard space to each subject’s native functional space and then binarized. Due to the risk of signal dropout in this specific region of the brain, seeds were refined using a whole brain binarized mask to eliminate regions with low signal intensity voxels. Subsequently, individual time courses were extracted.
Amygdala functional connectivity analysis
For each subject, GLM seed-region analyses using FSL FEAT were performed with the right and left amygdala simultaneously entered with WM, CSF, 6 motion parameters (i.e., 3 rotational and 3 translational), large motion artifact confound matrix (created using FSL Motion Outliers for motion <3mm) and added as variables of no interest. Once individual GLM FEAT analyses were completed, unpaired mixed-effects group analyses between patients and controls at Time 1 and paired mixed-effects group analyses from Time 1 (i.e., PPRC admission) to Time 2 (i.e., PPRC discharge) within patients and within controls were conducted for each seed region. The dependence between functional connectivity and fear of pain, after controlling for pain levels, was also examined within patients at Time 1 and Time 2. To analyze changes in the correlation of amygdala connectivity with pain related fear within patients from Time 1 to Time 2, a second level FEAT analysis of individual amygdala connectivity with FOPQ scores and pain level was performed. The analysis consisted of adding demeaned FOPQ scores and demeaned pain levels as explanatory variables to perform group comparisons of individual connectivity results with the amygdala across time points. Areas of significant positive results indicate a correlation of FOPQ scores with strength of connectivity with the amygdala, i.e., these brain areas have a reduced connectivity with the amygdala when the FOPQ scores were lower whereas negative results would suggest that higher FOPQ scores are associated weaker connectivity.
Gaussian mixture modeling
A seed based analysis, such as this one, is a massive univariate general linear model subject to excessive false positives if multiple comparison effects are not taken into consideration. If there are unmodeled signals or the model assumptions do not hold, the null distribution could be shifted and scaled [57]. In these cases, standard approaches to correct for multiple comparisons (e.g. Bonferroni, false-discovery-rate) are not applicable. Gaussian mixture modeling (GMM; [57]) deconstructs the overall statistical map into several optimized Gaussian distributions. This approach has been used previously for seed-based functional connectivity analyses [79]. Most statistical maps for these analyses indicated a non-zero mean and a standard deviation different from one, violating the assumption of normality and supporting the use of GMM for the current analysis. Thresholds were determined from a partition of the statistical map distribution using GMM conducted in MATLAB [1] to correct for multiple comparison effects as well as account for possible variations in the null distribution. Each voxel is associated with a vector of probabilities of association with each of the modeled Gaussian distributions. The map for the distribution associated with activation (or deactivation) was thresholded at a probability of p > 0.5 (meaning that the chances of a voxel belonging to that class was > 0.5); the corresponding z-value in the original z distribution was obtained from matching the center of the activation distribution and used as the threshold for determining activated voxels for each analysis (thus does not provide the standard p<.05 value).
Results
Participants
Six scans displayed excessive motion (> 3mm) resulting in the following final group numbers: Controls: Time 1 n=10, Time 2 n=10; Patients: Time 1 n=11, Time 2 n=11. For the covariate analysis, complete data on pain ratings and fear of pain symptoms were available for 10 CRPS patients.
Pain and Pain-related fear
Prior to treatment, 91% of patients reported moderate (73%) to severe (18%) average pain levels with 70% of patients reporting elevated or clinically significant pain-related fear (Figure 1). At the end of treatment, 55% reported moderate pain while the remaining patients reported only mild pain. Mean average pain ratings significantly decreased from 5.65 ± 0.54(SE) to 4.07 ± 0.67(SE) (t(10)=3.49, p<.01). For pain-related fear, average fear of pain scores significantly decreased from 43.8 ± 4.2(SE) to 22.9 ± 3.3(SE) (t(8)=3.58, p<.01). Pain-related fear and pain were not statistically significantly associated with one another at admission (r=.23, p=.53) nor at discharge (r=.48, p=.19).
Figure 1. Changes in pain-related fear from Time 1 to Time 2.

There was a significant mean decrease in fear of pain from Time 1 (pre-treatment) to Time 2 (post-treatment). Each red line reflects individual scores. Means scores with standard error bars at Time 1 and Time 2 are depicted in black. Although several patients continued to report modest levels of pain-related fear, only one patient reported clinically elevated fear scores at the end of treatment.
Resting state functional connectivity analyses
Results for group differences, paired analyses from Time 1 to Time 2, and fear of pain covariate analyses with clusters of activation greater than 2.0 cm3 are described in the text below. The tables provide greater detail with all significant clusters (> 26 voxels) listed.
Disease effect
Group differences at Time 1
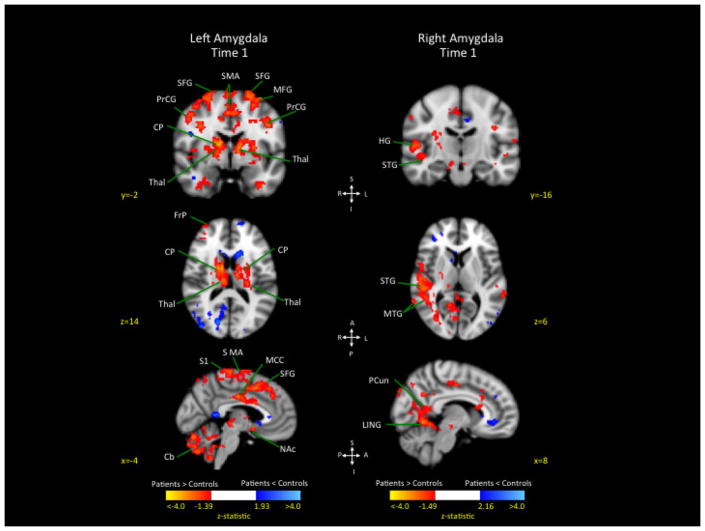
Analysis revealed greater connectivity of the left and right amygdala in patients compared to healthy controls in several cortical and subcortical areas, with weaker connectivity in patients compared to controls limited to a few brain regions (see Figure 2).
Figure 2. Connectivity differences between patients and controls at Time 1 from the left and right amygdala to the rest of the brain.
The left image represent significant correlated activity from left amygdala to the rest of the brain, contrasting patients and controls at Time 1. The image on the right represents synchronous activity from the right amygdala to the rest of the brain contrasting patients and controls at Time 1.
Key: SMA: Supplementary Motor Area; PrCG precentral gyrus; SFG: superior frontal gyrus; MFC: middle frontal cortex; CP: caudate-putamen; FrP: frontal pole; Thal: thalamus; MCC: middle cingulate cortex; STG: superior temporal gyrus; MTG: middle temporal gyrus; LING: Lingual Gyrus; Pcun: Precuneus; Cb: cerebellum; S1: primary somatosensory cortex; Ins: insula; MeFG: Medial Frontal Gyrus; NAc: nucleus accumbens.
Left amygdala
Greater connectivity in patients compared to controls was observed for the prefrontal cortex (right frontal pole, right middle, bilateral superior), motor cortices (bilateral paracentral lobule, bilateral precentral gyrus, right supplementary motor area), parietal lobe (right angular, right inferior, left primary somatosensory, right superior), and bilateral middle cingulate (see Table 2A). Greater connectivity was also observed in patients compared to controls from the left amygdala to several subcortical and cerebellar structures including the basal ganglia (bilateral caudate, bilateral putamen, left nucleus accumbens), bilateral thalamus, and bilateral cerebellum (with the largest segment in Crus 2). Weaker connectivity in patients compared to controls was observed for the left precuneus and occipital lobe (right calcarine, right cuneus, right middle, bilateral superior) (see Table 2B).
Table 2A.
Patients > Controls Time 1 (Negative Activation): Amygdala Left.
| Brain Region | Lat. | Z-stat | Coordinates (mm) |
Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Frontal Pole | R | 2.68 | 38 | 40 | 8 | 4.69 |
| R | 2.92 | 20 | 40 | 40 | 1.54 | |
| Middle Frontal | R | 2.89 | 42 | 24 | 34 | 1.35 |
| R | 3.46 | 34 | 22 | 44 | 3.19 | |
| R | 3.09 | 36 | 22 | 38 | 1.02 | |
| R | 2.99 | 34 | 12 | 36 | 1.48 | |
| R | 2.72 | 40 | 2 | 56 | 0.86 | |
| R | 2.70 | 34 | 2 | 38 | 0.37 | |
| Paracentral Lobule | R | 2.71 | 10 | −26 | 70 | 2.63 |
| R | 2.67 | 10 | −28 | 76 | 0.70 | |
| L | 2.71 | 0 | −18 | 68 | 2.35 | |
| L | 2.85 | −10 | −28 | 66 | 1.88 | |
| L | 2.70 | −2 | −30 | 72 | 0.68 | |
| Precentral | R | 3.67 | 48 | 4 | 44 | 7.26 |
| L | 2.80 | −44 | −2 | 34 | 2.90 | |
| L | 2.67 | −32 | −2 | 64 | 1.60 | |
| Superior | R | 3.22 | 6 | 32 | 46 | 5.57 |
| L | 2.76 | −22 | −2 | 68 | 3.03 | |
| R | 2.69 | 20 | 16 | 48 | 1.86 | |
| R | 2.92 | 14 | 14 | 54 | 0.39 | |
| R | 3.06 | 26 | 2 | 64 | 7.85 | |
| Supplementary Motor Area | R | 3.46 | 8 | 20 | 54 | 8.71 |
| R | 2.65 | 8 | 18 | 46 | 0.41 | |
| R | 2.72 | 2 | −8 | 70 | 1.47 | |
| R | 2.74 | 2 | −12 | 70 | 0.43 | |
| Parietal | ||||||
| Angular | R | 2.68 | 40 | −64 | 46 | 3.59 |
| Inferior | R | 3.16 | 34 | −46 | 52 | 1.58 |
| R | 2.70 | 52 | −46 | 52 | 2.68 | |
| Postcentral | L | 2.85 | −38 | −36 | 42 | 3.67 |
| Precuneus | L | 2.90 | −6 | −62 | 58 | 1.27 |
| Superior | R | 2.80 | 44 | −44 | 58 | 0.70 |
| R | 3.09 | 32 | −44 | 40 | 1.78 | |
| R | 2.80 | 40 | −48 | 60 | 0.66 | |
| SupraMarginal | R | 2.79 | 32 | −38 | 42 | 0.62 |
| Temporal | ||||||
| Pole Middle | L | 3.02 | −40 | 16 | −32 | 1.44 |
| Middle | L | 2.97 | −58 | −62 | 4 | 1.20 |
| Planum Polare | R | 2.82 | 50 | 2 | −4 | 0.78 |
| Cingulum | ||||||
| Middle | R | 2.69 | 6 | 12 | 30 | 2.05 |
| R | 2.79 | 6 | −16 | 36 | 1.29 | |
| L | 2.69 | −8 | −12 | 40 | 1.10 | |
| L | 2.78 | −4 | −14 | 36 | 1.05 | |
| Parahippocampus | ||||||
| Parahippocampal | R | 2.82 | 32 | −6 | −30 | 1.37 |
| Sub-Cortical | ||||||
| Caudate | R | 2.69 | 18 | 14 | 4 | 3.69 |
| R | 3.13 | 12 | 4 | 8 | 0.56 | |
| R | 3.46 | 14 | 0 | 12 | 2.94 | |
| Nucleus Accumbens | L | 3.22 | −6 | 6 | −12 | 2.30 |
| Pallidum | R | 2.71 | 16 | 0 | 2 | 0.70 |
| R | 2.87 | 14 | −6 | −4 | 0.67 | |
| Putamen | R | 2.63 | 24 | 0 | 6 | 1.66 |
| L | 2.63 | −26 | −4 | 10 | 5.62 | |
| Thalamus | R | 3.10 | 10 | −14 | 18 | 1.82 |
| R | 2.98 | 10 | −20 | 14 | 1.53 | |
| L | 3.29 | −10 | 0 | 8 | 2.87 | |
| L | 3.13 | −10 | −10 | 12 | 2.34 | |
| L | 2.96 | −10 | −10 | 2 | 0.82 | |
| L | 3.01 | −14 | −16 | −2 | 0.43 | |
| L | 3.11 | −16 | −20 | 0 | 1.82 | |
| Brainstem/Cerebellum | ||||||
| Cerebellum 4 5 | R | 2.77 | 30 | −32 | −30 | 1.42 |
| L | 2.81 | −16 | −40 | −26 | 4.62 | |
| Cerebellum 6 | R | 2.75 | 32 | −44 | −38 | 0.89 |
| R | 2.72 | 10 | −74 | −20 | 6.90 | |
| L | 2.72 | −34 | −40 | −32 | 0.66 | |
| L | 2.91 | −28 | −42 | −34 | 1.15 | |
| Cerebellum 7b | L | 3.05 | −46 | −44 | −42 | 0.28 |
| Cerebellum 8 | L | 2.65 | −30 | −56 | −52 | 2.03 |
| L | 3.28 | −14 | −64 | −50 | 2.46 | |
| Cerebellum 9 | R | 2.74 | 12 | −52 | −54 | 7.20 |
| L | 2.80 | −10 | −50 | −36 | 1.46 | |
| Cerebellum Crus1 | L | 3.62 | −42 | −44 | −38 | 2.78 |
| L | 2.80 | −36 | −60 | −32 | 3.98 | |
| Cerebellum Crus2 | R | 2.93 | 42 | −46 | −40 | 2.44 |
| L | 2.81 | −42 | −66 | −46 | 2.08 | |
| L | 3.01 | −38 | −74 | −46 | 3.32 | |
| L | 2.84 | −6 | −76 | −38 | 2.06 | |
| L | 2.76 | −4 | −78 | −24 | 4.83 | |
| V | L | 3.12 | −12 | −56 | −26 | 3.49 |
| VIIb | L | 2.87 | −2 | −72 | −46 | 3.72 |
Table 2B.
Controls > Patients Time 1 (Positive Activation): Amygdala Left
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Olfactory | R | 2.75 | 4 | 16 | −2 | 0.26 |
| Precentral | L | 2.42 | −36 | −24 | 62 | 0.92 |
| Frontal Pole | L | 2.69 | −18 | 64 | 12 | 0.44 |
| R | 2.73 | 16 | 54 | 32 | 0.25 | |
| Superior | L | 2.58 | −26 | 42 | 42 | 0.74 |
| Parietal | ||||||
| Angular | R | 2.77 | 42 | −60 | 24 | 0.26 |
| L | 2.59 | −50 | −64 | 32 | 0.34 | |
| Precuneus | R | 3.10 | 20 | −58 | 26 | 2.75 |
| L | 2.67 | −12 | −66 | 30 | 0.22 | |
| Occipital | ||||||
| Calcarine | R | 2.77 | 10 | −58 | 16 | 1.06 |
| R | 3.08 | 26 | −60 | 10 | 0.74 | |
| R | 2.69 | 12 | −66 | 16 | 0.43 | |
| R | 3.40 | 18 | −80 | 16 | 1.15 | |
| Cuneus | R | 2.66 | 8 | −80 | 28 | 0.63 |
| R | 3.37 | 12 | −90 | 30 | 3.10 | |
| Inferior | R | 2.86 | 40 | −72 | −12 | 0.54 |
| Lateral | R | 2.46 | 36 | −66 | 24 | 0.30 |
| R | 3.19 | 54 | −70 | 10 | 0.43 | |
| Middle | R | 2.96 | 44 | −80 | 20 | 1.95 |
| R | 2.80 | 30 | −88 | 24 | 1.02 | |
| L | 2.65 | −22 | −82 | 20 | 0.23 | |
| Rolandic Operculum | R | 2.81 | 52 | −24 | 20 | 0.34 |
| Superior | R | 2.63 | 30 | −80 | 38 | 0.30 |
| R | 2.87 | 24 | −88 | 34 | 0.22 | |
| R | 2.76 | 16 | −92 | 20 | 0.50 | |
| L | 2.71 | −22 | −80 | 26 | 0.33 | |
| L | 3.22 | −16 | −88 | 34 | 1.17 | |
| Temporal | ||||||
| Fusiform | R | 2.69 | 26 | −76 | −10 | 0.26 |
| L | 2.71 | −34 | −78 | −16 | 0.90 | |
| Heschl | R | 2.46 | 50 | −18 | 8 | 0.22 |
| Lingual | R | 2.40 | 14 | −48 | −6 | 0.23 |
| R | 3.21 | 20 | −82 | −6 | 0.72 | |
| Superior | R | 3.13 | 54 | −36 | 6 | 0.97 |
| Temporal Pole | R | 2.70 | 40 | 24 | −22 | 0.53 |
| Insula | ||||||
| Posterior | R | 2.81 | 42 | −12 | 6 | 0.32 |
| Sub-Cortical | ||||||
| Caudate | R | 3.35 | 14 | 22 | −4 | 0.79 |
| L | 2.81 | −18 | 24 | −2 | 0.58 | |
| Nucleus Accumbens | L | 2.56 | −6 | 16 | −4 | 0.23 |
| Brainstem/Cerebellum | ||||||
| Cerebellum Crus1 | L | 2.57 | −46 | −68 | −22 | 0.67 |
Right amygdala
Greater connectivity in patients compared to controls was observed for the right parietal lobe (precuneus, supramarginal), right occipital lobe (calcarine, superior), temporal lobe (bilateral middle, right superior), and right cerebellum (lobes IV, V) (see Table 2C). There were no brain regions that showed weaker connectivity in patients compared to controls (Table 2D).
Table 2C.
Patients > Controls Time 1 (Negative Activation): Amygdala Right
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Frontal Pole | L | 2.45 | −42 | 48 | 16 | 0.82 |
| Middle | L | 2.46 | −32 | 18 | 36 | 0.55 |
| Superior | L | 2.54 | −26 | 44 | 40 | 0.48 |
| L | 2.35 | −2 | 28 | 60 | 0.43 | |
| Supplementary Motor Area | R | 2.65 | 6 | −20 | 50 | 0.46 |
| Parietal | ||||||
| Precuneus | R | 2.49 | 12 | −54 | 8 | 2.15 |
| R | 3.11 | 12 | −56 | 30 | 0.49 | |
| R | 2.56 | 8 | −56 | 32 | 0.31 | |
| R | 2.66 | 16 | −58 | 24 | 1.10 | |
| SupraMarginal | R | 2.44 | 60 | −24 | 38 | 0.66 |
| R | 2.49 | 60 | −26 | 30 | 0.74 | |
| R | 2.76 | 50 | −46 | 32 | 2.80 | |
| Occipital | ||||||
| Calcarine | R | 2.54 | 20 | −80 | 14 | 3.92 |
| Inferior | R | 2.98 | 40 | −70 | −12 | 0.52 |
| Rolandic Operculum | R | 2.38 | 54 | 2 | 4 | 0.89 |
| R | 2.33 | 44 | −6 | 18 | 0.22 | |
| R | 2.37 | 40 | −8 | 18 | 0.26 | |
| L | 2.34 | −62 | −6 | 12 | 0.37 | |
| Superior | R | 2.74 | 26 | −64 | 26 | 2.44 |
| Temporal | ||||||
| Fusiform | R | 3.01 | 38 | −76 | −18 | 0.45 |
| Heschl | R | 2.60 | 50 | −18 | 8 | 0.76 |
| R | 2.45 | 38 | −28 | 14 | 0.62 | |
| Lingual | R | 3.32 | 14 | −48 | −8 | 0.55 |
| R | 3.08 | 28 | −54 | −4 | 0.86 | |
| R | 2.33 | 10 | −62 | 8 | 1.48 | |
| Middle | R | 2.36 | 48 | −44 | 6 | 1.48 |
| R | 2.30 | 64 | −48 | 4 | 0.67 | |
| L | 2.94 | −54 | −22 | 0 | 0.58 | |
| L | 2.28 | −64 | −42 | 6 | 0.47 | |
| Pole Superior | R | 2.88 | 40 | 22 | −24 | 0.96 |
| R | 2.29 | 40 | 14 | −26 | 1.14 | |
| Superior | R | 2.35 | 50 | −6 | −14 | 0.90 |
| R | 2.64 | 56 | −16 | 6 | 0.64 | |
| R | 2.89 | 56 | −22 | 6 | 0.49 | |
| R | 2.31 | 40 | −28 | 10 | 0.64 | |
| R | 2.80 | 52 | −32 | 6 | 1.74 | |
| Cingulum | ||||||
| Middle | R | 2.53 | 8 | −12 | 46 | 0.50 |
| L | 2.31 | −10 | 12 | 42 | 0.51 | |
| Posterior | R | 2.30 | 12 | −44 | 32 | 0.34 |
| Insula | ||||||
| Anterior | R | 2.32 | 38 | 12 | −6 | 0.54 |
| Posterior | R | 2.49 | 32 | −20 | 22 | 0.65 |
| Parahippocampus | ||||||
| Parahippocampal | L | 2.72 | −20 | −12 | −30 | 0.55 |
| Sub-Cortical | ||||||
| Caudate | L | 2.55 | −10 | 16 | 14 | 1.71 |
| Putamen | L | 2.45 | −20 | 16 | −2 | 0.46 |
| Thalamus | R | 2.30 | 16 | −28 | 6 | 0.36 |
| Brainstem/Cerebellum | ||||||
| Cerebellum 4 5 | R | 2.50 | 14 | −42 | −20 | 0.82 |
| R | 3.19 | 10 | −56 | −4 | 1.88 | |
| Cerebellum 8 | L | 2.59 | −36 | −58 | −46 | 0.79 |
| Cerebellum Crus1 | L | 2.31 | −48 | −68 | −22 | 0.74 |
| Vermis 4 5 | R | 2.69 | 6 | −48 | −6 | 0.52 |
| R | 2.59 | 4 | −52 | −6 | 0.64 | |
Table 2D.
Controls > Patients at Time 1 (Positive Activation): Amygdala Right
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Frontal Pole | R | 2.87 | 34 | 40 | 6 | 0.29 |
| L | 3.07 | −36 | 52 | −4 | 0.46 | |
| Middle | R | 3.09 | 36 | 12 | 36 | 0.58 |
| L | 3.07 | −46 | 14 | 36 | 0.25 | |
| Superior Orbital | R | 3.11 | 20 | 42 | 38 | 0.26 |
| Superior | R | 2.65 | 26 | −6 | 68 | 0.32 |
| Parietal | ||||||
| Postcentral | R | 2.99 | 38 | −38 | 56 | 0.52 |
| Occipital | ||||||
| Inferior | L | 2.72 | −50 | −68 | −12 | 0.27 |
| L | 2.51 | −22 | −98 | −10 | 0.39 | |
| Cingulum | ||||||
| Anterior | L | 3.00 | 0 | 46 | 0 | 1.42 |
| Posterior | 2.84 | −10 | −18 | 38 | 0.22 | |
| Brainstem/Cerebellum | ||||||
| Cerebellum 6 | R | 2.73 | 26 | −66 | −20 | 0.25 |
| Cerebellum Crus1 | L | 2.58 | −48 | −48 | −40 | 0.45 |
| L | 3.05 | −40 | −60 | −32 | 0.25 | |
Treatment effects
Within group changes over from Time 1 to Time 2
GLM-based within group paired functional connectivity analysis revealed several significant decreases in connectivity among patients for the left amygdala and only a few significant differences between controls in the right and left amygdala from Time 1 to Time 2.
Patients
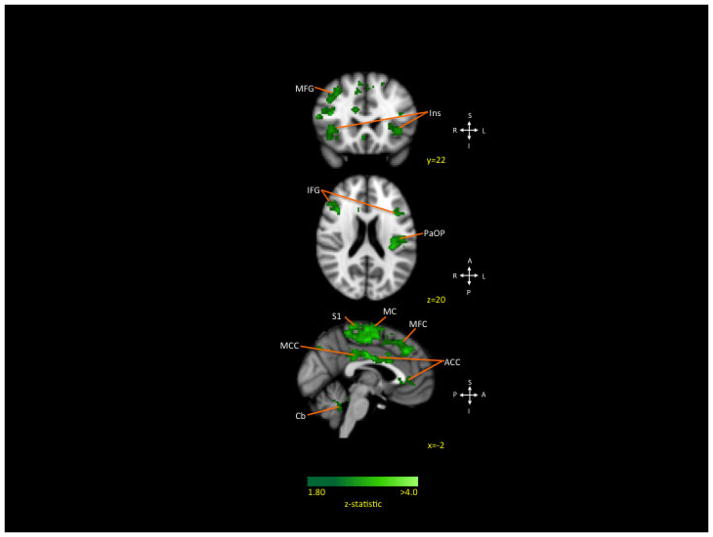
Decreased connectivity between the left amygdala and the prefrontal cortex (bilateral inferior, bilateral middle), motor cortex (bilateral paracentral lobule, bilateral precentral, right supplemental motor area), parietal lobe (right angular gyrus, left inferior gyrus, bilateral S1), bilateral cingulate (anterior, middle), bilateral anterior insula, and lobule IX of the cerebellum was observed from Time 1 to Time 2 (see Table 3A and Figure 3). No significant decreases were observed for the right amygdala nor in bilateral increased connectivity.
Table 3A.
Within Patients, Time 1 > Time 2 (Positive Activation): Amygdala Left
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Inferior Orbital | R | 3.00 | 36 | 34 | −8 | 0.24 |
| Inferior Triangular | R | 3.16 | 46 | 40 | 8 | 0.75 |
| R | 2.96 | 38 | 34 | 10 | 0.34 | |
| R | 2.92 | 54 | 22 | 16 | 0.47 | |
| R | 3.03 | 40 | 38 | 10 | 0.38 | |
| L | 2.92 | −42 | 42 | 14 | 1.10 | |
| Middle | L | 2.94 | −46 | 12 | 40 | 2.59 |
| L | 3.09 | −32 | 8 | 48 | 3.08 | |
| R | 3.04 | 34 | 20 | 42 | 1.07 | |
| R | 2.9 | 28 | 18 | 50 | 0.73 | |
| R | 2.84 | 44 | 16 | 42 | 0.42 | |
| R | 3.27 | 40 | 12 | 46 | 2.16 | |
| R | 3.07 | 28 | 8 | 52 | 1.06 | |
| R | 2.99 | 32 | 4 | 56 | 0.68 | |
| Paracentral Lobule | R | 2.85 | 10 | −28 | 64 | 0.49 |
| R | 2.91 | 6 | −30 | 70 | 0.44 | |
| L | 3.01 | −12 | −22 | 70 | 1.15 | |
| L | 3.2 | −4 | −36 | 70 | 1.38 | |
| L | 3.3 | −4 | −38 | 62 | 1.46 | |
| Precentral | R | 3.2 | 52 | 8 | 36 | 1.29 |
| R | 3.43 | 20 | −20 | 68 | 2.55 | |
| R | 3.34 | 30 | −20 | 56 | 1.05 | |
| R | 3.02 | 28 | −22 | 62 | 0.83 | |
| R | 2.85 | 20 | −32 | 70 | 0.56 | |
| L | 3.15 | −46 | −6 | 38 | 0.38 | |
| L | 3.12 | −50 | −6 | 32 | 0.84 | |
| L | 3.11 | −46 | −6 | 42 | 0.42 | |
| L | 3.19 | −40 | −10 | 38 | 0.63 | |
| L | 2.84 | −20 | −20 | 62 | 2.00 | |
| L | 3.04 | −20 | −24 | 72 | 0.79 | |
| Superior | L | 3.07 | −22 | 36 | 42 | 0.70 |
| Superior Medial | L | 3.54 | −2 | 34 | 38 | 4.31 |
| Supplementary Motor Area | R | 3.09 | 12 | −8 | 66 | 0.73 |
| R | 3.79 | 12 | −10 | 58 | 20.08 | |
| Parietal | ||||||
| Angular | R | 3.03 | 26 | −60 | 48 | 2.38 |
| R | 2.86 | 38 | −68 | 44 | 1.58 | |
| Inferior | L | 3.25 | −38 | −38 | 44 | 3.74 |
| L | 2.83 | −34 | −60 | 40 | 3.03 | |
| Postcentral | R | 3.11 | 50 | −24 | 54 | 1.33 |
| R | 3.09 | 14 | −36 | 72 | 0.56 | |
| R | 2.83 | 18 | −36 | 60 | 1.18 | |
| L | 3.37 | −44 | −10 | 48 | 0.58 | |
| L | 3.64 | −46 | −12 | 42 | 0.36 | |
| L | 2.94 | −50 | −12 | 48 | 0.98 | |
| L | 3.56 | −42 | −16 | 44 | 1.04 | |
| Precuneus | L | 2.86 | 0 | −74 | 42 | 0.52 |
| Superior | R | 2.96 | 46 | −44 | 56 | 1.85 |
| SupraMarginal | R | 3.17 | 52 | −24 | 28 | 1.02 |
| R | 2.92 | 52 | −44 | 42 | 0.26 | |
| Occipital | ||||||
| Rolandic Operculum | L | 3.14 | −46 | −18 | 20 | 1.58 |
| Temporal | ||||||
| Pole Middle | L | 3.12 | −30 | 10 | −34 | 0.36 |
| Superior | R | 3.04 | 60 | −22 | 8 | 0.32 |
| Cingulum | ||||||
| Anterior | R | 2.95 | 2 | 40 | 0 | 1.51 |
| L | 3.06 | −4 | 12 | 26 | 1.18 | |
| L | 2.92 | 0 | 4 | 28 | 0.58 | |
| Middle | R | 3.27 | 4 | −4 | 30 | 0.49 |
| R | 3.32 | 0 | −10 | 30 | 0.30 | |
| R | 3.49 | 4 | −14 | 34 | 0.58 | |
| L | 3.19 | 0 | 12 | 36 | 0.57 | |
| L | 3.85 | −6 | −18 | 36 | 2.43 | |
| L | 2.89 | −6 | −32 | 40 | 0.62 | |
| Post | L | 3.09 | 0 | −32 | 30 | 1.70 |
| Insula | ||||||
| Anterior | R | 2.89 | 40 | 24 | 4 | 1.46 |
| L | 2.93 | −40 | 18 | −4 | 1.89 | |
| Posterior | L | 3.1 | −36 | −18 | 18 | 0.34 |
| L | 3.56 | −34 | −30 | 22 | 1.23 | |
| Parahippocampus | ||||||
| Parahippocampal | R | 2.87 | 24 | 8 | −34 | 0.24 |
| Sub-Cortical | ||||||
| Caudate | L | 3.16 | −8 | 2 | 6 | 0.67 |
| Thalamus | L | 3.2 | −8 | −12 | 16 | 0.32 |
| Brainstem/Cerebellum | ||||||
| Cerebellum 9 | L | 2.96 | −12 | −44 | −42 | 2.26 |
Figure 3. Decreased connectivity within patients after treatment (Left Amygdala).
Weakened connections from the left amygdala to frontal, motor, and somatosensory cortices were observed at the end of treatment.
Key: MFG: middle frontal gyrus; Ins: insula; IFG: inferior frontal gyrus; S1: somatosensory cortex I; MC: motor cortex (paracentral lobule, precentral gyrus, supplemental motor area); PaOP: opercular cortex; ACC: anterior cingulate cortex; MCC: middle cingulate cortex.
Controls
Greater connectivity at Time 2 was limited to between the left amygdala and the right thalamus and bilateral cerebellum (with the largest segment in Crus 1) compared to Time 1 (see Appendix Table 3B, 3C, and 3D). No increases in connectivity of greater than 2 cm3 were observed for the right amygdala and no decreases in connectivity were observed bilaterally among controls.
Patient covariate analysis – Fear of Pain
The dependence between functional connectivity and fear of pain after controlling for pain levels was examined within patients to examine the unique association with fear of pain. At both Time 1 and Time 2, several regions key to fear circuitry were correlated with higher fear of pain scores.
Fear of Pain at Time 1
Higher fear of pain was associated with stronger connectivity between the left amygdala and the prefrontal cortex (bilateral frontal pole, bilateral superior), bilateral middle temporal lobe, bilateral anterior cingulate cortex, left anterior insula, left hippocampus, parahippocampal gyrus, brainstem, and cerebellum (see Figure 4 and Table 4A). No brain areas were correlated with lower pain-related fear scores. For the right amygdala, no areas exceeding 2 cm3 of positively or negatively correlated activity were observed (see Appendix Table 4B and 4C).
Figure 4. Connectivity strength by levels of pain-related fear in patients (Left Amygdala).
Across time, areas associated with fear circuitry were consistently associated with higher pain-related fear scores.
Key: MTG: middle temporal gyrus; BS: brain stem; Hi: hippocampus; Ins: insula; FrP: frontal pole; ACC: anterior cingulate cortex; SFG: superior frontal gyrus; Cb: cerebellum; MeFG: medial frontal gyrus.
Table 4A.
Time 1 Stronger Connectivity, Higher levels of pain-related fear: Amygdala Left
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Inferior Orbital | L | 2.34 | −32 | 34 | −10 | 0.23 |
| Inferior Triangular | R | 2.55 | 50 | 26 | 16 | 1.74 |
| Frontal Pole | L | 2.87 | −32 | 56 | 14 | 0.98 |
| R | 2.83 | 34 | 54 | 18 | 2.02 | |
| R | 2.86 | 30 | 48 | 38 | 2.23 | |
| R | 2.60 | 34 | 48 | 6 | 0.35 | |
| Superior | R | 2.44 | 28 | 32 | 50 | 0.26 |
| R | 2.80 | 14 | 68 | 18 | 0.30 | |
| L | 2.62 | −24 | 66 | 12 | 0.23 | |
| L | 2.56 | −24 | 58 | 18 | 0.43 | |
| L | 2.61 | −26 | 52 | 0 | 0.25 | |
| L | 2.37 | −20 | 50 | 20 | 0.33 | |
| L | 2.56 | −16 | 44 | 48 | 3.24 | |
| Parietal | ||||||
| Inferior | L | 2.44 | −54 | −54 | 38 | 0.37 |
| Precuneus | L | 2.50 | −8 | −70 | 34 | 0.28 |
| Occipital | ||||||
| Middle | L | 2.47 | −44 | −80 | 28 | 0.70 |
| Superior | L | 2.46 | 36 | −70 | 16 | 0.22 |
| Temporal | ||||||
| Fusiform | R | 2.42 | 22 | −54 | −14 | 0.78 |
| L | 2.43 | −26 | −58 | −8 | 0.22 | |
| L | 2.58 | −30 | −64 | −6 | 0.41 | |
| Inferior | L | 2.75 | −42 | −14 | −24 | 0.54 |
| L | 2.61 | −40 | −14 | −28 | 0.25 | |
| Middle | R | 2.50 | 58 | −18 | −12 | 0.97 |
| R | 2.64 | 58 | −24 | −14 | 0.30 | |
| L | 2.55 | −50 | 6 | −28 | 0.50 | |
| L | 2.49 | −44 | −2 | −18 | 0.62 | |
| L | 2.43 | −54 | −4 | −18 | 0.49 | |
| Cingulum | ||||||
| Anterior | R | 2.67 | 8 | 42 | 10 | 2.60 |
| L | 2.41 | −2 | 42 | 0 | 0.58 | |
| L | 2.71 | −8 | 28 | −4 | 0.91 | |
| L | 2.45 | −2 | −16 | 30 | 0.56 | |
| Posterior | R | 2.45 | −2 | −16 | 30 | 0.56 |
| Insula | ||||||
| Anterior | L | 2.45 | −38 | 18 | −6 | 0.34 |
| L | 3.29 | −30 | 16 | −12 | 1.04 | |
| L | 2.54 | −34 | 10 | 2 | 0.37 | |
| Parahippocampus | ||||||
| Parahippocampal | L | 2.88 | −26 | −2 | −30 | 0.65 |
| L | 2.89 | −20 | −4 | −36 | 0.50 | |
| Sub-Cortical | ||||||
| Hippocampus | L | 3.22 | −26 | −10 | −22 | 0.44 |
| L | 2.45 | −28 | −16 | −18 | 0.70 | |
| Putamen | R | 2.48 | 34 | 4 | 2 | 1.11 |
| Thalamus | R | 2.63 | 18 | −10 | 2 | 0.82 |
| Brainstem / Cerebellum | ||||||
| Brainstem | R | 2.71 | 6 | −20 | −26 | 1.02 |
| R | 2.34 | 6 | −36 | −22 | 0.63 | |
| Cerebellum 3 | L | 3.04 | −6 | −40 | −20 | 1.18 |
| Cerebellum 4,5 | R | 3.01 | 10 | −54 | −8 | 2.04 |
| L | 2.49 | −18 | −38 | −28 | 1.98 | |
| Cerebellum 6 | R | 2.38 | 10 | −58 | −20 | 2.37 |
| Cerebellum 7b | L | 2.39 | −40 | −58 | −48 | 1.26 |
| Cerebellum 9 | L | 2.47 | −10 | −48 | −54 | 0.40 |
| Crus1 | R | 2.56 | 36 | −78 | −26 | 0.77 |
| Crus2 | L | 3.03 | −46 | −62 | −40 | 2.41 |
| Vermis 3 | R | 2.57 | 6 | −46 | −20 | 0.77 |
| Vermis 4,5 | R | 2.69 | 6 | −50 | −22 | 0.87 |
Fear of Pain at Time 2
Although patients reported a significant decrease in pain-related fear at the end of intensive pain rehabilitation program, some residual levels of fear remained and were correlated with several brain areas and the left amygdala. Higher levels of fear of pain were associated with stronger connectivity between the left amygdala and the prefrontal cortex (bilateral frontal pole, right medial, bilateral middle, superior), bilateral middle temporal lobe, bilateral cingulate (anterior, middle), right hippocampus, and right putamen (see Figure 4 and Table 5A). For the right amygdala, higher levels of pain-related fear were associated with stronger connectivity with the cerebellum (with the largest segment Lobule VIII; see Appendix Table 5B and 5C).
Table 5a.
Time 2 Stronger Connectivity, Higher levels of pain-related fear: Amygdala Left
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Frontal Pole | R | 2.81 | 34 | 52 | −8 | 0.43 |
| R | 2.99 | 24 | 50 | −8 | 0.30 | |
| R | 2.65 | 34 | 56 | 14 | 0.51 | |
| R | 2.54 | 26 | 50 | 28 | 0.73 | |
| R | 2.65 | 26 | 58 | 16 | 0.61 | |
| R | 2.79 | 20 | 52 | 0 | 0.47 | |
| R | 2.51 | 22 | 50 | 14 | 0.24 | |
| R | 2.95 | 24 | 46 | 18 | 0.62 | |
| L | 3.61 | −26 | 50 | −8 | 0.57 | |
| L | 3.22 | −18 | 66 | 4 | 1.12 | |
| L | 2.78 | −14 | 56 | 36 | 1.01 | |
| L | 2.56 | −20 | 50 | 18 | 0.38 | |
| L | 3.14 | −10 | 58 | 10 | 1.14 | |
| Frontal Orbital | R | 3.47 | 28 | 38 | −8 | 0.82 |
| L | 2.62 | −22 | 14 | −24 | 0.22 | |
| Inferior Triangular | L | 2.78 | −40 | 40 | 4 | 0.70 |
| L | 2.51 | −56 | 24 | 10 | 0.26 | |
| Medial | R | 3.41 | 8 | 44 | −14 | 0.87 |
| R | 3.06 | 12 | 40 | −2 | 0.94 | |
| R | 2.93 | 6 | 46 | 0 | 1.27 | |
| L | 2.80 | −12 | 52 | 0 | 0.32 | |
| Middle | R | 2.57 | 40 | 34 | 34 | 0.49 |
| R | 3.20 | 34 | 14 | 36 | 0.60 | |
| R | 2.59 | 40 | 16 | 32 | 0.33 | |
| L | 2.86 | −26 | 28 | 34 | 0.70 | |
| Precentral | R | 2.49 | 12 | −30 | 76 | 0.50 |
| Superior | L | 3.05 | −6 | 62 | 26 | 1.97 |
| L | 2.75 | 2 | 52 | 32 | 0.26 | |
| L | 2.77 | −8 | 46 | 18 | 0.37 | |
| L | 2.71 | −8 | 40 | 36 | 1.35 | |
| Supplementary Motor Area | R | 2.98 | 2 | 0 | 70 | 0.39 |
| L | 2.53 | −10 | 6 | 70 | 0.64 | |
| Parietal | ||||||
| Angular | R | 2.90 | 52 | −50 | 32 | 1.58 |
| Postcentral | R | 2.56 | 40 | −28 | 42 | 0.43 |
| SupraMarginal | R | 2.54 | 48 | −42 | 36 | 0.22 |
| Temporal | ||||||
| Fusiform | R | 2.77 | 38 | −38 | −12 | 0.52 |
| Inferior | L | 3.07 | −46 | −38 | −14 | 0.51 |
| Middle | R | 2.74 | 60 | −44 | 8 | 1.89 |
| R | 2.57 | 60 | −54 | 8 | 0.66 | |
| R | 2.60 | 62 | −56 | −2 | 0.48 | |
| L | 2.74 | −60 | −56 | −4 | 1.38 | |
| L | 3.19 | −60 | −58 | 18 | 0.87 | |
| Pole | R | 3.26 | 34 | 12 | −34 | 0.74 |
| L | 2.83 | −34 | 16 | −36 | 0.43 | |
| Cingulum | ||||||
| Anterior | R | 2.83 | 4 | 20 | 26 | 0.65 |
| L | 2.79 | −8 | 52 | 0 | 0.33 | |
| L | 2.53 | −2 | 48 | 16 | 0.49 | |
| L | 2.78 | 0 | 42 | −2 | 0.48 | |
| L | 2.57 | 2 | 42 | 14 | 0.38 | |
| L | 2.54 | 0 | 38 | 6 | 1.20 | |
| L | 2.84 | −12 | 36 | 20 | 1.06 | |
| Middle | R | 2.50 | 4 | 30 | 34 | 0.62 |
| R | 2.59 | 12 | 22 | 36 | 1.06 | |
| L | 2.64 | 0 | 22 | 34 | 0.72 | |
| Insula | ||||||
| Anterior | R | 2.82 | 46 | 6 | −10 | 0.26 |
| R | 2.77 | 46 | 6 | 6 | 0.70 | |
| Parahippocampus | ||||||
| Parahippocampal | R | 2.80 | 32 | −40 | −6 | 0.43 |
| Sub-Cortical | ||||||
| Caudate | R | 3.32 | 6 | 8 | −4 | 0.50 |
| L | 2.78 | −16 | 2 | 22 | 0.22 | |
| Hippocampus | R | 3.71 | 30 | −18 | −14 | 2.18 |
| Nucleus Accumbens | L | 2.66 | −12 | 12 | −8 | 0.27 |
| Pallidum | L | 2.73 | −26 | −10 | −4 | 0.24 |
| Putamen | R | 3.10 | 24 | 18 | −2 | 0.66 |
| R | 3.17 | 30 | 16 | −2 | 0.70 | |
| R | 2.77 | 28 | −16 | 12 | 0.54 | |
| R | 2.80 | 18 | 16 | −6 | 0.42 | |
| Brainstem / Cerebellum | ||||||
| Crus1 | L | 3.03 | −46 | −72 | −36 | 0.66 |
| L | 2.89 | −42 | −74 | −24 | 0.24 | |
Change in Fear of Pain and Change in connectivity
The change in left amygdala resting state connectivity in the CRPS patients correlated with the change in fear of pain, indicating weakened connectivity between the left inferior frontal gyrus, left superior frontal gyrus, left supplementary motor, left anterior cingulate, left insula, left thalamus and the amygdala in patients who had decreases in pain-related fear after treatment (see Figure 5 and Table 6). No significant correlations between right amygdala changes and fear of pain changes were observed. Additionally, there were no significant correlations between left and right amygdala changes and changes in pain level.
Figure 5. Correlation between patient connectivity changes and pain-related fear changes (Left amygdala).
Weakened connectivity between the left amygdala and frontal, motor, somatosensory, and cingulate cortex is associated with greater decrease in pain-related fear scores after treatment.
Key: FOP: frontal opercular cortex; SFG: superior frontal gyrus; MC: motor cortex (precentral gyrus, supplemental motor area); ACC: anterior cingulate cortex; MCC: middle cingulate cortex; MeFG: medial frontal gyrus; Ins: insula; Thal: thalamus.
Table 6.
Decreases in connectivity, decreases in pain-related fear: Amygdala Left
| Brain Region | Lat. | Z-stat | Coordinates (mm) | Vol cm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Frontal | ||||||
| Inferior Operculum | R | 3.10 | 38 | 16 | 8 | 1.02 |
| L | 2.72 | −50 | 14 | 2 | 0.54 | |
| Inferior | L | 2.76 | −38 | 30 | 24 | 0.40 |
| L | 2.53 | −54 | 30 | 8 | 0.56 | |
| L | 2.80 | −40 | 20 | 16 | 0.36 | |
| Middle | L | 2.70 | −26 | 34 | 40 | 0.60 |
| L | 2.81 | −26 | 28 | 32 | 0.59 | |
| Middle Orbital | R | 2.53 | 26 | 24 | 42 | 0.43 |
| Precentral | L | 2.60 | −56 | 6 | 38 | 0.42 |
| L | 2.89 | −44 | −6 | 40 | 1.31 | |
| Superior | R | 2.52 | 4 | 44 | 38 | 0.50 |
| R | 2.83 | 8 | 20 | 42 | 0.96 | |
| L | 2.52 | −14 | 24 | 56 | 0.38 | |
| L | 2.88 | −22 | 0 | 64 | 1.51 | |
| Superior Medial | L | 3.19 | −10 | 58 | 6 | 1.02 |
| L | 2.52 | −4 | 44 | 42 | 0.27 | |
| L | 2.83 | −6 | 40 | 32 | 0.51 | |
| Superior Orbital | R | 2.62 | 20 | 8 | 62 | 0.66 |
| Supplementary Motor Area | L | 2.54 | 0 | 4 | 68 | 0.36 |
| L | 2.60 | 0 | 12 | 58 | 0.39 | |
| L | 2.54 | −12 | 8 | 66 | 0.46 | |
| L | 2.95 | −4 | 0 | 58 | 8.54 | |
| L | 2.51 | 0 | 0 | 70 | 0.26 | |
| Parietal | ||||||
| Postcentral | L | 2.50 | −48 | −8 | 48 | 0.24 |
| SupraMarginal | R | 2.67 | 64 | −46 | 26 | 0.34 |
| Occipital | ||||||
| Rolandic Operculum | L | 2.77 | −52 | 8 | 2 | 0.31 |
| Cingulum | ||||||
| Anterior | R | 2.55 | 10 | 42 | 6 | 0.33 |
| R | 2.70 | 4 | 22 | 26 | 0.43 | |
| R | 2.72 | 6 | 26 | 14 | 0.31 | |
| R | 2.52 | 6 | 48 | −2 | 0.22 | |
| L | 2.63 | −12 | 44 | 10 | 0.43 | |
| L | 3.00 | −2 | 42 | −2 | 0.72 | |
| L | 3.19 | −2 | 36 | 20 | 3.58 | |
| L | 2.94 | −2 | 12 | 26 | 0.98 | |
| Middle | R | 2.70 | 4 | 28 | 32 | 1.03 |
| L | 3.14 | 0 | −8 | 32 | 0.78 | |
| Insula | ||||||
| Insula Anterior | R | 2.70 | 40 | 2 | 4 | 0.34 |
| L | 2.90 | −38 | 22 | 2 | 0.90 | |
| L | 2.94 | −30 | 20 | 10 | 0.67 | |
| Parahippocampus | ||||||
| Parahippocampal | L | 2.94 | −22 | −8 | −36 | 0.27 |
| Sub-Cortical | ||||||
| Pallidum | R | 2.79 | 20 | 6 | −2 | 0.28 |
| R | 2.96 | 20 | −2 | 6 | 0.29 | |
| L | 2.59 | −14 | 6 | 2 | 0.28 | |
| Putamen | R | 2.94 | 30 | 16 | −2 | 0.67 |
| Thalamus | R | 2.53 | 18 | −12 | 12 | 0.82 |
| L | 2.82 | −34 | −4 | 6 | 0.85 | |
| L | 2.84 | −10 | −12 | 14 | 0.34 | |
| L | 2.70 | −20 | −20 | 12 | 0.26 | |
| L | 2.73 | −14 | −8 | 10 | 0.80 | |
Discussion
The amygdala is involved in fear [16], reward [76], and pain [54]. This study evaluated functional connectivity of the amygdala with cortical and subcortical regions in a group of pediatric patients with CRPS with age-gender matched controls before and after intensive pain rehabilitative treatment. To our knowledge, this is the first study to investigate amygdala resting state functional connectivity in children with CRPS.
Intensive psychophysical treatment of CRPS
CRPS is a debilitating, painful condition characterized by continued pain, disproportionate to any inciting event, with sensory, vasomotor, sudomotor, and motor/trophic changes [24,25]. Although CRPS in children is often reversible [47], some patients require intensive psychophysical treatment [45,70,74]. Outcomes from our intensive day hospital program show significant declines in disability, distress, pain intensity, and improvements in motor function [45]. In the current study, children prior to and after this intensive treatment were evaluated. Pain and fear significantly decreased, which is consistent with prior results [45,74].
Disease state
Significant differences in functional connectivity in patients compared with healthy controls at Time 1 (i.e., pre-treatment) were observed. Of the areas showing enhanced connectivity for the left amygdala, alterations were shown in cognitive/emotional (prefrontal, cingulate cortex, basal ganglia), sensorimotor (thalamus, somatosensory cortex) and integrative processing (cerebellum, parietal lobe, thalamus). Some of these regions have been implicated in the phenotypic presentation of CRPS and likely attribute to the disease state such as motor changes [40,66], altered cognitive and affective processing (prefrontal and cingulate cortex; [22]), and altered sensory processing (somatosensory cortex and thalamus [7]). Although the cerebellum-amygdala functional connectivity has not been well defined in humans, the cerebellum is an important region for pain processing [52]. In animal tracing studies, cerebellar axons are reported to extend to the amygdala [17]. Recent work has implicated the cerebellum-amygdala connection in fear memory and expression [64,81] showing strengthened functional connectivity between the cerebellum and amygdala in adolescents with generalized anxiety disorder [62], suggesting that this enhanced connection may be driven by both pain itself and the emotional aspects of pain. This pattern of heightened connectivity has been found previously in adults with CRPS [7].
Regarding differences observed in the right amygdala, changes were present in areas involved with visuo-spatial imagery, episodic memory retrieval and self-processing operations (precuneus; [12]) and complex sensory integration (temporal lobe). Neglect of the affected limb is often a consequence of CRPS and is consistent with heightened connectivity with the parietal lobe in prior research [35,51]. Although less is known regarding the temporal lobe, stronger connections the between the amygdala and temporal pole have also been observed in patients with migraine [23]. In a prior report on connectivity in recovered CRPS patients, alterations in pain-induced connectivity between the amygdala and a number of regions [43] was observed, including many of those noted in this study. Altogether alterations in amygdala connectivity observed in pediatric CRPS appear to reflect motor, sensoryintegrative, and cognitive-affective dimensions of the pain experience.
Treatment effects
Dampened functional connections were between the left amygdala and the prefrontal cortex (inferior, middle), motor cortex, parietal lobe, and bilateral cingulate cortex. Diminished connectivity observed in sensorimotor regions and the parietal lobe may reflect improved motor function associated with treatment. This corresponds with the intensive dose of physical and occupational therapy (3–4 hours daily) that patients receive and motor improvements that have been previously documented [45]. With regards to cognitive-affective regions (prefrontal cortex, cingulate), diminished connectivity may indicate psychological improvement in response to cognitive-behavioral treatment (CBT). CBT is an essential component of the PPRC and is intensive (2–3 hours daily) [45].
As described further below, decreases in amygdala connectivity associated with decreases in pain-related fear were found for several brain regions, suggesting that amygdala connectivity may serve as an indicator of psychological treatment response in pain patients. Recent research has also tied painevoked increases in prefrontal cortex activation in adults with fibromyalgia [30] and increases in gray matter in prefrontal, parietal, cingulate, sensorimotor, and hippocampal areas in adults with chronic pain [68] after cognitive-behavioral therapy. Thus, the intrinsic brain functional connectivity observed between the amygdala and other brain regions is likely altered by a physical and psychological treatment paradigm in children with CRPS.
Pediatric CRPS and pain-related fear
Beyond its emerging role in pain, the amygdala has a major role in fear processing [39,56]. Patients with CRPS avoid use of [71] and neglect [36] their affected limb(s), resulting in exacerbated symptoms, muscle atrophy, and ongoing pain-related disability. The brain network associated with fear extends beyond the amygdala and includes other brain areas such as the insula, hippocampus, striatum, thalamus, and anterior cingulate cortex, with selected frontal and motor-sensory cortices also implicated [67]. After controlling for pain levels, at both Time 1 and Time 2, several regions key of the fear circuitry were correlated with higher fear of pain scores, most notably from the left amygdala to the prefrontal cortex, bilateral middle temporal lobe, bilateral cingulate cortex, and hippocampus.
Decreases in functional connectivity between the motor cortex, cingulate, insula, frontal areas and the left amygdala were associated with less fear at the end of treatment (after controlling for changes in pain level), suggesting that several changes in intrinsic brain functional connectivity can be linked to pain-related fear symptom improvement and maps closely to the broad changes in connectivity that were observed in patients after treatment (see Figure 6). Although PPRC treatment does not include a specific exposure-based treatment paradigm, such a graded in-vivo exposure [14], the role of fear is explicitly discussed and the milieu focuses on progressively engaging in previously avoided activities. Notably, changes in pain did not correlate with changes in amygdala connectivity. It is possible that improvements in pain are associated with treatment response, but that when both pain and pain-related fear are accounted for the model, fear emerges as a stronger predictor, particularly of amygdala connectivity. Prior work supports the potential independence of pain level and persistent brain circuit abnormalities in chronic pain patients wherein almost complete remission in subjective pain scores in a cohort of pediatric CRPS patients was associated with persistent brain abnormalities [38] and alterations in amygdala connectivity observed in response to painful cold stimuli [43].
Figure 6. Treatment Response from Time 1 to Time 2: Decrease in functional connectivity and pain-related fear.
Paired analysis of amygdala connectivity changes within patients is depicted in green while connectivity changes that correlated with changes in pain-related fear are displayed in red. Many of the amygdala connectivity decreases were correlated with decreases in pain-related fear after treatment, suggesting that changes in intrinsic brain functional connectivity can be linked to symptom improvement. Key: MFG: middle frontal gyrus; Ins: insula; SMA: supplementary motor area; ACC: anterior cingulate cortex; MCC: middle cingulate cortex; FOP: frontal opercular cortex.
Lateralization of connectivity changes
Lateralization of functional connectivity in the amygdala was observed, with the left hemisphere dominant across all contrasts and analyses. In a recent meta-analysis examining the role of the amygdala in pain processing, the left amygdala was most commonly activated in patients with clinical pain conditions (e.g., fibromyalgia), with few clinical pain studies showing only right amygdala activation [72]. This is in contrast to experimental pain studies where the right amygdala predominates in human and animal models [11,32]. Potential reasons for these findings may be the left amygdala’s more prominent role in affective encoding [50], fear processes [4], or reflect gender differences in amygdala functional connectivity [34], given the preponderance of females in this sample. Despite the consistency of our findings, the sample was too small and homogenous for affected limb (75% left) to make firm conclusions on laterality effects.
Caveats
Several caveats must be considered. Data on a small number of patients is presented, but inclusion of matched controls and a within subject design to assess treatment effects allows conclusions to be drawn from a difficult to recruit and important group, children with CRPS. Although duration of pain differed substantially, all patients were enrolled in this intensive program due to non-response to standard treatment. Gender differences could not be examined because of the preponderance of females, which is consistent with the literature [15]. Although many patients were on medications, patients remained on the same regimen during treatment; thus, medication effects should be consistent across imaging sessions. Functional connectivity does not define the direction of change and can only be inferred based on our knowledge of anatomical afferent and efferent amygdala connections [61]. Lastly, early and late adolescence is a developmental stage known for potentially marked changes in hormone and brain function. All patients in the study were 10 years or older where the developmental shift in amygdala connectivity from positive to negative has been demonstrated to occur [21], but examination of developmental influences is warranted in future studies. Lastly, observed changes in amygdala connectivity may reflect greater neural plasticity in youth with CRPS, compared to adults with this difficult to treat condition. In order to test if youth are potentially more neurally ‘plastic’, future research should include both pediatric and adult CRPS patients.
Conclusions
CRPS patients report significantly higher levels of somatic symptoms and functional disability compared to patients with other chronic pain disorders [13,46]. Adults who were diagnosed with CPRS in childhood report lower quality of life compared to healthy adults [77]. Fear of pain is a major component of the behavioral consequences of the disease. By targeting treatments that affect specific components of the chronic pain state, direct and indirect benefits of treatment on specific brain pathways may prove to be a useful approach. Functional connectivity measures of such changes in areas such as the amygdala can produce objective evaluations of the efficacy of such treatments.
Supplementary Material
Summary.
Amygdala connectivity is altered in children with chronic neuropathic pain and is responsive to intensive interdisciplinary treatment with associated decreased in pain-related fear.
Acknowledgments
The work was supported by NIH Grants to DB (NINDS R01NS065051 and NINDS K24NS064050), LS (NICHD K23HD067202), and CB Sara Page Mayo Endowment for Pediatric Pain Research and Treatment.
Footnotes
Financial Disclosures
Dr. Simons, Melissa Pielech, Dr. Erpelding, Dr. Linnman, Dr. Moulton, Dr. Sava, Dr. Lebel, Paul Serrano, Dr. Sethna, Dr. Berde, Dr. Becerra, and Dr. Borsook reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MATLAB and Statistics Toolbox
- 2.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain research Brain research reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 7.Bolwerk A, Seifert F, Maihofner C. Altered Resting-State Functional Connectivity in Complex Regional Pain Syndrome. The journal of pain: official journal of the American Pain Society. 2013 doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- 9.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrasquillo Y, Gereau RWt. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 13.Cruz N, O’Reilly J, Slomine BS, Salorio CF. Emotional and neuropsychological profiles of children with complex regional pain syndrome type-I in an inpatient rehabilitation setting. Clin J Pain. 2011;27:27–34. doi: 10.1097/AJP.0b013e3181f15d95. [DOI] [PubMed] [Google Scholar]
- 14.de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116:264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 15.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrichs E. Divergent axon collaterals to cerebellum and amygdala from neurons in the parabrachial nucleus, the nucleus locus coeruleus and some adjacent nuclei. A fluorescent double labelling study using rhodamine labelled latex microspheres and fast blue as retrograde tracers. Anatomy and embryology. 1985;172:375–382. doi: 10.1007/BF00318986. [DOI] [PubMed] [Google Scholar]
- 18.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng P, Feng T, Chen Z, Lei X. Memory consolidation of fear conditioning: Bi-stable amygdala connectivity with dorsal anterior cingulate and medial prefrontal cortex. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdalaprefrontal circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, Caramia F, Tinelli E, Mainero C. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia: an international journal of headache. 2013;33:1264–1268. doi: 10.1177/0333102413490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Chont M, Vatine JJ. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, Barthel J, Costa B, Graciosa JR, Bruehl S Reflex Sympathetic Dystrophy Syndrome A. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain medicine. 2013;14:180–229. doi: 10.1111/pme.12033. [DOI] [PubMed] [Google Scholar]
- 26.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 30.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153:1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol. 2009;102:2253–2264. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 35.Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–3155. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolb L, Lang C, Seifert F, Maihofner C. Cognitive correlates of “neglect-like syndrome” in patients with complex regional pain syndrome. Pain. 2012;153:1063–1073. doi: 10.1016/j.pain.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- 39.LeDoux J. The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenz M, Hoffken O, Stude P, Lissek S, Schwenkreis P, Reinersmann A, Frettloh J, Richter H, Tegenthoff M, Maier C. Bilateral somatosensory cortex disinhibition in complex regional pain syndrome type I. Neurology. 2011;77:1096–1101. doi: 10.1212/WNL.0b013e31822e1436. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Wang J, Chen L, Zhang M, Wan Y. Basolateral amygdala lesion inhibits the development of pain chronicity in neuropathic pain rats. PloS one. 2013;8:e70921. doi: 10.1371/journal.pone.0070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linnman C, Becerra L, Borsook D. Inflaming the brain: CRPS a model disease to understand neuroimmune interactions in chronic pain. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013;8:547–563. doi: 10.1007/s11481-012-9422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linnman C, Becerra L, Lebel A, Berde C, Grant PE, Borsook D. Transient and persistent pain induced connectivity alterations in pediatric complex regional pain syndrome. PloS one. 2013;8:e57205. doi: 10.1371/journal.pone.0057205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2011 doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A Day-hospital Approach to Treatment of Pediatric Complex Regional Pain Syndrome: Initial Functional Outcomes. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logan DE, Williams SE, Carullo VP, Claar RL, Bruehl S, Berde CB. Children and adolescents with complex regional pain syndrome: more psychologically distressed than other children in pain? Pain research & management: the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2013;18:87–93. doi: 10.1155/2013/964352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. Journal of pediatric orthopedics. 2007;27:567–572. doi: 10.1097/BPO.0b013e318070cc4d. [DOI] [PubMed] [Google Scholar]
- 48.Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D. Migraine attacks the Basal Ganglia. Mol Pain. 2011;7:71. doi: 10.1186/1744-8069-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markowitsch HJ. Differential contribution of right and left amygdala to affective information processing. Behavioural neurology. 1998;11:233–244. doi: 10.1155/1999/180434. [DOI] [PubMed] [Google Scholar]
- 51.Molenberghs P, Sale MV, Mattingley JB. Is there a critical lesion site for unilateral spatial neglect? A meta-analysis using activation likelihood estimation. Front Hum Neurosci. 2012;6:78. doi: 10.3389/fnhum.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65:14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and rheumatism. 2012;64:2398–2403. doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M. Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC psychiatry. 2013;13:84. doi: 10.1186/1471-244X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Current opinion in neurobiology. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pendse G, Borsook D, Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. NeuroImage. 2009;47:231–261. doi: 10.1016/j.neuroimage.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrovic P, Carlsson K, Petersson KM, Hansson P, Ingvar M. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci. 2004;16:1289–1301. doi: 10.1162/0898929041920469. [DOI] [PubMed] [Google Scholar]
- 59.Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 60.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 61.Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magnetic resonance imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, Ernst M. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:290–299. e292. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sacchetti B, Sacco T, Strata P. Reversible inactivation of amygdala and cerebellum but not perirhinal cortex impairs reactivated fear memories. The European journal of neuroscience. 2007;25:2875–2884. doi: 10.1111/j.1460-9568.2007.05508.x. [DOI] [PubMed] [Google Scholar]
- 65.Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwenkreis P, Janssen F, Rommel O, Pleger B, Volker B, Hosbach I, Dertwinkel R, Maier C, Tegenthoff M. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61:515–519. doi: 10.1212/wnl.61.4.515. [DOI] [PubMed] [Google Scholar]
- 67.Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PloS one. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. The journal of pain: official journal of the American Pain Society. 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 70.Sherry DD, Wallace CA, Kelley C, Kidder M, Sapp L. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15:218–223. doi: 10.1097/00002508-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Simons LE, Kaczynski KJ, Conroy C, Logan DE. Fear of pain in the context of intensive pain rehabilitation among children and adolescents with neuropathic pain: associations with treatment response. The journal of pain: official journal of the American Pain Society. 2012;13:1151–1161. doi: 10.1016/j.jpain.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: Evidence from neuroimaging. Human brain mapping. 2012 doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. The journal of pain: official journal of the American Pain Society. 2011;12:677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What does it take? Comparing intensive rehabilitation to outpatient treatment for children with significant pain-related disability. Journal of pediatric psychology. 2013;38:213–223. doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan EC, van de Sandt-Renkema N, Krabbe PF, Aronson DC, Severijnen RS. Quality of life in adults with childhood-onset of Complex Regional Pain Syndrome type I. Injury. 2009;40:901–904. doi: 10.1016/j.injury.2009.01.134. [DOI] [PubMed] [Google Scholar]
- 78.Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–39. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 79.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhu L, Sacco T, Strata P, Sacchetti B. Basolateral amygdala inactivation impairs learning-induced long-term potentiation in the cerebellar cortex. PloS one. 2011;6:e16673. doi: 10.1371/journal.pone.0016673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.