Abstract
By selectively regulating the expression of the trans-dominant-negative mutant polypeptide UL9-C535C, of herpes simplex virus type 1 (HSV-1) origin binding protein UL9 with the tetracycline repressor (tetR)-mediated gene switch, we recently generated a novel replication-defective and anti-HSV-specific HSV-1 recombinant, CJ83193. The UL9-C535C peptides expressed by CJ83193 can function as a potent intracellular therapy against its own replication, as well as the replication of wild-type HSV-1 and HSV-2 in coinfected cells. In this report, we demonstrate that CJ83193 cannot initiate acute productive infection in corneas of infected mice nor can it reactivate from trigeminal ganglia of mice latently infected by CJ83193 in a mouse ocular model. Given that CJ83193 is capable of expressing the viral α, β, and γ1 genes but little or no γ2 genes, we tested the vaccine potential of CJ83193 against HSV-1 infection in a mouse ocular model. Our studies showed that immunization with CJ83193 significantly reduced the yields of challenge HSV in the eyes and trigeminal ganglia on days 3, 5, and 7 postchallenge. Like in mice immunized with the wild-type HSV-1 strain KOS, immunization of mice with CJ83193 prevents the development of keratitis and encephalitis induced by corneal challenge with wild-type HSV-1 strain mP. Delayed-type hypersensitivity (DTH) assays demonstrate that CJ83193 can elicit durable cell-mediated immunity at the same level as that of wild-type HSV-1 and is more effective than that induced by d27, an HSV-1 ICP27 deletion mutant. Moreover, mice immunized with CJ83193 developed strong, durable HSV-1-neutralizing antibodies at levels at least twofold higher than those induced by d27. The results presented in this report have shed new light on the development of effective HSV viral vaccines that encode a unique safety mechanism capable of inhibiting the mutant's own replication and that of wild-type virus.
Herpesviruses can cause both an acute productive infection and a long-term latent infection characterized by periodic recurrences (54). Among the human herpesviruses, herpes simplex virus types 1 and type 2 (HSV-1 and -2, respectively) are most closely related and share significant DNA sequence homology (15, 31, 32). Although HSV infections are often asymptomatic, their clinical manifestations include oral-facial infections, genital herpes, neonatal herpes, keratoconjunctivitis, and herpes encephalitis (48, 54). Despite the availability of various effective antiviral therapies, the spread of genital herpes infection continues to increase worldwide and has increased by 30% in the United States in the past two decades (19). The failure of antiviral therapy in effectively controlling the spread of HSV disease and the life-long nature of the infection present a strong need for developing safe and efficacious vaccines against HSV infections (48, 54).
HSV replicates in epithelial cells and establishes life-long latent infection in neuronal cell bodies within the sensory ganglia of infected individuals. During productive infection, HSV gene expression falls into three major classes based on the temporal order of their expression: immediate-early (α), early (β), and late (γ), with late genes being further divided into two groups, γ1 and γ2 (46). The expression of immediate-early genes is activated by the virion-associated protein VP16, together with cellular transcription factors when the viral DNA enters the nucleus (46). While the expression of viral α and β genes does not require viral DNA synthesis, expression of γ genes depends on de novo viral DNA synthesis. Specifically, expression of γ1 genes is enhanced by viral DNA replication, whereas expression of γ2 genes is inhibited in the absence of viral DNA synthesis. The protein products of the immediate-early genes are designated infected cell polypeptides ICP0, ICP4, ICP22, ICP27, and ICP47. Of the four immediate-early regulatory proteins, ICP0, ICP4, ICP22, and ICP27, only ICP0 can activate all classes of HSV genes (7, 8, 16, 17, 42, 43, 45). Although not essential for virus replication, ICP0 plays a major role in enhancing the reactivation of HSV from latency and confers a significant growth advantage on the virus at low multiplicities of infection (MOI) (7, 8, 29, 50). The major function of ICP47 during HSV infection appears to down-regulate the expression of the major histocompatibility complex (MHC) class I on the surface of infected cells (20, 23, 24, 62).
In the past three decades, various forms of HSV replication-defective viruses and neuroattenuated mutants have been generated (46). The efficacy of these viruses as potential vaccines against HSV infection has been evaluated in several different animal models (4, 5, 12, 18, 28, 33-35, 38, 41, 47, 52). For example, studies have shown that immunization with replication-defective mutants of HSV, such as an ICP8 or an ICP27 mutant virus capable of expressing viral α, β, and γ1 genes but not the γ2 genes, was effective in eliciting immune responses and in protecting against virulent HSV challenge (5, 12, 37, 38, 41). The ability of both replication-defective viruses and neuroattenuated mutants to establish life-long latent infection (9, 11, 14, 25, 26, 29, 30, 49, 51, 53) and the fact that they are replication competent in the context of wild-type virus do, however, pose a safety concern in using these recombinants in humans, particularly in individuals who harbor latent HSV infection.
With proper insertion of two tandem repeats of the tetracycline operator sequence (tetO) downstream of the TATA element in the human cytomegalovirus (hCMV) major immediate-early promoter, we recently established a novel tetracycline repressor (tetR)-mediated-gene switch (61) (T-REx, Invitrogen Inc., Calif.). By employing this gene switch, we constructed an HSV-1 recombinant virus (CJ83193) that encodes the dominant-negative mutant polypeptide UL9-C535C, of HSV-1 origin binding protein UL9 under control of the tetO-bearing hCMV major immediate-early promoter (57). It was demonstrated that the UL9-C535C peptide synthesized in CJ83193-infected non-tetR-producing cells or tetR-producing cells in the presence of tetracycline can function as a potent repressor of its own de novo viral production. Of particular significance is the demonstration that the UL9-C535C peptide expressed from CJ83193 can function in trans to inhibit the replication of wild-type HSV-1 and HSV-2 in cell cultures (56, 57). The ability of CJ83193 to inhibit the replication of wild-type HSV-1 has also been demonstrated in the central nervous systems of mice coinoculated with HSV-1 and CJ83193 (F. Yao and H. Augustinova, unpublished data). Given this unique dominant-negative effect of CJ83193 on the replication of wild-type HSV and its replication-defective nature in normal cells, we explored the vaccine potential of CJ83193 in a mouse ocular model of HSV-1 infection. The results demonstrate that CJ83193 can serve as an effective vaccine against HSV-1 infection in mice at levels comparable to those of wild-type HSV-1. Moreover, CJ83193 is capable of eliciting both durable humoral and cell-mediated immunity close to or equivalent to that induced by immunization with wild-type HSV-1 and is more effective than that induced by an ICP27 deletion mutant, d27.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) cells and the osteosarcoma line U2OS were grown and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (59). U2OS cells express a cellular activity that can substitute functionally for HSV-1 regulatory protein ICP0 (59). U2CEP4R11 cells, a tetR-expressing cell line derived from U2OS cells, were grown and maintained in the above-described growth medium in the presence of hygromycin B at a concentration of 50 μg/ml (61).
Wild-type HSV-1 (strains KOS and mP) was propagated and assayed on Vero cell monolayers (7). Strain mP was a gift from David M. Knipe (Harvard Medical School, Boston, Mass.). CJ83193 is a self-limiting and dominant-negative HSV-1 recombinant in which both copies of the ICP0 gene are replaced by DNA sequence encoding the trans-dominant-negative mutant polypeptide UL9-C535C under the control of the tet operator-bearing hCMV major immediate-early promoter (Fig. 1). CJ83193 was propagated and assayed in U2CEP4R11 cells (57). The plaque-forming efficiency of CJ83193 on 24-h-old Vero cell monolayers is reduced by 106-fold compared to that of U2CEP4R-11 (unpublished data). 7134, an ICP0-null mutant (7), was propagated and assayed on U2OS cell monolayers (59). d27 is a KOS-derived ICP27 deletion mutant (a kind gift from David M. Knipe), which can be propagated in the ICP27-expressing Vero cell line V827 (kindly provided by David M. Knipe) (40). To minimize concerns about a potential difference in physical particle/PFU ratio resulting from propagating viruses in different cell types, we established an ICP27-expressing U2CEP4R11 cell line, R27, which was generated by stable transfection of U2CEP4R11 cells with a plasmid encoding ICP27 (a gift from P. A. Schaffer, Harvard Medical School). While the plaque-forming efficiency of CJ83193 in R27 cells is reduced twofold compared to that in U2CEP4R11 cells, the plaque-forming efficiency of d27 on R27 cell monolayers is sixfold higher than that on V827 cell monolayers. Thus, d27 was propagated and the titer was determined in R27 cells. Given that deletion of ICP0 has no effect on ICP4 expression at an MOI of 1 PFU/cell compared to KOS infection (59), the similar levels of ICP4 detected in d27- and CJ83193-infected Vero cells (see Fig. 3) suggest that the physical particle/PFU ratios of these two viruses in their respective complementing cells are similar.
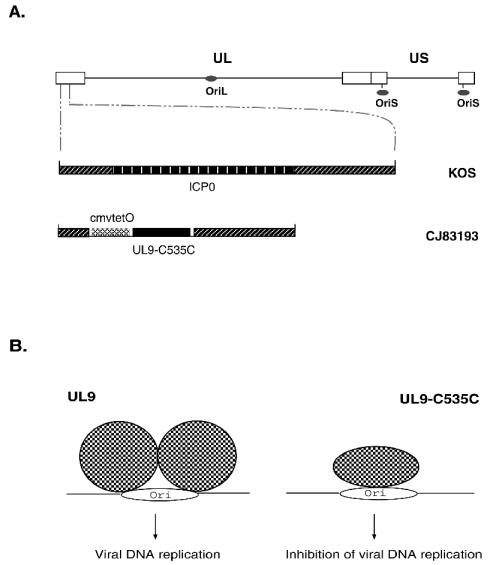
FIG. 1.
(A) A schematic diagram of genomes of the wild-type HSV-1 and the dominant-negative HSV-1 recombinant CJ83193. The top diagram shows the HSV genome, indicating the unique long (UL) region, the unique short (US) region, the inverted repeat regions (open boxes), and the three origins of DNA replication (gray ovals). The lower diagrams show an expanded SacI-PstI DNA fragment containing the ICP0 open reading frame with flanking sequences in wild-type HSV-1, strain KOS, and the DNA sequences encoding UL9-C535C under the control of the tetO-bearing hCMV major immediate-early promoter with the ICP0 flanking sequences in CJ83193. (B) A likely mechanism of UL9-C535C in preventing HSV viral DNA replication. Binding of UL9-C535C to the HSV-1 origin of DNA replication blocks the binding of wild-type HSV-1 UL9 origin binding protein to the origin of DNA replication, leading to inhibition of viral DNA replication.
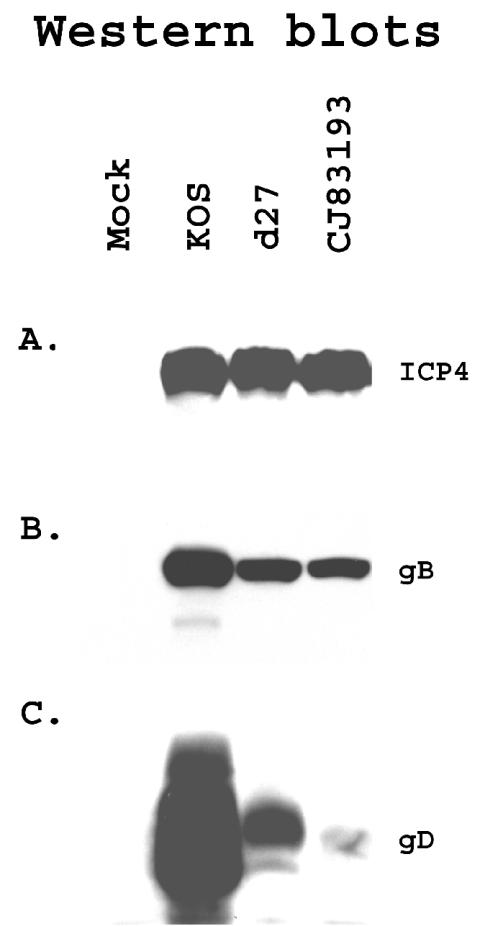
FIG. 3.
Selective detection of viral gene expression in CJ83193-infected cells. Vero cells were seeded at 3 × 106 cells per 100-mm dish. At 48 h postseeding, cells in duplicate dishes were mock infected or infected with KOS, d27, or CJ83193 at an MOI of 10 PFU/cell. Infected cell extracts were prepared at 15 h postinfection. Proteins in infected cell extracts were resolved on SDS-PAGE, followed by immunoblotting with monoclonal antibodies specific for ICP4 (A) and gB (B) and a polyclonal antibody specific for gD (C).
Mice.
CD-1 mice 7 to 8 weeks of age and female BALB/c mice 6 weeks of age were purchased from Taconic Laboratory (Germantown, N.Y.). Mice were housed in metal cages at four mice per cage and maintained on a 12-h light/dark cycle. Mice were allowed to acclimatize to the housing conditions for 1 week prior to experimentation.
Acute infection assays.
CD-1 mice were anesthetized with sodium pentobarbital, and both corneas were scarified. Wild-type HSV-1 and HSV-1 recombinant viruses at the indicated doses in a volume of 10 μl were then added to each eye according to the procedure of Leib et al. (29). On days 1, 3, 5, and 7 postinfection, both eyes of mice were swabbed with cotton swabs, and materials on the swabs were suspended in 1 ml of cell culture medium. For detection of acute viral replication in mouse trigeminal ganglia, mice were also scarified at the indicated time points, and their trigeminal ganglia were excised, frozen, and processed as previously described (29). Infectious viruses in eye swab material and ganglion homogenates were assessed by standard plaque assay on Vero or U2CEP4R11 cell monolayers.
Reactivation assays.
CD-1 mice were mock infected and infected with KOS, 7134, or CJ83193 as described in acute infection assays and sacrificed 30 days after infection (6, 29). Each trigeminal ganglion was removed, cut into eight pieces, and explanted onto a 60-mm-diameter cell culture dish containing either Vero cell monolayers or U2CEP4R11 cells at 4 times 105 cells per dish. The processed trigeminal ganglia of KOS-infected mice were explanted onto Vero cell monolayers, and trigeminal ganglia of mice infected with CJ83193 or 7134 were explanted onto U2CEP4R11 cell monolayers. After 5 days of cocultivation, explants were harvested, frozen and thawed three times, and homogenized (29). Following removal of large cellular debris by low-speed centrifugation, infectious viruses in the cocultivation medium were assayed on Vero cell monolayers for KOS and on U2CEP4R11 cell monolayers for CJ83193 and 7134. Cytopathic effects were scored (+ or −) on days 5 to 7 postinfection (6, 29).
Immunizations and challenges.
Six-week-old female BALB/c mice were randomly divided into several groups, and the hair on their left rear flanks was trimmed. Mice were immunized with either mock-infected cell lysate, KOS, d27, or CJ83193 at 2 × 106 PFU per mouse in a 20-μl volume subcutaneously (s.c.) with a 26-gauge needle. Mice were boosted s.c. with the same virus 2 weeks after primary immunization. Four weeks after the initial immunization, mice in all groups were challenged following corneal scarification with 2 × 105 PFU of HSV-1 strain mP per eye in a 10-μl volume (38). The ability of the challenge virus to cause acute infection in the eyes and trigeminal ganglia was assayed as described above.
For reactivation assays, following challenge with wild-type HSV-1 strain mP, both eyes of infected mice were swabbed with cotton swabs on days 1, 3, and 5 postinfection, and virus titers in eye swab material were determined by standard plaque assay on Vero cell monolayers. Mice were sacrificed 30 days after infection. Each trigeminal ganglion of mice from different groups was processed and cocultivated individually onto Vero cell monolayers. After 5 days of cocultivation, explants were harvested and assayed on Vero cell monolayers for the presence of infectious viruses.
Clinical observations.
Following immunization and subsequent challenge with wild-type HSV-1 strain mP, mice were observed daily during a 30-day follow-up period for signs of clinical illness, in particular, signs of encephalitis, including roughened fur, hunched posture, ataxia, and anorexia (38). Eyes were examined with an ophthalmoscope for evidence of keratitis every other day from days 1 to 9 and every third day from days 9 to 30. Individual eyes were rated for severity of disease (scale 0 to 4), and the mean keratitis score for each group of mice was determined. A score of 0 indicates a clear cornea; 1, 2, and 3 represent corneal opacity from mild (less than 25% of corneal surface) to moderate (25 to 50% of cornea surface) and severe (50 to 75% of corneal surface), respectively. A score of 4 indicates total opacity of cornea with no posterior view.
Detection of HSV-1 neutralization antibodies.
Blood was obtained from the tail veins of immunized or mock-infected cell lysate-immunized mice 1 day prior to immunization and 14 days or 1 to 6 months after primary immunization. Neutralizing serum antibody titers were determined (3, 36). In brief, serum from similarly infected mice was pooled and heat inactivated at 56°C for 15 min. A series of twofold dilutions of serum were made in normal growth medium containing amphotericin B at 50 μg/ml and Low Tox M rabbit C (Accurate Chemical and Scientific, Westbury, N.Y.) at 15-fold final dilution. A total of 250 PFU of wild-type HSV-1 was added to each tube containing diluted serum and to control tubes containing titration growth medium in the presence of Low Tox M rabbit C (1/15 final dilution) to achieve a final volume of 600 μl. Following incubation at 37°C for 1 h, HSV-1 titers in each tube were assayed on Vero cell monolayers. The neutralizing titer was expressed as the final serum dilution required to achieve a 50% reduction in HSV-1 PFU relative to the HSV-1 PFU obtained in medium plus complement alone.
DTH assay.
For the delayed-type hypersensitivity (DTH) assay, 6-week-old female BALB/c mice were immunized with either mock-infected cell lysate, KOS, d27, or CJ83193 as described. At 14 days or 6 months postvaccination, the left and right rear footpads of mice in all groups were challenged by injection of 106 PFU of HSV-1 strain mP and phosphate-buffered saline (PBS), respectively, in a volume of 10 μl. Footpad thickness was measured with a micrometer (Mitutoyo Manufacturing, Tokyo, Japan) at 40 to 44 h postchallenge (27). HSV-specific footpad swelling was expressed as the mean difference between the thickness of the left and the right footpads (10).
SDS-PAGE and Western blot analysis.
Dishes (100-mm diameter) of Vero cells were either mock infected or infected with KOS, d27, or CJ83193 at an MOI of 10 PFU/cell. Cell extracts were prepared at 15 h postinfection (60). Proteins in the cell extract were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9% acrylamide) and transferred to polyvinylidene difluoride membranes. Western blot analyses were performed with either monoclonal antibodies specific for HSV-1 immediate-early protein ICP4 and β/γ1 gene product glycoprotein B (gB), or a rabbit polyclonal antibody specific for gD (γ1) according to the procedures described previously (60). gB and gD are two major HSV-1-encoded glycoproteins. The anti-ICP4 and gB monoclonal antibodies were purchased from Fitzgerald Industries International, Inc. (Concord, Mass.). The anti-gD polyclonal antibody was a generous gift from Gary H. Cohen (University of Pennsylvania Philadelphia).
RESULTS
In vivo characterization of CJ83193 with a mouse ocular model.
One-step growth assays showed that overexpression of UL9-C535C in CJ83193-infected normal cells leads to severe impairment in de novo production of CJ83193 virus (57). To examine the replication efficiency of CJ83193 in vivo during acute infection and its ability to reactivate from latent infection, CD-1 mice were randomly assigned to three groups. Mice in the first group were inoculated with KOS at 106 PFU per eye. Considering the low plating efficiency of 7134 (7, 59) and severely impaired ability of CJ83193 to replicate on Vero cell monolayers (57), mice in the second and third groups were inoculated with CJ83193 and 7134 at 107 PFU per eye, respectively. For monitoring acute viral replication, both eyes of infected mice were swabbed on days 1, 3, and 5 postinfection. Significant amounts of KOS and 7134 viruses were detected in eye swab material from KOS- and 7134-infected mice at days 1, 3, and 5 postinfection (Fig. 2A). No CJ83193 was detectable in the eye swab material from CJ83193-infected mice at these three time points when assayed either on Vero cell monolayers or on U2CEP4R11 monolayers, indicating that CJ83193 cannot replicate in the mouse cornea under these experimental conditions. Consistent with this observation, we detected no infectious CJ83193 in trigeminal ganglia of mice on days 3 and 5 post-CJ83193 ocular inoculation at a dose of 107 PFU per eye (data not shown). The inability of CJ83193 to initiate acute productive infection in mice was further demonstrated by intracerebral inoculation with CJ83193 (unpublished data). Taken together, these results demonstrate that deletion of both copies of the ICP0 gene combined with high-level expression of UL9-C535C rendered CJ83193 replication defective in normal cells.
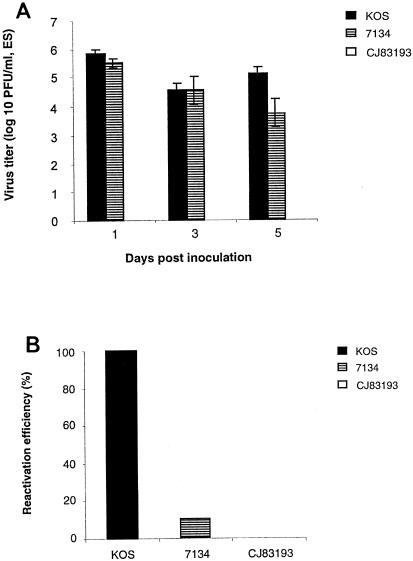
FIG. 2.
CJ83193 fails to establish reactivatable latent infection in a mouse ocular model. CD-1 mice were randomly assigned to three groups of six male and six female mice each. Following corneal scarification, individual groups of mice were inoculated with KOS at 106 PFU per eye or with CJ83193 and 7134 at 107 PFU per eye, respectively. (A) Acute viral replication in the eye of CD-1 mice. Both eyes of infected mice were swabbed on days 1, 3, and 5 postinfection. Infectious viruses in eye swab material were assessed by standard plaque assay on Vero cell monolayers for KOS and on U2CEP4R11 cell monolayers for CJ83193 and 7134. Virus titers are expressed as means ± standard error of eye swabs (n = 24) for 12 mice per group. (B) Reactivation of latent infection from trigeminal ganglia of mice following eye inoculation was determined by cocultivation at 30 days postinfection. Vero cell monolayers were used for KOS infection. Presence of reactivatable 7134 and CJ83193 viruses were assayed on U2CEP4R11 cell monolayers.
Although their efficiency is significantly reduced compared with wild-type virus, the replication-defective HSV-1 recombinants are capable of establishing latent infection in a mouse ocular model with no detectable reactivation (26). We examined whether CJ83193 can establish reactivatable latent infection in the infected mice described above. While the reactivation frequency for KOS was 100%, no CJ83193 virus can be reactivated from trigeminal ganglia of CJ83193-infected mice (Fig. 2B). The reactivation efficiency of 7134 was about 10%, consistent with the previous studies of Cai et al. (6) and Halford and Schaffer (22).
Selective detection of viral immediate-early, early, and delayed-early gene expression in CJ83193-infected cells.
Studies with replication-defective HSV-1 mutant viruses elegantly documented that the degree of humoral and cell-mediated immunity induced by replication-defective viruses correlates directly with the extent of viral gene expression occurring in infected cells (41). For example, HSV-1 mutant viruses such as an ICP27 mutant, which is capable of expressing viral α, β, and γ1 genes, but not γ2 genes, were more effective than an ICP4 mutant virus that expresses only viral α genes in protecting mice against a lethal challenge with wild-type HSV and the development of HSV-1-induced disease following eye inoculation. Because overexpression of UL9-C535C inhibits HSV-1 DNA replication, it is anticipated that infection of CJ83193 would lead to the expression of viral α, β, and γ1 genes with little or no γ2 gene expression in normal cells. As illustrated by Western blot analyses (Fig. 3), comparable levels of ICP4 were expressed among cells infected by KOS, d27, and CJ83193. While expression of gB was modestly reduced in d27- or CJ83193-infected cells relative to KOS infection, there is a significant reduction of expression of gD in d27- and CJ83193-infected cells. Levels of gD expressed in CJ83193-infected cells were further reduced compared with those in cells infected by d27. Recall that levels of gD expression in 7134-infected Vero cells at an MOI of 1 PFU/cell were similar to that detected in KOS-infected cells (59). This observation indicates that the marked reduction in gD expression in CJ83193-infected cells was the direct result of inhibition of de novo viral DNA replication by UL9-C535C.
Protection from HSV-1 infection in CJ83193-immunized mice in a mouse ocular model. (i) Effect of immunization with CJ83193 on acute viral replication of wild-type HSV-1.
Having demonstrated that, like the ICP27 mutant, CJ83193 is capable of expressing a broad spectrum of HSV-1 genes, the ability of CJ83193 to serve as an effective vaccine against wild-type HSV-1 infection was examined. Given the well-documented effect of immunization of the ICP27 mutant in protecting mice against wild-type HSV infection and that both CJ83193 and the ICP27 mutant are defective at the level of de novo viral DNA replication, herein, in addition to immunization of mice with either mock-infected cell lysate, KOS, or CJ83913, a group of mice was also immunized with the ICP27 deletion mutant, d27. Four weeks after the initial immunization, mice were challenged with HSV-1 strain mP following corneal scarification (38). As shown in Fig. 4A, the titers of challenge virus in eye swabs of mice immunized with KOS, d27, or CJ83193 were significantly lower than those of mice immunized with control cell lysate on day 3 postchallenge. No challenge virus was detectable in eye swabs collected from KOS-, d27-, or CJ83193-immunized mice on day 7 postchallenge. On day 5 after challenge, the yield of challenge virus in eye swabs of mice immunized with mock-infected cell lysate was about 26,500 PFU/ml and there was no challenge virus detected in eye swabs of mice immunized with KOS. An average of less than 1 PFU/ml of challenge virus was detected in mice immunized with d27 or CJ83193.
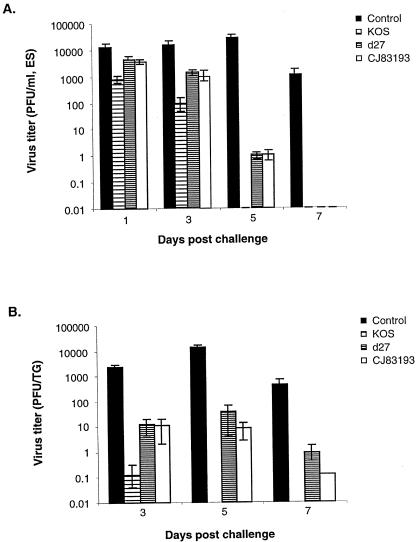
FIG. 4.
Reduction of challenge wild-type HSV-1 strain mP replication in the eye and trigeminal ganglion of mice immunized with CJ83193. Female BALB/c mice were immunized with either mock-infected cell lysate, KOS, d27, or CJ83193 at 2 × 106 PFU per mouse. Individual groups of mice (n = 12) were boosted with the same virus 2 weeks after primary immunization. At 4 weeks after primary immunization, mice in all groups were challenged following corneal scarification with HSV-1 strain mP. Eye swabs were taken on days 1, 3, 5, and 7 postchallenge, while mouse trigeminal ganglia (n = 8) were prepared on days 3, 5, and 7 postchallenge. Infectious viruses in individual eye swab materials (A) and trigeminal ganglia (B) were assessed by standard plaque assay on Vero cell monolayers. Viral titers are expressed as the mean ± standard error in individual eye swabs and trigeminal ganglia of mice per group.
The viral yields from trigeminal ganglia of mice immunized with mock-infected cell lysate, KOS, d27, or CJ83193 during acute infection are presented in Fig. 4B. Immunization with control cell lysate produced an increase in viral titer from day 3 to day 5 and a significant decrease in viral titer from day 5 to day 7. Such replication kinetics of wild-type HSV-1 in trigeminal ganglia of mice immunized with mock-infected cell lysate is consistent with studies of Morrison and Knipe (38). Mice immunized with KOS were best protected from infection by challenge virus in trigeminal ganglia. Little to no challenge virus was detectable in trigeminal ganglia of mice immunized with KOS on days 5 and 7 after challenge. The viral yields in trigeminal ganglia of CJ83193-immunized mice were reduced more than 1,500-fold on day 5 and 3,000-fold on day 7 postchallenge compared with such yields from mice immunized with mock-infected cell lysate. Notably, the viral yields in trigeminal ganglia of mice immunized with d27 were about 400-fold and 500-fold lower than those from mice immunized with mock-infected cell lysate on days 5 and 7 postchallenge, respectively. This degree of protection against wild-type HSV-1 infection in mice immunized with CJ83193 seems greater than that in mice immunized with d27. Collectively, the studies demonstrate that immunization with CJ83193 can significantly decrease acute replication of challenge wild-type HSV-1 in the corneal surface and trigeminal ganglion of immunized mice.
(ii) Effect of immunization with CJ83193 on wild-type challenge virus-induced disease following ocular inoculation.
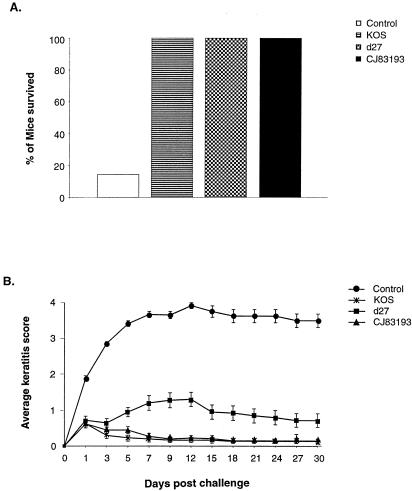
Following immunization of BALB/c mice with mock-infected cell lysate, KOS, d27, or CJ83193, mice were challenged with HSV-1 strain mP and observed daily for signs of clinical illness. It was observed that starting on day 5 postchallenge, all mice immunized with mock-infected cell lysate developed signs of encephalitis, and 24 of these 28 mice had died by day 12 postchallenge (Fig. 5A). Like mice immunized with KOS, mice immunized with CJ83193 showed no signs of virus-induced encephalitis. It was noted, however, that 2 of 20 d27-immunized mice showed brief signs of virus-induced encephalitis.
FIG. 5.
Prevention of mortality and herpetic keratitis in mice immunized with CJ83193 after corneal challenge with wild-type HSV-1 strain mP. Female BALB/c mice were randomly divided into four groups and immunized with either mock-infected cell lysate (n = 28), KOS (n = 16), d27 (n = 20), or CJ83193 (n = 20) as described. Four weeks after initial immunization, both eyes of mice were challenged with HSV-1 strain mP following corneal scarification. (A) Mortality of mice following ocular HSV-1 challenge during a 30-day follow-up period. (B) Ophthalmoscopic examination of mice eyes for signs of herpetic keratitis during the same period. Individual eyes were scored for severity of keratitis. The indicated values represent the mean score ± standard error of all eyes from each group of mice.
To assess HSV-induced keratitis by challenge virus, both eyes of mice mentioned above were examined with an ophthalmoscope (Fig. 5B). Similar to those immunized with KOS, mice immunized with CJ83193 were completely protected from developing HSV-1-induced keratitis. The results also indicated that the immune response elicited by immunization with CJ83193 was more efficacious than d27 in preventing HSV-induced keratitis from days 5 to 30 postchallenge (P < 0.003, Student's t test). Analysis of eye swabs of these mice showed again that immunization with CJ83193 significantly reduced shedding of challenge virus on days, 3, 5, and 7 postchallenge, and by day 5 after challenge, the shedding of challenge virus was reduced more than 15,000-fold compared with that seen in mice immunized with mock-infected cell lysate (data not shown). During the same period, immunization with d27 led only to about a 600-fold reduction in virus yields compared with those detected in mice immunized with mock-infected cell extract. By day 7, no virus was detected in eye swabs of mice immunized with KOS, d27, or CJ83193.
(iii) Reduction of establishment of latent infection by challenge virus in CJ83193-immunized mice.
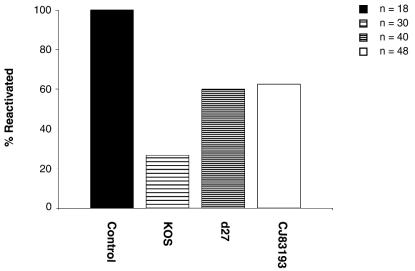
Immunization of mice with either wild-type virus or replication-defective HSV-1 recombinants leads to reduction of challenge virus in the establishment of latent infection (38, 39). On the basis that immunization with CJ83193 can significantly reduce the replication of challenge virus in the eyes and trigeminal ganglia of mice, we tested whether the efficiency of latent infection by challenge virus in trigeminal ganglia of mice could also be reduced by immunization with CJ83193. Individual groups of mice described in Fig. 5 were sacrificed 30 days after challenge. Trigeminal ganglia of mice from different groups were processed and assayed for the presence of reactivating viruses by cocultivation. Although not as effective as KOS, immunization with CJ83193 led to a 40% reduction in reactivation of challenge virus compared with that in control mice (Fig. 6). We, however, observed a 87.5% reduction in reactivation efficiency of challenge virus in mice receiving CJ83193 at an immunization dose of 2 × 107 PFU per mouse (data not shown), suggesting that the efficacy of CJ83193 as an effective vaccine against wild-type HSV infection could be elevated by increasing the immunization dose of CJ83193.
FIG. 6.
Reduction of latent infection by challenge virus in trigeminal ganglia of mice immunized with KOS, d27, or CJ83193. Mice described in experiments presented in Fig. 5 and from a similar experiment in which mice were immunized with either mock-infected cell lysate (n = 12) or CJ83193 at 2 × 106 PFU/mouse (n = 4) were sacrificed 30 days after challenge. Trigeminal ganglia of mice from different groups were processed and cocultivated individually onto Vero cell monolayers. After 5 days of cocultivation, explants were harvested, processed, and assayed on Vero cell monolayers for the presence of infectious viruses.
(iv) Induction of humoral and cell-mediated immunity by CJ83193.
DNA immunization with HSV-1 glycoproteins indicated that efficacy in inducing protective immune response correlated with the induction of HSV-1 neutralizing antibody. To investigate the efficiency of induction of HSV-1 neutralization antibody by CJ83193, mice were either mock immunized or immunized with KOS, d27, or CJ83193. Table 1 represents experiments focused on examining the induction of short-term HSV-1 specific neutralization antibody, whereas Table 2 investigated the efficacy of CJ83193 in eliciting durable neutralization antibody. These results indicate that although CJ83193 is not as effective as KOS, it is more effective than d27 at eliciting both short- and long-term humoral immunity.
TABLE 1.
Induction of short-term HSV-1 neutralization antibodies in mice immunized with mock-infected cell lysate, KOS, d27, or CJ83193a
| Day of serum collection | Neutralization antibody titer with immunogen:
|
|||
|---|---|---|---|---|
| Mock | KOS | d27 | CJ83193 | |
| −1 | <2 | <2 | <2 | <2 |
| 14 | <2 | 32-128 | 0-8 | 2-32 |
| 28 | <2 | 256-512 | 32-64 | 256-512 |
Blood was obtained from the tail veins of mice (results are presented in Fig. 4, 5, and 6) 1 day prior to immunization (−1) and 14 and 28 days after primary immunization. Serum from individual groups of mice (n = 16 to 28) was pooled and heat inactivated. A series of twofold dilutions of serum were made in normal growth medium in the presence of Low Tox M rabbit C. Wild-type HSV-1 (250 PFU) was added to each tube containing diluted serum and to control tubes containing titration growth medium and Low Tox M rabbit C in a final volume of 600 μl. The neutralizing antibody titers were calculated as the final serum dilution leading to a 50% reduction in the number of HSV-1 PFU compared to that obtained in medium plus complement alone.
TABLE 2.
Induction of long-term HSV-1 neutralization antibodies in mice immunized with KOS, d27, and CJ83193a
| Mo of serum collection | Neutralization antibody titer with immunogen:
|
|||
|---|---|---|---|---|
| Mock | KOS | d27 | CJ83193 | |
| 1 | <2 | 1,024 | 64 | 128 |
| 2 | <2 | 1,024 | 128 | 256 |
| 3 | <2 | 2,048 | 512 | 1,024 |
| 4 | <2 | 4,096 | 512 | 2,048 |
| 5 | <2 | 2,048 | 512 | 1,024 |
| 6 | <2 | 2,048 | 256 | 512 |
Six-week-old female BALB/C mice were randomly divided into four groups of eight mice each. Mice were immunized with either mock-infected cell lysate, KOS, d27, or CJ83193 at 2 × 106 PFU per mouse and boosted with the same virus 2 weeks later. Blood was obtained from tail veins of mice 1, 2, 3, 4, 5, and 6 months after primary immunization. Sera from individual groups of mice (n = 8) were pooled and heat inactivated, and HSV-1 neutralization antibody titers were assayed on Vero cell monolayers.
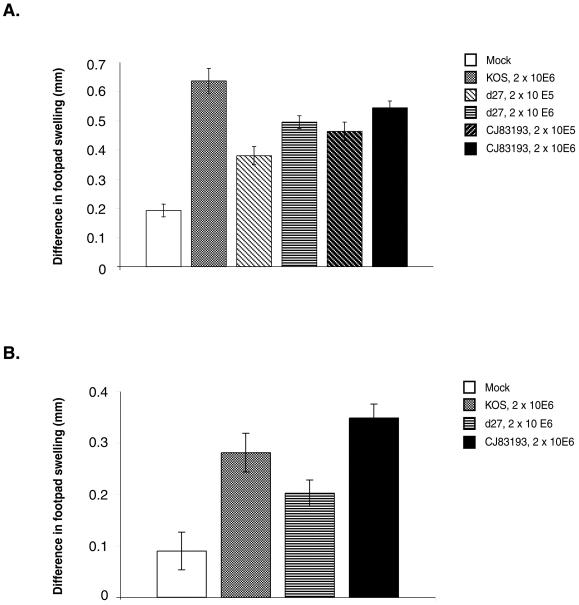
Cell-mediated immunity, predominantly mediated by CD4+ T cells, plays a primary role in controlling HSV infections of the skin and nervous system (48). To study the ability of CJ83193 to elicit CD4+ T-cell-mediated immune response, we measured DTH responses in groups of mice immunized with mock-infected cell lysate, KOS, d27, or CJ83193 first at 14 days postimmunization (Fig. 7A). The results showed that while similar levels of DTH responses were induced by CJ83193 and d27 at a dose of 2 × 106 PFU per mouse, CJ83193 induced a stronger DTH response than d27 at a dose of 2 × 105 PFU per mouse (P = 0.035, Student’s t test).
FIG. 7.
Induction of short- and long-term DTH responses by CJ83193. Six-week-old female BALB/c mice were randomly divided into several groups. (A) Mice were immunized with (i) either mock-infected cell lysate (n = 19) or KOS (n = 7), d27 (n = 13), and CJ83193 (n = 13) at a dose of 2 × 106 PFU per mouse and (ii) mock-infected cell lysate (n = 12) or d27 (n = 15) and CJ83193 (n = 15) at a dose of 2 × 105 PFU per mouse. At 14 days postvaccination, the left and right rear footpads of mice in all groups were challenged by injection of 106 PFU of HSV-1 strain mP and PBS, respectively. (B) At about 6 months postvaccination of the mice described in Table 2, the left and right rear footpads of mice in all groups were challenged with either HSV-1 strain mP or PBS as described. Footpad thickness was measured with a micrometer at 40 h postchallenge. HSV-specific footpad swelling was expressed as the mean difference ± standard error between the thickness of the left and right footpads.
Second, to assess the efficacy of CJ83193 in eliciting durable cell-mediated immunity, the mice described in Table 2 were assayed for DTH responses at 6 months post-primary immunization (Fig. 7B). The results demonstrate that CJ83193 is as effective as KOS in induction of durable DTH responses and is more effective than d27 (P = 0.007, Student's t test).
DISCUSSION
Safety and efficiency in eliciting an effective host immune response are two major criteria in developing recombinant viral vaccines against wild-type viral infections. An ideal recombinant viral vaccine would not only be replication defective and capable of eliciting a broad protective immune response but would also encode a safety mechanism that can inhibit de novo replication of endogenous wild-type virus if encountered within the same cells in the host. The dominant-negative effect of such a vaccine virus could minimize the potential outbreak of the vaccine virus in vaccinated populations that might result from the potential conversion of replication-defective virus to wild-type virus during vaccine virus production or recombination between vaccine virus and wild virus in the host.
Aiming to increase the safety of HSV recombinant vaccine virus while retaining its ability to express viral α, β, and γ1 gene products, we constructed a novel class of HSV-1 recombinant, CJ83193, with the employment of a newly developed viral replication switch strategy (57, 58). CJ83193 is self-limiting and replication defective in normal cells and can prevent the replication of wild-type HSV-1 and HSV-2 in in vitro coinfection assays (56, 57). The ability of CJ83193 to inhibit wild-type HSV-1 infection has recently been demonstrated in vivo by coinoculation assays (data not shown). This report shows that CJ83193 cannot replicate in corneas of infected mice, nor can it establish a reactivatable latent infection in mice latently infected by CJ83193. Da Costa et al. showed that, unlike HSV recombinants with a single essential gene defect, deletion of two viral essential genes whose function is required for HSV viral DNA replication could render the resulting viral recombinant, dl5-29, unable to establish a stable latent infection in trigeminal ganglia of mice infected intranasally (12). Whether CJ83193 or its future derivatives (discussed below) can establish stable latent infection following an ocular and other routes of inoculation remains to be determined.
In a mouse ocular model of HSV-1 infection, we demonstrate that immunization with CJ83193 leads to a marked reduction in the yields of challenge HSV in eyes and trigeminal ganglia on days 3, 5, and 7 postchallenge. Similar to mice immunized with wild-type HSV-1 strain KOS, mice immunized with CJ83193 were essentially completely protected from developing keratitis and encephalitis induced by corneal challenge with wild-type HSV-1 strain mP.
Using mouse strains deficient in either MHC class I or MHC class II, Ghiasi et al. demonstrate that CD4+ T-cell-mediated neutralization antibody induction is the most important immune response involved in the protection of mice against a lethal HSV-1 ocular challenge (21). DTH assays revealed that CJ83193 can effectively elicit cell-mediated immunity. Although similar levels of DTH responses were induced by CJ83193 and d27 at a higher dose at 2 weeks postimmunization, CJ83193 induced a stronger DTH response than d27 at a lower dose. It was further demonstrated that CJ83193 induces durable cell-mediated immunity more effectively than d27. Moreover, mice immunized with CJ83193 developed strong durable HSV-1-neutralizing antibodies at levels at least twofold higher than the neutralization antibody titers induced by d27. Considering that the levels of γ1 gene expression in CJ83193-infected cells, such as gD, a major antigen of HSV-1, is significantly lower than that expressed in KOS- and d27-infected cells (Fig. 3), this observation is very interesting.
Antigen dose and kinetics play important roles in determining the duration and extent of an immune response (63, 64). On the basis of the likely mechanisms by which de novo-expressed antigens are processed and presented in vivo to elicit effective immune response (13, 44, 63), it is reasonable to speculate that the degree of cytotoxicity of replication-defective HSV-1 vaccine viruses could directly influence the magnitude and type of the immune response elicited by these viruses. We noted that, unlike the highly toxic replication-defective ICP27 mutants (1, 2, 55), infection of Vero cells with CJ83193 exhibits little cytotoxicity. For example, by counting viable cells with trypan blue stain exclusion at 48 h postinfection, it was shown that while 95% of cells were killed in d27-infected dishes at an MOI of 10 PFU/cell and above, more than 80% of cells remained viable in dishes infected by CJ83193 at the same MOI. Furthermore, on day 5 postinfection, the number of viable cells at an MOI of 30 PFU/cell of CJ83193 infection was 76% of the number of uninfected control cells. The observed low cytotoxic effect of CJ83193 on infected cells could result in prolonged viral antigen expression in vaccinated mice relative to the previously described more toxic replication-defective HSV-1 mutants. The prolonged survival of infected cells might provide an extended reservoir of antigens to boost both arms of the adaptive host immune response. More in-depth analysis of CJ83193-induction of humoral and cell-mediated immune responses is currently under investigation.
Collectively, combined with the documented potent trans-dominant-negative effect of CJ83193 on the replication of wild-type HSV-1 and HSV-2 in coinfection assays (56, 57), we have established a new strategy for the potential development of a safe and effective recombinant HSV vaccine in eliciting protective host immunity against HSV-1 infection. Based on the characteristics of viral gene expression detected in CJ83193 infection of normal cells, we propose that the effectiveness of CJ83193 as an HSV vaccine could be enhanced by the increased expression of HSV-1 major antigens such as gD. In addition, although we have shown that CJ83193 cannot initiate productive viral infection in vivo, the safety of CJ83193 can be further secured by introducing an essential mutation, such as an ICP8 or UL9 deletion, without affecting its characteristics of expression of the viral α, β, and γ1 gene products.
Acknowledgments
This work was in part supported by Public Health Service grants 2RO1GM5144905 and 1RO1AI050880 from the National Institutes of Health.
REFERENCES
- 1.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne, N., G. N. Milligan, M. R. Schleiss, D. I. Bernstein, and L. R. Stanberry. 1996. DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine 14:1230-1234. [DOI] [PubMed] [Google Scholar]
- 4.Boursnell, M. E., C. Entwisle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, R. Jennings, D. Ni Challanain, I. Sobek, S. C. Inglis, and C. S. McLean. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm, M. A., R. H. Bonneau, D. M. Knipe, and S. S. Tevethia. 1997. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J. Virol. 71:3534-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, W., and P. A. Schaffer. 1989. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J. Virol. 63:4579-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. X., X. X. Zhu, and S. Silverstein. 1991. Mutational analysis of the sequence encoding ICP0 from herpes simplex virus type 1. Virology 180:207-220. [DOI] [PubMed] [Google Scholar]
- 9.Chiocca, E. A., B. B. Choi, W. Z. Cai, N. A. DeLuca, P. A. Schaffer, M. DiFiglia, X. O. Breakefield, and R. L. Martuza. 1990. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 2:739-746. [PubMed] [Google Scholar]
- 10.Chun, S., M. Daheshia, N. A. Kuklin, and B. T. Rouse. 1998. Modulation of viral immunoinflammatory responses with cytokine DNA administered by different routes. J. Virol. 72:5545-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobson, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tyler, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Haan, J. M., and M. J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437-441. [DOI] [PubMed] [Google Scholar]
- 14.Dobson, A. T., T. P. Margolis, F. Sedarati, J. G. Stevens, and L. T. Feldman. 1990. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron 5:353-360. [DOI] [PubMed] [Google Scholar]
- 15.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D. 1986. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J. Gen. Virol. 67:2507-2513. [DOI] [PubMed] [Google Scholar]
- 17.Everett, R. D. 1984. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell, H. E., C. S. McLean, C. Harley, S. Efstathiou, S. Inglis, and A. C. Minson. 1994. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J. Virol. 68:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 20.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 21.Ghiasi, H., S. Cai, A. B. Nesburn, and S. L. Wechsler. 1997. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr. Eye Res. 16:1152-1158. [DOI] [PubMed] [Google Scholar]
- 22.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 24.Hill, A. B., S. P. Lee, J. S. Haurum, N. Murray, Q. Y. Yao, M. Rowe, N. Signoret, A. B. Rickinson, and A. J. McMichael. 1995. Class I major histocompatibility complex-restricted cytotoxic T lymphocytes specific for Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell lines against which they were raised. J. Exp. Med. 181:2221-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson, J. G., D. A. Leib, D. J. Goldstein, C. L. Bogard, P. A. Schaffer, S. K. Weller, and D. M. Coen. 1989. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 173:276-283. [DOI] [PubMed] [Google Scholar]
- 26.Katz, J. P., E. T. Bodin, and D. M. Coen. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keadle, T. L., K. A. Laycock, J. K. Miller, K. K. Hook, E. D. Fenoglio, M. Francotte, M. Slaoui, P. M. Stuart, and J. S. Pepose. 1997. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J. Infect. Dis. 176:331-338. [DOI] [PubMed] [Google Scholar]
- 28.Keadle, T. L., L. A. Morrison, J. L. Morris, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 76:3615-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi, P., D. Krisky, T. Oligino, P. L. Poliani, R. Ramakrishnan, W. F. Goins, D. J. Fink, and J. C. Glorioso. 1996. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc. Natl. Acad. Sci. USA 93:11319-11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch, D. J., A. Dolan, and M. C. Frame. 1986. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 14:3435-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean, C. S., M. Erturk, R. Jennings, D. N. Challanain, A. C. Minson, I. Duncan, M. E. Boursnell, and S. C. Inglis. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 170:1100-1109. [DOI] [PubMed] [Google Scholar]
- 34.Meignier, B., R. Longnecker, and B. Roizman. 1988. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J. Infect. Dis. 158:602-614. [DOI] [PubMed] [Google Scholar]
- 35.Meignier, B., B. Martin, R. J. Whitley, and B. Roizman. 1990. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus). J. Infect. Dis. 162:313-321. [DOI] [PubMed] [Google Scholar]
- 36.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 37.Morrison, L. A., and D. M. Knipe. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315-326. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, L. A., and D. M. Knipe. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J. Virol. 68:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220:402-413. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen, L. H., D. M. Knipe, and R. W. Finberg. 1992. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J. Virol. 66:7067-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hare, P., and G. S. Hayward. 1985. Three trans-acting regulatory proteins of herpes simplex virus modulate immediate-early gene expression in a pathway involving positive and negative feedback regulation. J. Virol. 56:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 45.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 47.Spector, F. C., E. R. Kern, J. Palmer, R. Kaiwar, T. A. Cha, P. Brown, and R. R. Spaete. 1998. Evaluation of a live attenuated recombinant virus RAV 9395 as a herpes simplex virus type 2 vaccine in guinea pigs. J. Infect. Dis. 177:1143-1154. [DOI] [PubMed] [Google Scholar]
- 48.Stanberry, L. R., A. L. Cunningham, A. Mindel, L. L. Scott, S. L. Spruance, F. Y. Aoki, and C. J. Lacey. 2000. Prospects for control of herpes simplex virus disease through immunization. Clin. Infect. Dis. 30:549-566. [DOI] [PubMed] [Google Scholar]
- 49.Steiner, I., J. G. Spivack, S. L. Deshmane, C. I. Ace, C. M. Preston, and N. W. Fraser. 1990. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol. 64:1630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 51.Valyi-Nagy, T., S. L. Deshmane, J. G. Spivack, I. Steiner, C. I. Ace, C. M. Preston, and N. W. Fraser. 1991. Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in1814, that does not replicate in mouse trigeminal ganglia. J. Gen. Virol. 72:641-649. [DOI] [PubMed] [Google Scholar]
- 52.Walker, J., and D. A. Leib. 1998. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 16:1-5. [DOI] [PubMed] [Google Scholar]
- 53.Whitley, R. J., E. R. Kern, S. Chatterjee, J. Chou, and B. Roizman. 1993. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J. Clin. Investig. 91:2837-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitley, R. J., D. W. Kimberlin, and B. Roizman. 1998. Herpes simplex viruses. Clin. Infect. Dis. 26:541-555. [DOI] [PubMed] [Google Scholar]
- 55.Wu, N., S. C. Watkins, P. A. Schaffer, and N. A. DeLuca. 1996. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J. Virol. 70:6358-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao, F., and E. Eriksson. 2002. Inhibition of herpes simplex virus type 2 (HSV-2) viral replication by the dominant negative mutant polypeptide of HSV-1 origin binding protein. Antivir. Res. 53:127-133. [DOI] [PubMed] [Google Scholar]
- 57.Yao, F., and E. Eriksson. 1999. A novel anti-herpes simplex virus type 1-specific herpes simplex virus type 1 recombinant. Hum. Gene Ther. 10:1811-1818. [DOI] [PubMed] [Google Scholar]
- 58.Yao, F., and E. Eriksson. 1999. A novel tetracycline-inducible viral replication switch. Hum. Gene Ther. 10:419-427. [DOI] [PubMed] [Google Scholar]
- 59.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao, F., and P. A. Schaffer. 1994. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J. Virol. 68:8158-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao, F., T. Svensjo, T. Winkler, M. Lu, C. Eriksson, and E. Eriksson. 1998. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum. Gene Ther. 9:1939-1950. [DOI] [PubMed] [Google Scholar]
- 62.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]
- 63.Zinkernagel, R. M. 2000. Localization dose and time of antigens determine immune reactivity. Semin. Immunol. 12:163-171, 257-344. [DOI] [PubMed] [Google Scholar]
- 64.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]