Abstract
Stem cells, including mesenchymal stem cells and pluripotent stem cells, are becoming an indispensable tool for various biomedical applications including drug discovery, disease modeling, and tissue engineering. Bioprocess engineering, targeting large scale production, provides a platform to generate a controlled microenvironment that could potentially recreate the stem cell niche to promote stem cell proliferation or lineage-specific differentiation. This survey aims at defining the characteristics of stem cell populations currently in use and the present-day limits in their applications for therapeutic purposes. Furthermore, a bioprocess engineering strategy based on bioreactors and 3-D cultures is discussed in order to achieve the improved stem cell yield, function, and safety required for production under current good manufacturing practices.
Keywords: Stem cells, Bioprocess engineering, Bioreactor, Differentiation, Safety
Introduction
In recent years, stem cells have generated a lot of excitement for their potential biomedical applications. The discovery of these unique cell populations has overturned certain scientific dogmas. For instance, the existence of mesenchymal stem cells (MSCs) in vivo has considerably changed the perception of the stromal cells (Pittenger et al. 1999), which are now tested in more than 300 clinical trials for treating various diseases (www.clinicaltrials.org). Moreover, the isolation of embryonic stem cells (ESCs) has enabled the development of new tools to study embryonic tissue morphogenesis (Thomson et al. 1998). The derivation of induced pluripotent stem cells (iPSCs) opens the era of disease modeling and drug screening, as in the example of cardiac toxicity testing (Takahashi et al. 2007; Mandenius et al. 2011). Furthermore, the capacities of both ESCs and iPSCs, known as pluripotent stem cells (PSCs), have been demonstrated in the restoration of most cell types. Especially, the use of human ESC (hESC)-derived cells has been explored for treating patients with spinal cord injury and macular degeneration in Phase I clinical trials (Alper 2009; Schwartz et al. 2012). The possible use of PSCs in cell therapy has generated high hope for combatting incurable and degenerative diseases.
Consequently, there is an urgent need for large amounts of stem cells (e.g. 1010–1012 per batch of production for PSC-derived cardiomyocytes) in a functional and safe state for their cost-effective use in therapy, drug discovery, and disease modeling (Serra et al. 2012). Traditional practices of cell culture in 2-D plastic dishes are clearly inadequate to supply large numbers of cells. In addition, 2-D cultures are not able to recreate a physiological environment similar to that of the original niche or to promote sufficient signaling for stem cell differentiation with high efficiency. Additionally there are increasing concerns regarding the tumorigenicity as well as the potential immunogenicity of stem cell populations. Bioprocess engineering approaches resulting from well-established practice in recombinant protein production or environmental science (e.g. bioremediation) could offer rational tools to alleviate the above limitations of ex vivo stem cell cultivation.
Focusing on the potential applicability of stem cells for clinical uses, this survey summarizes the key characteristics of stem cell populations, as well as the current hurdles in their use in a clinical setting. To overcome these hurdles, the operational objective and the tools of a bioprocess engineering strategy are discussed for large scale production of stem cell-derived progeny under current good manufacturing practices (cGMP) and for controlling stem cell population behavior to enable safe transplantation.
Stem cells and stem cell niches
Stem cells are defined as a cell population sharing self-renewal and differentiation potential along various distinct lineages (Watt and Hogan 2000). For instance, ESCs display unlimited self-renewal and differentiation potential along the three embryonic germ layers: ectoderm, mesoderm, and endoderm (Thomson et al. 1998). From somatic cells or progenitor cells, iPSCs can be generated by reprogramming the cells with pluripotent genes or even small molecules and these reprogrammed cells display similar characteristics to ESCs (Hou et al. 2013; Stadtfeld and Hochedlinger 2010). MSCs are adult stem cell populations that at least have the in vitro differentiation potential into adipocytes, chondrocytes, and osteoblasts (Pittenger et al. 1999; Augello et al. 2010). MSCs are also able to secrete trophic factors which can stimulate the endogenous progenitors after in vivo MSC transplantation, including immune-regulatory proteins, growth factors, pro-angiogenic factors and anti-scarring factors (Caplan and Dennis 2006; Wagner et al. 2009). Stem cells may also exist in cancers. Some cancer stem cells (CSCs) were shown to be able to differentiate towards a phenotype resembling their tissue of origin, in reverting their tumorigenicity (Massard et al. 2006; Takehara et al. 2011).
The stem cell identity is closely linked to a proper dynamic “niche”, which is composed of a specific extracellular matrix (ECM) network, soluble endogenous and exogenous growth factors, and appropriate levels of metabolism-associated molecules such as oxygen to regulate cell proliferation or differentiation (Scadden 2006). Manipulating stem cell niches controls self-renewal versus lineage commitment as well as maintaining the differentiated cell functions (Lutolf and Blau 2009; Scadden 2006). For instance, modulation of mechanical forces (i.e. 1.5–15 dynes/cm2) through ECM and hydrodynamic environment was found to affect the degree of ESC self-renewal and lineage commitment into three-germ layers (Przybyla and Voldman 2012; Wolfe et al. 2012). Hence, recreating the stem cell niche in vitro has been the focus of recent investigations in developing new culture systems.
Current limitations in the use of stem cells for therapeutic applications
Capability of large scale production of stem cells in a functional state
Stem cells are supposed to constitute a scarce cell population, and the readily obtainable number of a stem cell population for therapeutic use is limited. For example, hematopoietic stem cells (HSCs) represent a small percentage (0.1–0.3 %) of the bone marrow (BM) cells (Spangrude et al. 1988). Similarly, BM-MSCs were reported to represent a minor fraction of a bone marrow aspirate (i.e. 0.001–0.01 %) (Pittenger et al. 1999). These primary stem cells have to be expanded in vitro to reach the clinical demand in cell number (e.g. 108–109 cells per patient). The initial derivation of ESCs and iPSCs was achieved from a small cell population as well. ESCs were derived from a minimal number of cells at the blastocyst stage, i.e. 14 inner cell masses were initially isolated to generate the first five ESC lines (Thomson et al. 1998). For iPSCs, the reprogramming efficiency is generally low, ranging from 0.001 to 1 % of the starting somatic cells (Stadtfeld and Hochedlinger 2010). After ESC and iPSC lines are established, they can be expanded indefinitely in theory. However, to fulfill the potential of the proliferation capacity of PSCs, large scale systems have to be developed to produce clinical-relevant quantity and quality of cells. Even for a low dose study (e.g. 106 cells per injection) using hESC-derived oligodendrocyte progenitor cells (OPCs), the cells required for multiple patients, safety testing, quality control, and the retained samples (e.g. samples used for the testing of sterility, endotoxin, identity/purity, impurity, stability, potency, etc.) would exceed a total of 109 cells for each batch of production. To use PSC-derived cardiomyocytes in drug screening or cell therapy (needs 109 cells per patient), large numbers of differentiated cells up to the order of 1010–1012 per production batch are clearly required (Sharma et al. 2011).
In addition to meeting the quantity demand, stem cells or their derivatives need to maintain the desired purity and function. Especially for PSCs, the degree and heterogeneity of the differentiation affect the efficiency of stem cell implantation and these cells’ in vivo therapeutic effects (Hedlund et al. 2008; Hwang et al. 2010). The difficulty of in vitro maturation of PSC-derived cells indicates that the currently used culture environment has not been able to recreate the in vivo-like stem cell niches, at least not fully predictably and consistently until now. Similarly, MSCs need to be primed in vitro to maximize their therapeutic effects, such as the secretion of trophic factors and pro-angiogenic factors (Wagner et al. 2009). Indeed, the limited performance of MSCs in clinical trials, e.g. for the treatment of chronic obstructive pulmonary disease (Weiss et al. 2013), indicates that the functions of MSCs have to be enhanced ex vivo. For instance, the MSC priming by hypoxia was found to enhance the pro-angiogenic properties when treating cardiovascular diseases (Wagner et al. 2009). All these considerations trigger the development of novel systems for large scale stem cell expansion and differentiation in a controlled manner.
The risks of immunogenicity and tumor formation
Stem cells have the risks of immunogenicity in transplantation. ESCs and iPSCs are not immune-privileged and are prone to immune rejection (Taylor et al. 2011; Dressel 2011; Zhao et al. 2011). Allogeneic MSCs have also been reported to retain some degree of immunogenicity by modifying the functions of innate and adaptive immunity (Griffin et al. 2010). Neural progenitors derived from teratocarcinoma have been shown to require strong immunosuppression (Hara et al. 2008). All these observations indicate that reducing the immune rejection is critical for the clinical applications of stem cells. The use of appropriate raw reagents (e.g. xeno-free) in culture systems, the characterization of stem cell populations prior to and after expansion, and the creation of stem cell banks based on human leukocyte antigens (HLA) should reduce HLA mismatching and immunogenicity (Unger et al. 2008).
ESCs and iPSCs, once injected in vivo without a complete in vitro differentiation, will form teratomas, a hallmark of pluripotency (Fong et al. 2010). Despite their high telomerase activity to allow indefinite growth, long-term culture-expanded PSCs are prone to chromosomal and genetic aberrations, leading to abnormal growth (Shay and Wright 2010; Blum and Benvenisty 2009; Knoepfler 2009). MSCs could be transformed by natural oncogenes (Lazennec and Jorgensen 2008). Recent studies indicate that mouse MSCs readily undergo chromosomal instability while human MSCs are more genetically stable (Rodriguez et al. 2012). However the trophic functions of human MSCs may stimulate tumor cell invasion, proliferation motility, and metastasis (Cuiffo and Karnoub 2012). Finally, HSC expansion was found to be associated with the increased expression of genes implicated in oncogenic transformation as well (Okamoto et al. 2007).
Consequently, an optimized bioprocess approach for large scale stem cell expansion and differentiation is required not only to reach the amount of primed cells necessary for therapeutic purposes, but also to control the cell population with reduced therapeutic risks. For example, process intensification to minimize the time in culture, and thus to limit the risk of cell genetic aberrations is necessary. Using xeno-free culture reagents, such as growth medium and substrates, is preferable to reduce the risk of pathogen transmission. Moreover, implementing the cell separation steps compatible with the upstream culture process is also important. The selection, purification, and quantification of suitable populations of stem cell-derived tissue-specific cells constitute an obligatory step to ensure their safe therapeutic use.
Regulations and guidelines for stem cell-derived products
In the United States, treatments with adult stem cells which have been minimally manipulated and are intended for autologous use need to comply with the PHS (Public Health Service) act 361 and the 21 code of federal regulations (CFR) 1271 regulated by the Food and Drug Administration (FDA) (FDA 2008; Sensebe et al. 2011). Part C of Title 21 CFR 1271 defines good tissue practices (GTPs), which includes the guidelines for the procurement, the processing storage, and the distribution of stem cells. Adult stem cells and PSC-derived products that are manipulated (e.g. by gradient selection, activation, large scale expansion, or genetic manipulation) or intended for allogeneic use must comply with the PHS act section 351, which includes the regulation of drugs, devices, and/or biological products. The safety studies of PSC-derived products include the evaluation of toxicity, tumorigenicity, and bio-distribution. The guidelines of cGMP as described in Title 21 CFR 210, 211, 312, and 600 provide regulations for screening, testing, processing, labeling, and packaging of the products (Carpenter et al. 2009).
In Europe, stem cell therapy is regulated by the directive on Advanced Therapy Medicinal Products (ATMPs) following the European Commission directive No 1394/2007. The directive 2004/23/EC delineates the unmodified or modified stem cells. Stem cell modification includes large-scale expansion, activation, genetic manipulation, and use for allogeneic purpose. Preclinical studies on stem cells must be performed according to the good laboratory practices (GLPs) following the directive 2004/10/EC, while the clinical trials must comply with the directive 2001/20/EC following the GMP guidelines defined in the 2003/94/EC and 91/356/EEC directives (Martin et al. 2014). GMPs require the documentation for all raw materials and equipment, the controlled production process, and the controlled product storage and release according to pre-established standards and specifications approved by the quality assurance system.
A bioprocess engineering approach for the controlled production of stem cells
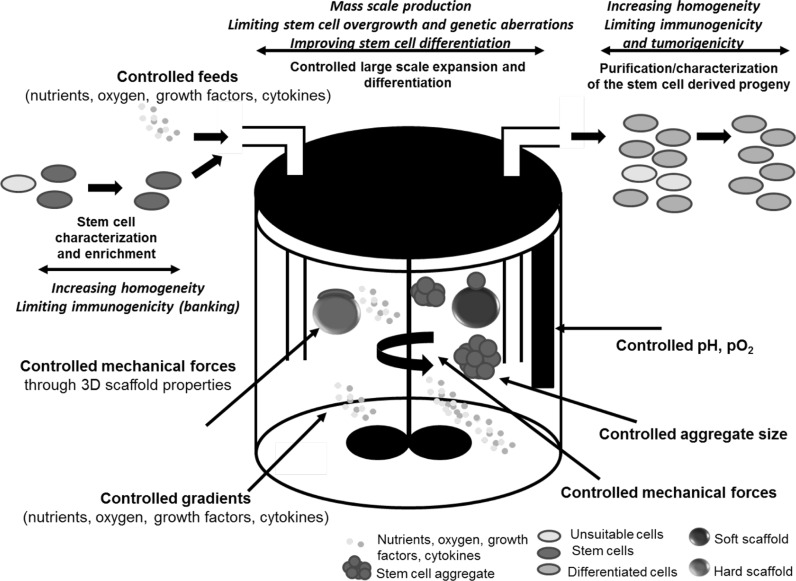
Bioprocess engineering aims at rationally designing the well-controlled equipment (e.g. bioreactors) and processes (e.g. efficient cell seeding and harvesting, microcarriers, serum-free medium, etc.) for mass scale production of stem cells and/or their derivatives (Godara et al. 2008). An analogy could be drawn from the well-established bioprocess engineering platforms applied to recombinant protein production from microbial and animal cells, although in the applications of tissue engineering and regenerative medicine the cell population itself instead of the secreted protein is the product. Thus the requirements for the control of process parameters during manufacturing are more stringent for stem cell bioprocessing (Fig. 1).
Fig. 1.
Illustration of a bioprocess engineering approach with bioreactors to alleviate current limitations in the use of stem cells for clinical application. Bioreactors enable the large scale stem cell expansion and differentiation in a controlled environment. Bioreactors provide the controlled mechanical forces, feeds, the gradients of nutrients and growth factors, pH/oxygen, as well as aggregate size. With the detailed characterizations of input cells and output cells, the homogeneity of the produced cell population will be increased
Automation devices for stem cell production
The immediate solution in stem cell bioprocessing to produce a large number of cells is the development of multi-layer culture vessels and flasks (e.g. CellFactory from Nunc, USA; CellSTACK from Corning, USA). However, the use of a large number of 2-D culture vessels (such as hyperstacks and hyperflasks) is labor intensive. Automated platforms have been developed to remove the human operator dependency and reduce the process variation for both MSC and human PSC cultures, such as the CompacT SelecT system (Thomas and Ratcliffe 2012; Ratcliffe et al. 2013) and the Cellhost system (Terstegge et al. 2007). The automation of partial processing can also be performed using devices such as the Pipeline Dispenser peristaltic pump for feeding (Essen Bioscience). However, this scale out approach is still limited in cell number and only suitable for a small number of patients and low dose studies. In particular, these systems are not amenable for 3-D stem cell cultivation.
Stem cell engineering for enhanced homogeneity, safety, and biological functions
To ensure the homogeneity of stem cell populations and avoid their potential tumorigenicity, stem cells should be characterized by the expression of cytogenetic, genetic, and surface/intracellular markers (e.g. by flow cytometry or proteomic analysis) (Bongso et al. 2008; Baker et al. 2007; Taylor et al. 2011). For example, hESC-derived OPCs are characterized by a set of surrogate purity markers NG2, Nestin, and PDGFRα, as well as the impurity markers Tra-1-60 and Oct-4 for residual undifferentiated cells (Li et al. 2013). Although the direct correlations of the surrogate markers with stem cell potency is yet to be established, these stem cell characterizations provide the filtering step for the follow up pre-clinical and clinical studies. The selective elimination of undifferentiated PSC populations could be performed via specific induction of apoptosis (e.g. mab 84 or ceramides) or cell depletion with magnetic-activated cell sorting (Bieberich et al. 2003; Tan et al. 2009; Diogo et al. 2012). The detection of histocompatibility complexes enables the creation of stem cell banks to preserve patient-specific cell lines (e.g. HLA-matched cell lines) (Fig. 1) (Heng et al. 2009; Bongso et al. 2008).
The development of defined serum-free culture systems constitutes a key tool to ensure the reproducibility of stem cell performance and to limit the putative introduction of pathogens. For instance, it has been reported that ESCs, cultured with animal-derived ingredients, might take up and express foreign non-human immunogenic sialoprotein, increasing the risk of immune rejection (Grinnemo et al. 2008). A number of serum-free media (e.g. E8 medium for human PSCs) have been developed for the expansion of PSCs and MSCs (Chen et al. 2011; Lennon et al. 1995), but relatively few exist for lineage-specific differentiation of stem cells (Outten et al. 2011).
Alternatively, a transgenic approach involving the modification of PSCs to enhance their specific properties such as promoting the expression of protective proteins (e.g. A20, Bcl-xL, HO-1, FasL, or IDO) could be a way to minimize rejection (Taylor et al. 2011). Similarly, the genetic modification of MSCs could enhance the therapeutic effect (e.g. in overexpressing insulin-like growth factor-1) as reported in the treatment of myocardial infarctions (Wagner et al. 2009) or in cancer therapy (e.g. overexpressing interferon-β, interferon-γ) (Lazennec and Jorgensen 2008). The GTP and GMP (i.e. Title 21 CFR 1271 and the European directive 1394/2007) compliances require the characterization of the stem cell population as well as any materials required to manipulate stem cells, such as the relevant plasmids (Horwitz et al. 2002). Engineering the input cells, the process of expansion and differentiation, and the output cells enhances the homogeneity, safety, and biological functions of stem cells for therapeutic uses.
Bioreactors to modulate the mechanical and biochemical niches of stem cells
Bioreactors enable the control of the physiological microenvironment of stem cells, and thus are well-placed to improve culture performance towards mass scale production (King and Miller 2007; Liu et al. 2013). Moreover, 3-D stem cell aggregates have been recently shown to increase the therapeutic potential and differentiation efficiency of stem cells through the sustainment of endogenous signaling (Sart et al. 2013c, d; Fridley et al. 2010). In addition, to meet the requirement for high dose cell therapy (e.g. 1012 cells per batch of production), multi-stack devices would require 1 × 104–1 × 105 layers, which is not a feasible process (Simaria et al. 2014). Bioreactors (e.g. spinner flasks) support the expansion of 3-D cellular organization and are well-placed for cost-effective systems towards potential commercial processes (Abbasalizadeh and Baharvand 2013; Sart et al. 2013d).
Various bioreactor systems can be used for stem cell expansion and differentiation (Table 1). For instance, perfusion reactors can continuously deliver fresh nutrients and at the same time expose cells to controlled fluid mechanical forces (Li et al. 2009). Stirred-tank bioreactors eliminate the concentration gradients of nutrients and create a homogeneous physicochemical environment (Kaiser et al. 2013). Rotating wall vessels display similar properties as the stirred culture vessel, while allowing the low shear stress and the control of gravity to mimic the in vivo tissue environment (Sheyn et al. 2010).
Table 1.
Summary of bioreactors and their effects on stem cell expansion or differentiation
| Stem cell type | Bioreactor type | Effect on stem cells | Reference |
|---|---|---|---|
| MSCs | Perfusion | Enhanced osteogenesis, proliferation and the secretion of vascular endothelial growth factor | Kreke et al. (2008) |
| MSCs | Perfusion | Enhanced chondrogenesis | Gonçalves et al. (2011) |
| MSCs | Biaxial spinner flask | Enhanced osteogenesis | Zhang et al. (2010) |
| MSCs | Rotating wall vessel | Enhanced adipogenesis | Meyers et al. (2005) |
| MSCs | Compression bioreactor | Enhanced chondrogenesis | Huang et al. (2004) |
| mESCs | Perfusion microfluidic bioreactor | Induced three-germ layer commitment | Przybyla and Voldman (2012); Wolfe et al. (2012) |
| mESCs | Spinner flask | Enhanced self-renewal | Gareau et al. (2012) |
| mESCs | Rotating wall vessel | Enhanced hematopoiesis | Fridley et al. (2010) |
| mESCs | Rotating wall vessel | Enhanced hepatic differentiation | Wang et al. (2012) |
| mESCs | Perfusion | Endothelial and hematopoietic differentiation | Wolfe and Ahsan (2013) |
| mESCs | Rotary orbital shaker | Enhanced cardiomyocyte differentiation | Sargent et al. (2009) |
| hESCs | Spinner flask | Induced three-germ layer commitment | Leung et al. (2010) |
| hESCs | Spinner flask; Rotating wall vessel |
Induced three-germ layer commitment | Yirme et al. (2008) |
| iPSCs | Spinner flask | Integrated iPSC derivation, expansion, and cardiomyocyte differentiation | Fluri et al. (2012) |
MSCs mesenchymal stem cells, mESCs mouse embryonic stem cells, hESCs human embryonic stem cells, iPSCs induced pluripotent stem cells
Stem cells are mechano-sensitive cell populations and bioreactors can provide controlled mechanical signaling to the cells (Table 1) (Sun et al. 2012). In addition to the commonly observed cell damage due to shear stress in bioreactors, shear stress could modulate stem cell fate decision for self-renewal or lineage commitment. For instance, perfusion-based bioreactors enhance MSC proliferation and osteogenic differentiation due to the presence of flow shear (Kreke et al. 2008; Liu et al. 2010). Shear stress (1.5–15 dynes/cm2) has also been shown as a potent inducer of mesodermal commitment of ESCs through the modulation of FLK1 membrane protein (Wolfe and Ahsan 2013). Conversely, lowering shear stress in rotating wall vessels was found to favor MSC adipogenesis (Meyers et al. 2005) as well as hepatogenic differentiation of ESCs (Fig. 1) (Wang et al. 2012).
The rational design of culture mode in bioreactors is also essential in order to meet the physiological requirements of a stem cell population for optimized proliferation and differentiation (Table 2) (Lo et al. 2011). While simple batch mode does not support efficient MSC and ESC expansion, adapted feeding of nutrients (e.g. glucose or glutamine) or growth factors (e.g. fibroblast growth factor) with a fed-batch mode significantly increases the stem cell yields in bioreactors (Fig. 1) (Sart et al. 2010; Chen et al. 2010; Schop et al. 2010). The regulation of oxygen tension (i.e. hypoxia vs. normoxia) in bioreactors also modulates the proliferation and differentiation of MSCs and PSCs (Table 2) (Dos Santos et al. 2010; Lovett et al. 2010; Niebruegge et al. 2009). Moreover, bioreactors enable the tight control of cytokine and growth factor gradients, leading to the enhanced stem cell differentiation (Fig. 1) (Cimetta et al. 2013).
Table 2.
Culture mode affecting stem cell proliferation or differentiation in bioreactors
| Stem cell type | Bioreactor type | Culture mode | Parameter controlled | Effect | Reference |
|---|---|---|---|---|---|
| MSCs | Spinner flask | Fed batch | Growth factor concentration | Enhanced stem cell yield | Sart et al. (2010) |
| MSCs | Perfusion bioreactor | Hypoxia (5 % oxygen) versus normoxia (20 % oxygen) | Oxygen tension | Adipogenesis (normoxia) Chondogenesis (hypoxia) |
Lovett et al. (2010) |
| mESCs | Microfluidic bioreactor | Gradient perfusion | Wnt3a, activin A, BMP-4 concentration | Mesodermal differentiation | Cimetta et al. (2013) |
| hESCs | Spinner flask | Hypoxia (4 % oxygen) | Oxygen tension | Cardiomyocyte differentiation | Niebruegge et al. (2009) |
| hESCs | Spinner flask | Fed batch | Glucose concentration | Enhanced stem cell yield | Chen et al. (2010) |
| hESCs | Spinner flask | Perfusion | Oxygen tension | Improved stem cell expansion | Serra et al. (2010) |
MSCs mesenchymal stem cells, mESCs mouse embryonic stem cells, hESCs human embryonic stem cells
Modulation of stem cell behavior through 3-D culture configuration in bioreactors
Bioreactors enable the cultivation of stem cells as functional 3-D constructs. The culture on 3-D scaffolds is amenable to scale up of stem cell production while recreating the stem cell niches to maintain the cellular functions (Li et al. 2003; Abranches et al. 2007; Singh et al. 2010; Stenberg et al. 2011). Scaffold or substrate biomaterials provide additional mechanical signaling to stem cells. The pattern and the stiffness of scaffolds/substrates regulate stem cell shape, proliferation, and differentiation potential. For instance, large ECM islands promoted proliferation and osteogenic differentiation of MSCs as well as sustained ESC self-renewal (McBeath et al. 2004; Peerani et al. 2009). Conversely, small ECM islands induced MSC chondrogenic differentiation and limiting ESC self-renewal ability (Gao et al. 2010; Peerani et al. 2009). The biomechanical property of the scaffold, e.g. modulus, regulated ESC self-renewal versus commitment (Zoldan et al. 2011), and MSC proliferation versus osteogenic differentiation (Shih et al. 2011). To grow the adherent stem cells in suspension, various types of microcarriers have been investigated to support cell expansion and differentiation. In bioreactors, microcarriers enhanced ESC differentiation potential into neural lineage compared to EB-based protocols (Bardy et al. 2013). Importantly, the physical and biochemical properties of microcarriers modulate MSC proliferation and differentiation through the control of cell shape and cytoskeleton re-organization (Table 3) (Sart et al. 2013a).
Table 3.
Three-dimensional culture configurations enhancing stem cell properties
| Stem cell type | Bioreactor type | 3-D culture configuration | Effect on stem cells | Reference |
|---|---|---|---|---|
| MSCs | Spinner flask | Aggregates | Enhanced cell survival, VEGF secretion | Bhang et al. (2011) |
| MSCs | Spinner flask | Aggregates | Enhanced IL-24 secretion (anti-tumor factor) | Frith et al. (2010) |
| MSCs | Spinner flask | Aggregates | Enhanced osteogenesis | Frith et al. (2010) |
| MSCs | Rotating wall vessel | Aggregates | Enhanced adipogenesis | Frith et al. (2010) |
| MSCs | Spinner flask | Cytopore-2 microcarriers | Enhanced chondrogenesis compared to 2-D | Sart et al. (2013a) |
| MSCs | Spinner flask | Cytodex-3 microcarriers | Enhanced osteogenesis compared to 2-D | Goh et al. (2013) |
| mESCs | Static | Aggregates | Enhanced cell survival | Sart et al. (2013b) |
| mESCs | Spinner flask | Aggregates | Enhanced osteoblast differentiation | Alfred et al. (2010) |
| mESCs | Spinner flask | CultiSpher-S | Supported stem cell expansion | Storm et al. (2010) |
| hESCs | Static | Aggregates | Enhanced self-renewal | Singh et al. (2010) |
| hESCs | Spinner flask | Tosoh 10 microcarriers | Enhanced cardiomyogenesis compared to EBs | Lecina et al. (2010) |
| hESCs hiPSCs |
Spinner flask | DE-53 microcarriers | Enhanced neurogenesis compared to EBs | Bardy et al. (2013) |
MSCs mesenchymal stem cells, mESCs mouse embryonic stem cells, hESCs human embryonic stem cells, hiPSCs human induced pluripotent stem cells
Both MSCs and PSCs can also self-assemble as 3-D aggregates and be expanded in suspension bioreactors. The scaffold-free stem cell aggregates were found to reinforce cell–cell contacts (Singh et al. 2010) and autocrine/paracrine signaling (Kabiri et al. 2012), leading to the desired stem cell survival and biological function (Table 3). MSC aggregates grown in bioreactors displayed higher differentiation efficiency and enhanced trophic factor secretion (e.g. IL-24) compared to static and 2-D cultures (Frith et al. 2010; Bhang et al. 2011). Using bioreactors, the collision of aggregates can be controlled through hydrodynamic mixing, which leads to homogeneous aggregate size distribution and improved mass transfer and/or diffusion in the aggregates.
To overcome the diffusion barriers in the multi-cellular aggregates, growth factor delivery using nanoscale or microscale bioactive particles has been explored. The effects of nano-/micro- scale particles have been observed for embryoid bodies (EBs), the aggregate-like structure mimicking embryonic development. EB formation has been extensively used for lineage-specific differentiation because of intimate cell–cell contacts and cell-ECM interactions (Bratt-Leal et al. 2009). However, EBs usually form a shell-like structure which limits the diffusion length within 100 μm. Engineering EBs through the incorporation of microparticles containing retinoic acid has been shown to replicate embryonic tissue development (Bratt-Leal et al. 2011; Ferreira et al. 2008). These novel approaches using 3-D culture configurations provide the appropriate biomimetic microenvironment in the corresponding bioreactors and bioprocesses, thus ensuring precise control of stem cell fate decisions.
Production of stem cell products under cGMP
Stem cell-based cellular products have been produced in cGMP facilities for large scale clinical applications, such as hESC-derived OPCs for Geron’s phase I clinical trial on spinal cord injury treatment (Geron, unpublished data). For MSCs, a cGMP process usually starts from the cell isolation, which requires the determination, screening, and testing of donor eligibility. For PSC-derived products, the process can start from a working cell bank (WCB). However, the source, history, and the generation of a WCB need to be well documented and the banks need to be cleared from adventitious agent testing. Similarly to recombinant protein production, the bioprocess of manufacturing pluripotent stem cell-derived products contains an upstream expansion stage, a differentiation process to produce the cell product (such as cardiomyocytes or neural progenitors), and a set of downstream purification and formulation processes (Fig. 2). To produce these novel cellular products, the process control aimed at reducing variability and the development of stringent bioreactor-based stem cell differentiation protocols to increase the production scale are becoming very critical. The process control includes the use of consistent and safe raw materials (such as replacing mouse tumor-derived Matrigel coated surface by synthetic peptide surface), the development of serum-free media for cell expansion and differentiation, and the implementation of in-process monitoring of parameters such as the metabolic activity (e.g. glucose consumption and lactate production) (Li et al. 2013). In bioreactors, as stem cells are exposed to shear stress, the impact of the hydrodynamic environment on stem cell differentiation needs to be better understood (Gareau et al. 2012; Leung et al. 2010). Stirred-tank bioreactors are more applicable for cGMP production compared to other types of bioreactors due to their simplicity and scale up feasibility. As some differentiation processes, such as the production of hESC-derived OPCs, involve multiple stages (i.e. suspension and adherent cultivation) over long-term periods (i.e. 6–8 weeks), novel strategies of process integration at the bioreactor level become critical (Liu et al. 2013, Oh and Choo 2006). To make the production successful, the peripheral processes such as reagent acquisition and preparation, personnel training, equipment specification and validation, and documentation system such as the history of batch production record (BPR) are needed to be in place to support the core production processes (Ratcliffe et al. 2011).
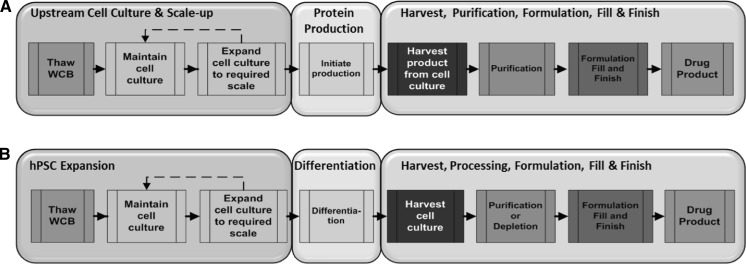
Fig. 2.
cGMP production process flow for pluripotent stem cell-derived products and recombinant protein production. a Bioprocess flow for recombinant protein production, b Bioprocess flow for the production of pluripotent stem cell-derived products. For both types of products, the bioprocess consists of three stages: upstream cell culture/expansion, protein production/differentiation, and downstream purification and formulation. WCB working cell bank, hPSC human pluripotent stem cell
Compared to recombinant protein production, the challenges of bioprocess engineering for stem cell-derived products include:
Reducing the variations from cell source and during the differentiation procedure. MSCs are primary cells typically isolated from various tissue types of the patients. For human PSCs, various culture methods have been developed by different groups. Hence, tight quality control for the starting cell population and the culture procedures has to be implemented to ensure consistent cell products.
Increasing the yield and purity of the differentiated cells. The differentiation protocols have been significantly improved in recent years, while most protocols still result in low purity (<30 %) of the lineage-specific cells. In addition, most protocols have not yet been evaluated in bioreactors. A good baseline protocol will ease the process development efforts to translate a laboratory procedure into a bioprocess in a cGMP facility.
Detailed characterization of the produced cell populations. Developing the clinically relevant product specifications is not trivial. New bioprocess and biomarker development to predict and/or eliminate the impure cells is critical for safe clinical use of stem cell products. The link of the stem cell products with the potential clinical outcome needs to be established before cGMP manufacturing.
Increasing the scale in bioreactors. Not every protocol in the laboratory can be translated into a bioreactor-based process. Understanding the change in culture environment is important and appropriate modifications of differentiation protocols are required.
Reducing the cost. Most current materials used in stem cell expansion and differentiation are still costly, such as serum-free media, growth factors, and the culture substrates. Understanding the signals to regulate stem cell self-renewal and lineage commitment using simplified reagents (e.g. E8 medium from Life Technologies) will be quite helpful to reduce the cost.
Conclusion
As learned from traditional bioprocesses in recombinant protein production, bioprocess engineering applied to stem cells and their products requires a horizontal approach: the selection of stem cell populations, bioreactor optimization, media development, and the detailed characterization of stem cell-derived progeny to improve safety. Such a strategy for a well-controlled process could rationally address the current issues and overcome the hurdles in stem cell-based clinical applications.
As analyzed above, the application of rational bioprocess engineering strategies to stem cell cultivation constitutes the best way to monitor their microenvironment. This should lead to the improvement in stem cell expansion and the priming ex vivo that should ultimately meet clinical demands with the proper safety considerations. Since various stem cells display different potential or physiological requirements, stem cell bioprocessing should be closely coupled to the specific physiological needs in both expansion and differentiation steps.
Acknowledgments
This work is supported by an IN-Wallonia-Brussels International bursary and a FSU start up fund. Partial support from the National Science Foundation (Grant No. 1342192) is also acknowledged.
References
- Abbasalizadeh S, Baharvand H (2013) Technological progress and challenges towards cGMP manufacturing of human pluripotent stem cells based therapeutic products for allogeneic and autologous cell therapies. Biotechnol Adv 31:1600–1623 [DOI] [PubMed]
- Abranches E, Bekman E, Henrique D, Cabral JMS. Expansion of mouse embryonic stem cells on microcarriers. Biotechnol Bioeng. 2007;96:1211–1221. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- Alfred R, Gareau T, Krawetz R, Rancourt D, Kallos MS (2010) Serum-free scaled up expansion and differentiation of murine embryonic stem cells to osteoblasts in suspension bioreactors. Biotechnol Bioeng 106:829–840 [DOI] [PubMed]
- Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW (2007) Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol 25:207–215 [DOI] [PubMed]
- Bardy J, Chen AK, Lim YM, Wu S, Wei S, Weiping H, Chan K, Reuveny S, Oh SK (2013) Microcarrier suspension cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng Part C Methods 19:166–180 [DOI] [PubMed]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS (2011) Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32:2734–2747 [DOI] [PubMed]
- Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG (2003) Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol 162:469–479 [DOI] [PMC free article] [PubMed]
- Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009;8:3822–3830. doi: 10.4161/cc.8.23.10067. [DOI] [PubMed] [Google Scholar]
- Bongso A, Fong C-Y, Gauthaman K. Taking stem cells to the clinic: major challenges. J Cell Biochem. 2008;105:1352–1360. doi: 10.1002/jcb.21957. [DOI] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC (2011) Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32:48–56 [DOI] [PMC free article] [PubMed]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Frey-Vasconcells J, Rao MS. Developing safe therapies from human pluripotent stem cells. Nat Biotechnol. 2009;27:606–613. doi: 10.1038/nbt0709-606. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen A, Woo TL, Choo AB, Reuveny S, Oh SK (2010) Investigations into the metabolism of two-dimensional colony and suspended microcarrier cultures of human embryonic stem cells in serum-free media. Stem Cells Dev 19:1781–1792 [DOI] [PubMed]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA (2011) Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8:424–429 [DOI] [PMC free article] [PubMed]
- Cimetta E, Sirabella D, Yeager K, Davidson K, Simon J, Moon RT, Vunjak-Novakovic G (2013) Microfluidic bioreactor for dynamic regulation of early mesodermal commitment in human pluripotent stem cells. Lab Chip 13:355–364 [DOI] [PMC free article] [PubMed]
- Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012;6:220–230. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo MM, da Silva CL, Cabral JM. Separation technologies for stem cell bioprocessing. Biotech Bioeng. 2012;109:2699–2709. doi: 10.1002/bit.24706. [DOI] [PubMed] [Google Scholar]
- Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM (2010) Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol 223:27–35 [DOI] [PubMed]
- Dressel R. Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Semin Immunopathol. 2011;33:573–591. doi: 10.1007/s00281-011-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, Guidance for FDA reviewers and sponsors (2008) Content and review of chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs). http://www.fda.gov
- Ferreira L, Squier T, Park H, Choe H, Kohane DS, Langer R (2008) Human embryoid bodies containing nano- and microparticulate delivery vehicles. Adv Mater 20:2285–2291
- Fluri DA, Tonge PD, Song H, Baptista RP, Shakiba N, Shukla S, Clarke G, Nagy A, Zandstra PW (2012) Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nat Methods 9:509–516 [DOI] [PMC free article] [PubMed]
- Fong C-Y, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem. 2010;111:769–781. doi: 10.1002/jcb.22775. [DOI] [PubMed] [Google Scholar]
- Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K (2010) Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A 16:3285–3298 [DOI] [PMC free article] [PubMed]
- Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.tec.2009.0432. [DOI] [PubMed] [Google Scholar]
- Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau T, Lara GG, Shepherd RD, Krawetz R, Rancourt DE, Rinker KD, Kallos MS (2012) Shear stress influences the pluripotency of murine embryonic stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med doi:10.1002/term.1518 [DOI] [PubMed]
- Godara P, McFarland CD, Nordon RE. Design of bioreactors for mesenchymal stem cell tissue engineering. J Chem Technol Biotechnol. 2008;83:408–420. doi: 10.1002/jctb.1918. [DOI] [Google Scholar]
- Goh TKP, Zhang ZY, Chen AKL, Reuveny S, Choolani M, Chan JKY, Oh SKW. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access. 2013;2:84–97. doi: 10.1089/biores.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Costa P, Rodrigues MT, Dias IR, Reis RL, Gomes ME (2011) Effect of flow perfusion conditions in the chondrogenic differentiation of bone marrow stromal cells cultured onto starch based biodegradable scaffolds. Acta Biomater 7:1644–1652 [DOI] [PubMed]
- Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- Grinnemo KH, Sylvén C, Hovatta O, Dellgren G, Corbascio M (2008) Immunogenicity of human embryonic stem cells. Cell Tissue Res 331:67–78 [DOI] [PubMed]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, Hess DC, Borlongan CV (2008) Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol 85:318–334 [DOI] [PubMed]
- Hedlund E, Pruszak J, Lardaro T, Ludwig W, Viñuela A, Kim KS, Isacson O (2008) Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells 26:1526–1536 [DOI] [PMC free article] [PubMed]
- Heng TS, Dudakov JA, Khong DM, Chidgey AP, Boyd RL (2009) Stem cells—meet immunity. J Mol Med 87:1061–1069 [DOI] [PubMed]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 99:8932–8937 [DOI] [PMC free article] [PubMed]
- Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H (2013) Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341:651–654. doi:10.1126/science.1239278 [DOI] [PubMed]
- Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22:313–323 [DOI] [PubMed]
- Hwang D-Y, Kim D-S, Kim D-W. Human ES and iPS cells as cell sources for the treatment of Parkinson’s disease: current state and problems. J Cell Biochem. 2010;109:292–301. doi: 10.1002/jcb.22411. [DOI] [PubMed] [Google Scholar]
- Kabiri M, Kul B, Lott WB, Futrega K, Ghanavi P, Upton Z, Doran MR (2012) 3-D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochem Biophys Res Commun 419:142–147 [DOI] [PubMed]
- Kaiser SC, Jossen V, Schirmaier C, Eibl D, Brill S, van den Bos C, Eibl R (2013) Investigations of fluid flow and cell proliferation of mesenchymal adipose-derived stem cells in small-scale, stirred, single-use bioreactors. Chem Ing Tech 85:95–102
- King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreke MR, Sharp LA, Lee YW, Goldstein AS. Effect of intermittent shear stress on mechanotransductive signaling and osteoblastic differentiation of bone marrow stromal cells. Tissue Eng Part A. 2008;14:529–537. doi: 10.1089/tea.2007.0068. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26:1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecina M, Ting S, Choo A, Reuveny S, Oh S (2010) Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng Part C Methods 16:1609–1619 [DOI] [PubMed]
- Lennon DP, Haynesworth SE, Young RG, Dennis JE, Caplan AI (1995) A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp Cell Res 219:211–222 [DOI] [PubMed]
- Leung HW, Chen A, Choo AB, Reuveny S, Oh SK (2010) Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng Part C Methods 17:165–172 [DOI] [PubMed]
- Li Y, Kniss DA, Lasky LC, Yang ST. Culturing and differentiation of murine embryonic stem cells in a three-dimensional fibrous matrix. Cytotechnology. 2003;41:23–35. doi: 10.1023/A:1024283521966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tang T, Lu J, Dai K. Effects of flow shear stress and mass transport on the construction of a large-scale tissue-engineered bone in a perfusion bioreactor. Tissue Eng Part A. 2009;15:2773–2783. doi: 10.1089/ten.tea.2008.0540. [DOI] [PubMed] [Google Scholar]
- Li Y, Gautam A, Yang J, Qiu L, Melkoumian Z, Weber J, Telukuntla L, Srivastava R, Whiteley EM, Brandenberger R (2013) Differentiation of oligodendrocyte progenitor cell differentiation from human embryonic stem cells on vitronectin-derived synthetic peptide acrylate surface. Stem Cells Dev 22:1497–1505 [DOI] [PubMed]
- Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010;9:659–670. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- Liu N, Zang R, Yang S-T, Li Y (2013) Stem cell engineering in bioreactors for large scale bioprocessing. Eng Life Sci. doi:10.1002/elsc.201300013
- Lo T, Ho JH, Yang M-H, Lee OK. Glucose reduction prevents replicative senescence and increases mitochondrial respiration in human mesenchymal stem cells. Cell Transpl. 2011;20:813–825. doi: 10.3727/096368910X539100. [DOI] [PubMed] [Google Scholar]
- Lovett M, Rockwood D, Baryshyan A, Kaplan DL. Simple modular bioreactors for tissue engineering: a system for characterization of oxygen gradients, human mesenchymal stem cell differentiation, and prevascularization. Tissue Eng Part C Methods. 2010;16:1565–1573. doi: 10.1089/ten.tec.2010.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Blau HM. Artificial stem cell niches. Adv Mater. 2009;21:3255–3268. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandenius CF, Steel D, Noor F, Meyer T, Heinzle E, Asp J, Arain S, Kraushaar U, Bremer S, Class R, Sartipy P (2011) Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J Appl Toxicol 31:191–205 [DOI] [PubMed]
- Martín PG, Martinez AR, Lara VG, Naveros BC (2014) Regulatory considerations in production of a cell therapy medicinal product in Europe to clinical research. Clin Exp Med 14:25–33 [DOI] [PubMed]
- Massard C, Deutsch E, Soria J-C. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620–1624. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6:483–495 [DOI] [PubMed]
- Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW (2009) Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng 102:493–507 [DOI] [PubMed]
- Oh S, Choo ABH. Human embryonic stem cell technology: large scale cell amplification and differentiation. Cytotechnology. 2006;50:181–190. doi: 10.1007/s10616-005-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto OK, Carvalho AC, Marti LC, Vêncio RZ, Moreira-Filho CA (2007) Common molecular pathways involved in human CD133+/CD34+ progenitor cell expansion and cancer. Cancer Cell Int 7:11 [DOI] [PMC free article] [PubMed]
- Outten JT, Cheng X, Gadue P, French DL, Diamond SL (2011) A high-throughput multiplexed screening assay for optimizing serum-free differentiation protocols of human embryonic stem cells. Stem Cell Res 6:129–142 [DOI] [PubMed]
- Peerani R, Onishi K, Mahdavi A, Kumacheva E, Zandstra PW (2009) Manipulation of signaling thresholds in “engineered stem cell niches” identifies design criteria for pluripotent stem cell screens. PLoS ONE 4:e6438 [DOI] [PMC free article] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed]
- Przybyla LM, Voldman J. Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal. Proc Natl Acad Sci USA. 2012;109:835–840. doi: 10.1073/pnas.1103100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe E, Thomas RJ, Williams DJ. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull. 2011;100:137–155. doi: 10.1093/bmb/ldr037. [DOI] [PubMed] [Google Scholar]
- Ratcliffe E, Hourd P, Guijarro-Leach J, Rayment E, Williams DJ, Thomas RJ (2013) Application of response surface methodology to maximize the productivity of scalable automated human embryonic stem cell manufacture. Regen Med 8:39–48 [DOI] [PubMed]
- Rodriguez R, Rubio R, Menendez P. Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res. 2012;22:62–77. doi: 10.1038/cr.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent CY, Berguig GY, McDevitt TC. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng Part A. 2009;15:331–342. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- Sart S, Schneider Y-J, Agathos SN. Influence of culture parameters on ear mesenchymal stem cells expanded on microcarriers. J Biotechnol. 2010;150:149–160. doi: 10.1016/j.jbiotec.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Sart S, Errachid A, Schneider Y-J, Agathos SN. Modulation of mesenchymal stem cell actin organization on conventional microcarriers for proliferation and differentiation in stirred bioreactors. J Tissue Eng Regen Med. 2013;7:537–551. doi: 10.1002/term.545. [DOI] [PubMed] [Google Scholar]
- Sart S, Ma T, Li Y. Cryopreservation of pluripotent stem cell aggregates in defined protein-free formulation. Biotechnol Prog. 2013;29:143–153. doi: 10.1002/btpr.1653. [DOI] [PubMed] [Google Scholar]
- Sart S, Agathos SN, Li Y (2013c) Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog 29:1354–1366 [DOI] [PubMed]
- Sart S, Tsai AC, Li Y, Ma T (2013d) Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B. doi:10.1089/ten.teb.2013.0537 [DOI] [PMC free article] [PubMed]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schop D, van Dijkhuizen-Radersma R, Borgart E, Janssen FW, Rozemuller H, Prins HJ, de Bruijn JD (2010) Expansion of human mesenchymal stromal cells on microcarriers: growth and metabolism. J Tissue Eng Regen Med 4:131–140 [DOI] [PubMed]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379:713–720 [DOI] [PubMed]
- Sensebe L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011;22:19–26. doi: 10.1089/hum.2010.197. [DOI] [PubMed] [Google Scholar]
- Serra M, Brito C, Sousa MF, Jensen J, Tostões R, Clemente J, Strehl R, Hyllner J, Carrondo MJ, Alves PM (2010) Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol 148:208–215 [DOI] [PubMed]
- Serra M, Brito C, Correia C, Alves PM (2012) Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol 30:350–359 [DOI] [PubMed]
- Sharma S, Raju R, Sui S, Hu WS. Stem cell culture engineering—process scale up and beyond. Biotechnol J. 2011;6:1317–1329. doi: 10.1002/biot.201000435. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010;584:3819–3825. doi: 10.1016/j.febslet.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheyn D, Pelled G, Netanely D, Domany E, Gazit D (2010) The effect of simulated microgravity on human mesenchymal stem cells cultured in an osteogenic differentiation system: a bioinformatics study. Tissue Eng Part A 16:3403–3412 [DOI] [PMC free article] [PubMed]
- Shih Y-RV, Tseng K-F, Lai H-Y, Lin C-H, Lee OK (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26:730–738 [DOI] [PubMed]
- Simaria AS, Hassan S, Varadaraju H, Rowley J, Warren K, Vanek P, Farid SS (2014) Allogeneic cell therapy bioprocess economics and optimization: single-use cell expansion technologies. Biotechnol Bioeng 111:69–83. doi:10.1002/bit.25008 [DOI] [PMC free article] [PubMed]
- Singh H, Mok P, Balakrishnan T, Rahmat SNB, Zweigerdt R (2010) Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res 4:165–179 [DOI] [PubMed]
- Spangrude GJ, Muller-Sieburg CE, Heimfeld S, Weissman IL. Two rare populations of mouse Thy-1lo bone marrow cells repopulate the thymus. J Exp Med. 1988;167:1671–1683. doi: 10.1084/jem.167.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg J, Elovsson M, Strehl R, Kilmare E, Hyllner J, Lindahl A (2011) Sustained embryoid body formation and culture in a non-laborious three dimensional culture system for human embryonic stem cells. Cytotechnology 63:227–237 [DOI] [PMC free article] [PubMed]
- Storm MP, Orchard CB, Bone HK, Chaudhuri JB, Welham MJ (2010) Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnol Bioeng 107:683–695 [DOI] [PMC free article] [PubMed]
- Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys. 2012;41:519–542. doi: 10.1146/annurev-biophys-042910-155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed]
- Takehara M, Hoshino T, Namba T, Yamakawa N, Mizushima T (2011) Acetaminophen-induced differentiation of human breast cancer stem cells and inhibition of tumor xenograft growth in mice. Biochem Pharmacol 81:1124–1135 [DOI] [PubMed]
- Tan HL, Fong WJ, Lee EH, Yap M, Choo A (2009) mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells 27:1792–1801 [DOI] [PubMed]
- Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstegge S, Laufenberg I, Pochert J, Schenk S, Itskovitz-Eldor J, Endl E, Brüstle O (2007) Automated maintenance of embryonic stem cell cultures. Biotechnol Bioeng 96:195–201 [DOI] [PubMed]
- Thomas R, Ratcliffe E. Automated adherent human cell culture (mesenchymal stem cells) Methods Mol Biol. 2012;806:393–406. doi: 10.1007/978-1-61779-367-7_26. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed]
- Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O (2008) Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet 17:R48–R53 [DOI] [PubMed]
- Wagner J, Kean T, Young R, Dennis JE, Caplan AI (2009) Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol 20:531–536 [DOI] [PubMed]
- Wang Y, Zhang Y, Zhang S, Peng G, Liu T, Li Y, Xiang D, Wassler MJ, Shelat HS, Geng Y (2012) Rotating microgravity-bioreactor cultivation enhances the hepatic differentiation of mouse embryonic stem cells on biodegradable polymer scaffolds. Tissue Eng Part A 18:2376–2385 [DOI] [PubMed]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP (2013) A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143:1590–1598 [DOI] [PMC free article] [PubMed]
- Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng. 2013;110:1231–1242. doi: 10.1002/bit.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RP, Leleux J, Nerem RM, Ahsan T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integr Biol (Camb) 2012;4:1263–1273. doi: 10.1039/c2ib20040f. [DOI] [PubMed] [Google Scholar]
- Yirme G, Amit M, Laevsky I, Osenberg S, Itskovitz-Eldor J. Establishing a dynamic process for the formation, propagation, and differentiation of human embryoid bodies. Stem Cells Dev. 2008;17:1227–1241. doi: 10.1089/scd.2007.0272. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Teoh SH, Teo EY, Khoon Chong MS, Shin CW, Tien FT, Choolani MA, Chan JK (2010) A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 31:8684–8695 [DOI] [PubMed]
- Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, Levenberg S (2011) The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 32:9612–9621 [DOI] [PMC free article] [PubMed]