Abstract
In this study, the antioxidant, antimicrobial, genotoxic and anticancer activities of Cetraria islandica methanol extract were determined by using free radical and superoxide anion scavenging activity, reducing power, determination of total phenolic compounds and flavonoid contents, broth microdilution minimal inhibitory concentration against five bacterial and five fungal species, cytokinesis block micronucleus (MN) assay on peripheral blood lymphocytes (PBLs) and the microculture tetrazolium test on FemX (human melanoma) and LS174 (human colon carcinoma) cell lines. As a result of the study, we found that C. islandica methanol extract exhibited moderate free-radical-scavenging activity with IC50 values 678.38 μg/ml. Moreover, the tested extract had effective reducing power and superoxide anion radical scavenging. The minimal inhibitory concentration values against the tested microorganisms ranged from 0.312 to 5 mg/ml. The extract increased MN frequency in a dose dependent manner, but it was significant in higher tested concentrations (50, 100 and 200 μg/ml). No significant differences were observed between NDI values in all treatments and untreated PBLs. In addition, the tested extract had strong anticancer activity towards both cell lines with IC50 values of 22.68 and 33.74 μg/ml. It can be concluded that the tested extract exhibited a certain level of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities.
Keywords: Cetraria islandica; Methanol extract; Antioxidant, antimicrobial, genotoxic, anticancer activities
Introduction
Lichens are symbiotic organisms consisting of a fungus partner and a photosynthetic organism, either algae or Cyanobacteria (Bates et al. 2011). More than 20,000 known species of lichens have been identified and inhabit diverse ecosystems ranging from arctic tundra to desert climates (Oboh and Ademosun 2006). They are ubiquitous on barks, stems, leaves and in soil but often grow in habitats less favorable for higher plants (Vrablikova et al. 2006). These organisms have historically been used as food, dyes, in production of alcohol and perfume industry. Lichens have also, for hundreds of years, been used in folk medicine in many countries (Bown 2001). Chemical studies on the secondary metabolites present in lichens have led to the isolation of many new substances, which today number over 800 (Huneck 1991; Muller 2001).
In recent years, there has been a renewed interest in lichens as potential drugs. In that respect, this report focuses on the lichen species Cetraria islandica.
Cetraria islandica (Iceland Moss) is a fruticose, or shrub-like and bushy, lichen growing loosely on the soil to a height of 3–4 inches. The thallus is channeled or rolled into thin, branched tubes, which terminate in flattened lobes fringed with minute papillae, rarely more than 5 mm wide. The whole lichen is very tough and springy. It varies considerably from pale chestnut to grayish white; the upper surface is darker, the under surface is lighter to whitish. There are white spots which have a chalky or mealy appearance, and which are lodged in little depressions of the thallus (Purvis et al. 1992; Dobson 2000).
Cetraria islandica is strongly antibiotic and expectorant. It soothes irritated tissues, especially mucous membranes and is often used in cough medications. It eases dry cough and helps in case of a sore throat. It has beneficial results in cases of tuberculosis and bronchitis. It also controls vomiting, has excellent effects in treatment of gastroenteritis, loss of appetite and food poisoning. Used externally, the lichen is an excellent remedy for vaginal discharge, boils and wounds (Chevallier 1996). On the basis of the aforesaid, we report antioxidant, antimicrobial, genotoxic and anticancer activities of methanol extract of lichen Cetraria islandica.
Materials and methods
Lichen sample
Lichen samples of C. islandica (L.) Ach., were collected from Kopaonik, Serbia, in September 2011. The demonstration samples were preserved at the Department of Biology and Ecology, Faculty of Science in Kragujevac. Determination of the investigated lichen was accomplished using standard methods.
Extraction
Finely dry ground thalli of the examined lichen (100 g) were extracted using methanol in a Soxhlet extractor. The extract was filtered and then concentrated under reduced pressure in a rotary evaporator. The dry extract was stored at −18 °C until it was used in the tests. The extract was dissolved in 5 % dimethyl sulphoxide (DMSO) for the experiments. DMSO was diluted in sterile distilled water to the desired concentration.
Antioxidant activity
DPPH Radical Scavenging
The free radical scavenging activity of the extract was measured by 1,1-diphenyl-2-picryl-hydrazil (DPPH). The method used was similar to Dorman et al. (2004) but was modified in some details. Stock solution of the extract was prepared in 5 % DMSO to achieve the concentration of 1,000 μg/ml. Further, two-fold dilutions were made to obtain concentrations of 500, 250, 125, 62.5 μg/ml. Diluted solutions of extract (1 ml each) were mixed with 2 ml of methanol solution of DPPH radical (0.05 mg/ml) in cuvettes. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm in a Jenway spectrophotometer (Bibby Scientific Limited, Stone, UK) against methanol as blank. Ascorbic acid was used as a standard. The DPPH radical concentration was calculated using the following equation:
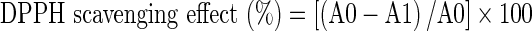 |
where A0 is the absorbance of the negative control (2 ml of methanol solution of DPPH radical + 1 ml of 5 % DMSO) and A1 is the absorbance of the reaction mixture or the standard.
The inhibitory concentration 50 (IC50) was the parameter used to compare the radical scavenging activity. A lower IC50 meant better radical scavenging activity.
Reducing power
The reducing power of extract was determined according to the method of Oyaizu (1986). Stock solution of the extract was prepared with 5 % DMSO to achieve the concentration of 1,000 μg/ml. Further, two-fold dilutions were made to obtain concentrations of 500, 250, 125, 62.5 μg/ml. One millilitre of each extract was mixed with 2.5 ml of phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1 %). The mixtures were incubated at 50 °C for 20 min. Then trichloroacetic acid (10 %, 2.5 ml) was added to the mixture and centrifuged. Finally, the upper layer (2.5 ml) was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml; 0.1 %). The absorbance of the solution reading was 700 nm using a spectrophotometer. The blank was prepared with all reaction agents without extract. Increased absorbance of the reaction mixture indicated an increase in the reducing power. Ascorbic acid was used as a positive control.
Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity of extract was detected according to the method of Nishimiki et al. (1972). Stock solution of the extract was prepared with 5 % DMSO to achieve the concentration of 1,000 μg/ml. Further, two-fold dilutions were made to obtain concentrations of 500, 250, 125, 62.5 μg/ml. Briefly, 0.1 ml of each extract was mixed with 1 ml nitroblue tetrazolium (NBT) solution (156 μM in 0.1 M phosphate buffer, pH 7.4) and 1 ml nicotinamide adenine dinucleotide (NADH) solution (468 μM in 0.1 M phosphate buffer, pH 7.4). The reaction was initiated by adding 100 μl of phenazine methosulphate (PMS) solution (60 μM in 0.1 M phosphate buffer, pH 7.4). The mixture was incubated at room temperature for 5 min, and the absorbance was measured spectrophotometrically at 560 nm against a blank sample (phosphate buffer). A decrease in the absorbance indicated increased superoxide anion radical scavenging activity. Ascorbic acid was used as a standard. The percentage inhibition of superoxide anion generation was calculated using the following formula:
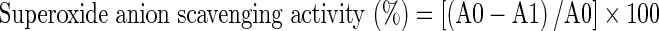 |
where A0 is the absorbance of the negative control (consisting of all reaction agents except the extract) and A1 is the absorbance of the reaction mixture or the standard.
The inhibitory concentration 50 (IC50) was the parameter used to compare the radical scavenging activity. IC50 is defined as the total antioxidant necessary to decrease the initial superoxide anion radical by 50 %. A lower IC50 meant better radical scavenging activity.
Determination of total phenolic compounds
Total soluble phenolic compounds in the extract were determined using the Folin-Ciocalteu reagent according to the method of Slinkard and Slingleton (1997) using pyrocatechol as a standard phenolic compound. Briefly, 1 ml of the extract (1 mg/ml) was diluted in distilled water (46 ml) in a volumetric flask. One millilitre of Folin-Ciocalteu reagent was added and the contents of the flask were mixed thoroughly. After 3 min, 3 ml of sodium carbonate (2 %) was added and then the mixture was allowed to stand for 2 h with intermittent shaking. The absorbance was spectrophotometrically measured at 760 nm against blank consisting of all reaction agents except the extract. The total concentration of phenolic compounds in the extract was determined as microgram of pyrocatechol equivalent (PE) per milligram of dry extract, using the equation obtained from standard pyrocatechol graph as follows:
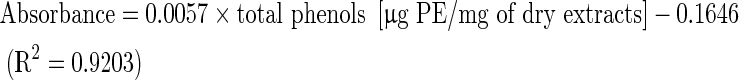 |
Total flavonoid content
The total flavonoid content was determined using the Dowd method (Meda et al. 2005). Two millilitres of 2 % aluminium trichloride (AlCl3) in methanol was mixed with the same volume of the extract solution (1 mg/ml). The mixture was incubated at room temperature for 10 min, and the absorbance reading was measured at 415 nm in a spectrophotometer. The negative control, without extract was used as the blank. The total flavonoid content determined as microgram of rutin equivalent (RE) per milligram of dry extract using an equation that was obtained from standard rutin graph as follows:
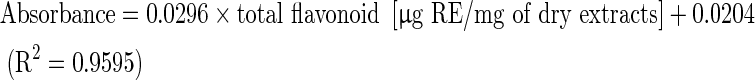 |
Antimicrobial activity
Microorganisms and media
The following bacteria were tested in this study: Staphylococcus aureus (ATCC 25923), Bacillus subtilis (ATCC 6633), Bacillus cereus (ATCC 10987), Escherichia coli (ATCC 25922) and Proteus mirabilis (ATCC 29906). The strains of bacteria used were obtained from the American Type Culture Collection (ATCC). Bacterial cultures were maintained on Müller-Hinton agar substrates (Torlak, Belgrade, Serbia). The fungi used throughout the study were: Aspergillus flavus (ATCC 9170), Candida albicans (ATCC 10259), Fusarium oxysporum (DBFS 292), Penicillium purpurescens (DBFS 418) and Trichoderma harsianum (DBFS 379). All fungi were from the mycological collection maintained at the Mycological Laboratory within the Department of Biology, Faculty of Science (DBFS), University of Kragujevac. The fungi were grown on potato dextrose (PD) agar except Candida albicans that was maintained on Sabouraud dextrose (SD) agar (Torlak). All cultures were stored at 4 °C and were subcultured every 15 days.
Bacterial inoculi were obtained from bacterial cultures incubated at 37 °C for 24 h on Müller-Hinton agar substrate and brought up by dilution according to the 0.5 McFarland standard to approximately 108 CFU/ml. Suspensions of fungal spores were prepared from fresh mature cultures (3- to 7-day-old) that grew at 30 °C on a PD agar substrate. Spores were rinsed with sterile distilled water, used to determine turbidity spectrophotometrically at 530 nm, and then further diluted to approximately 106 CFU/ml according to the procedure recommended by NCCLS (1998).
Minimal inhibitory concentration (MIC)
Minimal Inhibitory Concentration (MIC) was determined by the broth microdilution method using 96-well micro-titer plates (Sarker et al. 2007). A series of dilutions ranging from 40 to 0.156 mg/ml was tested in the experiment for every microorganism species. Starting solutions were prepared by measuring off a certain quantity of extract and dissolving it in DMSO. Two-fold dilutions of extracts were prepared in Müller-Hinton broth for bacterial cultures and SD broth for fungal cultures. The MIC was determined with resazurin. Resazurin is an oxidation–reduction indicator used for the evaluation of microbial growth. It is a blue non-fluorescent dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. The boundary dilution without any changing colour of resazurin was defined as the MIC for the tested microorganism at the given concentration. As a positive control of growth inhibition streptomycin was used in bacteria and ketoconazole in fungi. A DMSO solution was used as a negative control for the influence of the solvents.
In vitro cytokinesis-block micronucleus (MN) test
Peripheral venous blood was collected from three healthy donors aged 30–34 years, nonsmokers, who had not been exposed to known mutagens. All experiments conformed to the guidelines of the World Medical Assembly (Declaration of Helsinki) and informed consent was obtained from all donors.
Cytokinesis-block micronucleus (MN) assay was carried out using the protocol described by Fenech (2000). Briefly, heparinized whole blood (0.5 ml) was added to 5 ml of a fully-supplemented medium for lymphocyte—GIBCO™ PB-Max™ karyotyping medium (Invitrogen, Carlsbad, CA, USA). Cultures were incubated at 37 °C for 72 h. Five samples of methanol extract at different concentrations (12.5, 25, 50, 100 and 200 μg/ml) were added to lymphocyte cultures 24 h after the beginning of incubation. Forty-four hours after the beginning of incubation cytochalasin B (Sigma, St. Louis, MO, USA) was added at the final concentration of 4 μg/ml. Cultures were harvested 28 h later. The cells were collected by centrifugation, suspended in cold (4 °C) hypotonic solution (0.56 % KCl) and fixed with Carney’s fixative (methanol:glacial acetic acid = 3:1). The cell suspensions were dropped onto clean slides, air-dried and stained with 2 % Giemsa (Alfapanon, Novi Sad, Serbia).
MN analysis
The analysis of MN was performed using a light microscope (Nikon E50i, Otawara, Tochigi, Japan) at a magnification of ×400 following the criteria for MN scoring in binucleated (BN) cells as described by Fenech (2007). Three thousand BN cells per concentration (one thousand per donor) were examined for MN scoring and 500 cells per concentration were examined to determine the number of cells with 1, 2, 3 and 4 nuclei. NDI was calculated using the formula NDI = ((1 × M1 + (2 × M2) + (3 × M3) + (4 × M4))/N, where M1-M4 represent the number of cells with 1 to 4 nuclei and N is the total number of cells scored (Fenech 2000).
Cytotoxic activity
Cell lines
The human melanoma FemX and human colon carcinoma LS174 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). Both cancer cell lines were maintained in the recommended RPMI-1640 medium (PAA Laboratories, Pasching, Austria) supplemented with 10 % heat-inactivated (56 °C) fetal bovine serum, l-glutamine (3 mM), streptomycin (100 μg/ml), penicillin (100 IU/ml), and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and adjusted to pH 7.2 by bicarbonate solution. Cells were cultured at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air.
Treatment of cell lines
Stock solution (100 mg/ml) of test sample, made in dimethylsulfoxide (DMSO), was dissolved in the corresponding medium at the proposed working concentrations. Neoplastic FemX cells (5,000 cells per well) and neoplastic LS174 cells (7,000 cells per well) were seeded into 96-well microtiter plates, and 24 h later, after the cell adherence, five different, double diluted, concentrations of the to be investigated sample, were added to the wells. Final concentrations applied to target cells were 200, 100, 50, 25 and 12.5 μg/ml, except to the control wells, where only nutrient medium was added to the cells. The nutrient medium was RPMI 1640 medium, supplemented with l-glutamine (3 mM), streptomycin (100 μg/ml), penicillin (100 IU/ml), 10 % heat inactivated (56 °C) fetal bovine serum (FBS) and 25 mM HEPES, and was adjusted to pH 7.2 by bicarbonate solution. The cultures were incubated for 72 h.
Determination of cell survival (MTT assay)
The effect of test sample on cancer cell survival was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, according to Mosmann (1983) with modification by Ohno and Abe (1991), 72 h upon addition of the test sample, as described earlier. Briefly, 20 μl of MTT solution (Sigma, St. Louis, MO) (5 mg/ml PBS) was added to each well. The samples were further incubated for 4 h at 37 °C in 5 % CO2 and a humidified air atmosphere. Then, 100 μl of 10 % SDS was added to the extract of insoluble formazan product, resulting from MTT dye conversion by viable cells. The number of viable cells in each well was proportional to the intensity of light absorbance, which was then read in an ELISA plate reader at 570 nm. Absorbance (A) at 570 nm was measured 24 h later. To get the cell survival (%), A of a sample with cells grown in the presence of various concentrations of the investigated test sample, was divided by the optical density of the control (the A of control cells grown in nutrient-rich medium), and multiplied by 100. It was implied that A of blank was always subtracted from A of a corresponding sample with target cells. IC50 concentration was defined as the concentration of an agent inhibiting cell survival by 50 %, compared with a vehicle-treated control. As a positive control cis-diamminedichloroplatinum (cis-DDP) (Sigma Aldrich, St. Louis, MO, USA) was used. All experiments were done in triplicate.
Statistical analysis
The results are shown as mean ± standard deviation (S.D.). Statistically significant difference between mean baseline and induced MN frequencies was determined by Student’s t test. The level of significance was taken as p < 0.05. The relationship between the tested concentrations of extract and MN and NDI was determined by Pearson correlation coefficients.
Results
The results of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of methanol extract obtained from C. islandica are shown in Tables 1, 2, 3, 4, 5 and 6.
Table 1.
DPPH radical scavenging activity and superoxide anion scavenging activity of methanol extract of Cetraria islandica
| DPPH radical scavenging activity IC50 (μg/ml) | Superoxide anion scavenging activity IC50 (μg/ml) | |
|---|---|---|
| Cetraria islandica | 678.38 | 792.48 |
| Ascorbic acid | 6.42 | 115.61 |
Table 2.
Reducing power of methanol extract of Cetraria islandica
| Absorbance (700 nm) | 1,000 μg/ml | 500 μg/ml | 250 μg/ml | 125 μg/ml | 62.5 μg/ml |
|---|---|---|---|---|---|
| C. islandica | 0.4562 | 0.2979 | 0.1542 | 0.0873 | 0.0512 |
| Ascorbic acid | 2.1131 | 1.6543 | 0.9572 | 0.4784 | 0.2472 |
Table 3.
Total phenolics and flavonoid content of methanol extract of Cetraria islandica
| Phenolics content (μg PE/mg of extract) |
Flavonoid content (μg RE/mg of extract) |
|---|---|
| 38.08 | 25.81 |
Table 4.
Minimal inhibitory concentration (MIC) of methanol extract of Cetraria islandica
| Microorganisms | Methanol extract | S | K |
|---|---|---|---|
| Staphylococcus aureus | 1.25a | 31.25 | – |
| Bacillus subtilis | 0.625 | 15.62 | – |
| Bacillus cereus | 0.312 | 15.62 | – |
| Escherichia coli | 2.5 | 62.5 | – |
| Proteus mirabilis | 1.25 | 62.5 | – |
| Aspergillus flavus | 5 | – | 7.81 |
| Candida albicans | 1.25 | – | 3.9 |
| Fusarium oxysporum | 2.5 | – | 3.9 |
| Penicillium purpurescens | 5 | – | 15.62 |
| Trichoderma harsianum | 2.5 | – | 7.81 |
aMinimal inhibitory concentration (MIC); values given as mg/ml for extract and as μg/ml for antibiotics. Values are the mean of three replicates
Antibiotics: K ketoconazole, S streptomycin
Table 5.
Summarized results of micronuclei (MN) frequency and nuclear division index values (NDI) in cultured peripheral blood lymphocytes (PBLs) of healthy donors after the treatments with five concentrations of methanol extracts obtained from Cetraria islandica
| Treatments | Conc. (μg/ml) | Analyzed BN cells | MN/1000BN cells (X ± SD) | BN with MN (%) | Distribution of MN | NDI | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 MN (%) | 2 MN (%) | 3 MN (%) | 4 MN (%) | ||||||
| Control untreated cells | 0 | 3,000 | 9.00 ± 1.73 | 26 (0.86) | 25 (0.83) | 1 (0.03) | – | – | 1.65 ± 0.07 |
| C. islandica | 12.5 | 3,000 | 9.00 ± 1.00 | 25 (0.83) | 23 (0.77) | 2 (0.06) | – | – | 1.64 ± 0.13 |
| C. islandica | 25 | 3,000 | 9.33 ± 2.08 | 24 (0.80) | 20 (0.67) | 4 (0.13) | – | – | 1.63 ± 0.05 |
| C. islandica | 50 | 3,000 | 14.67 ± 3.51a | 40 (1.33) | 37 (1.23) | 2 (0.07) | 1 (0.03) | – | 1.60 ± 0.06 |
| C. islandica | 100 | 3,000 | 27.00 ± 8.19b | 63 (2.10) | 53 (1.77) | 5 (0.17) | 3 (0.10) | 2 (0.06) | 1.61 ± 0.12 |
| C. islandica | 200 | 3,000 | 20.33 ± 5.68c | 52 (1.73) | 46 (1.53) | 5 (0.17) | 1 (0.03) | – | 1.56 ± 0.12 |
a,b,cStatistically significant difference in the MN frequency between control untreated and treated PBL with 50, 100, 200 μg/ml C. islandica methanol extract (p < 0.05). % of cells with micronuclei in relation to total number of analyzed cells
Table 6.
Growth inhibitory effects of methanol extract of Cetraria islandica on FemX and LS 174 cell lines
| Test sample | FemX | LS 174 |
|---|---|---|
| IC50 (μg/ml) | ||
| Cetraria islandica | 22.68 ± 2.03 | 33.74 ± 0.36 |
| Cis-DDP | 0.94 ± 0.35 | 2.3 ± 0.31 |
The tested extract exhibited antioxidant activity against various oxidative systems in vitro as shown in Tables 1 and 2. DPPH radical scavenging and superoxide anion radical scavenging of the extract are summarized in Table 1, while reducing power is shown in Table 2. The tested extract revealed lower antioxidant activities than ascorbic acid. The IC50 values were 678.38 and 792.48 μg/ml for DPPH radicals and superoxide anion radicals scavenging activity, respectively. The absorbance for reducing power in the tested extract varied from 0.0512 to 0.4562. As shown in Table 2, reducing power was concentration-dependant (the higher the concentration, the higher the reducing power).
Total phenolic and flavonoid constituents of the extract are given in Table 3. The amounts of total phenolics and flavonoids in the extract were 38.08 μg PE/mg and 25.81 μg RE/mg, respectively.
Antimicrobial activity of the extract against the tested microorganisms is shown in Table 4. The methanol extract of the examined lichen showed relatively similar antibacterial and antifungal activity. The MIC values for the bacteria and fungi tested were 0.312–5 mg/ml. The lowest MIC value (0.312 mg/ml) was measured for the Bacillus cereus species.
The antimicrobial activity was compared to streptomycin (standard antibiotic) and ketoconazole (standard antimicotic). The results showed that streptomycin and ketoconazole had stronger activity than the tested extract as shown in Table 4. DMSO had no inhibitory effects against the tested organisms in the negative control.
The effects of the genotoxic potential of methanol extract obtained from C. islandica on genomic damage in cultured PBLs from healthy donors are shown in Table 5. The extract increased MN frequency when compared with control PBLs at concentrations of 25–200 μg/ml in a dose dependent manner (r = 0.724, p < 0.01), and significantly in the higher tested concentrations (50, 100 and 200 μg/ml) with p < 0.05. The analysis of MN distribution revealed that the extract concentrations ranging from 50 to 200 μg/ml significantly increased both the number of BN cells containing MNi and the number of MNi in BN cells. BN cells containing one MN were notably present in both treated and untreated PBL cultures, while BN cells with 2 MNi were less present. BN cells with 3 and 4 MNi were present only in PBLs treated with the highest concentrations of the extract tested. There were no significant differences between NDI values of all treatments and untreated PBLs (p > 0.05).
The anticancer activity of the lichen extract against the tested cell lines is shown in Table 6. The IC50 against FemX and LS174 cell lines was 22.68 and 33.74 μg/ml, respectively. As shown in the table, positive control (Cis-DDP) exhibited a slightly better anticancer activity than the tested sample.
Discussion
Different extraction solvents, according to their polarity, may extract various compounds which can participate in the great or weak antioxidant, antimicrobial, genotoxic and anticancer activities. It is necessary to understand that extracts are mixtures of natural compounds, and their antioxidant, antimicrobial, genotoxic and anticancer activities are not only a result of the different activities of individual components but may be the result of their interactions, which can have different effects on the overall activity of extracts. Often, the activity of the extracts may be the result of synergistic or antagonistic effects of several compounds. Previous literature data suggest that lichen Cetraria islandica contains: lichesterinic acid, protolichesterinic acid, fumarprotocetraric acid, arabitol, mannitol, lichenin, isolichenin, umbilicin, sucrose, trehalose, hemicellulose, ascorbic acid, vitamin B1, vitamin B12 and ergosterol (Culberson 1969; Brodo et al. 2001).
Antioxidant properties of methanol extracts of C. islandica were evaluated using different antioxidant tests, including free radical scavenging, superoxide anion radical scavenging and reducing power.
Free radical scavenging action is one of the numerous mechanisms for antioxidation (Sini and Devi 2004). Antiradical activity of the extract was studied by screening its ability to bleach the stable DPPH radical. The method is based on the formation of the non-radical form DPPH-H in the presence of alcoholic DPPH solution and hydrogen donating antioxidant (AH) by the reaction DPPH + AH → DPPH-H + A (Anandjiwala et al. 2008).
Reducing power of methanol extract of C. islandica may also indicate its potential antioxidant activity. The reducing features are mainly related with the presence of reductones. Gordan (1990) found that the antioxidant effect of reductones is based on the destruction of the free radical chain by donating a hydrogen atom. The reduction of ferrous ion (Fe3+) to ferric ion (Fe2+) is measured by the strength of the green-blue color of solution which absorbs at 700 nm. The result presented here indicates that the marked ferric reducing power activity of extract to be due to the presence of polyphenols which may act in a similar way as reductones react with free radicals to turn them into more stable products and abort free radical chain reactions (Sasikumar et al. 2010).
The superoxide radical scavenging activity of methanol extract of C. islandica was estimated based on its ability to destroy the superoxide radical produced from the PMS/NADH reaction. The decrease of absorbance at 560 nm with antioxidants indicates that superoxide anion in the reaction mixture disappeared (Gulcin et al. 2004).
Antioxidative nature of methanol extract of C. islandica might depend on its phenolics. Phenolic components are potential antioxidants. Phenolic compounds can donate hydrogen to free radicals and thus stop the chain reaction of lipid oxidation at the initial stage. This ability of phenolic compounds to scavenge radicals is due to the presence of their phenolic hydroxyl groups (Sawa et al. 1999). Flavonoids are widely distributed natural compounds and also the most important natural phenolics. In most lichens, phenols are important antioxidants because of their ability to scavenge free radicals such as singlet oxygen, superoxide and hydroxyl radicals (Shanab et al. 2011). Numerous researches reported a significant correlation between antioxidative activities of lichens and phenolic content (Behera et al. 2009; Ranković et al. 2012). However, some authors believe that the antioxidant activity of extracts may not necessarily correlate with the content of polyphenolics (Odabasoglu et al. 2004), suggesting that the antioxidant activity of different lichens may also depend on other, non-phenol components.
Gulcin et al. (2002) investigated aqueous extract of C. islandica which showed effective reducing power, superoxide anion radical scavenging and free radical scavenging activities. Ivanov et al. (2007) found moderate antioxidant activities for various aqueous-alcoholic extracts of the lichen C. islandica. Compared with their results, we suggest that the methanol extract of C. islandica showed a relatively powerful antioxidant activity.
The antioxidant effect of some other lichens was also studied by other researchers. For example, Luo et al. (2006) determined the antioxidant activity of methanol extracts from the lichen Thamnolia vermicularis. Praveen Kumar et al. (2010) determined the antioxidant activity of the extracts of the lichen Ramalina hossei and Ramalina conduplicans. Manojlović et al. (2010a) explored the antioxidant properties of Laurera benguelensis. Kosanić et al. (2012a, b) found that the lichens Umbilicaria crustulosa, U. cylindrica, U. polyphylla, Parmelia caperata, P. sulcata, and P. saxatilis have a strong antioxidant effect.
Few researchers examined lichen C. islandica for its antimicrobial activity in search for new antimicrobial agents. For example, Dulger et al. (1998) investigated ethyl acetate, acetone, chloroform and ethanol extracts of C. islandica against different microorganisms by disc-diffusion method. They have found that C. islandica exhibited antimicrobial activity against some gram-positive bacteria, but had no antimicrobial activity against gram-negative bacteria and fungi. Differences in the results related to some microorganisms can be explained by different sensitivity of tested microorganisms, samples from different locations, different methods of testing and the solvents used.
In these experiments, the examined extract at the same concentrations showed a slightly stronger antibacterial than antifungal activity. These results could be expected due to the fact that numerous tests proved that bacteria are more sensitive to the antibiotic compared to fungi (Hugo and Russell 1983). The reason for different sensitivity between fungi and bacteria can be found in different transparency of the cell wall. The cell walls of gram-positive bacteria consist of peptidoglycan (murein) and teichoic acids, while the cell walls of gram-negative bacteria consist of lipopolysaccharides and lipopoliproteins (Heijenoort 2001). On the other hand, the cell walls of fungi consist of polysaccharides such as chitin and glucan (Farkaš 2003).
Different lichens were screened for antimicrobial activity in search of new antimicrobial agents. Kosanić et al. (2012a) found antimicrobial activity for the acetone extract of the lichens Umbilicaria crustulosa, U. cylindrica, and U. polyphylla. Similar results were reported by Karthikai Devi et al. (2011) for different extracts extracted from the lichen Roccella belangeriana. Goel et al. (2011) found that the lichen Parmotrema reticulatum had a strong antimicrobial effect.
Cytokinesis-block micronucleus (MN) test in peripheral blood lymphocytes (PBLs) is a well established method to biomonitor human exposure to genotoxic agents in vivo (Milošević-Djordjević et al. 2007; Speit et al. 2012; Vrndić et al. 2013) or in vitro (Milošević-Djordjević et al. 2011; Turkez and Aydin 2012; Milošević-Djordjević et al. 2013). MNi are small cytoplasmic bodies formed during cell division originating from chromosome/chromatid fragments or whole chromosome loss. MN presents index of cytogenetic damage in the analyzed cells while nuclear division index (NDI) is the parameter that shows the number of cell cycles through which the treated cells have passed in culture (Surralles et al. 1995; Fenech 2000).
The present results show that only higher extract concentrations (50, 100 and 200 μg/ml) induced genotoxic effects in human PBLs of healthy donors. We detected a dose dependent relation among all tested extract concentrations and mean frequencies of MN, as an index of cytogenetic damage in analyzed cells.
This finding can be explained by the fact that higher concentrations of flavonoids may have pro-oxidative effects on DNA damage. Similar results were obtained in other experiments (Yang et al. 2009; Eroglu et al. 2010).
In the present study, the results clearly demonstrate that methanol extracts of the examined lichen induced significant anticancer effects on the tested cancer cell lines. In the literature there are no data on the anticancer activity of C. islandica, but anticancer activities of some other lichens have been studied. Kosanić et al. (2013) reported significant anticancer effect for Evernia prunastri and Pseudoevernia furfuraceae. Manojlović et al. (2010b) explored anticancer properties of Thamnolia vermicularis. Triggiani et al. (2009) found strong anticancer activity for Xanthoria parietina. Compared with them, the results of this research suggest that the methanol extract of C. islandica exhibited a relatively powerful anticancer activity.
The importance of lichens as anticancer agents was confirmed in recent years, which suggests that lichens can be used as biological agents in the treatment of cancer. The mechanism of action of the tested extract remains to be tested. Further research will be necessary for fractionation in order to identify compounds responsible for the observed anti-tumor effect, to establish the opportunities for reinforcing activities, and to improve the selectivity.
Numerous studies showed that lichens have anti-proliferative and cytotoxic effects on various human cancer lines, including those on A2780 and HT-29 human cancer cell lines. These effects of lichens can be explained by action of their secondary metabolites. So far however, the exact molecular mechanisms of the action of lichen secondary metabolites are almost entirely unknown (Bačkorova et al. 2011, 2012).
In our experiment we tested only the methanol extract on antioxidant, antimicrobial, genotoxic and anticancer activities in order to see whether it is active. Since we have good results, we plan to continue with a more detailed analysis. Further research will be related with fractionation in order to identify compounds responsible for the obtained antioxidant, antimicrobial, genotoxic and anticancer effects.
In conclusion, it can be stated that the tested extract has a certain level of antioxidant, antimicrobial and anticancer activities in vitro. In addition, our results suggest that the use of the lower tested concentrations of C. islandica extract (12.5 and 25 μg/ml) is safe. Further studies should be done on the isolation and characterization of pure lichen compounds responsible for its antioxidant, antimicrobial, genotoxic and anticancer activities.
Acknowledgments
The study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. III41010; 173032, 175011).
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- Anandjiwala S, Bagul MS, Parabia M, Rajani M. Evaluation of free radical scavenging activity of an ayurvedic formulation, Panchvalkala. Indian J Pharm Sci. 2008;70:31–35. doi: 10.4103/0250-474X.40328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bačkorova M, Bačkor M, Mikeš J, Jendželovsky R, Fedoročko P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol In Vitro. 2011;25:37–44. doi: 10.1016/j.tiv.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Bačkorova M, Jendželovsky R, Kello M, Bačkor M, Mikeš J, Fedoročko P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol In Vitro. 2012;26:462–468. doi: 10.1016/j.tiv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Bates ST, Cropsey GW, Caporaso JG, Knight R, Fierer N. Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol. 2011;77:1309–1314. doi: 10.1128/AEM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera B, Verma N, Sonone A, Makhija U. Optimization of culture conditions for lichen Usnea ghattensis G. Awasthi to increase biomass and antioxidant metabolite production. Food Technol Biotechnol. 2009;47:7–12. [Google Scholar]
- Bown D. Encyclopedia of herbs and their uses. London: Dorling Kindersley; 2001. [Google Scholar]
- Brodo LM, Sharnoff D, Sharnoff S. Lichens of North America. New Haven: Yale University Press; 2001. [Google Scholar]
- Chevallier A. The encyclopedia of medicinal plants. London: Dorling Kindersley; 1996. [Google Scholar]
- Culberson CF. Chemical and botanical guide to lichen products. Chapel Hill: University of North Carolina Press; 1969. [Google Scholar]
- Dobson F. Lichens an illustrated guide. England: The Richmond publishing Co. Ltd.; 2000. [Google Scholar]
- Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- Dulger B, Gucin F, Aslan A. Antimicrobial activity of Cetraria islandica (L.) Ac. Turk J Biol. 1998;22:111–118. [Google Scholar]
- Eroglu EH, Hamzaoglu E, Aksoy A, Budak U, Ozkul Y. In vitro genotoxic effects of four Helichrysum species in human lymphocytes cultures. Biol Res. 2010;43:177–182. [PubMed] [Google Scholar]
- Farkaš V. Structure and biosynthesis of fungal cell walls: methodological approaches. Folia Microbiol. 2003;48:469–478. doi: 10.1007/BF02931327. [DOI] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Goel M, Dureja P, Rani A, Uniyal PL, Laatsch H. Isolation, characterization and antifungal activity of major constituents of the Himalayan lichen Parmelia reticulate Tayl. J Agric Food Chem. 2011;59:2299–2307. doi: 10.1021/jf1049613. [DOI] [PubMed] [Google Scholar]
- Gordan MH. Food antioxidants. New York: Elsevier; 1990. [Google Scholar]
- Gulcin I, Oktay M, Kufrevioglu OI, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol. 2002;79:325–329. doi: 10.1016/S0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Kurfrevioglu OI, Oktay M, Buyukokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 2001;11:25–36. doi: 10.1093/glycob/11.3.25R. [DOI] [PubMed] [Google Scholar]
- Hugo WB, Russell AD (1983) Pharmaceutical microbiology, 3rd edn. Blackwell Scientific Publications, Oxford
- Huneck S. New results in the chemistry of lichens. Symbiosis. 1991;11:225–248. [Google Scholar]
- Ivanov D, Kiselova Y, Ivanova D (2007) Cetraria islandica as a natural source of antioxidants, “Ovidius” University Annals of Medical Science–Pharmacy 5:80–83
- Karthikai Devi G, Anantharaman P, Kathiresan K, Balasubramanian T. Antimicrobial activities of the lichen Roccella belangeriana (Awasthi) from mangroves of Gulf of Mannar. Indian J Geo-Mar Sci. 2011;40:449–453. [Google Scholar]
- Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial and anticancer activity of 3 Umbilicaria species. J Food Sci. 2012;77:T20–T25. doi: 10.1111/j.1750-3841.2011.02459.x. [DOI] [PubMed] [Google Scholar]
- Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J Sci Food Agric. 2012;92:1909–1916. doi: 10.1002/jsfa.5559. [DOI] [PubMed] [Google Scholar]
- Kosanić M, Manojlović N, Janković S, Stanojković T, Ranković B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem Toxicol. 2013;53:112–118. doi: 10.1016/j.fct.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Luo H, Ren M, Lim KM, Koh YJ, Wang LS, Hur JS. Antioxidative activity of lichen Thamnolia vermicularis in vitro. Mycobiology. 2006;34:124–127. doi: 10.4489/MYCO.2006.34.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manojlović N, Vasiljević P, Gritsanapan W, Supabphol R, Manojlović I. Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res. 2010;43:169–176. [PubMed] [Google Scholar]
- Manojlović N, Vasiljević P, Jusković M, Najman S, Janković S, Milenković-Andjelković A. HPLC analysis and cytotoxic potential of extracts from the lichen, Thamnolia vermicularis var. Subuliformis. J Med Plants Res. 2010;4:817–823. [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Milošević-Djordjević O, Grujičić D, Arsenijević S, Brkić M, Ugrinović S, Marinković D. Micronuclei in cord blood lymphocytes as a biomarker of transplacental exposure to environmental pollutants. Tohoku J Exp Med. 2007;213:231–239. doi: 10.1620/tjem.213.231. [DOI] [PubMed] [Google Scholar]
- Milošević-Djordjević O, Grujičić D, Joksić G, Marinković D. In vitro evaluation of the genotoxicity of ritodrine and verapamil in human lymphocytes. Hum Exp Toxicol. 2011;30:398–405. doi: 10.1177/0960327110372404. [DOI] [PubMed] [Google Scholar]
- Milošević-Djordjević O, Stošić I, Stanković M, Grujičić D (2013) Comparative study of genotoxicity and antimutagenicity of methanolic extracts from Teucrium chamaedrys and Teucrium montanum in human lymphocytes using micronucleus assay. Cytotechnology 65:863–869. doi:10.1007/s10616-012-9527-1 [DOI] [PMC free article] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol. 2001;56:9–16. doi: 10.1007/s002530100684. [DOI] [PubMed] [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards) (1998) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-forming Filamentous Fungi: Proposed Standard M38-P. NCCLS, Wayne, PA, USA
- Nishimiki M, Rao NA, Yagi K. The occurrence of super-oxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–853. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Oboh G, Ademosun AO. Comparative studies on the ability of crude polyphenols from some nigerian citrus peels to prevent lipid peroxidation: in vitro. Asian J Biochem. 2006;1:169–177. doi: 10.3923/ajb.2006.169.177. [DOI] [Google Scholar]
- Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, Halici M, Bayir Y. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res. 2004;18:938–941. doi: 10.1002/ptr.1488. [DOI] [PubMed] [Google Scholar]
- Ohno M, Abe T. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6) J Immunol Methods. 1991;145:199–203. doi: 10.1016/0022-1759(91)90327-C. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–314. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Praveen Kumar SV, Prashith Kekuda TR, Vinayaka KS, Sudharshan SJ. Anthelmintic and antioxidant efficacy of two Macrolichens of Ramalinaceae. Phcog J. 2010;1:169–176. [Google Scholar]
- Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM (1992) The lichen flora of Great Britain and Ireland, Natural History Museum. Publications in association with the British Lichen Society, London
- Ranković B, Kosanić M, Stanojković T, Vasiljević P, Manojlović N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int J Mol Sci. 2012;13:14707–14722. doi: 10.3390/ijms131114707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar JM, Mathew GM, Teepica PDD. Comparative studies on antioxidant activity of methanol extract and flavonoid fraction of Nyctanthes arbortristis leaves. EJEAFChe. 2010;9:227–233. [Google Scholar]
- Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor promoter effect of vegetables. J Agric Food Chem. 1999;47:397–492. doi: 10.1021/jf980765e. [DOI] [PubMed] [Google Scholar]
- Shanab SMM, Shalaby EA, El-Fayoumy EA. Enteromorpha compressa exhibits potent antioxidant activity. J Biomed Biotechnol. 2011;2011:726405. doi: 10.1155/2011/726405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sini H, Devi KS. Antioxidant activities of chloroform extract of Solanumtrilobatum. Pharm Biol. 2004;42:462–466. doi: 10.1080/13880200490886238. [DOI] [Google Scholar]
- Slinkard K, Slingleton VL. Total phenolic analyses: automation and comparison with manual methods. Am J Enol Viticult. 1997;28:49–55. [Google Scholar]
- Speit G, Linsenmeyer R, Schutz P, Kuehner S. Insensitivity of the in vitro cytokinesis-block micronucleus assay with human lymphocytes for the detection of DNA damage present at the start of the cell culture. Mutagenesis. 2012;27:743–747. doi: 10.1093/mutage/ges041. [DOI] [PubMed] [Google Scholar]
- Surralles J, Xamena N, Creus A, Marcos R. The suitability of the micronucleus assay in human lymphocytes as a new biomarker of excision repair. Mutat Res. 1995;342:43–59. doi: 10.1016/0165-1218(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Triggiani D, Ceccarelli D, Tiezzi A, Pisani T, Munzi S, Gaggi C, Loppi S. Antiproliferative activity of lichen extracts on murine myeloma cells. Biologia. 2009;64:59–62. doi: 10.2478/s11756-009-0005-y. [DOI] [Google Scholar]
- Turkez H, Aydin E. The effects of taurine on permethrin-induced cytogenetic and oxidative damage in cultured human lymphocytes. Arh Hig Rada Toksikol. 2012;63:27–34. doi: 10.2478/10004-1254-63-2012-2114. [DOI] [PubMed] [Google Scholar]
- Vrablikova H, McEvoy M, Solhaug KA, Bartak M, Gauslaa Y. Annual variation in photo acclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J Photochem Photobiol. 2006;83:151–162. doi: 10.1016/j.jphotobiol.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Vrndić O, Milošević-Djordjević O, Mijatović LJ, Jeremić M, Stošić I, Grujičić D, Živančević-Simonović S. Correlation between micronuclei frequency in peripheral blood lymphocytes and retention of 131-I in thyroid cancer patients. Tohoku J Exp Med. 2013;229:115–124. doi: 10.1620/tjem.229.115. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83:11–21. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


