Abstract
Cervical facet joint injury induces persistent pain and central sensitization. Preventing the peripheral neuronal signals that initiate sensitization attenuates neuropathic pain. Yet, there is no clear relationship between facet joint afferent activity, development of central sensitization, and pain, which may be hindering effective treatments for this pain syndrome. This study investigates how afferent activity from the injured cervical facet joint affects induction of behavioral sensitivity and central sensitization. Intra-articular bupivacaine was administered to transiently suppress afferent activity immediately or 4 days after facet injury. Mechanical hyperalgesia was monitored after injury, and spinal neuronal hyperexcitability and spinal expression of proteins that promote neuronal excitability were measured on day 7. Facet injury with saline vehicle treatment induced significant mechanical hyperalgesia (p<0.027), dorsal horn neuronal hyperexcitability (p<0.026), upregulation of pERK1/2, pNR1, mGluR5, GLAST, and GFAP, and downregulation of GLT1 (p<0.032). However, intra-articular bupivacaine immediately after injury significantly attenuated hyperalgesia (p<0.0001), neuronal hyperexcitability (p<0.004), and dysregulation of excitatory signaling proteins (p<0.049). In contrast, intra-articular bupivacaine at day 4 had no effect on these outcomes. Silencing afferent activity during the development of neuronal hyperexcitability (4hr, 8hr, 1 day) attenuated hyperalgesia and neuronal hyperexcitability (p<0.045) only for the treatment given 4 hours after injury. This study suggests that early afferent activity from the injured facet induces development of spinal sensitization via spinal excitatory glutamatergic signaling. Peripheral intervention blocking afferent activity is only effective over a short period of time early after injury and before spinal modifications develop, and is independent of modulating spinal glial activation.
Keywords: Facet joint, glutamate, neuronal hyperexcitability, hyperalgesia, pain, bupivacaine
Introduction
Chronic pain from neck trauma or spinal pathology is a common clinical problem, with a 12-month prevalence of 30-50% in the general population [23]. The cervical facet joints are at risk for injury during abnormal neck motions and have been implicated in up to 60% of chronic pain cases [3,38,41]. Current treatments for facet joint-mediated pain include intra-articular injection of analgesics or corticosteroids, medial nerve block, and radiofrequency neurotomy of the nerve innervating the joint [2,31,39,42]. Although these treatments can provide relief in some patients, it is often only temporary. It remains unclear whether the effectiveness of local interventions for spinal joint pain depends on the timing of the treatment relative to the onset of trauma and/or pain or the development of central changes that mediate persistent pain.
Interrupting the neuronal signals that initiate central sensitization has been a focus of therapeutic approaches to preemptively reduce postoperative pain [7,55,59] and alleviate neuropathic pain following nerve injury [49,60]. For nerve injury, early intervention that blocks discharges from the injured fibers is more effective at reducing or preventing neuropathic pain than treatments initiated after central sensitization has already developed [1,20,49-51,60]. Injury to the cervical facet joint and its capsule is primarily a ligamentous injury but because the facet capsule is innervated there may also be neuropathic injury [6,29,44,61]. In fact, capsule stretch in several animal models induces both transient increases in firing of joint-innervating afferents similar to the injury discharge that accompanies nerve injury [6,25,40] and also the later development of ectopic firing and hyperexcitability in dorsal horn neurons [11,46]. Despite evidence of enhanced facet capsule afferent activity and spinal plasticity after joint injury, the temporal relationship between capsule afferent activity, the development of central sensitization, and pain after mechanical facet joint injury has not been defined and would inform timing of effective treatments.
This study investigates the role of afferent activity from the facet joint in inducing behavioral sensitivity and central sensitization in a rat model of facet capsule trauma. We hypothesize that the afferent activity from the facet occurring early after joint injury is critical for initiating central sensitization, and that quieting peripheral inputs from the joints after it is established is ineffective at attenuating central sensitization. To test this, painful facet capsule stretch was imposed in separate groups followed by intra-articular injection of bupivacaine at two times after injury – prior to and after neuronal hypersensitivity is established [11]. Behavioral sensitivity was measured for 7 days and then extracellular potentials were recorded from dorsal horn neurons to assess neuronal excitability. Indicators of glutamate signaling, the primary excitatory neurotransmitter in spinal nociceptive circuits, and astrocytic activation were quantified in the spinal cord, since both contribute to the spinal hyperexcitability associated with central sensitization [32,54]. Based on that work, a separate study administered bupivacaine to quiet joint afferent activity at different times throughout the period when sensitization develops in order to determine the extent to which the timing of afferent inputs modulates the transition to sustained pain.
Methods
All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [64]. Male Holtzman rats (395±30g) were housed under USDA- and AAALAC-compliant conditions with free access to food and water.
In order to investigate the effect of afferent discharge following joint injury in the initiation of central sensitization, rats underwent either a painful facet capsule injury or sham injury, followed by intra-articular injection of bupivacaine or a saline vehicle. Bupivacaine injections were made immediately after surgery (at day 0), to silence the immediate signaling of the afferents at the time of their injury and signaling to the spinal cord, or at 4 days after injury, a time when spinal hyperexcitability has already developed but afferent activity may still remain. Sensitization was assessed at day 7 by measuring mechanical hyperalgesia, neuronal excitability in the spinal dorsal horn, and spinal protein expression of glutamate receptors and transporters, as well as pERK and astrocytic activation, whose dysregulation can promote neuronal hyperexcitability. Based on findings of reduced neuronal firing and excitatory signaling in the spinal cord after immediate bupivacaine treatment in the joint, and the fact that both hyperalgesia and dorsal horn neuronal hyperexcitability have been shown to develop between 6 hours and 1 day after joint injury [11], quieting joint afferent firing at any time before the onset of neuronal hyperexcitability may attenuate sensitization. To test that hypothesis, separate groups of rats underwent intra-articular bupivacaine injections at 4 hours, 8 hours, or 1 day after injury to determine if blocking joint afferent activity at those time points during the development of spinal sensitization reduces the mechanical hyperalgesia and dorsal horn neuronal hyperexcitability that are induced by painful facet capsule injury.
Facet capsule injury with immediate or delayed intra-articular bupivacaine
Separate groups of rats received a bilateral intra-articular injection of bupivacaine either immediately after injury (inj-BP0h; n=12) or delayed at 4 days after injury (inj-BPd4; n=12). Similarly, separate groups of rats received control injections of the saline vehicle immediately following either the injury (inj-VEH0h, n=13) or a sham surgery (sham-VEH0h, n=12); as with the delayed bupivacaine treatment, groups also received saline vehicle at 4 days after injury (inj-VEHd4, n=11) or sham surgery (sham-VEHd4, n=12). Mechanical hyperalgesia was assessed prior to and at days 1, 3, 5, and 7 after surgery, and compared to baseline and between groups using a repeated-measures ANOVA with post-hoc Tukey's HSD tests. On day 7, extracellular electrophysiological recordings were acquired from a subset of rats that received intra-articular injections of bupivacaine (n=6 inj-BP0h, inj-BPd4) or saline (n=6 sham-VEH0h, inj-VEHd4, sham-VEHd4; n=7 inj-VEH0h). Spinal cord tissue was collected after electrophysiology testing for Western blot analysis; spinal cord samples were collected from the remaining rats in each group to assess region-specific expression of mGluR5, pNR1, and GFAP by immunohistochemical analysis in particular. Evoked neuronal firing and protein expression were compared between groups for each injection time by ANOVA with a post-hoc Tukey's HSD test.
Facet joint capsule injury
The surgical methods used to impose painful facet joint injury have been previously described [17,33,36]. Briefly, rats were anesthetized with isoflurane (4% induction, 2-3% maintenance) and placed in a prone position. An incision was made along the posterior midline of the back of the neck to expose and separate the paraspinal musculature. The bilateral vertebral laminae and facet joints from C5 to T1 were exposed, soft tissue resected, and the interspinous ligaments cut to enable attachment of the C6 and C7 vertebrae to a custom loading device [17,33]. The C6 vertebrae was distracted 0.7mm rostrally while the C7 vertebra was held fixed to stretch the facet capsules across the bilateral C6/C7 joints [17]. Separate rats underwent surgical procedures for a sham injury with attachment to the loading device but no joint distraction applied. Following surgery, incisions were closed using 3-0 polyester suture and surgical staples, and rats were monitored during recovery in room air.
Intra-articular bupivacaine injections
Intra-articular injections of 0.5% bupivacaine or 0.9% saline solution were given in a volume of 10μL injected into the left and right facet joints using a 10μL microsyringe with a 33G beveled needle (Hamilton; Reno, NV). The microsyringe was held in the joint for at least 30 seconds after injection before it was slowly removed in order to prevent fluid leakage from the joint space. For those injections given immediately after injury at day 0, intra-articular injections were administered following the injury or sham facet capsule loading, prior to closing the surgical incisions. For the intra-articular injections made on day 4, rats were anesthetized with isoflurane (4% induction, 2-3% maintenance), the paraspinal musculature was separated to re-expose the C6/C7 facet joints and injections performed as described above. All incisions were closed using 3-0 polyester suture and surgical staples, and rats were monitored during recovery in room air.
Assessment of mechanical hyperalgesia
Mechanical hyperalgesia was evaluated in all rats prior to and after injury and/or treatment, using methods detailed previously [16,29]. A series of weighted von Frey filaments was applied to the left and right forepaws in increasing weight (1.4, 2, 4, 6, 8, 10, 15, and 26g). Each filament was applied five times before advancing to the next strongest filament. If a rat responded to two consecutive filament weights by withdrawing, licking, or shaking the forepaw, the lower of those filament weights was recorded as the paw withdrawal threshold, with a maximum threshold of 26g. Testing was repeated in three rounds and the average of all rounds was calculated for each rat, by averaging the left and right paw withdrawal thresholds (mean±SD) [16,29].
Spinal cord electrophysiology & analysis of dorsal horn neuronal excitability
On the day of extracellular electrophysiological recordings, rats were anesthetized with sodium pentobarbital (45mg/kg, i.p.) and given supplementary doses (5-10mg/kg, i.p.), as needed based on toe pinch reflexes. The C6/C7 spinal cord was exposed by a bilateral laminectomy and dural resection, and the rat was immobilized on a stereotaxic frame using ear bars and a vertebral clamp at T2 to stabilize the cervical spine (David Kopf Instruments; Tujunga, CA). Core temperature was maintained at 35-37°C using a heating plate with a rectal probe (Physitemp; Clifton, NJ), and the spinal cord was bathed in 37°C mineral oil to prevent drying. Extracellular potentials were recorded using carbon fiber electrodes (Carbostar 1, Kation Scientific; Minneapolis, MN). Potentials were amplified with a gain of 103 and conditioned using a bandpass filter between 0.3kHz and 3kHz (World Precision Instruments; Sarasota, FL) and a 60Hz HumBug adaptive filter (Quest Scientific; North Vancouver, BC). Signals were then digitally sampled at 25kHz (Micro1401, CED; Cambridge, UK), and monitored with a speaker for audio feedback (A-M Systems; Carlsborg, WA).
The electrode was lowered by a micropositioner (Narishige; Tokyo, Japan) in the spinal dorsal horn by entering the pial surface of the C6 or C7 spinal cord. Neurons were selected for recording if they were responsive to mechanical stimulation of the forepaw by brushing with a cotton swab [9,46]. Once selected, a neuron was mechanically stimulated at the forepaw with a series of non-noxious and noxious stimuli, including a 2-second baseline period before each stimulus, 10 seconds of light brushing, five consecutive 1-second stimulations at 1-second intervals with a series of von Frey filaments (1.4, 4, 10, and 26g), and concluding with 10 seconds of noxious pinch by a 60g vascular clip [21,46]. Each stimulus was separated by at least 30 seconds to prevent windup of mechanically sensitive neurons. The von Frey filaments were chosen to represent the range of stimuli used to evaluate mechanical hyperalgesia and their application was synchronized with the electrophysiological recordings.
Individual potentials were sorted with Spike2 software (CED; Cambridge, UK) to ensure that spikes from only one neuron were considered for each recording site. Evoked firing was counted for a given stimulus (light brush, each von Frey filament, or noxious pinch) by totaling the number of spikes during mechanical stimulation and subtracting spontaneous firing as determined by the firing rate during the preceding baseline period [21]. Spike counts for each stimulus were log-transformed due to a positive skew in the distributions of the spike totals. Residuals were plotted after transformation to confirm a normal distribution.
Western blot analysis of spinal cord
Rats were transcardially perfused with 300mL of chilled phosphate-buffered saline (PBS). The C6/C7 spinal cord tissue was collected and homogenized in lysis buffer (50mM Tris-HCl, 1% Triton X-100, 150mM NaCl, 1mM EDTA, pH 8.0) with protease and phosphatase inhibitors (Sigma-Aldrich Corp; St. Louis, MO). SDS-PAGE was performed by loading spinal cord protein (50μg per well) on a polyacrylamide gel (Invitrogen; Carlsbad, CA) and running for 75 minutes at 150V. Protein was transferred to a polyvinylidene difluoride (PVDF) membrane using an iBlot (Invitrogen). Gels were cut into strips corresponding to high molecular weight (75-250kDa) and low molecular weight proteins (25-75kDa). Multiple high molecular weight or low molecular weight strips were transferred onto single PVDF membranes to allow synchronous detection of numerous protein targets from the same spinal cord samples and comparative analysis across multiple gels that were run simultaneously [27]. Membranes were blocked for 1 hour in 5% dry-milk blocking reagent in Tris-buffered saline (TBS).
The membranes were incubated overnight at 4°C with primary antibodies for the secondary signaling molecule, ERK, as an indicator of neuronal activation (mouse, 1:2000; Cell Signaling; Boston, MA), phosphorylated ERK (pERK; rabbit, 1:1000; Cell Signaling; Boston, MA), the ionotropic glutamate receptor NMDA subunit, NR1 (rabbit, 1:1000; Millipore; Billerica, MA) and its phosphorylated form (pNR1; rabbit, 1:667; Millipore; Billerica, MA), the metabotropic glutamate receptor mGluR5 (rabbit, 1:1250; Millipore; Billerica, MA), the astrocytic glutamate transporters GLAST (rabbit, 1:2000; Abcam; Cambridge, MA) and GLT1 (rabbit, 1:500, Abcam; Cambridge, MA), glial fibrillary acidic protein for activated astrocytes (GFAP; rabbit, 1:1000; Dako; Carpinteria, CA), or β-tubulin as a loading control (mouse, 1:2000; Covance; Princeton, NJ). The PVDF membrane was washed in TBS with 0.1% Tween, followed by 2 hour incubation at room temperature with goat anti-rabbit 800 and goat anti-mouse 680 fluorescent secondary antibodies (1:10,000; Li-Cor Biosciences; Lincoln, NE). Each membrane was imaged using an Odyssey Imaging System (Li-Cor). The fluorescence intensity of each target protein band was analyzed using the Odyssey 2.1 software and normalized to the corresponding -tubulin fluorescence to control for the amount of protein loaded.
Fluorescent immunohistochemistry of spinal cord
Rats were transcardially perfused first with 250mL of chilled PBS, then 300mL of 4% paraformaldehyde (PFA). The C6/C7 spinal cord was dissected out, post-fixed overnight in 4% PFA, and then submerged in 30% sucrose solution for 5-7 days for cryoprotection. Spinal cords were embedded in OCT medium and frozen, and then 14μm cryosections were mounted on Fisher Superfrost slides. Sections were blocked for 2 hours at room temperature with 10% normal goat serum with 0.3% Triton-X, and then labeled overnight at 4°C with primary antibodies for pNR1 (rabbit, 1:500; Abcam; Cambridge, MA), mGluR5 (rabbit, 1:1000; Millipore; Billerica, MA), or GFAP (mouse, 1:500; Millipore; Billerica, MA). Slides were rinsed with PBS and labeled with goat anti-mouse Alexa 488 and goat anti-rabbit Alexa 568 secondary antibodies, then coverslips were mounted with Fluorogel medium (Electron Microscopy Sciences; Hatfield, PA). The spinal dorsal horns were imaged at 200x using an Olympus BX51 microscope, and analyzed using densitometry techniques in a customized MATLAB code to quantify the percentage of pNR1-, mGluR5-, or GFAP-positive pixels in each image [16,30]. The percentage of positive pixels in the injury groups with vehicle or bupivacaine treatment were normalized to sham values and compared by one-way ANOVA with post-hoc Tukey's HSD test.
Facet capsule injury with intra-articular bupivacaine during spinal sensitization onset
The goal of the second study was to determine if and how blocking afferent activity at times during the development of spinal sensitization affects the onset of hyperalgesia and spinal neuronal hyperexcitability. Intra-articular bupivacaine injections were used to temporarily quiet facet joint afferent firing in separate groups with injections at additional times early after the injury (4 hours, 8 hours, and 1 day), These times are all before the development of hyperalgesia and neuronal hyperexcitability in the spinal cord [11]. Rats underwent facet joint capsule injury as described above and received bilateral intra-articular doses of bupivacaine at 4 hours (inj-BP4h; n=5), 8 hours (inj-BP8h; n=6), or 1 day (inj-BPd1; n=6) later. Mechanical hyperalgesia was assessed prior to injury and at days 1 and 7 after injury; responses were compared to pre-injury baseline responses by repeated-measures ANOVA with post-hoc Tukey's HSD test. On day 7, electrophysiological recordings were made to quantify dorsal horn neuronal activity as described above. Evoked neuronal firing was compared between bupivacaine-treated groups by ANOVA with a post-hoc Tukey's HSD test. Behavioral and electrophysiological data from the groups in the first study that received intra-articualr bupivacaine immediately or 4 days after injury were included in these statistical comparisons.
Results
Immediate, but not delayed, intra-articular bupivacaine attenuates mechanical hyperalgesia
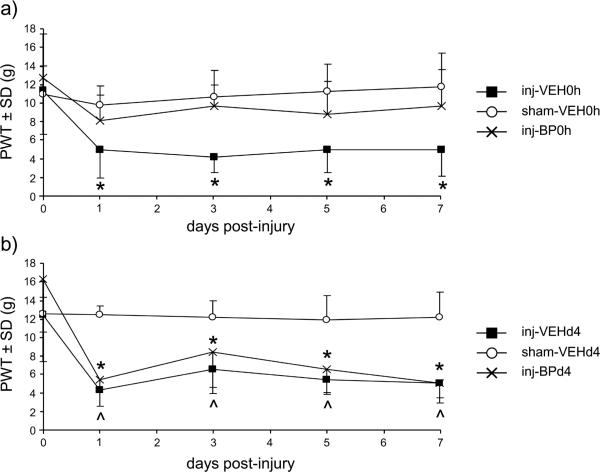
Facet capsule stretch with immediate saline vehicle treatment (inj-VEH0h) induces a significant decrease from the baseline paw withdrawal threshold (PWT) (p<0.027) at all test days after injury. PWT is not different from baseline on any day after the sham injury with vehicle treatment (sham-VEH0h) (Fig. 1a). However, rats receiving intra-articular bupivacaine immediately after facet injury (inj-BP0h) do not develop sensitivity at any day, with paw withdrawal thresholds remaining at baseline levels (Fig. 1a). The overall effect of treatment is also significant, with PWTs for the injVEH0h group being lower than those for the sham-VEH0h (p<0.0001) and inj-BP0h groups (p<0.0001). The behavioral response after either injury or sham with vehicle injection at day 4 is the same as when a vehicle injection is given immediately. Specifically, the inj-VEHd4 group exhibits significant decreases from baseline (p<0.003) on all days after injury and the paw withdrawal threshold remains at baseline levels in the sham-VEHd4 group (Fig. 1b). Unlike the bupivacaine treatment given at the time of injury (Fig. 1a), intra-articular bupivacaine given at 4 days after injury (inj-BPd4) does not attenuate sensitivity, with significant decreases from the baseline PWT at all testing days (p<0.001) (Fig. 1b). Furthermore, the PWTs are significantly lower overall for both the inj-VEHd4 and inj-BPd4 groups relative to the sham-VEHd4 group (p<0.0001).
Figure 1.
Behavioral sensitivity after intra-articular bupivacaine given either at injury or 4 days later. (a) Paw withdrawal threshold (PWT) decreases from baseline on all days (*p<0.027) after injury with immediate intra-articular saline (inj-VEH0h), but PWT after bupivacaine treatment at the time of injury (inj-BP0h) is not different from baseline on all days, as is observed with sham (sham-VEH0h). (b) In contrast, intra-articular bupivacaine given at 4 days after injury (inj-BPd4) has no effect on PWT, with decreased PWT compared to baseline on all days (*p<0.003), similar to the injury with a saline vehicle injection (inj-VEHd4) (^p<0.0001).
Dorsal horn neuronal hyperexcitability is prevented by immediate bupivacaine injection
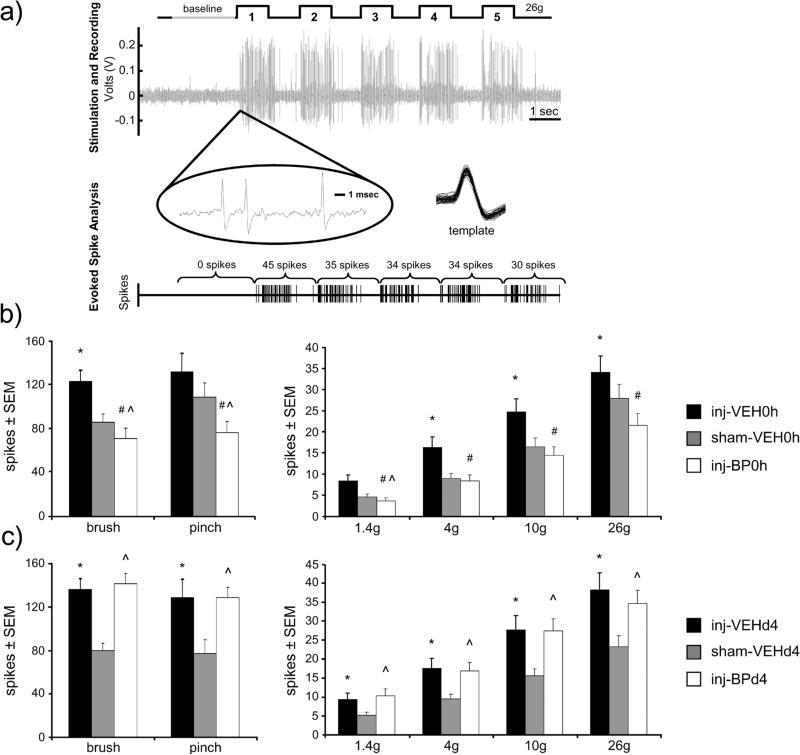
Neuronal activity in the dorsal horn was recorded from a total of 370 neurons (62±4 neurons per group) at an average depth of 638±177μm below the pial surface. In general, at day 7, neuronal activity increases after facet capsule injury in response to mechanical stimulation of the forepaw, but is reduced by bupivacaine administered at the time of injury (Fig. 2). Rats receiving saline vehicle at either time after injury exhibit increases in evoked firing of dorsal horn neurons relative to sham, with significant increases during light brush, and 4g, 10g, and 26g von Frey filament stimulation in the inj-VEH0h group (p<0.026) and increases during all stimuli in the inj-VEHd4 group (p<0.045) (Figs. 2b & 2c). However, immediate bupivacaine treatment (inj-BP0h) significantly reduces the number of spikes evoked on day 7 by light brushing (p<0.0001), noxious pinch (p=0.004), and all of the different strength von Frey filaments (p<0.0001) compared to the inj-VEH0h group (Fig. 2b). In fact, immediate bupivacaine reduces neuronal firing levels below that of sham firing levels during brush, pinch, and 1.4g von Frey filament stimulation (p<0.0007). Firing is also increased after injury with a day 4 vehicle treatment (inj-VEHd4) during all stimuli (p<0.045). In contrast to immediate bupivacaine treatment, bupivacaine given 4 days after injury (inj-BPd4) does not alter neuronal firing from those responses of the matching vehicle group (inj-VEHd4) at day 7 (Fig. 2c). In fact, evoked firing is increased in the inj-BPd4 group over sham in response to all mechanical stimuli (p<0.035) (Fig. 2c).
Figure 2.
Extracellular spike activity in the spinal dorsal horn 7 days after facet capsule injury. (a) Traces indicate the filament application, raw extracellular voltage recording, and neuron identification and spike counts. Neuronal firing was sorted and spikes were counted during a baseline period and mechanical stimulation of the forepaw; a representative response to five applications of a 26g von Frey filament 7 days after facet capsule injury is shown. (b) Firing increases after injury (inj-VEH0h) over sham (sham-VEH0h) during light brush, 4g, 10g, and 26g von Frey filament stimulation (*p<0.026). Immediate bupivacaine attenuates firing in response to all stimuli after injury (#p≤0.004). Bupivacaine treatment (inj-BP0h) also reduces activity below that of sham for brush, pinch, and the 1.4g von Frey filament (^p<0.0007). (c) During all stimuli, for injections at day 4, neuronal firing is increased over sham (sham-VEHd4) after injury with either vehicle injection (inj-VEHd4) (*p<0.045) and after bupivacaine injection (inj-BPd4) (^p<0.035).
Excitatory signaling is modified by immediate bupivacaine injection
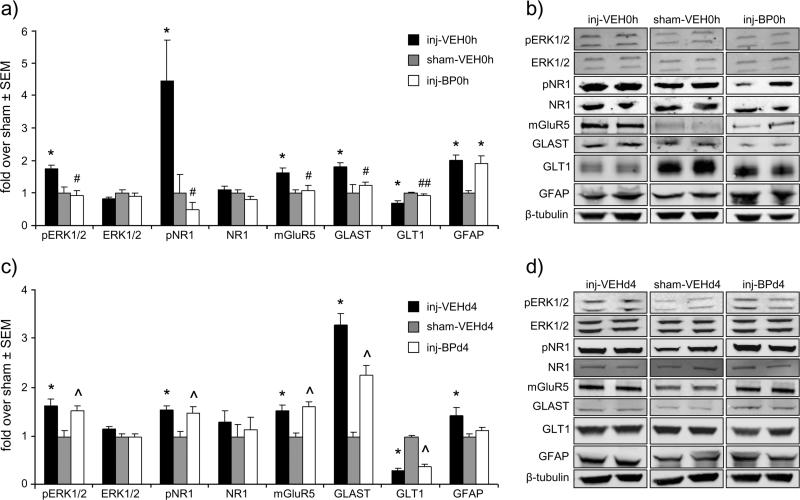
Several components of the glutamatergic system are increased in the spinal cord at day 7 after painful facet capsule injury and are attenuated with immediate bupivacaine administration in the joint (Fig. 3). Phosphorylated ERK1/2 (p<0.012), pNR1 (p<0.029), mGluR5 (p<0.026), and GLAST expression (p<0.023) all increase after injury, regardless of whether vehicle treatment is given at the time of injury or at day 4 (Fig. 3). However, immediate bupivacaine treatment prevents those increases in the expression of pERK1/2, pNR1, mGluR5, and GLAST (Figs. 3a & 3b), with expression in the inj-BP0h group being significantly different for each protein compared to that of the inj-VEH0h group (p<0.029), and not different from sham levels (Figs. 3a & 3b). GLT1 expression significantly decreases after injury with immediate (p=0.032) or day 4 (p=0.0001) vehicle injection. Immediate bupivacaine also prevents the decrease in GLT1, with expression significantly higher than the inj-VEH0h group (p=0.049) and not different from sham. Bupivacaine given at day 4 after injury does not prevent the increases in pERK1/2 (p=0.048), pNR1 (p=0.034), mGluR5 (p=0.0003), and GLAST (p=0.0006), or the decrease in GLT1 (p=0.0001), that are typically evident over sham (Figs. 3c & 3d). Total ERK1/2 and NR1 protein levels are not different from sham in any injury or treatment group. Immunolabeling localizes increases in pNR1 and mGluR5 after injury with immediate vehicle treatment (p≤0.046), and increases in pNR1, mGluR5, and GFAP after injury with day 4 vehicle treatment (p≤0.003) to the dorsal horn of the spinal cord (Fig. 4). However immediate bupivacaine treatment decreases labeling of pNR1 and mGluR5 to sham levels (p<0.0001), but treatment at day 4 does not change the injury-induced increases in pNR1 and mGluR5 (p≤0.0009) (Fig. 4). GFAP labeling also remains elevated over sham when injury is followed by immediate (p<0.0001) or day 4 (p=0.024) bupivacaine treatment (Fig. 4).
Figure 3.
Western blot of spinal cord at day 7. (a,b) Facet capsule injury (inj-VEH0h) increases pERK1/2, pNR1, mGluR5, GLAST and GFAP, and decreases expression of GLT1 (*p≤0.032). Immediate bupivacaine treatment (inj-BP0h) prevents such increases, with significantly lower expression (#p<0.029) of pERK1/2, pNR1, mGluR5, and GLAST than inj-VEH0h and no differences from sham (sham-VEH0h). Immediate bupivacaine also prevents the decrease in GLT1 expression that is evident after injury (##p=0.049). However, GFAP expression remains significantly elevated over sham (*) and is not different from the inj-VEH0h group. (c,d) Injury with vehicle treatment at day 4 (inj-VEHd4) induces the same changes relative to sham as does injury with vehicle treatment at the time of injury (inj-VEH0h) (*p≤0.046). However, pERK1/2, pNR1, mGluR5, GLT1, and GLAST remain at injury levels with bupivacaine treatment at day 4 (inj-BPd4), and are significantly elevated over sham (^p≤0.048). Total ERK1/2 and NR1 expression is unchanged in all groups.
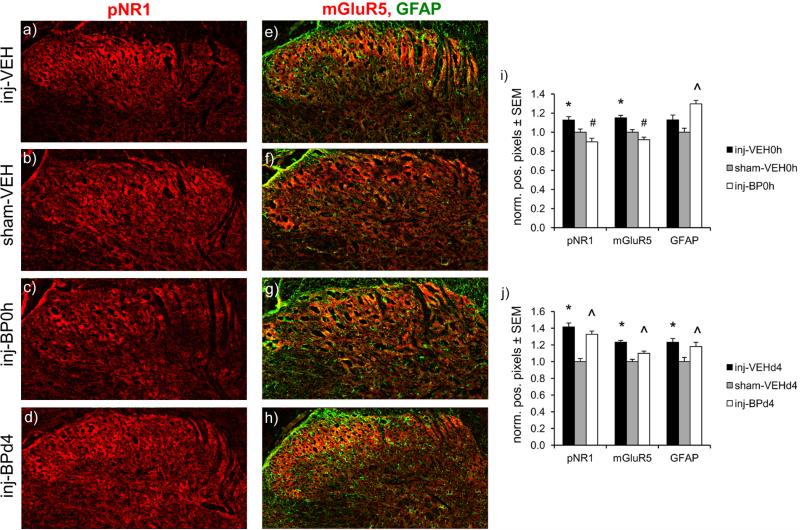
Figure 4.
Immunolabeling of phosphorylated NR1 (pNR1), mGluR5, and GFAP in the spinal dorsal horn at day 7. Representative images are shown for (a-d) pNR1 (red) and (e-h) mGluR5 (red) and GFAP (green) after injury, sham, and injury with immediate or day 4 bupivacaine treatment. (i) The percentage of pNR1- and mGluR5-positive pixels normalized to sham levels increases in the dorsal horn after injury with immediate vehicle treatment (inj-VEH0h) (*p 0.046). Immediate bupivacaine treatment (inj-BP0h) prevents those increases in both mGluR5 and pNR1 (#p<0.0001), although GFAP remains increased (^p<0.0001). (j) The normalized percentages of pNR1-, mGluR5-, and GFAP-positive pixels increase after injury with day 4 vehicle treatment, relative to sham levels (*p≤0.003). However, bupivacaine administered 4 days after injury (inj-BPd4) does not prevent the increases in pNR1, mGluR5, and GFAP (^p≤0.024). Scale bar=100μm.
GFAP expression, unlike the other proteins quantified in this study that are involved in excitatory signaling, is not attenuated by immediate bupivacaine treatment (Figs. 3a & 3b). In fact, GFAP significantly increases over sham after facet capsule injury with immediate vehicle injection (p=0.002), and is similarly increased even after immediate bupivacaine treatment (p=0.006) (Fig. 3a). For vehicle treatment given at day 4, GFAP expression is increased over sham (p=0.046), but is not different from sham levels with bupivacaine treatment given at day 4 (Fig. 3c). The Western blot findings are supported by the immunolabeling of GFAP in the dorsal horn (Fig. 4).
Intra-articular bupivacaine within 8 hours after injury attenuates spinal sensitization
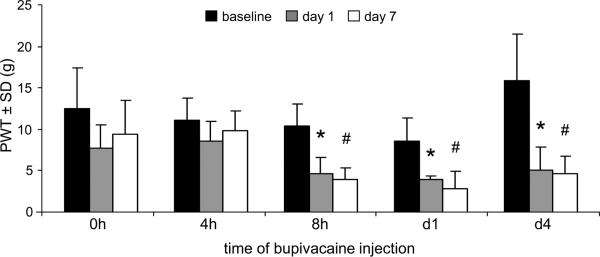
Bupivacaine treatment administered 4 hours after facet capsule injury prevents the development of mechanical hyperalgesia. Paw withdrawal thresholds at day 1 and day 7 are not different from baseline when rats receive intra-articular bupivacaine up to 4 hours after injury (Fig. 5); this is consistent with the PWT responses of the group receiving bupivacaine treatment immediately after injury in the first study (Figs. 1a & 5). However, paw withdrawal thresholds decrease from baseline values on both day 1 (p<0.006) and day 7 (p<0.0002) if intra-articular bupivacaine is given at either 8 hours or 1 day after facet capsule injury (Fig. 5). That finding is similar to the PWT pattern for the group (inj-BPd4) with bupivacaine treatment at 4 days after injury (Figs. 1b & 5).
Figure 5.
Bupivacaine administered immediately (0h) or 4 hours (4h) after facet capsule injury prevents any change from baseline paw withdrawal threshold (PWT) at day 1 and day 7 after injury. However, when bupivacaine treatment is given at 8 hours (8h), 1 day (d1), or 4 days (d4) after injury the PWT is significantly decreased from baseline levels on day 1 (*p<0.006) and day 7 (#p<0.0002).
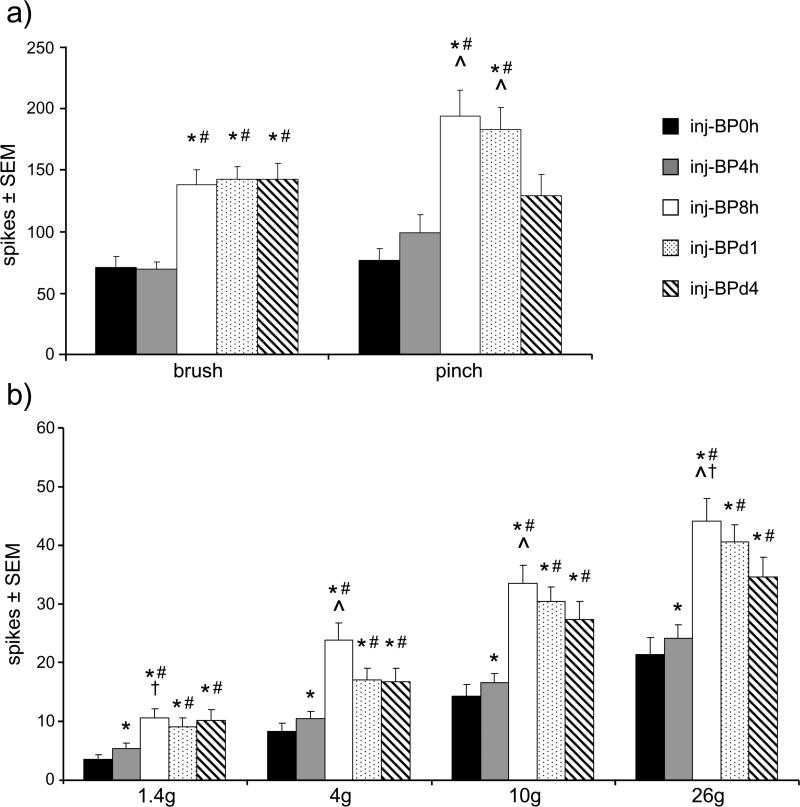
Evoked firing in dorsal horn neurons on day 7 exhibits the same general pattern as the behavioral responses (Figs. 5 & 6), with attenuation of the number of spikes by intra-articular bupivacaine injections that are given before 8 hours. Firing evoked by light brush is significantly lower in the inj-BP0h (p<0.0001) and inj-BP4h (p<0.0001) groups than in the groups receiving bupivacaine at later time points (inj-BP8h, inj-BPd1, inj-BPd4) (Fig. 6). The evoked neuronal response to noxious pinch is lower in the groups receiving bupivacaine immediately (p<0.0001), 4 hours (p<0.002), or 4 days after injury (p<0.018) than in either of the groups receiving intraarticular injections at either 8 hours or 1 day. Neuronal firing evoked by all of the von Frey filaments is also significantly lower after bupivacaine treatments either given immediately after injury (p<0.0001) or 4 hours later (p<0.045) compared to the firing evoked in the groups receiving bupivacaine injection at later times: 8 hours, 1 day, or 4 days post-injury (Fig. 6). However, immediate bupivacaine does attenuate firing significantly more than the 4 hour treatment at each von Frey filament strength (p<0.0001). In addition, firing is significantly greater in the group receiving bupivacaine at 8 hours after injury compared to those groups with treatment at 1 day and 4 days after injury, for isolated filament strengths. (Fig. 6b).
Figure 6.
Evoked spike activity in the dorsal horn on day 7 after facet capsule injury varies with timing of intra-articular bupivacaine. (a) Neuronal firing evoked by light brush is significantly higher when bupivacaine is administered at time later than 4 hours after injury than when it is given immediately (inj-BP0h) (*p<0.0001) or at 4 hours (inj-BP4h) (#p<0.0001). Firing during a noxious pinch is increased in the inj-BP8h and inj-BPd1 groups compared to the inj-BP0h (*p<0.0001), inj-BP4h (#p<0.002), and inj-BPd4 (^p<0.018) groups. (b) Similarly, firing evoked by stimulation by all of the magnitudes of von Frey filaments is significantly greater when bupivacaine is administered 8 hours (inj-BP8h), 1 day (inj-BPd1), or 4 days after injury (inj-BPd4) than when it is given immediately (inj-BP0h) (*p<0.0001) or at 4 hours (inj-BP4h) (#p<0.045). Firing is also greater after treatment given at 4 hours (inj-BP4h) than it is when given at the time of injury (inj-BP0h) for all von Frey filament stimuli (*p<0.0001). There is also significantly more evoked firing in the inj-BP8h group than the inj-BPd1 group for stimulation by the 1.4g and 26g filaments (†p<0.0003) and the inj-BPd4 group for the 4g, 10g, and 26 filaments (^p<0.0001).
Discussion
Behavioral sensitivity and neuronal hyperexcitability after painful facet capsule injury are induced within hours by afferent activity from the injured joint. The fast-acting anesthetic bupivacaine, given intra-articularly immediately after painful facet joint injury, prevents the development of behavioral sensitivity (Fig. 1) and also reduces neuronal hyperexcitability and dysregulation of excitatory glutamate signaling in the dorsal horn at day 7 (Figs. 2-4). The same is observed for intra-articular bupivacaine treatments given 4 hours after injury but not for later times (Figs. 5 & 6). Collectively, these data are the first to our knowledge utilizing peripheral joint injections in a controlled study in which the time after injury is known, and support the notion that the timing of joint afferent fiber blocks with respect to the development of spinal sensitization is critical for preventing the transition to persistent pain.
Intra-articular bupivacaine given at the time of facet capsule injury prevents the onset of mechanical hyperalgesia and neuronal hyperexcitability on day 7 (Figs. 1 & 2) suggesting that afferent inputs from the joint immediately after capsule loading are critical to induce sustained central sensitization. The painful joint injury used in this model induces joint capsular strains with the same magnitude as those strains that increase firing and afterdischarge for up to 30 minutes in unmyelinated C-fibers that innervate the capsule [6,33,40]. Stimulation of C-fibers induces sensitization of dorsal horn neurons that long outlasts the initial stimulation [10,53]. Furthermore, both capsule transection that removes any loading of the capsule and selective chemical ablation of spinal C-fiber populations prior to facet capsule injury each prevent the development of hyperalgesia [57,58]. So, C-fiber afferents in the facet likely play an important role in transducing mechanical loading of that joint's capsule into nociceptive signals regulating spinal sensitization. Small-diameter, peptidergic fibers have been identified in the rat cervical facet capsules indicating joint innervation by C-fibers that may be blocked by bupivacaine after intra-articular injection [24,29]; however, bupivacaine does not selectively affect C-fibers [19,52]. As such, additional studies that selectively and reversibly block C-fiber activity from the facet joint after injury are needed to determine if, and how, C-fiber firing after painful facet loading directly contributes to central sensitization.
Early afferent activity from the facet joint potentiates excitatory signaling that may cause neuronal hyperexcitability, as is evident in the attenuation of neuronal firing and glutamate signaling on day 7 by bupivacaine at the time of injury (Figs. 2-4). Peripheral afferent firing due to facet capsule loading may depolarize dorsal horn neurons sufficiently to remove the voltage-dependent magnesium block on ionotropic NMDA glutamate receptors, allowing rapid calcium influx that may induce numerous downstream effects leading to dorsal horn hyperexcitability [14,26,32,43]. For example, calcium-dependent activation of the secondary signaling molecule, PKC, [16,26] may increase NR1 phosphorylation in the dorsal horn after facet injury (Figs. 3 & 4). Activation of NR1, together with increases in pERK1/2 and mGluR5 (Figs. 3 & 4), can potentiate glutamate signaling and neuronal excitability [5,8,26,32]. Furthermore, decreases in spinal GLT1 (Fig. 3), the primary transporter responsible for synaptic glutamate clearance, may impair glutamate uptake, increasing synaptic glutamate and excitatory signaling [12,47]. Although this study did not classify phenotypes of the neurons from which recordings were made, the increased response to both innocuous and noxious stimuli (Figs. 2 & 6) suggests that there is enhanced excitatory signaling at the synapses between primary afferents and wide dynamic range neurons that integrate both non-nociceptive and nociceptive signals [9]. Since bupivacaine was used at a 0.5% concentration in the current study, it is expected to have a duration of action of several hours [18,28]. Unfortunately, the specific time between 4 and 8 hours when peripheral intervention becomes ineffective is not determined here since each bupivacaine administration likely blocks afferent activity for several hours. Nonetheless, silencing afferent firing for a period of several hours before the development of spinal plasticity likely returns dorsal horn neurons to resting membrane potential and allows NMDA receptors to recover their magnesium block, slowing the influx of calcium and the resulting potentiation of excitatory signaling [14].
In contrast to the robust effects of immediate bupivacaine injection, blocking afferent firing 8 hours or later after facet injury does not prevent mechanical hyperalgesia or neuronal hyperexcitability (Figs. 1-6). This suggests that once central sensitization has developed after joint injury it persists despite a transient absence of input from the joint. Despite being ineffective (Figs. 1 & 3), bupivacaine at day 4 reduces spinal neuronal firing compared to both the 8 hour and 1 day treatment groups (Fig. 6). This partial attenuation of selected components of neuronal hyperexcitability may be due to effects of the treatment that was given only 3 days before the measurements, rather than the 6-7 days earlier for the other groups. Studies observing extended post-treatment periods would better assess the persistence of sensitization after bupivacaine treatment. However, mechanical hyperalgesia (Fig. 1b), dorsal horn neuronal firing (Fig. 2c), and spinal expression of excitatory signaling proteins (Fig. 3b) after bupivacaine treatment at day 4 all remain elevated over sham at day 7, suggesting that despite decreased neuronal excitability after bupivacaine treatment at day 4, delayed treatment does not attenuate sensitization.
The differential effects of early (0h, 4h) and delayed (8h, 1d, 4d) bupivacaine treatment after facet injury (Figs. 5 & 6) suggest that there is a critical period lasting several hours after injury during which afferent activity from the joint is necessary to initiate central sensitization. Though, that timing identified in the rodent should not be taken as directly translating to that in the human due to lifespan differences between species. That critical period corresponds to the time before ectopic afferent activity develops, which occurs between 6 and 24 hours after painful facet injury in the rat [11]. Central hypersensitivity after whiplash injury is maintained in part by nociceptive input to the spinal cord [22]. Many studies suggest that aberrant spontaneous activity is also important for the maintenance of central sensitization, and treatments that suppress that spontaneous activity can reduce sensitivity for time when the treatment is active, after which the ectopic discharge resumes and restores sensitization [11,13,15,37,60]. The onset of spontaneous firing likely represents a temporal threshold after which sensitization persists despite blockade of joint afferent activity with nerve blocks or neurotomy [39,42]. The results of our study are consistent with previous reports of pain attenuation by anesthetic treatment administered early after nerve injury, before the onset of ectopic activity [1,36,51,60,63]. Xie et al. [60] found that local nerve block reduces mechanical hypersensitivity when started immediately after nerve injury and continued for 3-5 days, but not when started 10 days after injury. Although our findings (Figs. 5 & 6) support a transition to centrally-mediated responses, after which peripheral nerve block is ineffective, this study used a local, short-duration anesthetic block. It remains unclear whether other clinically relevant joint interventions or nerve blocks with longer durations of action could be more effective at reversing sensitization after it has already developed. However, clinical studies and data from other models of persistent pain do suggest that delayed treatment is not likely to permanently reverse pain regardless of treatment mode or duration of action [1,42,48,51,60].
Despite early intra-articular bupivacaine reducing behavioral and neuronal hypersensitivity, GFAP expression remains increased at day 7 after facet injury (Figs. 3 & 4), similar to previously reported increases in GFAP in dorsal horn astrocytes after facet injury [33,56]. Once activated, astrocytes regulate and mediate neuronal signaling through release of molecules including pro-inflammatory cytokines and glutamate [54]. It is possible that different hallmarks of astrocyte activation, like hypertrophy of processes and vimentin expression [45], or production of interleukin-1, that were not measured in this study, may more directly correlate to pain [30,34,54]. Spinal glial activation also may be insufficient to induce joint pain; spinal astrocytes and microglia have been reported to be in activated states despite analgesic treatments that attenuate joint or neuropathic pain [62]. Facet joint loading does induce spinal inflammation as early as 1 day after injury [30,34], so astrocytic activation observed even after attenuation of sensitization by early bupivacaine treatment may result from inflammatory signals that are independent of spinal sensitization [4].
In summary, these findings suggest that silencing afferent activity from the facet joint early after its injury is critical to block the spinal sensitization following painful facet capsule injury. Local intervention at the facet joint to block afferent activity can prevent sensitization, but only when initiated early after injury, before the development of spinal sensitization and spontaneous firing in injured capsule-innervating fibers. Our study indicates that it is crucial to consider the timing and type of local anesthetic joint injection for blocking the development and/or maintenance of persistent sensitivity. Indeed, this study suggests reasoning for why it is that such joint injections have varied and ineffective outcomes clinically. Perhaps better treatment strategies could be developed to determine if and when early interventions could be provided following traumatic joint injury to more effectively manage, or prevent, chronic joint-mediated pain.
Summary.
Joint afferent activity induces central sensitization after facet injury that can be prevented by transiently blocking afferent firing within a critical period early after injury.
Acknowledgements
This work was supported by grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288 & BIRT Supplement) and a Fellowship from the Ashton Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Araujo MC, Sinnott CJ, Strichartz GR. Multiple phases of relief from experimental mechanical allodynia by systemic lidocaine: responses to early and late infusions. Pain. 2003;103:21–29. doi: 10.1016/s0304-3959(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 2.Barnsley L, Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Region Anesth. 1993;18(6):343–350. [PubMed] [Google Scholar]
- 3.Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20(1):20–26. doi: 10.1097/00007632-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste EN. Inflammatory cytokines within the central nervous sytem: sources, function, and mechanism of action. Am J Physiol. 1992;263(1):C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 5.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20(2):375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Lu Y, Kallakuri S, Patwardhan A, Cavanaugh JM. Distribution of A-delta and C-fiber receptors in the cervical facet joint capsule and their response to stretch. J Bone Joint Surg. 2006;88:1807–1816. doi: 10.2106/JBJS.E.00880. [DOI] [PubMed] [Google Scholar]
- 7.Chen DW, Hu CC, Chang YH, Lee MS, Chang CJ, Hsieh PH. Intra-articular bupivacaine reduces postoperative pain and meperidine use after total hip arthroplasty: a randomized double-blind study. J Arthroplasty. 2014 doi: 10.1016/j.arth.2013.12.021. in press. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Huang LM. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 9.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotraum. 1997;14(8):517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 10.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 11.Crosby ND, Weisshaar CL, Winkelstein BA. Spinal neuronal plasticity is evident within 1 day after a painful cervical facet joint injury. Neurosci Lett. 2013;542:102–106. doi: 10.1016/j.neulet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Devor M. Ectopic discharge in A-beta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 14.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–62. [PubMed] [Google Scholar]
- 15.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. Neurobiol Dis. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L, Quindlen JC, Lipschutz DE, Winkelstein BA. Whiplash-like facet joint loading initiates glutamatergic responses in the DRG and spinal cord associated with behavioral hypersensitivity. Brain Res. 2012;1461:51–63. doi: 10.1016/j.brainres.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotraum. 2010;27(1):163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyhre H, Lang M, Wallin R, Renck H. The duration of action of bupivacaine, levobupivacaine, ropivacaine and pethidine in peripheral nerve block in the rat. Acta Anaesthesiol Scand. 1997;41:1346–1352. doi: 10.1111/j.1399-6576.1997.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 19.Gissen AJ, Covino BG, Gregus J. Differential sensitivity of fast and slow fibers in mammalian nerve: effect of etidocaine and bupivacaine on fast/slow fibers. Anesth Analg. 1982;61(7):570–575. [PubMed] [Google Scholar]
- 20.Gonzales-Darder JM, Barbera J, Abellan MJ. Effect of prior anesthesia on autotomy following sciatic transection in rats. Pain. 1986;24:87–91. doi: 10.1016/0304-3959(86)90029-1. [DOI] [PubMed] [Google Scholar]
- 21.Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23(26):8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di Stefano G, Radanov BP, Curatolo M. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med. 2004;5(4):366–376. doi: 10.1111/j.1526-4637.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 23.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Cote P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. The burden and determinants of neck pain in the general population. Eur Spine J. 2008;17:S39–S51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- 24.Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine. 2004;29(11):1182–1186. doi: 10.1097/00007632-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kallakuri S, Singh A, Lu Y, Chen C, Patwardhan A, Cavanaugh JM. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556–563. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Ven Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24(38):8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyatkin A, Aksamitiene E. Multistrip Western blotting to increase quantitative data output. Methods Mol Biol. 2009;536:149–161. doi: 10.1007/978-1-59745-542-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein SM, Greengrass RA, Steele SM, D'Ercole FJ, Speer KP, Gleason DH, DeLong ER, Warner DS. A comparison of 0.5% bupivacaine, 0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial plexus block. Anesth Analg. 1998;87:1316–1319. doi: 10.1097/00000539-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Kras JV, Tanaka K, Gilliland TM, Winkelstein BA. An anatomical and immunohistochemical characterization of afferents innervating the C6/C7 facet joint after painful joint loading in the rat. Spine. 2013;38(6):E325–E331. doi: 10.1097/BRS.0b013e318285b5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kras JV, Dong L, Winkelstein BA. Increased interleukin-1 & prostaglandin E2 expression in the spinal cord at day 1 after painful facet joint injury: evidence of early spinal inflammation. Spine. 2014;39(3):207–212. doi: 10.1097/BRS.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lander P. Selective cervical nerve blocks. In: Schweitzer ME, Laredo JD, editors. New techniques in interventional musculoskeletal radiology. first edition Informa Healthcare; New York: 2007. [Google Scholar]
- 32.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: Mechanical implications for whiplash & neck pain. Stapp Car C. 2004;48:373–396. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 34.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotraum. 2008;25(11):1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 35.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10(4):436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Lin SC, Yeh JH, Chen CL, Chou SH, Tsai YJ. Effects of local lidocaine treatment before and after median nerve injury on mechanical hypersensitivity and microglia activation in rat cuneate nucleus. Eur J Pain. 2011;15:359–367. doi: 10.1016/j.ejpain.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:203–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- 38.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash: a placebo-controlled prevalence study. Spine. 1996;21(15):1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 39.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. New Engl J Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanisms. Stapp Car C. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 41.Manchikanti L, Boswell MV, Singh V, Pampati V, Damron KS, Beyer CD. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord. 2004;5:15. doi: 10.1186/1471-2474-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manchikanti L, Singh V, Falco FJE, Cash KM, Fellows B. Cervical medial branch blocks for chronic cerical facet joint pain. Spine. 2008;33(17):1813–1820. doi: 10.1097/BRS.0b013e31817b8f88. [DOI] [PubMed] [Google Scholar]
- 43.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 44.McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine. 1994;19(5):495–501. doi: 10.1097/00007632-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 46.Quinn KP, Dong L, Golder FJ, Winkelstein BA. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–421. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169(4):1888–1900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy DF, Fleury J, Fontaine SB, Dussault RG. Clinical evaluation of cervical facet joint infiltration. J Can Assoc Radiol. 1988;39:118–120. [PubMed] [Google Scholar]
- 49.Seltzer Z, Beilin B, Ginzburg R, Paran Y, Shimko T. The role of injury discharge in the induction of neuropathic pain behavior in rats. Pain. 1991;46:327–336. doi: 10.1016/0304-3959(91)90115-E. [DOI] [PubMed] [Google Scholar]
- 50.Seltzer Z, Cohn S, Ginzburg R, Beilin B. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain. 1991;45:69–75. doi: 10.1016/0304-3959(91)90166-U. [DOI] [PubMed] [Google Scholar]
- 51.Shankarappa SA, Tsui JH, Kim KN, Reznor G, Dohlman JG, Langer R, Kohane DS. Prolonged nerve blockade delays the onset of neuropathic pain. P Natl Acad Sci USA. 2012;109(43):17555–17560. doi: 10.1073/pnas.1214634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabatabai M, Booth AM. Mechanism of action of local anesthetics on synaptic transmission in the rat. Anesth Analg. 1990;71:149–157. doi: 10.1213/00000539-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Wall PD, Woolf CJ. The brief and the prolonged facilitatory effects of unmyelinated afferent input on the rat spinal cord are independently influenced by peripheral nerve section. Neuroscience. 1986;17(4):1199–1205. doi: 10.1016/0306-4522(86)90087-4. [DOI] [PubMed] [Google Scholar]
- 54.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 55.Wei J, Yang HB, Qin JB, Kong FJ, Yang TB. Single-dose intra-articular bupivacaine after knee arthroscopic surgery: a meta-analysis of randomized placebo-controlled studies. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2543-7. DOI:10.1007/s00167-013-2543-7. [DOI] [PubMed] [Google Scholar]
- 56.Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27(12):2261–2271. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 57.Weisshaar CL, Winkelstein BA. Ablating spinal NK1-bearing neurons eliminates the development of pain and reduces spinal neuronal hyperexcitability and inflammation from mechanical joint injury in the rat. J Pain. 2014;15(4):378–386. doi: 10.1016/j.jpain.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkelstein BA, Santos DG. An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine. 2008;33(8):856–862. doi: 10.1097/BRS.0b013e31816b4710. [DOI] [PubMed] [Google Scholar]
- 59.Woolf CJ, Chong MS. Preemptive analgesia – treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 60.Xie W, Stron JA, Meij JTA, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamashita T, Cavanaugh JM, el-Bohy AA, Getchell TV, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72(6):865–870. [PubMed] [Google Scholar]
- 62.Yang F, Whang J, Derry WT, Vardeh D, Scholz J. Analgesic treatment with pregabalin does not prevent persistent pain after peripheral nerve injury in the rat. Pain. 2014;155(2):356–366. doi: 10.1016/j.pain.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Xie W, Xie Y. Spinal cord injury triggers sensitization of wide dynamic range dorsal horn neurons in segments rostral to the injury. Brain Res. 2005;1055(1):103–110. doi: 10.1016/j.brainres.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 64.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]