Abstract
BACKGROUND
While inspiratory muscle weakness is common in prolonged mechanical ventilation, inspiratory muscle strength training (IMST) can facilitate strengthening and ventilator weaning. However, the inspiratory load compensation (ILC) responses to threshold loads are not well characterized in patients. We retrospectively compared ILC responses according to the clinical outcomes of IMST (ie, maximum inspiratory pressure [PImax], weaning outcome), in difficult-to-wean ICU patients.
METHODS
Sixteen tracheostomized subjects (10 weaned, 6 unweaned) from a previous clinical trial underwent IMST 5 days/week, at the highest tolerated load, in conjunction with daily, progressive spontaneous breathing trials. PImax and ILC with a 10 cm H2O load were compared in the subjects before and after IMST. Changes in ILC performance were further characterized (5, 10, 15 cm H2O loads) in the trained subjects who weaned.
RESULTS
Demographics, respiratory mechanics, and initial PImax (52 ± 26 cm H2O vs 42 ± 13 cm H2O) did not significantly differ between the groups. Upon enrollment, PImax significantly correlated with flow ILC responses with the 10 cm H2O load (r = 0.64, P = .008). After IMST, PImax significantly increased in the entire sample (P = .03). Both before and after IMST, subjects who weaned generated greater flow and volume ILC than subjects who failed to wean. Additionally, ILC flow, tidal volume, and duty cycle increased upon ventilator weaning, at loads of 5, 10, and 15 cm H2O.
CONCLUSIONS
Flow ILC at a threshold load of 10 cm H2O in ventilated, tracheostomized subjects positively correlated with PImax. Although PImax improved in both groups, the flow and volume ILC responses of the weaned subjects were more robust, both before and after IMST. The results suggest that ILC response is different in weaned and unweaned subjects, reflecting dynamic inspiratory muscular efforts that could be influential in weaning.
Keywords: respiratory failure, respiratory muscle training, respiratory muscles, ventilator weaning
Introduction
Prolonged mechanical ventilation (MV) is associated with an imbalance between the respiratory load that must be overcome to produce air flow and the capacity of the inspiratory muscles to generate pressure.1,2 Patients who require MV have excessive ventilatory load that perturbs breathing. Resistive ventilatory load may occur due to airway obstruction by edema, bronchoconstriction, or mucus plugging, whereas pulmonary congestion, effusion, as-cites, or skeletal injury can create elastic loads. Additionally, chronic comorbidities such as COPD and obesity compound the acute resistive and elastic ventilatory loads.3,4 Patients with repeated weaning failure may have a tidal pressure load that exceeds 40% of the maximum transdiaphragmatic pressure.1
Both increased tidal breathing load and insufficient muscular capacity contribute to difficult weaning. Ventilatory muscle weakness impedes a patient's ability to compensate for unexpected breathing load, such as from an airway obstructed by mucus.5 Inspiratory weakness is prevalent yet potentially treatable in difficult-to-wean patients,6,7 and may influence the breathing pattern response to pulmonary intrinsic loads.2,6,8 Inspiratory muscle strength training (IMST) has shown promise to counteract inspira-tory muscle weakness.9,10 A controlled clinical trial from our laboratory demonstrated that IMST increased static maximum inspiratory pressure (PImax) and facilitated ventilator weaning in MV-dependent adults.11 A recent systematic review of IMST also found that higher-intensity training with pressure-threshold devices conferred greater strengthening benefits.12
During IMST the applied inspiratory load elicits an inspiratory load compensation (ILC) response, in an effort to maintain minute ventilation during the exercise sets. ILC is a term used to describe changes in ventilatory timing, flow, pressure, and volume in response to mechanical disturbances in breathing. The diaphragm and accessory muscles execute ILC motor responses. Healthy adults typically increase inspiratory flow and volume during repeated, moderate pressure-threshold loading, but inspiratory flow decreases with high-magnitude loads.13-15 However, ILC responses and the effect of training are not well understood in ventilated patients.
Therefore, the purpose of this study was to determine the ILC response to pressure-threshold load in difficult-to-wean, ventilated ICU patients who underwent IMST. We hypothesized that ILC volume and flow responses would be greater in patients who weaned, and that weaned patients would utilize different ILC strategies after IMST.
Methods
This retrospective cohort study was conducted with data from a subset of subjects from a larger, randomized, single-blind clinical trial of IMST and ventilator weaning (NCT00419458, University of Florida trial 420-1998).11 In that randomized trial, subjects were randomly assigned to either IMST or sham training. An initial spontaneous breathing trial (SBT) to failure was conducted, and baseline PImax was measured. On subsequent days, subjects received daily, protocolized ventilator weaning trials as well as their assigned training exercises, 5 days per week. The experimental period lasted up to 28 consecutive days or until weaning occurred.
Subject Recruitment and Screening
The subjects were adults with acute, prolonged MV dependence16 in the adult medical, surgical, and burn ICUs of a university teaching hospital. After resolution of the acute factors contributing to respiratory failure, tracheostomized subjects who failed to wean from MV with usual care were identified. The inclusion and exclusion criteria for the clinical trial have been detailed elsewhere,11 and include: age ≥ 18 years; stabilization of the underlying cause of respiratory failure; no evidence of hemodynamic deterioration with low doses of intravenous vasopressors; awake; spontaneously triggering the ventilator; able to follow one-step motor commands; required MV support with continuous spontaneous ventilation mode or pressure- or volume-controlled intermittent mandatory ventilation (≤ 6 breaths/min) mode; required pressure support of ≤ 15 cm H2O and PEEP ≤ 10 cm H2O; ≥ 3 previous failed attempts to breathe without MV support for 72 consecutive hours; no radiological evidence of hemi-diaphragm elevation; no progressive neuromuscular disease; anticipated life expectancy of at least 12 months; no musculoskeletal pathology that impeded chest wall movement; no preexisting MV dependence; and body mass index ≥ 40 kg/m2. The subjects or his or her designee consented to participate in the clinical trial.
We studied a subset of subjects from a previous clinical trial of IMST and ventilator weaning.11 Of the 35 clinical trial participants randomized to receive IMST, we retrospectively examined all subjects who completed ILC performance testing with standardized pressure threshold loads on their first and last training sessions. In 19 subjects, breath-by-breath ILC respiratory parameters were not available for at least one session. In the remaining 16 subjects the PImax and ILC responses of the 10 subjects who subsequently weaned from MV were compared to the 6 subjects who failed to wean. We separately compared the demographic characteristics from all 35 IMST subjects to the 16-subject subset and found no significant differences between the groups.
Inspiratory Muscle Strength Training
Subjects trained 5 days per week until they weaned from MV, for up to 28 calendar days. The IMST sessions were implemented by physical and respiratory therapists, and consisted of 4 sets of 6–10 best-effort breaths, using a positive expiratory pressure device (Threshold PEP, Philips Respironics, Murrysville, Pennsylvania), inverted to deliver an inspiratory threshold training load (Fig. 1). The subjects were encouraged to take very deep and rapid breaths, but they self-selected the breathing frequency of the training breaths. The IMST sets lasted < 1 min. Between sets the subjects rested on their baseline MV settings for 2–3 min. The IMST load was set at the highest setting that allowed inspiratory valve opening on every breath, with stable vital signs, and ≥ 50% of the inspired tidal volume (VT) achieved on resting MV settings.
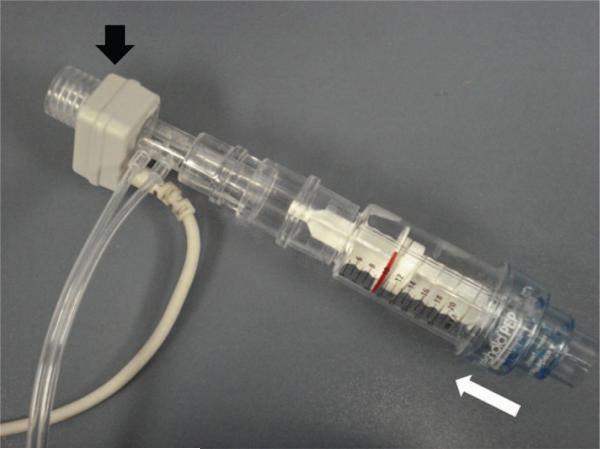
Fig. 1.
For inspiratory load compensation testing, this threshold positive expiratory pressure (PEP) training device was inverted and connected to a respiratory sensor (black arrow). The subject was briefly disconnected from the ventilator, and the sensor was connected directly to the tracheostomy tube. The valve of the training device remained closed until sufficient inspiratory pressure was generated to overcome the 10 cm H2O load. Once the threshold pressure was reached, the valve opened, permitting inspiratory air flow (white arrow). Expiration was unimpeded by the training device.
Ventilator Weaning Activities
Physicians, nurses, and therapists coordinated concomitant, protocolized ventilator weaning trials for the subjects, 7 days per week. An initial, unsupported SBT confirmed the diagnosis of failure to wean and determined the time goals for subsequent SBTs. The SBT protocol was used to standardize decision-making for daily weaning activities. The rate of progression for the prescribed daily SBT duration was developed to provide progressive respiratory muscle endurance conditioning (Fig. 2). Each morning the attending physician determined whether or not the subject was medically stable for an SBT. Respiratory care initiated each SBT. The subject was disconnected from MV and attached to supplemental oxygen via T-piece (FIO ≥ 0.4 to maintain SpO2 ≥ 92%). Heart rate and breathing frequency, blood pressure, electrocardiogram, and SpO2 were continuously monitored during the SBT. The SBT continued until the subject exhibited one or more physiological signs of weaning failure (Table 1) or the subject could not subjectively tolerate further unassisted breathing and requested to resume MV.
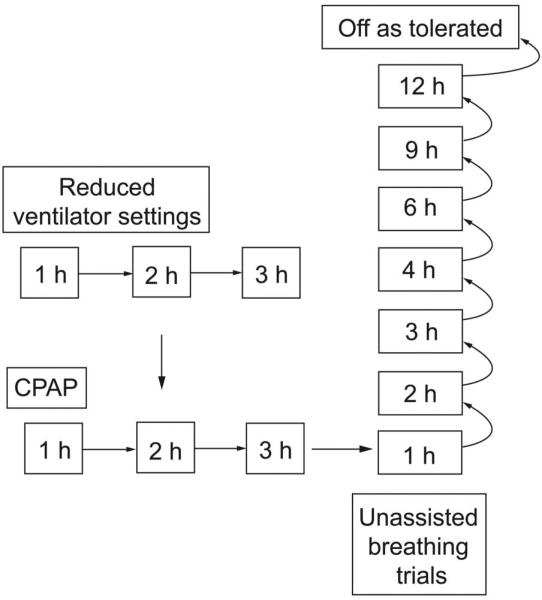
Fig. 2.
Progression of spontaneous breathing trials administered during inspiratory muscle strength training study interventions. Subjects who could not initially tolerate at least 1 hour of spontaneous, unassisted breathing (right) underwent trials of reduced pressure support or CPAP (left).
Table 1.
Criteria Used to Conclude a Spontaneous Breathing Trial
| Physiologic signs of spontaneous breathing trial failure |
| Increase in heart rate by ≥ 30 beats/min above resting, or heart rate 80% of age-predicted maximum |
| Systolic blood pressure > 180 mm Hg or < 90 mm Hg |
| SpO2 sustained < 90% for ≥ 5 min |
| Breathing frequency > 35 breaths/min for ≥ 5 min |
| Serious dysrhythmia |
| Evidence of impending fatigue: accessory muscle use, substernal retraction, sternocleidomastoid activation, paradoxical breathing, nasal flaring |
| Diaphoresis, pallor changes |
| Patient felt unable to continue, requested assisted ventilation |
The duration of subsequent SBTs was based on the subject's performance in the most recent SBT. Subjects who completed the assigned SBT were advanced to the next, longer scheduled time. Subjects who could not tolerate the assigned SBT duration attempted a shorter duration in the subsequent SBT. Occasionally, some subjects could not complete an SBT without MV support. These subjects underwent trials with reduced MV support (see Fig. 2). Subjects were considered successfully weaned when they could tolerate 72 continuous hours of breathing without ventilatory support (invasive or noninvasive). Subjects who could not complete the SBT protocol and did not achieve 72 ventilator-free hours at day 28 were considered unweaned.
Maximum Inspiratory Pressure
PImax was a primary clinical end point, and was measured using a 20-second inspiratory occlusion method previously validated for ventilated subjects.17 Briefly, the subject is removed from the ventilator and connected to a manometer and a one-way valve that blocks inspiration. Between the 20-second PImax efforts the subject rests on his or her baseline MV settings. The PImax measurement is repeated 3 times, and the most negative value is recorded.
Inspiratory Load Compensation
ILC responses were evaluated to determine their role in strengthening and weaning outcome. With the tracheostomy cuff inflated, the subject took 10–12 best-effort breaths using a threshold training device. The tests were first conducted during the initial IMST session (pre). Weaned subjects were tested again the morning prior to the protocolized liberation from MV (post). This corresponded to the day a 12-hour SBT trial was completed (see Fig. 2). ILC was not tested on the morning of liberation, to remove the risk that the performance test could acutely fatigue the subject. Subjects who did not reach 72 ventilator-free hours after liberation were considered not weaned, and resumed IMST and protocolized weaning trials. For subjects who could not breathe without MV for 72 continuous hours by day 28 (unweaned subjects), their final IMST session was treated as the “post” condition.
A 10 cm H2O load was used to determine whether ILC strategies differed between subjects who weaned and those who failed to wean. This load was selected because it was systematically administered to most subjects on their first IMST session, and all of the unweaned subjects used the 10 cm H2O pressure threshold load during their final training session. To further discern how the ILC responses to loads of different magnitude changed with IMST in weaned subjects, ILC performance was characterized using 5, 10, and 15 cm H2O threshold loads.
Data Analysis
Respiratory mechanics, PImax, and ILC performance were recorded with a respiratory monitor (Capnostat or CO2SMO, Philips Respironics, Murrysville, Pennsylvania) placed in series with the training equipment. Respiratory mechanics were measured for at least 15 min while the subject was in a rested state in the morning, prior to any respiratory testing or training. For ILC we recorded inspired tidal volume (VT), peak inspiratory flow (PIF), inspiratory time (TI), and expiratory time (TE). For each 10–12 breath ILC set, the 6 breaths with the highest combined inspiratory pressure and volume were averaged for statistical analysis.
The data were first tested to determine whether assumptions of normality were met. Based upon these results, group demographics and respiratory mechanics were compared with either the independent t test or the Mann-Whitney U test. The relationships between the PImax and ILC responses were assessed using Pearson correlation. Two-way repeated-measures analysis of variance (ANOVA) for training (pre, post), and weaning outcome (unweaned, weaned) was used to examine group differences in PImax and ILC. To specifically evaluate the effect of the load magnitude on ILC responses of weaned subjects, we conducted 2-way repeated measures ANOVA for training (pre-post) and load (5, 10, 15 cm H2O). Cell means contrasts were used to explore differences when significant interactions were present in ANOVA. The calculations were made with statistics software (SPSS 17.0, SPSS, Chicago, Illinois). Normally distributed data are reported as mean ± SD. Median and IQR are reported for demographic data that did not meet the assumption of normality. To illustrate the spread of the ILC responses we created box and whisker plots. Statistical significance was considered P < .05.
Results
Subject Demographics
Group characteristics are summarized in Table 2. Individual causes leading to prolonged MV can be found in the supplementary materials at http://www.rcjournal.com. There were no significant group differences in the demographics, respiratory mechanics, number of completed IMST sessions, or total days of study participation. Un-weaned subjects were ultimately discharged (n = 1), underwent surgery (n = 2), or experienced unrelated medical complications (n = 3).
Table 2.
Subject Demographics and Clinical Characteristics
| Weaned n = 10 | Unweaned n = 6 | P | |
|---|---|---|---|
| Age | 66 ± 16 | 68 ± 9 | .77 |
| Male/female, no. | 4/6 | 3/3 | |
| Prior mechanical ventilation days | 44 ± 23 | 48 ± 32 | .77 |
| Medical Research Council sum score | 31 ± 10 | 25 ± 10 | .23 |
| Simplified Acute Physiology Score II score | 35 ± 8 | 33± 6 | .56 |
| Organ failure, median (IQR) no. | 3 (2–3) | 3 (3–5) | .18* |
| Smokers, %, pack-years | 50, 57 ± 6 | 67, 63 ± 36 | .78 |
| Dynamic compliance, mL/cm H2O | 52.9 ± 18.1 | 55.9 ± 19.8 | .76 |
| Dynamic inspiratory resistance, cm H2O/L/min | 7.4 ± 3.4 | 8.3 ± 2.3 | .55 |
| Dynamic expiratory resistance, cm H2O/L/min | 7.7 ± 3.5 | 8.8 ± 2.5 | .51 |
| Days of IMST | 9.4 ± 4.2 | 9.2 ± 3.0 | .91 |
| Days IMST deferred, median (IQR) | 2 (1–3) | 8 (3–9) | .13* |
| Days of study participation, median (IQR) | 18 (11–23) | 25 (16–28) | .16* |
± Values are mean ± SD.
Via Mann-Whitney U test.
IMST = inspiratory muscle strength training
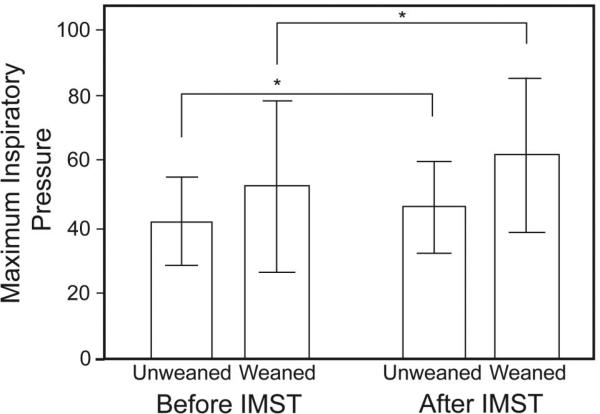
Initial PImax was comparable between the weaned (52.3 ± 25.8 cm H2O) and unweaned (41.8 ± 13.2 cm H2O) subjects, and PImax increased after training in both groups (weaned 61.9 ± 23.0 cm H2O, unweaned 46.3 ± 13.8 cm H2O, P = .03) (Fig. 3).
Fig. 3.
Maximum inspiratory pressure did not significantly differ between the weaned and unweaned groups (P = .24). In both groups the maximum inspiratory pressure increased after inspira-tory muscle strength training (IMST). * Analysis of variance main effect P = .03. The data bars represent the mean values and the whisker bars represent the standard deviations.
Relationship Between PImax and ILC Responses
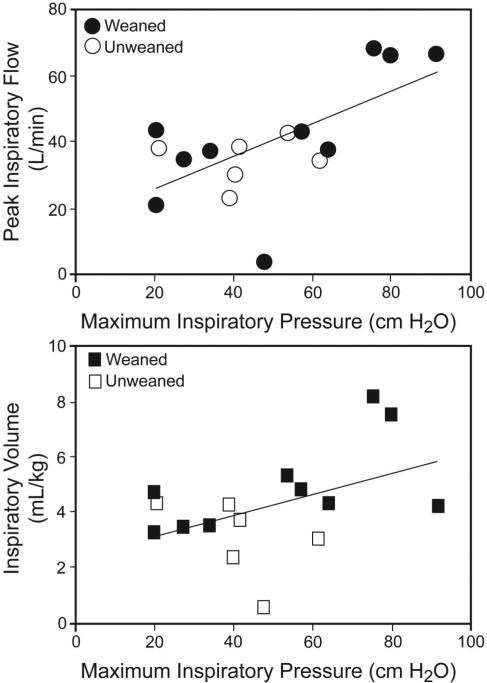
To investigate the relationship between PImax and ILC in difficult-to-wean subjects, separately from the influence of training, we conducted correlations in the 16-subject subgroup (Fig. 4) pre-IMST. With prolonged MV and prior to IMST, PImax significantly correlated only with PIF (r = 0.64, P = .008), indicating that the baseline flow ILC responses were greater in stronger subjects.
Fig. 4.
Correlation between maximum inspiratory pressure and inspiratory load compensation (ILC) ventilatory variables in the 16 difficult-to-wean subjects, prior to inspiratory muscle strength training (IMST), with a 10 cm H2O load. Before IMST the maximum inspiratory pressure in the subjects who ultimately weaned from mechanical ventilation after IMST (shaded data points) significantly correlated with peak inspiratory flow (r = 0.64, P = .008), and there was trend in inspired VT (r = 0.45, P = .08). The white data points represent the subjects who failed to wean. In contrast, no linear relationship was found between maximum inspiratory pressure and inspiratory or expiratory timing during ILC.
Load Compensation Differences Between Weaned and Unweaned Subjects
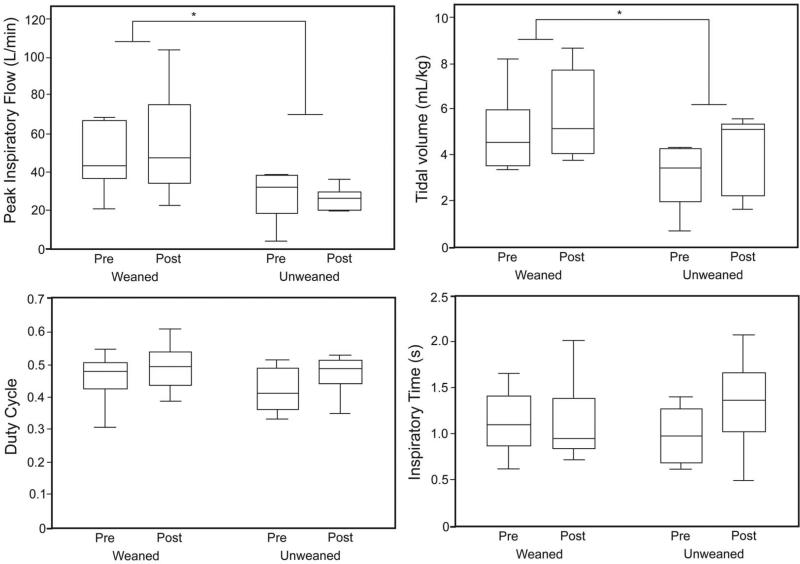
Next we looked at the influences of training and weaning outcome on ILC performance variables. An imposed 10 cm H2O ILC load represented a similar proportion of the average PImax at the outset of training (weaned group 25 (15)%, unweaned group 27 (11)% of PImax) as well as at its conclusion (weaned group 18 (6)%, unweaned group 24 (8)% of PImax). However, subjects who ultimately weaned from MV had higher PIF both before and after IMST: weaned pre-IMST 46.7 ± 16.1 L/min, weaned post- IMST 54.9 ± 28.7 L/min, unweaned pre-IMST 28.5 ± 13.4 L/min, unweaned post-IMST 25.9 ± 5.9 L/ min (P = .02). Likewise, the inspired VT response was larger in the weaned group before and after IMST: weaned pre-IMST 5.0 ± 1.7 mL/kg, weaned post-IMST 5.7 ± 1.9 mL/kg, unweaned pre-IMST 3.1 ± 1.4 mL/kg, unweaned post-IMST 4.2 ± 1.7 mL/kg (P = .10) than in subjects who failed to wean. There were no group or training differences in the ILC timing responses (TI, TE, ratio of TI to total respiratory cycle time [TI/Ttotal, aka duty cycle]) against the 10 cm H2O load (Fig. 5).
Fig. 5.
Inspiratory load compensation responses before and after inspiratory muscle strength training (IMST) in the unweaned versus weaned subjects, with a 10 cm H2O load. In the weaned subjects peak inspiratory flow was significantly greater (P = .02) and inspired tidal volume was significantly larger (P = .03), both before and after IMST. The duty cycle (ratio of inspiratory time to total respiratory cycle time), inspiratory time, and expiratory time (not shown) were similar between the groups. * Main effect for weaning outcome P < .05. In each data bar the horizontal line represents the median, the top and bottom of the bar represent the IQR, and the whisker bars represent the 95th percentiles.
After IMST the off-ventilator minute ventilation values were similar between the groups (weaned 7.3 ± 1.2 L/min, unweaned 7.9 ± 1.3 L/min), but breathing frequency trended slower in the weaned subjects: 25 ± 4 breaths/min vs 30 ± 4 breaths/min (P = .07).
Inspiratory Load Compensation Upon Ventilator Weaning
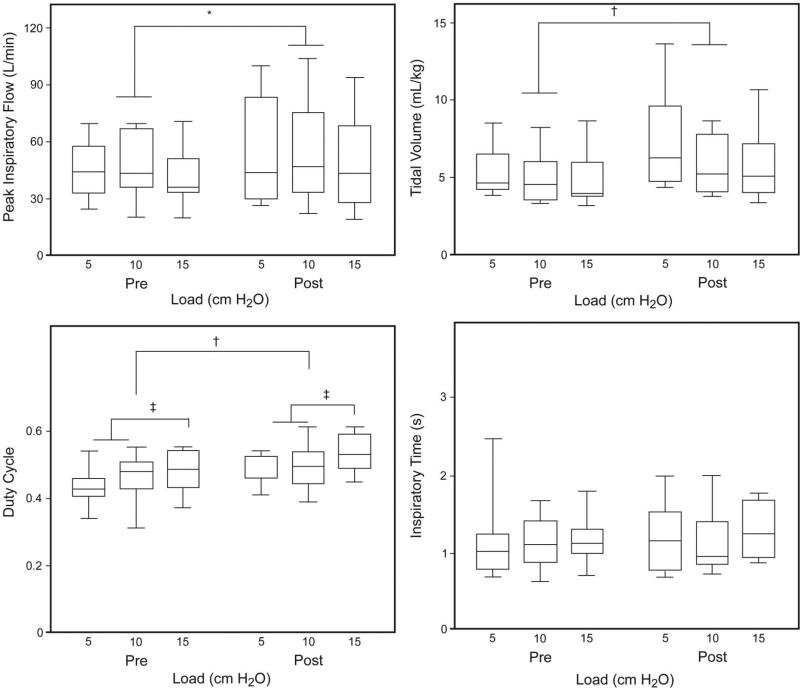
For the 10 subjects who weaned we evaluated whether the size of the inspiratory load was a significant influence on the magnitude of ILC training responses (Fig. 6). The average IMST training load of the 10 weaned subjects started at 10.2 ± 2.5 cm H2O and improved to 13.7 ± 3.0 cm H2O by the time of weaning. Post-IMST the weaned subjects had significantly higher PIF at all the loads (Table 3, main effect P = .03). Inspired VT was also larger after IMST (Table 4, main effect P = .007). Before and after IMST, TI/Ttotal was consistently greater with the highest threshold load (P = .048), and TI/Ttotal increased after IMST (P = .049). There were no individual differences in TI or TE.
Fig. 6.
Detailed inspiratory load compensation performance before and after inspiratory muscle strength training (IMST) in the weaned subjects. After IMST the peak inspiratory flow was significantly larger (* main effect P = .03), tidal volumes of all breaths were significantly larger († P = .007), and duty cycle was significantly longer († main effect for IMST P = .049). Duty cycle was also significantly longer with greater loads (‡ P = .048). In contrast, inspiratory time did not change after IMST. In each data bar the horizontal line represents the median, the top and bottom of the bar represent the IQR, and the whisker bars represent the 95th percentiles.
Table 3.
Peak Inspiratory Flow Before and After Inspiratory Muscle Strength Training
| Peak Inspiratory Flow* L/min |
||
|---|---|---|
| Before IMST | After IMST | |
| Load, cm H2O | ||
| 5 | 45.7 ± 14.4 | 54.1 ± 25.6 |
| 10 | 46.7 ± 16.1 | 54.9 ± 28.7 |
| 15 | 41.6 ± 15.4 | 48.3 ± 23.1 |
Values are mean ± SD.
P = .03 for main effect of training.
IMST = inspiratory muscle strength training
Table 4.
Inspired Tidal Volume Before and After Inspiratory Muscle Strength Training
| Inspired Tidal Volume* mL/kg |
||
|---|---|---|
| Before IMST | After IMST | |
| Load, cm H2O | ||
| 5 | 5.3 ± 1.7 | 7.5 ± 3.3 |
| 10 | 5.0 ± 1.7 | 5.7 ± 1.9 |
| 15 | 4.8 ± 1.7 | 5.7 ± 2.2 |
Values are mean ± SD.
P = .007 for main effect of training.
IMST = inspiratory muscle strength training
Discussion
Summary of New Findings
This study identified the characteristics of ILC responses to threshold loads, as a method to assess inspiratory muscle performance. Although we failed to find distinctions in PImax between weaned and unweaned subjects, we found that the flow and volume ILC responses were greater in subjects who ultimately weaned. There was a positive significant relationship between PImax and flow ILC. In subjects who weaned, IMST increased the flow, volume, and duty cycle of ILC responses increased across a range of different threshold load magnitudes.
ILC Responses and PImax
A methodological issue with PImax is that it measures static “strength” of the breathing pump. PImax is roughly analogous to a maximum voluntary contraction in the limb muscles.18 While this practical and noninvasive bedside technique is validated to estimate inspiratory muscle strength in clinical research and practice, the maneuver does not generate inspiratory air flow, so it does not measure dynamic motor performance (ie, muscle force-velocity) or take into account the effect of airway and pulmonary mechanics. Further, PImax is influenced by the lung volume and diaphragmatic conformation, which could make it more prone to measurement error.
In contrast to static PImax maneuvers, ILC specifically examines the capacity to generate repeated, dynamic muscle contractions. Although the flow response to inspiratory threshold loads was positively correlated to PImax, ILC tests may provide additional useful information beyond PImax about a patient's ability to detect breathing loads and generate pressure. This characteristic of ILC is related to the pressure-threshold load provided by the valve. In ILC the initial phase of a threshold-loaded inspiratory effort is occlusive, until sufficient pressure has been generated to open the inspiratory valve.19 The inspiratory timing of pressure-threshold ILC is relatively constant, because pressure-threshold loads are flow-independent,20,21 unlike inspiratory resistive loads.
ILC and Weaning Success
While this study was not specifically designed to examine predictors of ventilator weaning, significantly larger flow and volume ILC responses were apparent in the weaned subjects, even prior to IMST. ILC performance data in healthy adults indicated that the inspira-tory and expiratory times are preserved during loaded breaths, while inspired VT increases.13,15 Although the absolute flow and volume ILC of the weaned subjects remained well below the values reported in healthy subjects,14,15,19 the results indicated that weaned subjects were able to generate greater inspiratory muscle tension for ILC, producing larger inspired flows and volumes within the same TI. These findings may reflect a greater ability of weaned subjects to produce appropriate motor responses during an elevated work of breathing. Further study is required before we can determine whether specific ILC loads can best differentiate weaning outcomes.
Since strength and respiratory mechanics were similar for both weaning outcomes, other aspects of critical illness may have affected ILC performance of the un-weaned subjects, such as cardiac insufficiency. In a similar group of subjects selected for weaning, the presence of left ventricular diastolic dysfunction during SBT has been associated with weaning failure.22 The un-weaned subjects did not have significantly different other organ failure during their period of MV-dependence (see the supplementary materials at http://www.rcjournal.com). Alternatively, the unweaned subjects may have developed more extensive skeletal muscle cachexia as a result of their prolonged critical illness. The Medical Research Council sum scores revealed widespread ICU-acquired muscle weakness in both groups.23 While there were no group differences in the body mass index or Medical Research Council sum score, we cannot rule out the possibility that unweaned subjects had less overall muscle mass.
Relationship Between IMST and ILC Performance
This study was not designed to measure the training effectiveness of IMST. Since training did not significantly change ILC with the 10 cm H2O threshold load for either weaning outcome, it is possible that IMST identifies only patients with the available reserve needed to wean from MV. However, weaned subjects had greater flow, volume, and duty cycle ILC responses across a range of load magnitudes (5–15 cm H2O) after training. A recent systematic review indicated IMST increased inspiratory muscle strength in subjects weaning from MV.12 There is evidence that, much like strength training in limb muscles, IMST induces a multitude of compensatory neuromuscular responses that can be improved with repetition (training).3,24-28 A broader range of test loads may be needed to identify whether changes in ILC can help make distinctions in the effectiveness of IMST.
Besides the changes in the ILC response curve, the weaned subjects improved their unassisted breathing pattern during SBTs. After IMST the weaned subjects tended to have a lower breathing frequency, suggesting subjects sustained ventilation with a slower breathing pattern. The precise mechanisms by which IMST potentiates chronic ventilatory motor responses require future study.
Limitations
The magnitude of the findings could be limited by the small sample size available for analysis, and therefore IMST and weaning may yield additional differences in ILC that were not detected by this report. Additionally, the dependent variables were conscious, maximal-effort motor behaviors. Transdiaphragmatic pressure responses to evoked contractions provide the most specific assessment of diaphragm activation.18 However, the equipment and specialized training required for these non-volitional techniques are not available in many clinical weaning settings. The PImax occlusion test has been validated for ventilated adults,17 and we provided strong encouragement to standardize maximal subject efforts. We cannot rule out a familiarization effect in the subjects. However, threshold loading performance appears less susceptible to learning among subjects with existing lung disease than among healthy adults.29 Patient-generated dynamic inspiratory muscle contraction is the primary method to reliably achieve ventilator independence. Consequently, ILC performance tests may provide additional information for determining what patients will actually be able to accomplish during unassisted breathing trials for progressing ventilator weaning.
Conclusions
In tracheostomized subjects undergoing a clinical trial of IMST effects on liberation from MV,11 flow and volume ILC responses started significantly higher in weaned subjects and remained significantly greater after IMST. In contrast, PImax measurements were similar between weaned and unweaned subjects. ILC required subjects to generate dynamic contractions against inspiratory loads that differed from static PImax maneuvers to estimate strength. Among subjects who weaned, increased volume and flow ILC responses across a range of load magnitudes after IMST suggest a carryover effect. Thus flow and volume ILC measurements may provide additional insights when testing the muscular capacity of difficult-to-wean patients, including their eligibility for IMST. Future research is needed to determine whether ILC could offer any predictive value for weaning.
Supplementary Material
QUICK LOOK.
Current knowledge
Respiratory muscle weakness and ventilator-induced diaphragmatic dysfunction are increasingly recognized as complications of mechanical ventilation. The roles of the ventilation mode, spontaneous breathing, and inspiratory muscle training in prevention or treatment of ventilator-induced diaphragmatic dysfunction are not well defined.
What this paper contributes to our knowledge
In a small group of tracheostomized subjects, inspira-tory muscle strength training improved inspiratory load compensation response. Inspiratory load compensation response to an inspiratory threshold may identify patients who are ready to wean after prolonged mechanical ventilation.
Acknowledgments
This research was partly supported by National Institutes of Health grant R01HD42705 to Dr Martin, and training support grant NIH K12 HD055929 to Dr Kellerman Smith.
The University of Florida and Drs Martin and Gabrielli have applied for a patent to modify ventilators to provide threshold inspiratory muscle training to ventilated subjects.
Footnotes
Supplementary material related to this paper is available at http://www.rcjournal.com.
The other authors have disclosed no conflicts of interest.
Contributor Information
Barbara Kellerman Smith, Department of Physical Therapy, University of Florida, Gainesville, Florida.
Andrea Gabrielli, Department of Anesthesiology, University of Florida, Gainesville, Florida; Department of Surgery, University of Florida, Gainesville, Florida.
Paul W Davenport, Department of Physiological Sciences, University of Florida, Gainesville, Florida.
A Daniel Martin, Department of Physical Therapy, University of Florida, Gainesville, Florida; Department of Anesthesiology, University of Florida, Gainesville, Florida.
REFERENCES
- 1.Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1115–1123. doi: 10.1164/ajrccm.161.4.9812160. [DOI] [PubMed] [Google Scholar]
- 2.Vassilakopoulos T, Routsi C, Sotiropoulou C, Bitsakou C, Stanopoulos I, Roussos C, et al. The combination of the load/force balance and the frequency/tidal volume can predict weaning outcome. Intensive Care Med. 2006;32(5):684–691. doi: 10.1007/s00134-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 3.Hill K, Eastwood P. Effects of loading on upper airway and respiratory pump muscle motoneurons. Respir Physiol Neurobiol. 2011;179(1):64–70. doi: 10.1016/j.resp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 4.BaHammam A. Acute ventilatory failure complicating obesity hypoventilation: update on a ‘critical care syndrome’. Curr Opin Pulm Med. 2010;16(6):543–551. doi: 10.1097/MCP.0b013e32833ef52e. [DOI] [PubMed] [Google Scholar]
- 5.Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 6.Brochard L, Thille AW. What is the proper approach to liberating the weak from mechanical ventilation? Crit Care Med. 2009;37(10 Suppl):S410–S415. doi: 10.1097/CCM.0b013e3181b6e28b. [DOI] [PubMed] [Google Scholar]
- 7.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, et al. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37(4):1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jubran A, Tobin MJ. Passive mechanics of lung and chest wall in patients who failed or succeeded in trials of weaning. Am J Respir Crit Care Med. 1997;155(3):916–921. doi: 10.1164/ajrccm.155.3.9117026. [DOI] [PubMed] [Google Scholar]
- 9.Sprague SS, Hopkins PD. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther. 2003;83(2):171–181. [PubMed] [Google Scholar]
- 10.Chang AT, Boots RJ, Henderson R, Paratz JD, Hodges PW. Case report: inspiratory muscle training in chronic critically ill patients: a report of two cases. Physiother Res Int. 2005;10(4):222–226. doi: 10.1002/pri.14. [DOI] [PubMed] [Google Scholar]
- 11.Martin AD, Smith BK, Davenport P, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15(2):R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moodie L, Reeve J, Elkins M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. J Physiother. 2011;57(4):213–221. doi: 10.1016/S1836-9553(11)70051-0. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood PR, Hillman DR, Finucane KE. Ventilatory responses to inspiratory threshold loading and role of muscle fatigue in task failure. J Appl Physiol. 1994;76(1):185–195. doi: 10.1152/jappl.1994.76.1.185. [DOI] [PubMed] [Google Scholar]
- 14.Yan S, Bates JH. Breathing responses to small inspiratory threshold loads in humans. J Appl Physiol. 1999;86(3):874–880. doi: 10.1152/jappl.1999.86.3.874. [DOI] [PubMed] [Google Scholar]
- 15.Yanos J, Banner A, Stanko R, Gentry S, Greenawalt K. Ventilatory responses to inspiratory threshold loading in humans. J Appl Physiol. 1990;68(6):2511–2520. doi: 10.1152/jappl.1990.68.6.2511. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 17.Truwit JD, Marini JJ. Validation of a technique to assess maximal inspiratory pressure in poorly cooperative patients. Chest. 1992;102(4):1216–1219. doi: 10.1378/chest.102.4.1216. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society/European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 19.Huang CH, Martin AD, Davenport PW. Effects of inspiratory strength training on the detection of inspiratory loads. Appl Psychophysiol Biofeedback. 2009;34(1):17–26. doi: 10.1007/s10484-008-9073-y. [DOI] [PubMed] [Google Scholar]
- 20.Gosselink R, Wagenaar RC, Decramer M. Reliability of a commercially available threshold loading device in healthy subjects and in patients with chronic obstructive pulmonary disease. Thorax. 1996;51(6):601–605. doi: 10.1136/thx.51.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson PH, Cowley AJ, Kinnear WJ. Evaluation of the Threshold trainer for inspiratory muscle endurance training: comparison with the weighted plunger method. Eur Respir J. 1996;9(12):2681–2684. doi: 10.1183/09031936.96.09122681. [DOI] [PubMed] [Google Scholar]
- 22.Papanikolaou J, Makris D, Saranteas T, Karakitsos D, Zintzaras E, Karabinis A, et al. New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med. 2011;37(12):1976–1985. doi: 10.1007/s00134-011-2368-0. [DOI] [PubMed] [Google Scholar]
- 23.De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 24.Hawkes EZ, Nowicky AV, McConnell AK. Diaphragm and intercostal surface EMG and muscle performance after acute inspiratory muscle loading. Respir Physiol Neurobiol. 2007;155(3):213–219. doi: 10.1016/j.resp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Duiverman ML, van Eykern LA, Vennik PW, Koeter GH, Maarsingh EJ, Wijkstra PJ. Reproducibility and responsiveness of a noninvasive EMG technique of the respiratory muscles in COPD patients and in healthy subjects. J Appl Physiol. 2004;96(5):1723–1729. doi: 10.1152/japplphysiol.00914.2003. [DOI] [PubMed] [Google Scholar]
- 26.Romer LM, McConnell AK. Specificity and reversibility of inspiratory muscle training. Med Sci Sports Exerc. 2003;35(2):237–244. doi: 10.1249/01.MSS.0000048642.58419.1E. [DOI] [PubMed] [Google Scholar]
- 27.Smith BK, Martin AD, Vandenborne K, Darragh BD, Davenport PW. Chronic intrinsic transient tracheal occlusion elicits diaphragmatic muscle fiber remodeling in conscious rodents. PLoS One. 2012;7(11):e49264. doi: 10.1371/journal.pone.0049264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166(11):1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 29.Sturdy GA, Hillman DR, Green DJ, Jenkins SC, Cecins NM, Eastwood PR. The effect of learning on ventilatory responses to inspiratory threshold loading in COPD. Respir Med. 2004;98(1):1–8. doi: 10.1016/j.rmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.