Abstract
Background
Despite institutional studies that suggest that radical hysterectomy for cervical cancer is well tolerated in the elderly, little population-level data is available on the procedure’s outcomes in older women. We performed a population-based analysis to determine the morbidity, mortality, and resource utilization of radical hysterectomy in elderly women with cervical cancer.
Methods
Patients recorded in the Nationwide Inpatient Sample with invasive cervical cancer who underwent abdominal radical hysterectomy between 1998–2010 were analyzed. Patients were stratified by age: <50, 50–59, 60–69, and ≥70 years. We examined the association between age and the outcomes of interest using chi square tests and multivariable generalized estimating equations.
Results
A total of 8199 women were identified, including 768 (9.4%) women age 60–69 and 462 (5.6%) women ≥70 years of age. All cause morbidity increased from 22.1% in women <50, to 24.7% in those 50–59 years, 31.4% in patients 60–69 years and 34.9% in women >70 years of age (P<0.0001). Compared to women < 50, those >70 were more likely to have intraoperative complications (4.8% vs. 9.1%, P=0.0003), surgical site complications (10.9% vs. 17.5%, P<0.0001), and medical complications (9.9% vs. 19.5%, P<0.0001). The risk of non-routine discharge (to a nursing facility) was 0.5% in women <50 vs.12.3% in women ≥70 (P<0.0001). Perioperative mortality women ≥70 years of age was 30 times greater than that of women <50 (P<0.0001)
Conclusion
Perioperative morbidity and mortality are substantially greater in elderly women who undergo radical hysterectomy for cervical cancer. Non-surgical treatments should be considered in these patients.
Keywords: Cervical cancer, cervical carcinoma, radical hysterectomy, hysterectomy, elderly, surgery, early-stage
Introduction
Cervical cancer remains a major cause of cancer-related morbidity and mortality in women1. In the United States, it is estimated that approximately 12,000 new cases of cervical cancer and 4,000 deaths from the disease occurred in 2013.2 While effective treatment is available for cervical cancer, curative intent therapy is often associated with substantial morbidity.3
For women with stage IB-IIA tumors, treatment consists of either surgery with radical hysterectomy or primary radiotherapy.3 Although survival is similar for the two treatments, radical hysterectomy is often considered the treatment of choice when feasible. Radical hysterectomy consists of removal of the uterus in conjunction with en bloc resection of the upper vagina, parametria, and uterosacral ligaments. Despite the oncologic benefits of the procedure, radical hysterectomy is associated with significant morbidity including blood loss, bladder dysfunction, lymphedema, and sexual dysfunction.3
Cervical cancer has a bimodal peak and is common in the elderly. An abundance of data suggests that the outcomes of elderly women with cervical cancer are inferior to younger patients.4 Prior work has shown that elderly women less often receive curative intent therapy, are less frequently treated with surgery, and are less likely to receive adjuvant radiation and chemotherapy.5–9 However, even after adjusting for disparities in care, elderly women with cervical cancer are more likely to die from their tumors.4
The optimal treatment for elderly women with early-stage cervical cancer remains unknown. Elderly patients often have significant underlying comorbidity, poor performance status and impaired functional ability that place them at increased risk for perioperative complications. Although elderly patients are at increased risk for perioperative morbidity, several single institution observational studies have suggested that the procedure is well tolerated in older women.10–15 Given the limited data available to guide the treatment of elderly women with cervical cancer, we performed a population-based analysis to determine the morbidity, mortality, and resource utilization of radical hysterectomy in elderly women with invasive cervical cancer.
Materials and Methods
The Nationwide Inpatient Sample (NIS) was utilized for analysis. NIS is a nationwide datasource maintained by the Agency for Healthcare Research and Quality (AHRQ) that captures inpatient hospitalizations. Each year NIS collects a random sample of approximately 20% of hospital discharges from facilities throughout the United States. The sampling frame for NIS includes nonfederal, general and specialty-specific hospitals within the U.S. NIS includes academic and community hospitals and hospitals of all sizes. The sampling scheme represents approximately 97% of hospitals in the U.S. and NIS is the largest all-payer inpatient care database.16 In 2007, NIS recorded 8 million hospital stays from 40 states. Institutional review board exemption was obtained from Columbia University.
Patients with a diagnosis of invasive cervical (ICD-9 180.x) who underwent surgery between 1998 and 2010 were selected. The cohort included only those women who underwent radical hysterectomy (ICD-9 68.6, 68.60, 68.69). Women who underwent laparoscopic radical hysterectomy or vaginal hysterectomy were excluded. Patients were stratified by age into the following groups: <50 years, 50–59 years, 60–69 years, and ≥70 years. Performance of concomitant lymphadenectomy at the time of the operation was noted for each patient.
Clinical and demographic data analyzed included race (white, black, Hispanic, other, unknown), household income (low, medium, high, highest, unknown), and primary insurance payor (private, Medicare, Medicaid, self pay, other, and unknown).16 Geographic variation was captured through region of the country in which the patient resided (northeast, midwest, south, west), and the area of residence (metropolitan and non-metropolitan). Hospital characteristics including hospital size (small, medium, or large) and teaching status (teaching, non-teaching, unknown) were also recorded. Risk adjustment for comorbid medical conditions was performed using the Elixhauser comorbidity index. Patients were classified based on the number of medical comorbidities into the following groups: 1, 2, ≥3.17 Hospital procedure volume was assessed as the annualized number of radical hysterectomies performed and grouped into approximately equal volume-based quartiles. Finally, we report the categorization of the inpatient admission during which time the radical hysterectomy was performed (elective, emergent, urgent).
The outcomes of interest were perioperative morbidity and mortality as well as resource utilization. Morbidity was classified based on a previously reported system in which complications were classified into the following categories: i) intraoperative complications (bladder, ureteral, intestinal, vascular or other operative injury), ii) surgical site complications (wound complications, abscess, hemorrhage, bowel obstruction, and ileus), and iii) medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, respiratory failure, renal failure, stroke, bacteremia/sepsis, shock, pneumonia).18–21 A composite category of any complication was also recorded as any of the above complications.18–21 Metrics for resource utilization included transfusion, reoperation, and length of hospital stay of ≥4 days. Non-routine discharge was defined as discharge to a skilled nursing facility, nursing home, acute or sub-acute rehabilitation unit. Perioperative mortality was defined as death during the index hospitalization.18–21
Frequency distributions across the age groups were compared using χ2 tests. To examine the independent association between age and morbidity, mortality, and resource utilization, we developed multivariable generalized estimating equations (GEE). These models included the clinical and demographic characteristics of interest and account for patient clustering within hospitals. Data are reported as risk ratios with 95% confidence intervals. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
A total of 8199 women who underwent radical hysterectomy for cervical cancer were identified. The cohort included 5551 (67.7%) <50 years of age, 1418 (17.3%) age 50–59 years, 768 (9.4%) age 60–69 years, and 462 (5.6%) ≥70 years of age.
Table 1 displays the clinical and demographic characteristics of the patient population. Compared to younger women, those ≥70 years of age who underwent radical hysterectomy were more often white (P<0.0001), and had higher household incomes (P=0.006). Elderly women more frequently had a greater number of comorbid medical conditions; among women ≥70 years of age, 56.9% had a comorbidity score of ≥3 compared to 16.1% of those <50 years of age (P=0.01). Elderly women were more likely to undergo surgery at a non-teaching hospital (26.2% for women ≥70 years of age vs. 15.6% for those <50) (P<0.0001) and at low volume centers (33.8% of women ≥70 years of age vs. 22.4% for those <50) (P=0.004) and less likely to undergo lymphadenectomy 88.1% vs. 94.8%) (P<0.0001).
Table 1.
Clinical and demographic characteristics of the cohort stratified by age.
| <50 years | 50–59 years | 60–69 years | ≥70 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | P-value | ||
| 5551 | (67.7) | 1418 | (17.3) | 768 | (9.4) | 462 | (5.6) | |||
| Year | 0.12 | |||||||||
| 1998 | 357 | (6.4) | 79 | (5.6) | 45 | (5.9) | 18 | (3.9) | ||
| 1999 | 451 | (8.1) | 102 | (7.2) | 49 | (6.4) | 35 | (7.6) | ||
| 2000 | 373 | (6.7) | 82 | (5.8) | 52 | (6.8) | 26 | (5.6) | ||
| 2001 | 411 | (7.4) | 79 | (5.6) | 52 | (6.8) | 32 | (6.9) | ||
| 2002 | 494 | (8.9) | 110 | (7.8) | 69 | (9.0) | 45 | (9.7) | ||
| 2003 | 389 | (7.0) | 112 | (7.9) | 57 | (7.4) | 29 | (6.3) | ||
| 2004 | 491 | (8.9) | 129 | (9.1) | 61 | (7.9) | 37 | (8.0) | ||
| 2005 | 506 | (9.1) | 124 | (8.7) | 62 | (8.1) | 49 | (10.6) | ||
| 2006 | 436 | (7.9) | 127 | (9.0) | 68 | (8.9) | 49 | (10.6) | ||
| 2007 | 452 | (8.1) | 119 | (8.4) | 62 | (8.1) | 34 | (7.4) | ||
| 2008 | 394 | (7.1) | 118 | (8.3) | 52 | (6.8) | 29 | (6.3) | ||
| 2009 | 392 | (7.1) | 104 | (7.3) | 71 | (9.2) | 36 | (7.8) | ||
| 2010 | 405 | (7.3) | 133 | (9.4) | 68 | (8.9) | 43 | (9.3) | ||
| Race | <0.0001 | |||||||||
| White | 2735 | (49.3) | 701 | (49.4) | 389 | (50.7) | 242 | (52.4) | ||
| Black | 412 | (7.4) | 122 | (8.6) | 80 | (10.4) | 39 | (8.4) | ||
| Hispanic | 670 | (12.1) | 156 | (11.0) | 76 | (9.9) | 34 | (7.4) | ||
| Other | 330 | (5.9) | 120 | (8.5) | 68 | (8.9) | 37 | (8.0) | ||
| Unknown | 1404 | (25.3) | 319 | (22.5) | 155 | (20.2) | 110 | (23.8) | ||
| Income | 0.05 | |||||||||
| Low | 137 | (2.5) | 21 | (1.5) | 20 | (2.6) | * | * | ||
| Medium | 561 | (10.1) | 136 | (9.6) | 75 | (9.8) | 47 | (10.2) | ||
| High | 707 | (12.7) | 142 | (10.0) | 95 | (12.4) | 66 | (14.3) | ||
| Highest | 985 | (17.8) | 227 | (16.0) | 112 | (14.6) | 77 | (16.7) | ||
| Unknown | 3161 | (56.9) | 892 | (62.9) | 466 | (60.7) | * | * | ||
| Insurance | <0.0001 | |||||||||
| Private | 3801 | (68.5) | 970 | (68.4) | 336 | (43.8) | 31 | (6.7) | ||
| Medicare | 78 | (1.4) | 48 | (3.4) | 272 | (35.5) | 399 | (86.4) | ||
| Medicaid | 1034 | (18.6) | 204 | (14.4) | 85 | (11.1) | * | * | ||
| Self pay | 302 | (5.4) | 93 | (6.6) | 39 | (5.1) | * | * | ||
| Other | 316 | (5.7) | * | * | 36 | (4.7) | * | * | ||
| Unknown | 20 | (0.4) | * | * | 0 | - | * | * | ||
| Elixhauser Comorbidity | <0.0001 | |||||||||
| 1 | 3024 | (54.5) | 467 | (32.9) | 179 | (23.3) | 73 | (15.8) | ||
| 2 | 1632 | (29.4) | 453 | (31.9) | 237 | (31.0) | 126 | (27.3) | ||
| ≥3 | 892 | (16.1) | 498 | (35.2) | 351 | (45.7) | 263 | (56.9) | ||
| Region | 0.01 | |||||||||
| Northeast | 852 | (15.4) | 231 | (16.3) | 131 | (17.1) | 80 | (17.3) | ||
| Midwest | 1243 | (22.4) | 251 | (17.7) | 146 | (19.0) | 88 | (19.1) | ||
| South | 2109 | (38.0) | 555 | (39.1) | 293 | (38.2) | 182 | (39.4) | ||
| West | 1347 | (24.3) | 381 | (26.9) | 198 | (25.8) | 112 | (24.2) | ||
| Area of residence | 0.39 | |||||||||
| Metropolitan | 5476 | (98.7) | 1397 | (98.5) | 755 | (98.3) | 451 | (97.6) | ||
| Non-metropolitan | 75 | (1.4) | 21 | (1.5) | 13 | (1.7) | 11 | (2.4) | ||
| Hospital size | 0.88 | |||||||||
| Small | 462 | (8.3) | 123 | (8.7) | 63 | (8.2) | 46 | (10.0) | ||
| Medium | 1059 | (19.1) | 277 | (19.5) | 149 | (19.4) | 94 | (20.4) | ||
| Large | 4030 | (72.6) | 1018 | (71.8) | 556 | (72.4) | 322 | (69.7) | ||
| Hospital teaching status | <0.0001 | |||||||||
| Non-teaching | 867 | (15.6) | 241 | (17.0) | 140 | (18.2) | 121 | (26.2) | ||
| Teaching | 3234 | (58.3) | 856 | (60.4) | 428 | (55.7) | 241 | (52.2) | ||
| Unknown | 1450 | (26.1) | 321 | (22.6) | 200 | (26.0) | 100 | (21.7) | ||
| Hospital volume | 0.004 | |||||||||
| Lowest | 1356 | (24.4) | 360 | (25.4) | 187 | (24.4) | 156 | (33.8) | ||
| Second | 1391 | (25.1) | 349 | (24.6) | 205 | (26.7) | 108 | (23.4) | ||
| Third | 1385 | (25.0) | 357 | (25.2) | 202 | (26.3) | 99 | (21.4) | ||
| Highest | 1419 | (25.6) | 352 | (24.8) | 174 | (22.7) | 99 | (21.4) | ||
| Admission type | 0.36 | |||||||||
| Elective | 4178 | (75.3) | 1039 | (73.3) | 568 | (74.0) | 344 | (74.5) | ||
| Emergent | 584 | (10.5) | 148 | (10.4) | 77 | (10.0) | 42 | (9.1) | ||
| Unknown | 789 | (14.2) | 231 | (16.3) | 123 | (16.0) | 76 | (16.5) | ||
| Lymphadenectomy | <0.0001 | |||||||||
| No | 289 | (5.2) | 92 | (6.5) | 53 | (6.9) | 55 | (11.9) | ||
| Yes | 5262 | (94.8) | 1326 | (93.5) | 715 | (93.1) | 407 | (88.1) | ||
Cell count <10 suppressed.
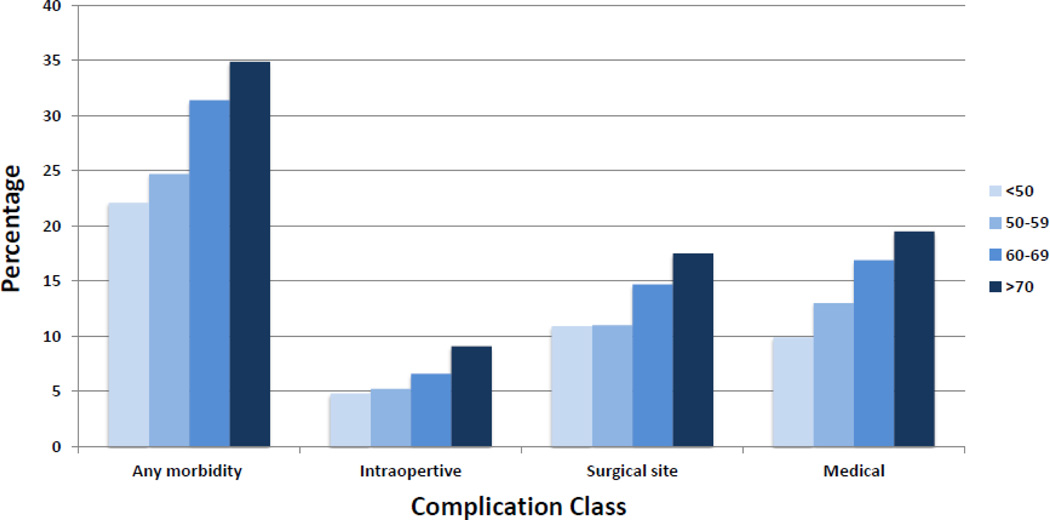
Complications increased with age (Table 2, Figure 1). The overall complication rate was 22.1% for women <50 years of age, 24.7% for those aged 50–59, 31.4% for patients age 60–69, and 34.9% for those ≥70 years old (P<0.0001). The adjusted risk ratio for any morbidity in women ≥70 compared to those <50 years of age was 1.23 (95% CI, 1.07–1.41). The rate of intraoperative complications was 4.8% for women <50 vs. 9.1% for those ≥70 years of age (P=0.003) (adjusted RR=1.62; 95% CI, 1.14–2.30) (Table 3). Similarly, surgical site complications were noted in 10.9% of those <50 years of age compared to 17.5% in women ≥70 years old (P<0.0001) (adjusted RR=1.19; 95% CI, 0.95–1.48). Finally, the rate of medical complications rose with age from 9.9% in patients <50 to 19.5% in women ≥70 years old (P<0.0001) (adjusted RR=1.45; 95% CI, 1.18–1.77).
Table 2.
Perioperative morbidity, mortality and resource utilization in patients undergoing radical hysterectomy for cervical cancer stratified by age.
| <50 years | 50–59 years | 60–69 years | >70 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | P-value | |
| 5551 | (67.7) | 1418 | (17.3) | 768 | (9.4) | 462 | (5.6) | ||
| Any complication | 1229 | (22.1) | 350 | (24.7) | 241 | (31.4) | 161 | (34.9) | <0.0001 |
| Intraoperative complications | 265 | (4.8) | 74 | (5.2) | 51 | (6.6) | 42 | (9.1) | 0.003 |
| Surgical site complications | 604 | (10.9) | 156 | (11.0) | 113 | (14.7) | 81 | (17.5) | <0.0001 |
| Medical complications | 549 | (9.9) | 184 | (13.0) | 130 | (16.9) | 90 | (19.5) | <0.0001 |
| Utilization Transfusion | 611 | (11.0) | 191 | (13.5) | 137 | (17.8) | 107 | (23.2) | <0.0001 |
| Length of stay ≥ 4 days | 3300 | (59.5) | 911 | (64.3) | 534 | (69.5) | 370 | (80.1) | <0.0001 |
| Non-routine discharge | 25 | (0.5) | 17 | (1.2) | 18 | (2.3) | 57 | (12.3) | <0.0001 |
| Death | * | * | * | * | * | * | * | * | <0.0001 |
Cell count <10 and suppressed.
Figure 1.
Unadjusted rate of complications stratified by age.
Table 3.
Multivariable models of the association between age and morbidity and mortality.
| Any complication |
Intraoperative complication |
Surgical site complication |
Medical complication |
Transfusion | Non-routine discharge |
|
|---|---|---|---|---|---|---|
| <50 years | Referent | Referent | Referent | Referent | Referent | Referent |
| 50–59 years | 0.98 (0.88–1.10) | 1.03 (0.79–1.33) | 0.86 (0.72–1.03) | 1.11 (0.93–1.31) | 0.94 (0.80–1.10) | 1.92 (1.02–3.60)* |
| 60–69 years | 1.17 (1.03–1.33)* | 1.27 (0.92–1.74) | 1.07 (0.87–1.32) | 1.32 (1.09–1.59)* | 1.14 (0.93–1.40) | 3.15 (1.65–6.01)* |
| ≥70 years | 1.23 (1.07–1.41)* | 1.62 (1.14–2.30)* | 1.19 (0.95–1.48) | 1.45 (1.18–1.77)* | 1.41 (1.17–1.70)* | 13.74 (7.96–23.71)* |
P<0.05
Models adjusted for age, year of diagnosis, race, performance of lymphadenectomy, hospital type, comorbidity, hospital volume and hospital-level clustering.
The perioperative transfusion rate rose with age from 11.0% in women <50 to 13.5% for those 50–59 years of age, 17.8% for those 60–69 and 23.2% for those ≥70 (P<0.0001) (Table 2). The adjusted risk ratio for women ≥70 compared to those <50 was 1.41 (95% CI, 1.17–1.70). Non-routine discharge rose from 0.5% to 12.3% in women ≥70 years of age (P<0.0001) (adjusted RR=13.74; 95% CI, 7.96–23.71). The overall mortality rate for the cohort was 0.2%. The mortality rate for women ≥70 years of age was 30 times greater than that of women <50 (P<0.0001) (raw data suppressed as cells <10 patients).
Discussion
Our findings suggest that radical hysterectomy for cervical cancer is associated with substantial morbidity. Compared to younger women, elderly patients who undergo radical hysterectomy are at increased risk for perioperative morbidity. The in-hospital mortality rate for women ≥70 years of age who undergo the procedure is 30-fold higher than that of younger women.
Elderly women with cervical cancer are treated less aggressively than their younger counterparts.4–9,22 In an analysis of over 28,000 women recorded in the Surveillance, Epidemiology, and End Results database, Sharma and colleagues noted that women with cervical cancer younger than age 50 years of age (82%) were more likely to undergo primary surgery than those aged 70–79 (55%) and those aged ≥80 (33%). Among those women who were managed surgically, performance of both radical hysterectomy and lymphadenectomy decreased with advancing age, and, older women were less likely to receive guideline-recommended adjuvant therapy. Even after accounting for disparities in treatment, this study noted that elderly women with cervical cancer were more likely to die from their cancer than younger patients.4 Other reports have also noted that elderly women with early-stage cervical cancer are less likely to undergo surgery.5,22
Prior single and multi-institutional observational studies have reported the outcomes of radical hysterectomy in elderly women. In general, these studies have suggested that the procedure is well tolerated and that the outcomes in elderly women are similar to their younger counterparts.10–15,23 An analysis that compared 22 patients aged ≥60 years to a younger cohort of 128 patients who underwent radical hysterectomy for early stage cervical cancer noted that although comorbidities were more common in the elderly, there was no significant difference in either major or minor postoperative complications. The authors reported that mean length of stay (5 vs. 3 days) and duration of bladder catheterization were longer in the elderly patients.15 Similarly, a matched case control study of 62 women >65 years of age and 124 <50 years of age with stage IB cervical cancer who underwent radical hysterectomy and lymphadenectomy found no difference in perioperative morbidity or mortality between the groups.13
For women with early-stage cervical cancer, primary radiotherapy is an alternative management strategy. A randomized controlled trial comparing radiation to radical hysterectomy for women with stage IB-IIA cervical cancer reported similar survival for the two treatment modalities.3 Radiation therapy, both external beam as well as brachytherapy, are well tolerated by the elderly.24–29 In a cohort of over 1600 patients with pelvic malignancies treated with radiotherapy in the European Organization for Research and Treatment of Cancer (EORTC) group’s therapeutic trials, there were no differences in either early or late toxicity based on age.28 It also appears that combination chemoradiation is well tolerated in older women.24 Despite the tolerance to radiotherapy, elderly women often receive non-optimal therapeutic courses. One report found that among women >75 years of age nearly one-third did not complete the prescribed course and 42% experienced treatment disruptions.30
Our data describing radical hysterectomy in the elderly is similar to many other high-risk oncologic procedures in which population-based data conflict with institutional series and suggests that outcomes are inferior in elderly patients.20,21,31,32 We previously reported that morbidity and mortality were higher in elderly women with endometrial cancer and ovarian cancer undergoing surgery compared to their younger counterparts.20,21 Similar data has been described for other solid tumor surgeries, including colectomy and pancreatectomy.31,32 The disparate outcomes may be due in part to inadequate power of small studies to detect age-related differences in outcomes. Alternatively, studies reporting outcomes of highly selected elderly patients from experienced centers may not be generalizable to the general population.
While radical hysterectomy remains the surgical procedure of choice for stage IB-IIA cervical cancer, newer surgical alternatives may reduce the morbidity associated with the operation. First, minimally invasive radical hysterectomy, both laparoscopic and robotically assisted, has been reported for cervical cancer. Although long-term outcomes data is lacking, these minimally invasive options have been shown to reduce acute perioperative morbidity.19,33 Second, reports have investigated less radical surgical alternatives to radical hysterectomy, including local excision and simple hysterectomy.34 These more conservative approaches are predicated on the fact that the parametrium is rarely involved in women with small tumors while removal of the parametrium at the time of radical hysterectomy adds substantial morbidity.34,35 These surgical alternatives warrant further investigation in the elderly.
Although our study benefits from the inclusion of a large sample of women from across the U.S., we recognize a number of important limitations. First, NIS lacks data on tumor characteristics, including stage, which may have impacted both treatment selection and outcome. Second, NIS lacks longitudinal follow-up data and, as such, we are unable to capture long-term toxicity, 30-day mortality, or assess the impact of age on receipt of adjuvant therapy. Third, while we included measurable comorbidity on in our analysis, we were unable to capture more subjective measures of physical functioning, such as performance status. Lastly, unmeasured confounding factors, including patient and physician preferences undoubtedly influenced the allocation of treatment.
Our data suggests that the treatment of early-stage cervical cancer in the elderly should be individualized. In addition to patient preferences and chronologic age, medical comorbidities and functional status are important considerations for elderly women.36 The comprehensive geriatric assessment (CGA) is a multidimensional and multidisciplinary diagnostic instrument to guide treatment that has been described for elderly patients with other solid tumors.37 While data describing the use of the CGA in gynecologic oncology and for gynecologic surgery, the instrument may be of use for elderly cervical cancer patients and deserves further study.38–40 Despite the fact that our cohort likely represents a selected group of elderly women chosen to undergo surgery, perioperative morbidity and mortality were substantially greater than in younger women. Given that survival is similar for radiation and surgery, non-surgical treatment options should be considered in elderly women with cervical cancer.
Research Highlights.
-
-
Perioperative morbidity and mortality are substantially greater in elderly women who undergo radical hysterectomy for cervical cancer.
-
-
Non-surgical treatments should be considered in these patients.
Acknowledgements
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA166084) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or disclosures.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Sharma C, Deutsch I, Horowitz DP, et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer. 2012;118:3618–3626. doi: 10.1002/cncr.26589. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Gibb RK, Geevarghese S, et al. Cervical carcinoma in the elderly: an analysis of patterns of care and outcome. Cancer. 2005;103:85–91. doi: 10.1002/cncr.20751. [DOI] [PubMed] [Google Scholar]
- 6.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. Jama. 2010;303:2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin JS, Samet JM, Hunt WC. Determinants of survival in older cancer patients. J Natl Cancer Inst. 1996;88:1031–1038. doi: 10.1093/jnci/88.15.1031. [DOI] [PubMed] [Google Scholar]
- 8.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. Jama. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 9.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28:2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YS, Kim YH, Kang S, et al. Feasibility of radical surgery in the management of elderly patients with uterine cervical cancer in Korea. Gynecol Obstet Invest. 2005;59:165–170. doi: 10.1159/000083681. [DOI] [PubMed] [Google Scholar]
- 11.Fuchtner C, Manetta A, Walker JL, Emma D, Berman M, DiSaia PJ. Radical hysterectomy in the elderly patient: analysis of morbidity. Am J Obstet Gynecol. 1992;166:593–597. doi: 10.1016/0002-9378(92)91681-y. [DOI] [PubMed] [Google Scholar]
- 12.Geisler JP, Geisler HE. Radical hysterectomy in patients 65 years of age and older. Gynecol Oncol. 1994;53:208–211. doi: 10.1006/gyno.1994.1117. [DOI] [PubMed] [Google Scholar]
- 13.Geisler JP, Geisler HE. Radical hysterectomy in the elderly female: a comparison to patients age 50 or younger. Gynecol Oncol. 2001;80:258–261. doi: 10.1006/gyno.2000.6044. [DOI] [PubMed] [Google Scholar]
- 14.Levrant SG, Fruchter RG, Maiman M. Radical hysterectomy for cervical cancer: morbidity and survival in relation to weight and age. Gynecol Oncol. 1992;45:317–322. doi: 10.1016/0090-8258(92)90312-7. [DOI] [PubMed] [Google Scholar]
- 15.Mousavi A, Karimi Zarchi M, Gilani MM, et al. Radical hysterectomy in the elderly. World journal of surgical oncology. 2008;6:38. doi: 10.1186/1477-7819-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) Rockville, MD: 2007–2009. [Accessed January 26, 2014]. Agency for Healthcare Research and Quality. at http://www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 17.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 18.Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341–1347. doi: 10.1097/AOG.0b013e3181fca8c5. [DOI] [PubMed] [Google Scholar]
- 19.Wright JD, Herzog TJ, Neugut AI, et al. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Wright JD, Lewin SN, Barrena Medel NI, et al. Morbidity and mortality of surgery for endometrial cancer in the oldest old. Am J Obstet Gynecol. 2011;205:66 e1–68 e1. doi: 10.1016/j.ajog.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 21.Wright JD, Lewin SN, Deutsch I, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2011;123:467–473. doi: 10.1016/j.ygyno.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 22.van der Aa MA, Siesling S, v d Poll-Franse LV, Schutter EM, Lybeert ML, Coebergh JW. Age-specific differences in the treatment of cervical cancer in the east and the south of The Netherlands 1989–2004. European journal of obstetrics, gynecology, and reproductive biology. 2009;147:78–82. doi: 10.1016/j.ejogrb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Parker DY, Burke JJ, 2nd, Gallup DG. Gynecological surgery in octogenarians and nonagenarians. Am J Obstet Gynecol. 2004;190:1401–1403. doi: 10.1016/j.ajog.2004.01.065. [DOI] [PubMed] [Google Scholar]
- 24.Goodheart M, Jacobson G, Smith BJ, Zhou L. Chemoradiation for invasive cervical cancer in elderly patients: outcomes and morbidity. Int J Gynecol Cancer. 2008;18:95–103. doi: 10.1111/j.1525-1438.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 25.Ikushima H, Takegawa Y, Osaki K, et al. Radiation therapy for cervical cancer in the elderly. Gynecol Oncol. 2007;107:339–343. doi: 10.1016/j.ygyno.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 26.Magne N, Mancy NC, Chajon E, et al. Patterns of care and outcome in elderly cervical cancer patients: a special focus on brachytherapy. Radiother Oncol. 2009;91:197–201. doi: 10.1016/j.radonc.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Minagawa Y, Kigawa J, Itamochi H, Terakawa N. The outcome of radiation therapy in elderly patients with advanced cervical cancer. Int J Gynaecol Obstet. 1997;58:305–309. doi: 10.1016/s0020-7292(97)00124-0. [DOI] [PubMed] [Google Scholar]
- 28.Pignon T, Horiot JC, Bolla M, et al. Age is not a limiting factor for radical radiotherapy in pelvic malignancies. Radiother Oncol. 1997;42:107–120. doi: 10.1016/s0167-8140(96)01861-0. [DOI] [PubMed] [Google Scholar]
- 29.Zachariah B, Balducci L, Venkattaramanabalaji GV, Casey L, Greenberg HM, DelRegato JA. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39:1125–1129. doi: 10.1016/s0360-3016(97)00552-x. [DOI] [PubMed] [Google Scholar]
- 30.Grant PT, Jeffrey JF, Fraser RC, Tompkins MG, Filbee JF, Wong OS. Pelvic radiation therapy for gynecologic malignancy in geriatric patients. Gynecol Oncol. 1989;33:185–188. doi: 10.1016/0090-8258(89)90548-9. [DOI] [PubMed] [Google Scholar]
- 31.Dimick JB, Cowan JA, Jr, Upchurch GR, Jr, Colletti LM. Hospital volume and surgical outcomes for elderly patients with colorectal cancer in the United States. J Surg Res. 2003;114:50–56. doi: 10.1016/s0022-4804(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 32.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–194. doi: 10.1016/j.ygyno.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez PT, Pareja R, Rendon GJ, Millan C, Frumovitz M, Schmeler KM. Management of low-risk early-stage cervical cancer: Should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JD, Grigsby PW, Brooks R, et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007;110:1281–1286. doi: 10.1002/cncr.22899. [DOI] [PubMed] [Google Scholar]
- 36.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 37.Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, Machteld Wymenga AN. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Partridge JS, Harari D, Martin FC, Dhesi JK. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: a systematic review. Anaesthesia. 2014;69(Suppl 1):8–16. doi: 10.1111/anae.12494. [DOI] [PubMed] [Google Scholar]
- 39.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 40.Tredan O, Geay JF, Touzet S, et al. Carboplatin/cyclophosphamide or carboplatin/paclitaxel in elderly patients with advanced ovarian cancer? Analysis of two consecutive trials from the Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. Ann Oncol. 2007;18:256–262. doi: 10.1093/annonc/mdl400. [DOI] [PubMed] [Google Scholar]