Abstract
Left ventricular assist device (LVAD) support has been used in the treatment of end-stage heart failure (HF), however use of anti-fibrotic co-therapies may improve prognosis. Natriuretic peptides (NPs) possess anti-fibrotic properties through their receptors, GC-A/GC-B/NPR-C. We sought to evaluate cardiac fibrosis and the endogenous NP system in end-stage HF with and without LVAD therapy and to assess the anti-fibrotic actions of the dual GC-A/-B activator CD-NP in vitro. Collagen (Col) protein content was assessed by Picrosirius Red staining and NPs, NP receptors, and Col I mRNA expression were determined by qPCR in LV tissue from patients in end-stage HF (n=13), after LVAD support (n=5) and in normal subjects (n=6). Col I mRNA and protein levels in cardiac fibroblasts (CFs) pretreated with CD-NP were compared to BNP or CNP pretreatment. The LV in end-stage HF was characterized by higher Col I mRNA expression and Col protein deposition compared to normal which was sustained after LVAD support. ANP and BNP mRNA expressions were higher while CNP was lower in end-stage HF LV. GC-A expression did not change while GC-B and NPR-C increased compared to normal LV. The changes in NP system expression were not reversed after LVAD support. In vitro, CD-NP reduced Col I production stimulated by TGF-beta 1 greater than BNP or CNP in CFs. We conclude that the failing LV is characterized by increased fibrosis and reduced CNP gene expression. LVAD support did not reverse Col deposition nor restore CNP production, suggesting a therapeutic opportunity for CD-NP.
Keywords: heart failure, fibrosis, heart-assist device, natriuretic peptides
1. INTRODUCTION
Cardiac fibrosis is a hallmark of end-stage heart failure (HF) and contributes to both HF progression and the need for replacement therapy with cardiac transplantation and mechanical assist devices. No new drugs have been approved for HF since 2001 and none have been approved for preventing or suppressing on-going cardiac fibrosis in HF.
The cardiac natriuretic peptide (NP) system is represented by peptides of myocardial or endothelial cell origin [1]. Specifically, atrial NP (ANP) and B-type NP (BNP) are endogenous activators of the particulate guanylyl cyclase A (GC-A) receptor and are produced in cardiomyocytes while C-type NP (CNP) is produced in endothelial cells and activates the GC-B receptor. All three peptides increase production of cyclic guanosine monophosphate (cGMP) with downstream activation of protein kinase G. All NPs also bind NP receptor-C (NPR-C) which is thought to serve as a clearance receptor as well as possessing anti-fibrotic properties [2]. In the heart, human cardiac fibroblasts (CFs) possess both GC-A and GC-B, the later in high abundance [2]. Activating these highly abundant GC-B receptors in rodents by continuous infusion of CNP for two weeks post myocardial infarction suppressed cardiac fibrosis and preserved left ventricular (LV) function [3]. In addition, we recently reported that 8 weeks of twice-daily subcutaneous injection of BNP to humans with stable HF resulted in reductions in LV mass and lower LV volumes by MRI and improved diastolic function by echocardiography [4].
A potential innovative therapy therefore for cardiac fibrosis in end-stage HF could be a novel dual GC-A and GC-B activating peptide. Indeed, we designed a dual NP receptor activating peptide, CD-NP, which has entered clinical testing in HF [5]. We have previously reported that CD-NP activates cGMP in CFs and reduces fibroblast proliferation [6]. Further, two week subcutaneous infusion of CD-NP in experimental cardiac fibrosis prevented fibrosis and improved diastolic function [7]. In addition to having direct actions on CFs, CD-NP also suppresses aldosterone via GC-A, which is highly expressed in the adrenal gland and regulates aldosterone production and release [7].
The current study was designed with several objectives and a special focus on end-stage human HF and cardiac fibrosis. First, we sought to investigate the presence of LV fibrosis in end-stage human HF by determining the content and production of the major extracellular matrix protein, collagen (Col) in hearts from patients undergoing cardiac transplantation and from myocardium obtained from patients before and following LV assist device (LVAD), as recent studies suggest cardiac fibrosis persists and/or increases with LVAD therapy [8-10]. We also examined gene expression of the endogenous cardiac NP system in end-stage HF LV myocardium, including NPR-C which possesses anti-proliferative properties [2]. Finally, we defined in vitro in cultured CFs, the anti-fibrotic actions of the GC-A agonist BNP, the GC-B agonist CNP, and the dual GC-A/B agonist CD-NP, hypothesizing CD-NP would possess greater fibro-inhibiting properties through dual GC-A/B receptor activation. Thus, these studies provide new insights into cardiac fibrosis in human end-stage HF and a potential anti-fibrosis treatment using the NP system, with an innovative first generation dual GC-A/B activator, CD-NP.
2. MATERIAL AND METHODS
2.1. Study population
Patients with New York Heart Association class III to IV HF who underwent cardiac transplantation or were implanted with LVADs (unsupported) and patients who underwent heart transplantation following LVAD therapy (supported) were studied. The study was approved by the Mayo Foundation Institutional Review Board.
2.2. LV tissue from normal and patients with end-stage HF
LV tissue from failing hearts was obtained from patients who were undergoing cardiac transplantation or receiving LVAD implantation (End-stage HF, n=13) and at the time of heart transplantation in the setting of LVAD therapy (post-LVAD, n=5). End-stage HF tissues were obtained from LV apical core which was removed at the time of LVAD implantation or LV lateral or antero-lateral wall, which did not have obvious myocardial infarction scar. Post LVAD tissues were LV lateral or antero-lateral wall which also did not have obvious scar from myocardial infarction. LV tissue from age- and gender matched normal subjects was obtained from ILS bio Inc. (Chestertown, MD) (normal, n=6). These tissues were snap frozen in liquid nitrogen and stored at −80°C or fixed for histology.
2.3. Quantifying interstitial fibrosis in LV tissues
For histology, fixed LV were dehydrated, embedded in paraffin, and sectioned at a thickness of 4 μm. Sections were stained with Picrosirius Red and analyzed as previously described [7, 11, 12].
2.4. Cell culture and treatment
CFs (Cell Applications Inc., San Diego, CA) were cultured in fibroblast growth media (Cell Applications Inc.). Passages 2 to 4 at 80% confluency were used. After serum deprivation for 24 hours, cells were stimulated with 5 ng/ml TGF-beta 1 (TGF; R&D systems, Minneapolis, MN) in DMEM (Gibco, Grand Island, NY) for 24 hours for mRNA expression or for 48 hours for protein expression, with or without 30 min preincubation of equimolar doses (10−8 and 10−6 M) of BNP (Phoenix Pharmaceutics Inc., Burlingame, CA), CNP (Phoenix Pharmaceutics Inc.) or CD-NP (Capricor Therapeutics Inc., Beverly Hills, CA).
2.5. Quantative RT-PCR
Total RNA was isolated from frozen tissue and CFs using the Trizol method and reverse transcribed to cDNA. For quantitative comparison, real-time RT-PCR with Universal ProbeLibrary hydrolysis dual-color probe sets was performed using a LightCycler480 System (Roche, Indianapolis, IN). Both negative and positive controls were included in each PCR reaction. All assays were carried out three times as independent PCR runs for each cDNA sample, and normalized by GUSB expression, which was determined to be the most stable housekeeping gene using the RealTime ready Human Reference Gene Panel (Roche). Sequences of primers are shown in Table 1.
Table 1. Human primer sequences.
| mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| ANP | 5′- CAG GAT GGA CAG GAT TGG AG -3′ | 5′- TCC TCC CTG GCT GTT ATC TTC -3′ |
| BNP | 5′- GCT TTG GGA GGA AGA TGG AC -3′ | 5′- GCA GCC AGG ACT TCC TCT TA -3′ |
| CNP | 5′- GGC TCG CCT TCT GCA AGA G -3′ | 5′- GCT TGA GGC CGA AGC AGC CC -3′ |
| GC-A | 5′- CGG CAT TCT GAT TGT CTC C -3′ | 5′- ACA GCT CCG AGG CCA GTT -3′ |
| GC-B | 5′- CTG CTT TGA TGC CAT AAT TGA C -3′ | 5′- CCA TGT AAG CAT CCC CAA TC -3′ |
| NPRC | 5′- ATT GCC ATG ACT GAT GTG GA -3′ | 5′- CGC ATT TCA AAA CGA CCT TC -3′ |
| Col I | 5′- GGG ATT CCC TGG ACC TAA AG -3′ | 5′- GGA ACA CCT CGC TCT CCA -3′ |
| GUSB | 5′- CCA TTC CTA TGC CAT CGT GT -3′ | 5′- TGG ACC AGG TTG CTG ATG T -3′ |
ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CNP, C-type natriuretic peptide; GC-A, Guanylyl cyclase receptor-A; GC-B, Guanylyl cyclase receptor-B; NPR-C, Natriuretic peptide receptor-C; Col I, Collagen type I; GUSB, Beta-glucuronidase.
2.6. Immunocytochemistry
Immunocytochemistry was performed on CFs fixed by 3% paraformaldehyde. A commercially available indirect immunoperoxidase kit (Vector Stain, Vector Laboratories, Inc. Burlingame, CA) was used as described previously [13]. Briefly, after blocking, cells were incubated overnight with a primary antibody for GC-A (1:100, Abcam, Cambridge, MA), GC-B (1:50 Novus Biologicals, Littleton, CO), NPR-C (1:25, Abcam), or Col I (1:100, Abcam). The sample incubated in non-immune horse serum (NHS) without primary antibody served as a negative control. The specificity was further confirmed by substitution of non-immune horse serum or PBS for primary antibody.
2.7. Cyclic GMP assay
Cells were incubated with NPs (10−8 and 10−6 M) for 10 min to assess intracellular cGMP activity. Briefly, cells were incubated in Hank’s balanced salt solution (Invitrogen, Carlsbad, CA) containing 20mmol/L N-[2-hydroxyethyl]piperazine-N′[2-ethanesulfonic acid], 0.1% bovine serum albumin, and 0.5 mmol/L 3-isobutyl-1-methylzanthine (Sigma, St. Louis, MO). Cells were lysed in 6% TCA and sonicated for 10 min. The samples were ether extracted four times in 4 volumes of ether, dried, and reconstituted in 300μl cGMP assay buffer and measured as previously described [2, 7].
2.8. Col I enzyme-linked immunosorbent assay
Protein concentrations of Col I were measured by a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instruction (Cosmo Bio. Co., LTD, Japan). In brief, cells were removed from dishes and incubated with 0.1mg/mL pepsin with acetic acid overnight at 4°C. After centrifugation, the supernatants were neutralized with 200mM Tris; 150 mM NaCl solution and used in the Col I ELISA. Samples and standards were mixed with biotin-labeled human anti-Col I polyclonal antibody, added to microtiter plate wells precoated with human Col I, and incubated for 1 hour. Each well was washed with washing buffer and incubated with HRP-labeled avidin for 1 hour. Following washes to remove unbound antibody-enzyme reagent, a substrate of peroxidase solution (3, 3′, 5, 5′-tetramethylbenzidine) was added to each well. After incubation for 20 minutes at room temperature, the enzyme reaction was stopped. Col I concentration was determined by comparing the optical density results to the standard curves. Intra-assay and inter-assay variations were 10.1 % and 9.4%, respectively.
2.9. Statistical analysis
The primary analyses of quantitative studies are expressed as means ± SEM. For comparison of normal distribution data, un-paired student’s t-tests for equal variance data, Welch test for non-equal variance data were used between groups. For non-nominal distributed data, Wisconsin rank sum test was performed. Nominal distribution of respective data was assessed by Shapiro-Wilk test. Nominal variables between groups were analyzed by Chi-square test. Statistical significance was accepted at p<0.05.
3. RESULTS
3.1. Patient characteristics
Table 2 reports patient characteristics. All patients in the post LVAD group received continuous flow LVAD (VentAssist) for 158.8 ± 33.5 days. Plasma BNP levels in post LVAD patients were significantly lower than those in end-stage HF patients. There were significant differences in medication between end-stage HF and post LVAD patients specifically related to more use of phosphodiesterase-5 inhibitors and anti-platelet agents in post-LVAD patients.
Table 2. Patient characteristics.
| Normals (%) (n=6) |
End-stage HF (%) (n=13) |
Post LVAD (%) (n=5) |
p* | |
|---|---|---|---|---|
| Age (yo) | 43 ± 6 | 57 ± 3 | 47 ± 5 | 0.10 |
| Male | 5 (83) | 11 (85) | 5 (100) | 0.35 |
| Dilated cardiomyopathy | — | 9 (69) | 3 (60) | 0.71 |
| Ejection fraction (%) | — | 17 ± 1 | 18 ± 4 | 0.69 |
| Plasma BNP (pg/ml) | — | 1761 ± 296 | 696 ± 369 | 0.04 |
| Past history | ||||
| Diabetes | — | 3 (23) | 0 (0) | 0.23 |
| Hypertension | — | 3 (23) | 2 (40) | 0.47 |
| Hyperlipidemia | — | 6 (46) | 1 (20) | 0.31 |
| Coronary artery disease | — | 3 (23.1) | 0 (0) | 0.24 |
| Arrhythmia | — | 4 (31) | 2 (40) | 0.71 |
| Chronic renal failure | — | 2 (15) | 1 (20) | 0.81 |
| Pulmonary disease | — | 4 (31) | 1 (20) | 0.71 |
| Medication | ||||
| Inotrope | — | 4 (31) | 0 (0) | 0.16 |
| Beta-blocker | — | 11 (85) | 4 (80) | 0.81 |
| ACEI/ARB | — | 9 (69) | 3 (60) | 0.71 |
| Spironolactone | — | 8 (62) | 1 (20) | 0.11 |
| Loop diuretics | — | 9 (69) | 6 (60) | 0.89 |
| Anti-arrythimics | — | 10 (77) | 4 (80) | 0.89 |
| Nitrate | — | 4 (31) | 1 (20) | 0.65 |
| PDE5 inhibitor | — | 0 (0) | 2 (40) | 0.02 |
| Anti-platelets | — | 6 (46) | 5 (100) | 0.04 |
| Statin | — | 6 (46) | 2 (40) | 0.81 |
| Warfarin | — | 8 (62) | 5 (100) | 0.10 |
BNP, B-type natriuretic peptide; ACE, angiotensin converting enzyme inhibitor; ARB,angiotensin receptor blockade; PDE5, phosphodiesterase-5.
p: End-stage HF vs post LVAD.
3.2. Col I mRNA expression and fibrosis in LV myocardium
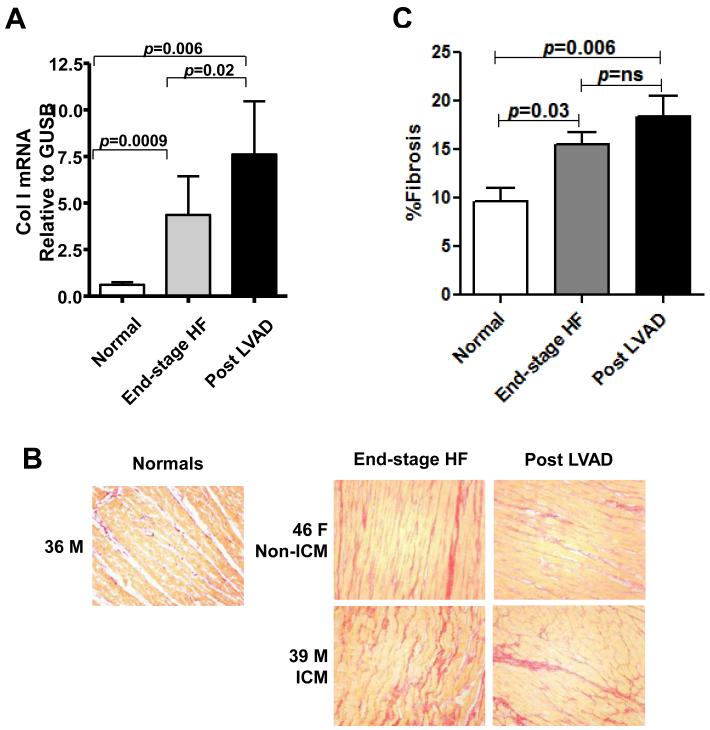
We assessed Col I mRNA expression (Fig. 1A) in LV tissues from normal, end-stage HF and post LVAD patients. As expected, Col I mRNA expression was significantly higher in end-stage HF patients compared to normal. Col I mRNA expression was significantly higher in LV from post LVAD compared to end-stage HF patients and normal, suggesting continued high production of ECM proteins in patients with LVAD support.
Figure 1. Fibrosis in normal, end-stage HF and post LVAD LV tissues.
A:Collagen (Col) I mRNA expression in normal, end-stage HF, and post LVAD LV tissues. B: Representative Picrosirius Red staining at × 20 objective magnification. 36 M, 36 years old male; Non-ICM, non-ischemic cardiomyopathy; 46 F, 46 years old female; 39 M, 39 years old male. C: Quantification of Picrosirius Red staining for interstitial Col deposition for normal, end-stage HF and post LVAD LV tissues.
We then quantified the area of fibrosis in LV from normals, end-stage HF and post LVAD patients. Representative staining for fibrosis (Col content) is shown in Figure 1B. We observed that Col area was significantly lower in normals compared to end-stage HF and post LVAD, and was comparable between post LVAD and end-stage HF subjects (Fig. 1C).
Taken together, these data suggest that increased Col production is a hallmark of end-stage HF which is sustained in the setting of LVAD therapy.
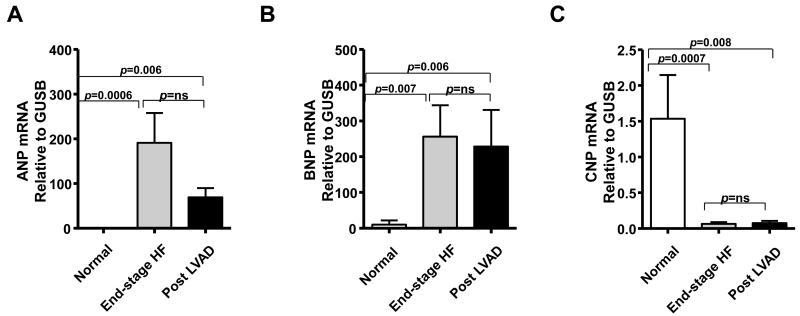
3.3. NPs mRNA expression in LV myocardium
We examined NP mRNA expression in the LV. Figure 2 illustrates ANP (Fig. 2A), BNP (Fig. 2B), and CNP (Fig. 2C) mRNA expressions in LV tissue from the 3 groups. ANP and BNP mRNA expressions in normal LV were low and increased as expected in end-stage HF patients. ANP mRNA tended to decrease in post LVAD subjects while BNP mRNA levels were sustained post LVAD (Figs. 2A and 2B). In contrast, CNP mRNA expression in both end-stage HF and post LVAD patients was lower than normal, with no significant difference between end-stage HF and post LVAD CNP (Fig. 2C). These results suggest that CNP production may be suppressed in end-stage HF, together with a trend for activated BNP and ANP production which tended to be suppressed after LVAD support.
Figure 2. NP mRNA expression in normal, end-stage HF and post LVAD LV tissues.
ANP (A), BNP (B), and CNP (C) mRNA expression in LV tissues.
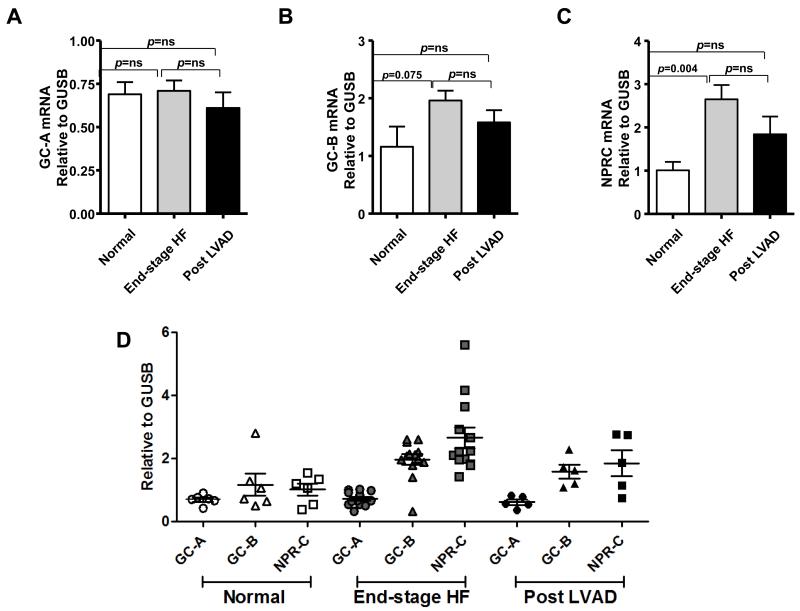
3.4. NP receptor mRNA expressions in LV myocardium
GC-A (Fig. 3A), GC-B (Fig. 3B), and NPR-C (Fig. 3C) mRNA expression was detected in normal LV. There was no significant difference in GC-A mRNA expression among the groups (Fig.3A). GC-B and NPR-C mRNA expression were higher in end-stage HF than in normal, with a trend to decrease for both post LVAD. (Figs. 3B and 3C). Figure 3D illustrates NP receptor expressions according to patient groups, which better demonstrates the reduction of GC-B and NPR-C mRNA expression post LVAD and also highlights the greater variability in expression of these two receptors in HF and post LVAD compared to normal and particularly compared to GC-A. These results suggest that GC-B and NPR-C may be involved in end-stage HF progression and while LVAD support improves the expression levels towards normal, it is insufficient to return these two receptors to completely normal levels.
Figure 3. NP receptor mRNA expression in normal, end-stage HF and post LVAD LV tissues.
GC-A (A), GC-B (B), and NPR-C (C) mRNA expression in LV tissues. D: Distribution of NP receptor mRNA expressions in each group.
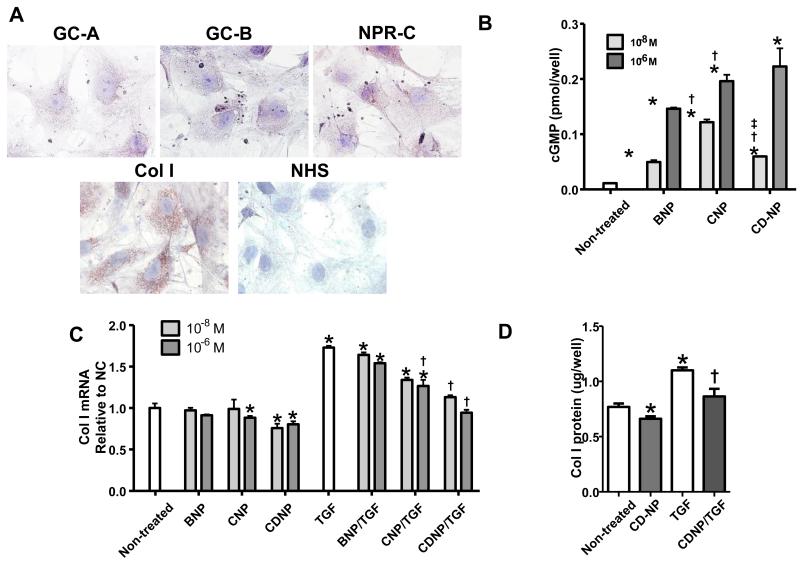
3.5. In vitro study: the anti-fibrotic effects of NPs in human CFs
We investigated the presence of NP receptor protein and Col I protein by immunohistochemistry in CFs. In Figure 4A, positive staining in CFs was observed for GC-A, GC-B, NPR-C and Col I, with relatively high levels of Col I and NPR-C. Next we examined cGMP activation in CFs by exogenous BNP; CNP and CD-NP (Fig. 4B). Cyclic GMP generation was increased in CFs by all NPs compared to non-treated cells. CD-NP and CNP have equal potency while BNP induced lower cGMP generation (Fig. 4B).
Figure 4. In vitro study in CFs.
A: Immunocytochemical staining for GC-A, GC-B, NPR-C, and Col I protein expression in CFs at × 100 objective magnification. B: cGMP generation in CFs stimulated by 10−8 and 10−6 M BNP, CNP, or CD-NP. Values are from three experiments and the mean of three samples per treatment. *p<0.05 vs non-treated cells, †p<0.05 vs BNP at the same concentration, ‡p<0.05 vs CNP at the same concentration. C: Col I mRNA expression with or without TGF beta-1 (5 ng/ml) treatment in CFs pretreated with or without 10−8 and 10−6 M BNP, CNP, or CD-NP. Values are from three experiments and the mean of three samples per treatment. *p<0.05 vs non-treated cells, †p<0.05 vs TGF. D: Col I protein expression with or without TGF beta-1 (5 ng/ml) treatment in CFs pretreated with or without 10−6 M CD-NP, determined by Col I ELISA. Values are from three experiments and the mean of three samples per treatment. *p<0.05 vs non-treated cells, †p<0.05 vs TGF.
Finally, we assessed Col I mRNA and protein expression with or without NPs pretreatment or TGF stimulation in CFs. At the mRNA level, pretreatment with CD-NP (10−8 M and 10−6 M) significantly suppressed Col I mRNA expression, CNP reduced Col I mRNA but only with 10−6 M pretreatment, while BNP had little effect on Col I mRNA. TGF stimulation significantly activated Col I mRNA expression compared to non-treated cells. Pretreatment with CD-NP at both dose concentrations and CNP at 10−6 M treatment suppressed TGF stimulated Col I mRNA, while BNP again had little effect (Fig. 4C). CD-NP also reduced Col I protein expression both in the absence and presence of TGF stimulation (Fig. 4D). These results suggest that CD-NP is a potent anti-fibrotic peptide regulating Col I production in human CFs in vitro.
4. DISCUSSION
As is well known, we observed that the LV myocardium of patients with end-stage HF is characterized by the presence of fibrosis. While less reported, we also observed the presence of cardiac fibrosis in a small number of patients with LVAD therapy, with widespread Col deposition and increased of Col I mRNA expression. In both end-stage HF and with LVAD therapy, the gene expression of GC-A remained preserved compared to normal human LV myocardium, while GC-B and NPR-C showed a trend to increase in HF which was partially improved in the presence of LVAD therapy. Myocardial ANP and BNP mRNA expression increased in end-stage HF, however CNP, which is the most anti-fibrotic of the NPs, is reduced in the failing LV and remains reduced with LVAD support. A novel anti-fibrotic therapy CD-NP was more potent than BNP, a GC-A activator, and CNP, a GC-B activator, in reducing Col I production in vitro in CFs. Thus, these highly translational studies provide new insights into cardiac fibrosis in failing human LV and the role the NP system may play in the progression of HF with and without LVAD support. Our studies also support the potential anti-fibrotic properties of CD-NP, which is in clinical trials in HF, as a novel pharmacological co-therapy to LVAD in end-stage HF.
The clinical significance of cardiac fibrosis in HF is that fibrosis contributes to impaired myocardial function, as well as arrhythmogenesis, which may lead to worsening clinical outcomes [14]. In the current study, end-stage HF was characterized by high Col I production and cardiac fibrosis in the LV myocardium compared to normals, even in the presence of LVAD therapy. Our findings reinforce recent studies that reported LVAD therapy showed continued progression of cardiac fibrosis by an increase in Col content [8, 9] or by increased Col cross-linking resulting in myocardial stiffness, although some studies have also observed decreases of collagen after LVAD therapy [10, 15, 16].
Previously, Kuhn and colleagues reported that ANP and BNP mRNA levels increased in HF with significantly lower levels during LVAD support. In addition they reported that GC-A mRNA stayed relatively level between normal, HF, and LVAD supported patients while NPR-C mRNA expression significantly increased in HF before LVAD and markedly decreased to near normal levels during LVAD [17]. We in part confirm their seminal report, showing similar results for ANP, BNP, GC-A and NPR-C mRNA expression, however in our studies, although BNP and NPR-C trended lower following LVAD, they did not achieve significance or a return to normal levels. It should be noted that it has been reported that LVAD implantation resulted in decreased circulating levels of BNP and/or NT-proBNP [18, 19] and that BNP protein is present in the LVAD supported heart [19]. Our results showed decreased circulating BNP levels (Table 2) and sustained BNP mRNA expression in LV tissues after LVAD support compared to end-stage HF (Figure 2A). Previously, we reported that BNP mRNA expression was observed strongly in atria in early and chronic HF canine models, whereas BNP mRNA expression was seen only in chronic – severe HF LV [20, 21]. In an experimental cardiac unloading model, the atrial BNP mRNA expression decreased but LV BNP mRNA expression did not [22]. These results suggest that the main source of circulating BNP may be atrium in response to cardiac loading and unloading.
Extending the study, we investigated CNP and GC-B mRNA expression, recognizing that CNP is the most potent native NP in inhibiting fibroblast proliferation and Col production in CFs[3]. CNP mRNA expression was much less in the failing LV, both with and without LVAD support (Fig. 2C) while GC-B mRNA expression tended to be higher in end-stage HF patients compared to normals (Fig. 3B). This reduction in CNP production in the LV myocardium may play a key role in the development of cardiac fibrosis as we reported previously in aging rat hearts [11]. LVAD support alone does not compensate for this deficiency, therefore, replacement therapy with CNP or a CNP-like peptide may be an effective anti-fibrotic strategy alone or in combination with LVAD. Although it has been reported that plasma CNP or NT-proCNP is increased in HF [23, 24] while the CNP precursor proCNP is unchanged [25], the source of elevated circulating CNP remains undefined, as CNP is released from not only the heart, but also from vascular endothelial cells, brain, heart, and lung [26]. In addition, different forms of CNP are expressed depending on cell type and species [27] with CNP53 predominating in tissues and CNP in plasma, suggesting the loss of CNP in HF LV may be heart specific [28]. Of note, all NP receptors are present in end-stage HF LV myocardium, either in the end-stage failing heart or post LVAD support, suggesting the end-stage failing LV myocardium may be responsive to NP therapy. Interestingly, there is differential regulation of NP production in the failing LV, with ANP and BNP, GC-A agonists, increasing while CNP, a GC-B agonist, decreases. The differential regulation of the NPs in the failing heart is associated with differential regulation of the proNP convertases corin and furin [13]. Specifically, in the experimental HF failing heart, corin, the proNP convertase for ANP and BNP, is reduced while furin, which processes proCNP to CNP, is increased. The mechanism of this differential regulation of the ANP, BNP and CNP genes and of corin and furin remain to be defined.
Currently, CNP is not considered a feasible therapeutic agent because of its short half-life [29]. For GC-B activation in humans, only CD-NP is approved for clinical investigation and is entering a Phase 2 trial in post acute HF to reduce rehospitalization [30]. CD-NP has potent properties to reduce CF proliferation in vitro and chronic subcutaneous delivery in a rodent model of early cardiac fibrosis and diastolic dysfunction in vivo inhibited cardiac fibrosis and preserved diastolic function [7]. This strong fibro-inhibiting property of CD-NP may in part be explained by its design as the only dual GC-A/B receptor agonist which would engage fibro-suppressing actions from both GC linked NP receptors. Our current study establishes that CD-NP is the most potent in the suppression of Col I production, as compared to BNP or CNP in vitro. Interestingly, cGMP stimulation by CD-NP was weaker (Figure 4B), however Col I inhibition by CD-NP was stronger than BNP or CNP (Figure 4C). The reason may be that CD-NP is more resistant to degradation which results in a longer half-life and bioactivity than BNP or CNP [31]. These findings support a potential therapeutic role for CD-NP in targeting the GC-A/GC-B/cGMP signaling pathway to suppression cardiac fibrosis. Indeed, a safety study in patients with LVAD support with subcutaneous CD-NP is currently underway at the Mayo Clinic (Clinicaltrials.gov: NCT01750905).
While the current study provides new insights into cardiac fibrosis and possible co-therapy in end-stage HF using LVAD combined with CD-NP, our study has limitations. Our study population was small and compared each mRNA expression by un-paired comparison among normal, end-stage HF and post-LVAD LV myocardium. Of note, end-stage LV tissues were obtained from LV free wall or apical core which has heterogeneity in fibrosis [32]. Finally, our findings regarding anti-Col I production by CD-NP is based on in vitro studies, and needs to be confirmed in an in vivo HF cardiac fibrosis model.
In conclusion, the human failing LV myocardium is characterized by an activated NP system with loss of CNP gene expression and an increase in cardiac fibrosis. Importantly, the NP/GC-A/GC-B system appears intact in end-stage HF and the novel dual GC-A/B activator CD-NP is a potent inhibitor of Col production in CFs compared to BNP or CNP. Thus CD-NP may represent a potential novel peptide therapeutic for the treatment of cardiac fibrosis. The findings lay the foundation into new directions for NP biology and potential therapeutics in HF.
Highlights.
LV in end-stage HF and with LVAD support had more fibrosis than normals.
CNP mRNA was deficient in end-stage HF which was not improved after LVAD.
CD-NP had more anti-fibrotic effects than BNP or CNP in vitro.
CD-NP may be potential CNP replacement and anti-fibrotic therapy in HF.
Acknowledgements
National Institute of Health (R01 HL36634 and P01 HL76611) awarded to Dr. John C. Burnett Jr., American Heart Association Post-Doctoral Fellowship (10POST3600045) and Scientist Development Grant (12SDG11460017) awarded to Dr. Tomoko Ichiki, and the Mayo Foundation.
Nonstandard Abbreviations and Acronyms
- HF
heart failure
- NP
natriuretic peptide
- GC
guanylyl cyclase
- cGMP
cyclic guanosine monophosphate
- NPR-C
natriuretic peptide receptor C
- CFs
cardiac fibroblasts
- LV
left ventricle
- Col
collagen
- LVAD
left ventricular assist device
- TGF
TGF beta-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Mayo Clinic has licensed CD-NP to Capricor Therapeutics.
REFERENCES
- [1].Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009:341–66. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209:943–9. doi: 10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- [3].Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, Mori K, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45:608–16. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- [4].Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. Journal of the American College of Cardiology. 2012;60:2305–12. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dickey DM, Burnett JC, Jr., Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–9. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–8. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin FL, Sangaralingham SJ, Huntley BK, McKie PM, Ichiki T, Chen HH, et al. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLOS ONE. 2012;7:e52422. doi: 10.1371/journal.pone.0052422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klotz S, Danser AH, Foronjy RF, Oz MC, Wang J, Mancini D, et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–74. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- [9].Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. Journal of the American College of Cardiology. 2010;56:382–91. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klotz S, Foronjy RF, Dickstein ML, Gu A, Garrelds IM, Danser AH, et al. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–74. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- [11].Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–7. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ichiki T, Boerrigter G, Huntley BK, Sangaralingham SJ, McKie PM, Harty GJ, et al. Differential Expression of the Pro-natriuretic Peptide Convertases Corin and Furin in Experimental Heart Failure and Atrial Fibrosis. Am J Physiol Regul Integr Comp Physiol. 2012;304:R102–9. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ichiki T, Boerrigter G, Huntley BK, Sangaralingham SJ, McKie PM, Harty GJ, et al. Differential expression of the pro-natriuretic peptide convertases corin and furin in experimental heart failure and atrial fibrosis. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R102–9. doi: 10.1152/ajpregu.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen TP, Qu Z, Weiss JN. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J Mol Cell Cardiol. 2014;70C:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- [16].Kato TS, Chokshi A, Singh P, Khawaja T, Cheema F, Akashi H, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circulation Heart failure. 2011;4:546–53. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kuhn M, Voss M, Mitko D, Stypmann J, Schmid C, Kawaguchi N, et al. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc Res. 2004;64:308–14. doi: 10.1016/j.cardiores.2004.07.004. [DOI] [PubMed] [Google Scholar]
- [18].Kemperman H, van den Berg M, Kirkels H, de Jonge N. B-type natriuretic peptide (BNP) and N-terminal proBNP in patients with end-stage heart failure supported by a left ventricular assist device. Clinical chemistry. 2004;50:1670–2. doi: 10.1373/clinchem.2003.030510. [DOI] [PubMed] [Google Scholar]
- [19].Bruggink AH, de Jonge N, van Oosterhout MF, Van Wichen DF, de Koning E, Lahpor JR, et al. Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2006;25:174–80. doi: 10.1016/j.healun.2005.09.007. [DOI] [PubMed] [Google Scholar]
- [20].Luchner A, Stevens TL, Borgeson DD, Redfield M, Wei CM, Porter JG, et al. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. American Journal of Physiology. 1998;274:H1684–9. doi: 10.1152/ajpheart.1998.274.5.H1684. [DOI] [PubMed] [Google Scholar]
- [21].Jougasaki M, Leskinen H, Larsen AM, Luchner A, Cataliotti A, Tachibana I, et al. Ventricular cardiotrophin-1 activation precedes BNP in experimental heart failure. Peptides. 2003;24:889–92. doi: 10.1016/s0196-9781(03)00163-3. [DOI] [PubMed] [Google Scholar]
- [22].Lisy O, Redfield MM, Schirger JA, Burnett JC., Jr. Atrial BNP endocrine function during chronic unloading of the normal canine heart. Am J Physiol Regul Integr Comp Physiol. 2005;288:R158–62. doi: 10.1152/ajpregu.00444.2004. [DOI] [PubMed] [Google Scholar]
- [23].Del Ry S, Cabiati M, Stefano T, Catapano G, Caselli C, Prescimone T, et al. Comparison of NT-proCNP and CNP plasma levels in heart failure, diabetes and cirrhosis patients. Regul Pept. 2011;166:15–20. doi: 10.1016/j.regpep.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [24].Zakeri R, Sangaralingham SJ, Sandberg SM, Heublein DM, Scott CG, Burnett JC., Jr. Urinary C-type natriuretic peptide: a new heart failure biomarker. JACC Heart Fail. 2013;1:170–7. doi: 10.1016/j.jchf.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nielsen SJ, Rehfeld JF, Pedersen F, Kastrup J, Videbaek R, Goetze JP. Measurement of pro-C-type natriuretic peptide in plasma. Clinical chemistry. 2005;51:2173–6. doi: 10.1373/clinchem.2005.053488. [DOI] [PubMed] [Google Scholar]
- [26].Palmer SC, Prickett TC, Espiner EA, Yandle TG, Richards AM. Regional release and clearance of C-type natriuretic peptides in the human circulation and relation to cardiac function. Hypertension. 2009;54:612–8. doi: 10.1161/HYPERTENSIONAHA.109.135608. [DOI] [PubMed] [Google Scholar]
- [27].Sellitti DF, Koles N, Mendonca MC. Regulation of C-type natriuretic peptide expression. Peptides. 2011;32:1964–71. doi: 10.1016/j.peptides.2011.07.013. [DOI] [PubMed] [Google Scholar]
- [28].Del Ry S, Passino C, Emdin M, Giannessi D. C-type natriuretic peptide and heart failure. Pharmacol Res. 2006;54:326–33. doi: 10.1016/j.phrs.2006.06.011. [DOI] [PubMed] [Google Scholar]
- [29].Lumsden NG, Khambata RS, Hobbs AJ. C-type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr Pharm Des. 2010;16:4080–8. doi: 10.2174/138161210794519237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, et al. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–73. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Cardiol. 2011;51:67–71. doi: 10.1016/j.yjmcc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Segura AM, Radovancevic R, Aguayo A, Frazier OH, Buja LM. Variability in fibrosis in tissue samples obtained during diaphragmatic and apical LVAD implantation. Cardiovasc Pathol. 2014;23:121–5. doi: 10.1016/j.carpath.2013.12.002. [DOI] [PubMed] [Google Scholar]