Abstract
Human dermal fibroblasts (HDFs) are a potential source of somatic cells for genetic manipulation and tissue engineering. Confirmation of cytogenetic stability of these cells is an essential step for cell nuclear transfer and generation of a suitable and functional induced pluripotent stem cells line. HDF cells were isolated and cultured from human foreskin samples. Cytogenetic stability of these cells was evaluated in early (3–4) and late (10–15) passages using karyotype test and alkaline comet assay techniques. HDF cells in early and late passages showed normal karyotype but by comet assay abnormality and DNA damages in late passages of HDFs were observed. Also, the parameters of alkaline comet assay in early passages of HDFs compared with late passages and positive control groups more significantly were different (p < 0.05). These findings indicate that single-strand breaks or DNA damage after many passages may have occurred in HDF cells. Our results demonstrate that only early passages of HDF cells maintain cytogenetic stability and are good candidates for gene reprogramming. In conclusion, karyotype testing alone can not be used for detection of all signs of cytogenetic abnormality and DNA damages of cells. So, for precise evaluation of DNA damage and cytogenetic instability of fibroblast cells comet assay and karyotype techniques could complement each other.
Keywords: HDF, Cytogenetic analysis, Karyotype test, Comet assay, IPSC
Introduction
Human dermal fibroblasts (HDFs) are the first cell type that has been reprogrammed to form induced pluripotent stem (iPS) cells. HDFs are found in all connective tissues with mesenchymal origin and could be obtained from the dermis of normal human adult skin or neonatal foreskin (Chang et al. 2002; French et al. 2004). These cells are still an interesting source because they are available, easy to access with relatively good kinetics of growth and can be easily cultured in vitro with a high yield of cells using standard cell culture protocols with minimal invasive cellular procedures (El-Ghalbzouri et al. 2002; Takahashi et al. 2007; Wong et al. 2007). They synthesize and secrete extracellular matrix proteins (including laminin and fibronectin) and collagen under cell culture conditions (Mizuno and Glowacki 1996; Wong et al. 2007).
Dermal fibroblasts have many roles in epithelial-mesenchymal interactions, and wound healing. They have various functions in proliferation and migration in response to chemotactic, mitogenic and modulatory cytokines, and also autocrine and paracrine interactions (Jongkind and Verkerk 1984; Mastromonaco et al. 2006). The future application of dermal fibroblasts for gene therapy also offers great potential in providing new strategies for treating some of the severe skin genodermatoses (Wong et al. 2007). Fibroblasts have been used in tissue engineering, cell nuclear transfer, and reprogramming (Mastromonaco et al. 2006).
Increasing of chromosomal abnormality, level of phosphorylated histones, and cytogenetic instability of cells were observed after prolonged time in culture. These abnormal changes are important in somatic cell nuclear transfer and gene reprogramming (Chu 1962; Mastromonaco et al. 2006). There are many techniques available for detecting DNA damage, chromosomal abnormality, and human mutagens (Anderson et al. 1998; Tice et al. 2000) such as Karyotype test and single cell gel electrophoresis assay (also known as comet assay). Comet assay is an uncomplicated and sensitive method used for the detection of single-strand breaks (SSBs), genetic instability, and DNA damage of fibroblast cells (McKelvey-Martin et al. 1998; Anderson and Plewa 1998; Speit et al. 2009). Karyotype analysis is another technique to identify the number, evaluate the size, and shape of the chromosomes. This test is useful to analyze chromosomal errors and detection of abnormality and position of a centromere. G-banding of the karyotype test can be used to detect insertions, deletions, duplications, inversions, and translocations of chromosomes (Gagos et al. 2008; Paskulin et al. 2011).
Many reports indicated changes in methylation, DNA patterns and cytogenetic instability of fibroblast with time in culture and after many passages (Reis and Goldstein 1982). So, evaluation and characterization of cytogenetic and chromosomal stability of fibroblasts in the first and aftermany passages are important for subsequent manipulation. In the present study, we evaluated chromosomal and cytogenetic stability of HDF cells isolated from human foreskin samples during early (3–4) and late (10–15) passages using both karyotype test and alkaline comet assay techniques.
Materials and methods
HDF cell culture
The foreskins samples of healthy male newborns were obtained from the Kashani Hospital (Shahrekord City, Iran) and transferred to the Cellular and Molecular Research Center. Neonatal HDF cells were isolated from human foreskin tissue by a combination of mechanical disaggregation and enzymatic digestion (0.25 % Trypsin–EDTA solution, 100 U/mL Collagenase type IV and 100 μg/mL DNase). Then, single cells were cultured at 37 °C in a 5 % CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) with 10 % fetal bovine serum (FBS), and 1 % penicillin/streptomycin antibiotics (all Gibco, Grand Island, NY, USA).
HDF cells preparation for experiments
Cells obtained from early (3–4) and late passages (10–15) of HDFs cultured in vitro were washed with phosphate-buffered saline (PBS) twice and dissociated with 0.25 % Trypsin–EDTA solution at 37 °C for 5 min. The enzyme was neutralized by DMEM containing 15 % FBS. HDF cells were counted by in a Neubauer chamber using microscopy. Then, cells were seeded into 6-well plates at a concentration of 150 × 103 in 2 mL of DMEM 15 % FBS per well, and incubated in a CO2 incubator at 37 °C for 24 h. After confluence of the cultures, DMEM containing 0.1 % FBS was added to the cells for synchronization. After 48 h, the HDF cells were ready to be used in karyotype test and comet assay.
Cell growth rate of HDFs
HDF cells between passages 5–7 were cultured in a 6-well plate with a concentration of 4 × 104 per well. The culture medium was again changed every 3 days. Through the 6 days the growth curves of HDF cells were obtained by counting the number of cells for each triplicate wells every 24 h.
Karyotype test
Karyotype analysis via Giemsa-banding (G-banding) was performed in triplicate on each early and late passage of HDFs. When cell confluence reached to 80–90 % in the 6-well plates, culture medium was replaced with media containing 0.1 μg/mL Karyomax Colcemid® solution for each well (Cat. no. 15212-012. Invitrogen, Carlsbad, CA, USA) and then culture was returned to the CO2 incubator. After 20 min cells were collected and suspended in 5 mL of 0.075 M KCl solution. Then the suspension was incubated in 37 °C for 20 min. 1 mL of cold Carnoy’s Fixative (methanol/acetic acid, 3:1) was added and mixed together. The cells were centrifuged at 900 rpm for 10 min at room temperature and cell pellet were collected. After two rounds of fixations (add 5 mL fixative and centrifuge at 900 rpm for 10 min), the pellets were fixed via suspending in 200 μL of cold fixative and cells from each suspension were dispensed onto glass slides and baked at 75 °C for 3 h. Routine chromosome G-banding analysis was then carried out. Twenty karyotypes per slide were examined.
Comet assay
Positive control
For positive control in comet assay we used γ-radiation treatment. Cells were seeded in a multi-well plate include 2 mL at 150 × 103 cells/mL in DMEM. Cells cultured in medium supplemented with 10 % FBS, 2 mM glutamine, and 1 % (100 μg/mL/100 U/mL) penicillin/streptomycin antibiotics and allowed to attach for 24 h at 37 °C in a CO2 incubator. HDF cells γ-radiation was performed in Shahrekord Omid Nuclear Medicine Center with 277.48 ± 11.51 CGy/min dose to induce DNA damage according to the method described by Benhusein et al. (2010). The plate was incubated at 37 °C for 60 min. At the end of incubation the cells were used as positive control (DNA damage) of comet assay analysis.
The alkaline comet assay
The alkaline comet assay was performed following standard protocol described by McKelvey-Martin et al. with a few modifications (McKelvey-Martin et al. 1998). All slides were washed with methanol and heated to remove the proteins. Dakin microscope slides covered with 250–300 μL of 0.1 % normal melting point agarose (Gibco, Carlsbad, Ca, USA) prepared in PBS at 50 °C and allowed to be fully frosted. To solidify agarose, a coverslip was placed on top and the slide was kept on ice. Each pellet of cells (prepared as described above) was re-suspended in 80 μL of low melting point agarose at 37 °C. After gently removing the coverslip, the cell suspension was quickly pipetted onto the first agarose layer and the coverslip replaced on top and the slide left on ice to solidify the agarose. The coverslip was gently removed and the third layer of 250–300 μL of 0.1 % normal melting point agarose was put on them. After removing the coverslips, slides were quickly immersed in freshly prepared cold lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, pH 10, with 1 % Triton X-100 and DMSO 10 % added just before use) for 12 h at 4 °C. After lysis, the slides were drained and placed side by side in a horizontal gel electrophoresis tank. The tank was filled with fresh and cold electrophoresis buffer (300 mM NaOH, 1 mM Na2EDTA, pH > 13) to a level of approximately 0.25 cm above the slides. The slides were left in the alkaline buffer for 30 min to allow that DNA unwinding occurs. Electrophoresis was performed at 25 V (0.66 V/cm) and 300 mA for 30 min at room temperature. After electrophoresis, the slides were drained and placed on a tray and flooded slowly with three changes of neutralization buffer (0.4 M Tris, pH 7.5) for 5 min to remove alkali and detergents. All slides were just once washed with ethanol 95 % for 5 min. Slides were drained and stained with 2 μg/mL ethidium bromide and left in a humidified chamber at 4 °C prior to analysis. All slides were prepared under UV light and duplicate slides for each treatment were prepared. The parameters of alkaline comet assay include head area, tail area, head DNA, tail DNA, tail length, comet length, and tail moment of positive control and HDF cells in early and late passages were evaluated by CaspLab (Comet Assay Software Project) software version 1.0.0.
Statistical analysis
Each experiment performed at least three times. Data of all experiments were collected in Statistics programs for the Social Sciences software, version 17 (SPSS, Inc., Chicago, IL, USA). The alkaline comet assay parameters were evaluated by CaspLab software version 1.0.0 and the within groups variance was calculated by ANOVA (Analysis of variance) test. The 0.05 % (5 %) is considered as statistically significant.
Results
HDF cells preparation
We prepared HDF cells in early (passages 3–4) and late passages (10–15) for evaluation of cytogenetic stability, DNA damages, and karyotyping properties using karyotype test and comet assay. Figure 1 shows the HDF cells isolated from human foreskin samples in early and late passages.
Fig. 1.
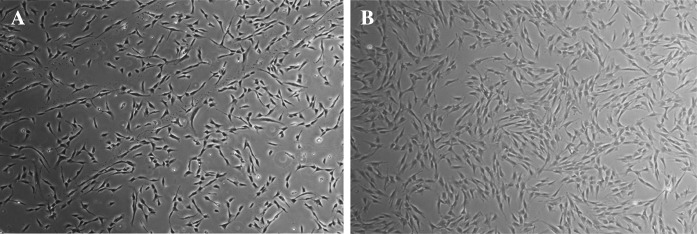
a HDF cells in early passage (P3) and b late passage (P10) isolated from human foreskin samples
Cell growth rate
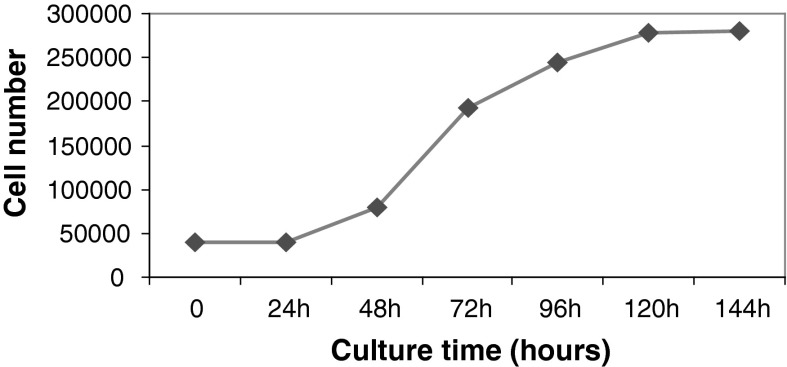
After HDF cells (P5 through P7) reached 80–90 % confluence, they were passaged to 6 wells plate to calculate the cell growth rate. The growth curve of HDF cells showed that the number of HDF cells did not increase in the first 24 h after passage. From 24 to 120 h, HDF cells showed normal exponential growth and 120–144 h after passage HDF cells entered stationary phase (Fig. 2).
Fig. 2.
Growth curves of HDF cells through 6 days. The growth curve shows no change in number of cells at 0–24 h, exponential growth phase at 24–120 h, and stationary phase at 120–144 h after HDF cell passage
Karyotype analysis
G-banding was conducted to determine the karyotypes of HDF cells in early and late passages. 20 karyotypes per slide were analyzed and showed that HDFs had normal karyotype (20/20, 46 chromosomes, XY) in early (3–4) and late (10–15) passages (Figs. 3, 4). The karyotype analysis did not show any abnormalities including insertions, deletions, and duplications in karyotypes of HDF cells in late passages.
Fig. 3.
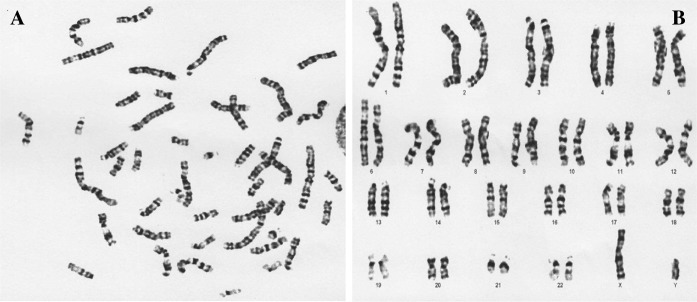
a The metaphase and b karyotype of HDF cells in early passages
Fig. 4.
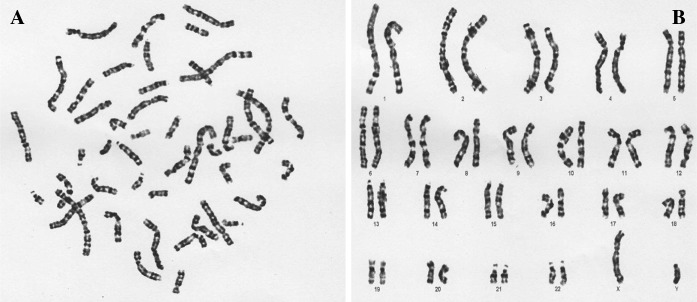
a The metaphase and b karyotype of HDF cells in late passages
Comet assay
Alkaline comet assay was performed on 70 HDF cells exposed to γ-radiation and also 100 and 94 HDF cells in early and late passages, respectively. The γ-radiation treatment was used as positive control for DNA damage of early and late passages of HDF cells in comet assay (Fig. 5).
Fig. 5.

The γ-radiation treatment (positive control) to create DNA damage in HDFs
After alkaline comet assay of HDF cells in early passages, no damaged DNA and DNA SSBs were observed (Fig. 6a). But the alkaline comet assay analysis of HDF cells in late passages showed a long tail like positive control (Fig. 6b).
Fig. 6.
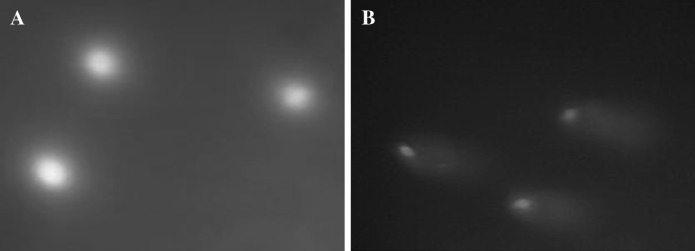
a Three HDF cells in early passages after alkaline comet assay showed normal cells and no DNA damage. b Alkaline comet assay analysis of three HDF cells in late passages showed long tail like positive control
The mean values of head area, tail area, head DNA, tail DNA, tail length, comet length, and tail moment of positive control and early and late passages of HDFs were calculated by CaspLab software version 1.0.0 to compare the cytogenetic status (Table 1). The relationship and analysis of variance between groups were evaluated by analysis of variance (ANOVA) test (Table 2). The p < 0.05 was considered significant.
Table 1.
The details of alkaline comet assay analysis on HDF cells (early and late passages) and positive control (cells exposed by γ-radiation)
| Parameters | Early passage of HDF | Late passage of HDF | γ-radiation |
|---|---|---|---|
| No. of cells (100) | No. of cells (94) | No. of cells (70) | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Head area | 1512.29 ± 725.854 | 805.63 ± 369.86 | 784.41 ± 140.26 |
| Tail area | 102.75 ± 99.158 | 2486.78 ± 676 | 8504 ± 2487.915 |
| Head DNA | 209.271 ± 101.533 | 86.887 ± 35.621 | 68.562 ± 28.461 |
| Tail DNA | 3.631 ± 4.137 | 120.777 ± 40.118 | 510.02 ± 90.75 |
| Head DNA % | 98.257 ± 1.8 | 41.436 ± 9.22 | 11.36 ± 3.62 |
| Tail DNA % | 1.743 ± 1.8 | 58.564 ± 9.22 | 88.64 ± 3.62 |
| Tail length | 3.44 ± 0.978 | 53.45 ± 8.928 | 116 ± 33.05 |
| Comet length | 48.46 ± 9.784 | 86.81 ± 11.125 | 148.66 ± 31.96 |
| Tail moment | 0.068 ± 0.0802 | 31.575 ± 8.638 | 102.24 ± 27.21 |
Head area: area of the comet head in pixels, Tail area: area of the comet tail in pixels, Head DNA: amount of DNA in the comet head, Tail DNA: amount of DNA in the comet tail, Tail Length: length of the comet tail measured from right border of head area to end of tail (in pixels), Comet length: length of the entire comet from left border of head area to end of tail (in pixels), Tail moment: tail DNA % × tail length [(percent of DNA in the tail) × (tail length)]
SD Standard deviation
Table 2.
The relationship of alkaline comet assay parameters between early and late passages of HDFs and the positive control analyzed by ANOVA test
| Parameters | Early passage of HDFs compare with positive control (γ-radiation) | Early passage compare with late passage of HDFs | ||
|---|---|---|---|---|
| Mean difference ± SE | p value | Mean difference ± SE | p value | |
| Head area | 727.876* ± 109.743 | 0.000 | 706.662* ± 100.713 | 0.000 |
| Tail area | −8401.25* ± 171.3 | 0.000 | −2384.027* ± 169.311 | 0.000 |
| Head DNA % | 86.9* ± 0.766 | 0.000 | 56.821* ± 0.725 | 0.000 |
| Tail DNA % | −86.9* ± 0.766 | 0.000 | −56.821* ± 0.725 | 0.000 |
| Tail length | −112.56* ± 2.22 | 0.000 | −50.007* ± 2.285 | 0.000 |
| Comet length | −100.197* ± 2.57 | 0.000 | −38.348* ± 2.616 | 0.000 |
| Tail moment | −102.177* ± 1.89 | 0.000 | −31.506* ± 1.93 | 0.000 |
*The mean difference is significant at p < 0.05
SE Standard error
The comparison of alkaline comet assay parameters and analysis of variance between early passages of HDFs and groups (positive control and late passages of HDFs) by ANOVA test showed that head area, tail area, head DNA %, tail DNA %, tail length, comet length, and tail moment were significantly different (p < 0.05).
Discussion
Cytogenetic and chromosome stability of HDF cells during early and late passages must first be characterized before nuclear transfer and reprogramming them to iPS cells (Chu 1962; Takahashi et al. 2007; Lowry et al. 2008; Xue et al. 2010). In 2007, Takahashi et al. showed that adult human fibroblasts are a good and safe source for reprogramming to produce iPS cells (Takahashi et al. 2007). In 2013, Bisson et al. indicated that irradiated HDFs provide a good human feeder layer for an effective expansion of keratinocytes in vitro that are to be used for clinical purposes (Bisson et al. 2013).
In this study karyotype analysis and alkaline comet assay performed to determine the HDFs cytogenetic instability and chromosome abnormality in early and late passages. We used γ-radiation treatments to induce DNA damage on HDF cells culture as a positive control of comet assay. The results of the present study demonstrated that HDFs have a normal karyotype in early passages (3–4) and late passages (10–15). The alkaline comet assay of HDFs in early passages showed normal DNA but in late passages showed damaged DNA and DNA SSBs like the positive control. The analysis of cytogenetic stability of HDFs in early passages compared with late passages and positive control group by ANOVA test were significantly different (p < 0.05) in the alkaline comet assay parameters including head area, tail area, head DNA %, tail DNA %, tail length, comet length, and tail moment. These results indicated that chromosome abnormality and DNA damage in HDF cells may occur after many passages. According to these findings karyotype testing alone can not detect all signs of cytogenetic abnormality and DNA damages of cells.
There are many studies performed on determination of cytogenetic instability and karyotyping of fibroblast cells. Reis and Goldstein examined DNA methylation in diploid human fibroblasts, early and late in their replicative life-span, using restriction enzyme. Their study showed that the pattern of methylation in endogenous gene regions appeared to undergo random drift during replication of diploid fibroblasts (Reis and Goldstein 1982). The studies of Ford et al. (1959), Hsu and Moorhead (1957), and Chu (1962) using conventional karyotyping, showed that the chromosomal composition of some cell lines cultured in vitro deviates from the diploid content of in vitro tissues. Their findings indicated that stable diploidy was maintained in rat cell cultures (Hsu and Moorhead 1957; Ford et al. 1959; Hsu and Kellogg 1960), but that mouse cells began to change their chromosomal constitution even in primary cultures (Chu 1962).
Previous studies on in vitro HDF cells culture after long term passages (usually after 5–7 passages) report abnormal karyotypes including chromosomal aneuploidy and multiploidy karyotypes in spontaneously mutated clones in most species, especially in rodents (Xue et al. 2010; Junker et al. 2010). In the present research we did not find any definitively abnormal karyotypes through the standard G-banding karyotyping in early and late passages of HDF cells. But we observed abnormality and damaged DNA by comet assay in late passages. In 2008, Ponzinibbio et al. in 2008 analyzed the delayed DNA damage induced by ionizing radiation in MRC-5 human fibroblasts cultured in vitro. Their study showed that low doses of ionizing radiation (10–50 mGy) could induce delayed damage. They indicated that comet assay, micronucleus analysis and γ-H2AX focus analysis are useful techniques to detect DNA damage and might be also sensitive for the study of delayed events as genomic instability (Ponzinibbio et al. 2008). The findings of the present study showed that comet assay technique is sensitive and could detect DNA damage of fibroblast cells after many passages. Xue et al. in 2010 characterized biological properties of female human dermal fibroblasts in terms of cell-growth rate, cytogenetic stability, and the number of inactive X chromosomes during long-term passaging. They did not find any definitively abnormal karyotypes through the standard G-banding karyotyping. But their findings showed that female HDF cultures exhibit a high risk of genetic anomalies such as carrying an increased number of X chromosomes including both active and inactive X chromosomes at a high passage (≥P10) (Xue et al. 2010). In our study we used HDF cells from male newborn foreskin samples and thus genetic anomalies including an increased number of X chromosomes were not observed. Our results indicated cytogenetic instability of HDFs after many passages could occur and detection of chromosome stability and cytogenetic parameters must be evaluated by karyotyping and comet assay techniques.
Conclusion
In conclusion, our present study demonstrates that only early passages of HDF cells culture are cytogenetically stable and could be used for nuclear transfer, genetic manipulation, and cell reprogramming to produce iPS cells. These results indicated SSBs or DNA damage in HDFs after many passages may occur. Also, karyotype test could not show all signs of DNA damages and abnormality of HDF cells after long term passages. As suggestion for precise, rapid, and sensitive evaluation of cytogenetic instability and DNA damage of fibroblast cells we can use both comet assay and karyotype techniques together.
Acknowledgments
This study was supported by Cellular and Molecular Research Center of Shahrekord University of Medical Sciences, Shahrekord, Iran (Grant No. 91-10-5). The authors would like to express their deepest gratitude to the staffs of Shahrekord Omid Nuclear Medicine Center for sincere cooperation.
Abbreviations
- HDFs
Human dermal fibroblasts
- IPSC
Induced pluripotent stem cell
- ECM
Extracellular matrix
- LMA
Low melting point agarose
References
- Anderson D, Plewa MJ. The international comet assay workshop. Mutagen. 1998;13:67–73. doi: 10.1093/mutage/13.1.67. [DOI] [PubMed] [Google Scholar]
- Anderson D, Yu T-W, McGregor DB. Comet assay responses as indicators of carcinogenic exposure. Mutagen. 1998;13:539–555. doi: 10.1093/mutage/13.6.539. [DOI] [PubMed] [Google Scholar]
- Benhusein GM, Mutch E, Aburawi S, Williams FM. Genotoxic effect induced by hydrogen peroxide in human hepatoma cells using comet assay. Libyan J Med. 2010;5:4637. doi: 10.3402/ljm.v5i0.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson F, Rochefort E, Lavoie A, Larouche D, Zaniolo K, Simard-Bisson C, Damour O, Auger FA, Guérin SL, Germain L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int J Mol Sci. 2013;14:4684–4704. doi: 10.3390/ijms14034684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Chi J-T, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EH. Chromosomal stabilization of cell strains. Natl Cancer Inst Monogr. 1962;7:55–71. [PubMed] [Google Scholar]
- El-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol. 2002;147:230–243. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]
- Ford DK, Wakonig R, Yerganian G (1959) Further observations on the chromosomes of Chinese hamster cells in tissue culture. J Natl Cancer Inst 22:765–799 [PubMed]
- French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32:50–56. doi: 10.1023/B:ABME.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- Gagos S, Papaioannou G, Chiourea M, Merk-Loretti S, Jefford CE, Mikou P, Irminger-Finger I, Liossi A, Blouin JL, Dahoun S. Unusually stable abnormal karyotype in a highly aggressive melanoma negative for telomerase activity. Mol Cytogenet. 2008;1:20. doi: 10.1186/1755-8166-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TC, Kellogg DS. Mammalian chromosomes in vitro. XII. Experimental evolution of cell populations. J Natl Cancer Inst. 1960;24:1067–1093. [PubMed] [Google Scholar]
- Hsu TC, Moorhead PS. Mammalian chromosomes in vitro. VII. Heteroploidy in human cell strains. J Natl Cancer Inst. 1957;18:463–471. [PubMed] [Google Scholar]
- Jongkind JF, Verkerk A. Cell sorting and microchemistry of cultured human fibroblasts: applications in genetics and aging research. Cytometry. 1984;5:182–187. doi: 10.1002/cyto.990050212. [DOI] [PubMed] [Google Scholar]
- Junker JP, Sommar P, Skog M, Johnson H, Kratz G. Adipogenic, chondrogenic and osteogenic differentiation of clonally derived human dermal fibroblasts. Cells Tissues Organs. 2010;191:105–118. doi: 10.1159/000232157. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromonaco GF, Perrault SD, Betts DH, King WA. Role of chromosome stability and telomere length in the production of viable cell lines for somatic cell nuclear transfer. BMC Dev Biol. 2006;6:41–53. doi: 10.1186/1471-213X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey-Martin VJ, Ho ETS, McKeown SR, Johnston SR, McCarthy PJ, Rajab NF, Downes CS. Emerging applications of the single cell gel electrophoresis (Comet) assay. I. Management of invasive transitional cell human bladder carcinoma. II. Fluorescent in situ hybridization comets for the identification of damaged and repaired DNA sequences in individual cells. Mutagen. 1998;13:1–8. doi: 10.1093/mutage/13.1.1. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Glowacki J. Chondroinduction of human dermal fibroblasts by demineralized bone in three-dimensional culture. Exp Cell Res. 1996;227:89–97. doi: 10.1006/excr.1996.0253. [DOI] [PubMed] [Google Scholar]
- Paskulin GA, Lorenzen MB, Rosa RFM, Graziadio C, Zen PRG. Importance of the fibroblast chromosomal analysis in suspected cases of mosaicism: experience of a clinical Genetics service. Rev Paul Pediatr. 2011;29:73–79. doi: 10.1590/S0103-05822011000100012. [DOI] [Google Scholar]
- Ponzinibbio MV, Crudeli C, Peral García P, Seoane A. Cytogenetic and cytomolecular delayed damage induced in human fibroblasts by low X-ray doses. J Basic Appl Genet. 2008;19:35–41. [Google Scholar]
- Reis RJS, Goldstein S. Variability of DNA methylation patterns during serial passage of human diploid fibroblasts. Proc Natl Acad Sci USA. 1982;79:3949–3953. doi: 10.1073/pnas.79.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speit G, Vasquez M, Hartmann A. The comet assay as an indicator test for germ cell genotoxicity. Mutat Res. 2009;681:3–12. doi: 10.1016/j.mrrev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Takahashi KTK, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–221. doi: 10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wong T, McGrath JA, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- Xue ZG, Shi ZP, Dong J, Liao TT, Wang YP, Sun XP, Yan ZJ, Qian XQ, Cui YG, Chen J, Liu JY, Fan G. Evaluation of X-Inactivation status and cytogenetic stability of human dermal fibroblasts after long-term culture. Int J Cell Biol. 2010;2010:1–5. doi: 10.1155/2010/289653. [DOI] [PMC free article] [PubMed] [Google Scholar]