Abstract
In recent years, water pollution has been converted to a challenging discussion in health area of human being. Heavy elements are one of the most important water pollutants and their negative adverse effects on body systems have been confirmed. In this study, investigation of effects of two heavy elements including lead (Pb) and copper (Cu) on expression of interlukin-4 (IL-4) and interferon-gamma (IFN-γ) as humoral and cellular immunity biomarkers, respectively, was aimed and PCR, real-time PCR and electrophoresis techniques were used. In this study, BALB/c mice were studied that had free access to drinking water which contained Cu or Pb salts. After 2 weeks, spleens of mice were removed, RNA extracted, and cDNA was prepared for RT-PCR. Then the expression of IL-4 and IFN-γ genes were assessed by real-time PCR. The expression of IFN-γ was up-regulated in both treated groups and the expression of IL-4 was only up-regulated in the group treated with Cu and down-regulated in the group treated with Pb. This study shows that the presence of heavy elements as drinking-water pollutants results in a disproportion of natural cytokines balances, and thus may result in a negative effect on immune system.
Keywords: Drinking-water pollution, Cytokine, Copper, Lead, Real-time PCR
Introduction
Water is the most widely used substance and precious resource in the whole of universe and plays a vital role in our daily lives. Access to a safe water supply with high quality is one of the first rights of the human being and is always a big concern.
Water has unique chemical properties due to its polarity and hydrogen bonds. This means it is able to dissolve, absorb, adsorb or suspend many different compounds. These include contaminants that may represent hazards in themselves or that may be able to react with intended product substances, resulting in hazards to health.
Drinking-water quality is covered by the World Health Organization (WHO) drinking-water guidelines, standards from the International Organization for standardization (ISO) and other regional and national agencies (World Health Organization 2011).
Where drinking-water is stored and distributed by the user, the storage systems have to preclude degradation of the water quality before use. Pollution of drinking-water with heavy metals has become a concern in view of their toxicity to human and other biological systems (Chakrabarty and Sarma 2011). Most of the heavy metal ions are toxic or carcinogenic in nature and pose a threat to human health and the environment (Gillialand et al. 1999). Heavy metals are absorbed via the intestine, as far as they are fairly water-soluble, are transmitted to several organs through the circulatory system and are absorbed by different systems. This form of absorption is particularly significant to the overall heavy metal load in the human body as higher concentrations of these metals can be absorbed (Marth et al. 2001, Markevièius and Dringeliene 2004).
The immune system consists of a complex network of cells and molecules scattered throughout the body of all multicellular organisms and is able to recognize and neutralize potentially harmful agents, conferring to the organism resistance to infectious and malignant diseases (Markevièius and Dringeliene 2004, Marth et al. 2001). The immune system is a complex network of multiple organs, tissues and cells consisting of many points for heavy metals to attack and influence the function of the immune system, either by inhibition or stimulation and leading to pathological consequence (Kelley et al. 1995).
CD4+ T helper cells have been classified into two major subsets (TH1 and TH2) based on the production of different cytokines which promote distinct functions, result in polarizing of immune responses, cellular or humoral. TH1 cells produce mainly IFN-γ which skews the immune responses to cell-mediated responses, while TH2 cells secrete IL-4 shifting to antibody production and humoral immune responses. The Th1/Th2 paradigm provides a valuable model for understanding the effects of stimulators (substances, antigens and pathogens) on immune responses (Muraille and Leo 1998). Evaluation of IFN-γ and IL-4 levels is a good measurement to assess the TH1/TH2 responses and ongoing responses in different conditions. Real time PCR techniques has provided a powerful detection system (van Oosterhout and Motta 2005).
IFN-γ (Type II interferon) is a homodimeric glycoprotein that is produced by activated T, B and NK cells. IFN-γ is produced by CD8+ TH1 cells and inhibits the proliferation of TH2 cells. IFN-γ functions as an anti-viral and anti-parasitic agent and also acts in synergy with other cytokines, such as TNFα to inhibit the proliferation of normal and transformed cells. IFN-γ induces immunomodulatory effects in a wide range of cell types that includes being a potent activator of mononuclear phagocytes, augmentation of endocytosis and phagocytosis by monocytes, and activation of macrophages to kill tumor cells. Additionally, it enhances the proliferation of activated B cells and can act synergistically with IL-2 to increase immunoglobulin light-chain synthesis. Finally, IFN-γ activates neutrophils, NK cells and vascular endothelial cells. The role of IFN-γ has been identified as a cell mediated immune response (Sen 2001).
IL-4 is a TH2 cytokine that is secreted by activated TH2 and NKT cells. IL-4 is a potent inducer that directs differentiation of CD4+naive T cells into TH2 effector cells. In B cells, IL-4 promotes proliferation and differentiation, and promotes immunological class switching to IgE and IgG1 isotypes. In T and B lymphocytes, mast cells, and endothelial cells, IL-4 promotes survival, growth, and differentiation. Totally IL-4 is known as a marker for humoral immune responses (Overbergh et al. 1999).
Lead and copper are two of important heavy metals and are most discussed in drinking-water. Lead is proposed as a strong neurotoxin and might leak from water pipes made of lead. Chakrabarty showed long term exposure to lead results in accumulation of lead in bones leading to sever disorders. Lead may adsorb into liver, kidney, heart and testis and affects the immune system (Chakrabarty and Sarma 2011). The decrease of B and T lymphocytes and natural killer (NK) cells has been found in people who were exposed to lead (Skoczynska et al. 2002). Other imbalances, such as the increase of the number of T CD8+, decrease of B lymphocytes and increase of IgA level have been reported (Kelley et al. 1995; Kvietkauskaite et al. 2004; Ercal et al. 2000; Singh et al. 2008).
Copper ions exist in many enzymes as cofactor including cytochrome c oxidase, dopamine β-monoxygenase and in peptidyl α-amidating monooxygenase which play important roles in the body. However the release of copper radicals in the body results in toxicity and affects liver, blood cells and the immune system (Kvietkauskaite et al. 2004; Markevièius and Dringeliene 2004). High-dose uptake of copper may result in a fatal consequence. Mutagenesis, carcinogenicity, infertility and immune suppression are caused by low-dose uptake of copper ions. Exposure to copper ions leads to sever decrease of NK cells (Kvietkauskaite et al. 2004).
Cytokines are important mediators of the immune system (Yin et al. 2001) and important immune response modulators that may be affected by extrinsic factors such as heavy metal exposure (Faith et al. 1979). Quantification of these products is an acceptable method to assess the effects on immune processes (Overbergh et al. 1999). Quantitative polymerase chain reaction (PCR) is a great tool to quantify gene expression. Real-time PCR (comparative real-time PCR) is currently the most accurate and sensitive method for quantifying the mRNA expression of cytokines, which are often expressed at very low levels, it is working with a fluorescent dye (e.g., SYBR Green) and fluorogenic sequence-specific probes (TaqMan™, molecular beacons, scorpions and hybridization probes) (Giulietti et al. 2001).
The objective of the present study was to investigate the effects of drinking-water pollution with copper, and lead on the expression of cytokines, interleukin-4 (IL-4) and interferon gamma (IFN-γ) genes as markers of the humoral and cellular immune system, respectively in mice by real-time RT-PCR and semi-quantitative techniques.
Materials and methods
Solutions preparation
Cu(NO3)2 (2 mg/l) and Pb(NO3)2 (0.3 mg/l) salts were dissolved in distilled water separately and were used for copper and lead exposure, respectively. These concentrations are two times of the upper safety limits of Cu and Pb ions in drinking water, reported by EPA (Environmental Protection Agency). The salts were reagent grade and purchased from Merck Company, Darmstadt, Germany.
Mice and drinking protocols
Fifteen female BALB/c mice 6–7 weeks of age with an average weight of about 25 g, were obtained from the Razi Vaccine and Serum Research Institute (RVSRI), Mashhad, Iran. The mice were maintained in a temperature (23–25 °C) controlled environment. The mice were allowed free access to standard laboratory chow and water throughout the experiment.
The animals were divided to three groups, each comprising five mice treated as follows:
Group 1, was considered as control group and accessed to distilled water for drinking. Groups 2 and 3 were accessed to water containing copper [Cu(NO3)2] and lead [Pb(NO3)2] ions solutions, respectively.
After 15 days, the weights of mice were measured and the spleens of mice were removed and subjected to RNA extraction. The weights of spleens were recorded too. The mice were treated ethically in compliance with the RVSRI Ethics Committee.
RNA extraction and reverse-transcription (RT)-PCR
Total RNA extraction from spleen was performed using Trizol (Invitrogen, Carlsbad, CA, USA), treated with RNase-free DNase (Ambion, Austin, TX, USA) to remove any residual genomic DNA that may be present in the RNA. Then RNA was reverse transcribed as following:
Reverse transcription of target RNA (1–5 μg) was carried out in a final volume of 12.5 μl using 1.5 μl of 10X MULV Buffer, 0.5 μl of 0.2 mM dNTP mix (Fermentas—VWR International—Germany GmbH, Darmstadt, Germany), 0.25 μl of 40 U/μl ribonuclease inhibitor (Fermentas), 0.5 μl of 0.5 μg Oligo dT primers (Fermentas), 0.25 μl of 200 U/μl MULV reverse transcriptase (Life Technologies, Carlsbad, CA, USA) and 8 μl of Diethylpyrocarbonate (DEPC)-treated water.
For every reaction set, one RNA sample was performed without MULV reverse transcriptase to provide a negative control in subsequent PCR reactions.
Primers
Primers were designed with Primer Premier Software on the basis of mRNA sequences available on NCBI and previously published studies for the relative quantification of the expression of the target genes (IL-4 and IFN-c), and the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) was used as the reference gene. The primers were synthesized by the Bioneer Company, Daejeon, (Korea). A brief description of the target genes and primers are listed in Table 1.
Table 1.
Sequences of primers used for quantitative real time-PCR
| Target gene | Sequence (5′-3′) | Product size (bp) |
|---|---|---|
| GAPDH | Forward TTCACCACCATGGAGAAGGC | 236 |
| Reverse GGCATGGACTGTGGTCATGA | ||
| IL-4 | Forward ACAGGAGAAGGGACGCCAT | 95 |
| Reverse GAAGCCCTACAGACGAGCTCA | ||
| IFN-γ | Forward TCAAGTGGCATAGATGTGGAAGAA | 92 |
| Reverse TGGCTCTGCAGGATTTTCATG |
Real-time RT-PCR
Reverse-transcription PCR quantification of cytokine gene expression was done using standard curves. mRNA levels were assessed by conventional and quantitative RT-PCR. Amplification was performed with Step one AB (Applied Biosystems—Life Technologies) using SYBR Green PCR Master Mix (Fermentas) in a total volume of 12.5 μl, containing 0.5 μl cDNA sample, and 0.25 μM each primer and 5.25 μl ddH2O. Quantitative PCR amplification was performed for 40 cycles at 95 °C for 10 s, specific annealing temperature for 30 s and 72 °C for 30 s. Amplification specificity was checked using melting curve. The endogenous housekeeping gene, GAPDH, was used to normalize target gene expression. A no-template control cDNA was included to detect contamination or non-specific reactions.
Amplified real-time PCR products were separated electrophoretically on 1.5 % agarose gel (HiMedia, Mumbai, India) in TAE (Tris–acetate-EDTA) buffer containing 1 μl syber Green. The mRNA expression was visualized using a Gel–Doc system (Syngene, Cambridge, UK).
Data analysis and calculations for relative quantification
Quantification of cytokine gene expression was calculated by the comparative CT method as described by Peinnequin et al. (2004) and by Schmittgen and Livak (2008). This method compares test samples to a comparator sample (non treated sample, control group) and uses results obtained with a uniformly expressed internal control gene (GAPDH) to correct for differences in the amounts of RNA present in the two samples being compared to generate a ΔCT value [ΔCT = (CT gene of interest—CT internal control)]. Results are expressed as the degrees of difference between ΔCT values of test and comparator samples [ΔΔCT = ΔCT (test sample)—ΔCT (comparator sample)] and relative quantification was calculated as 2−ΔΔCT. If the fold-change is greater than 1, then the result may be reported as a fold up-regulation. If the fold-change is less than 1, then the negative inverse of the result may be reported as a fold down-regulation.
Statistical analyses were performed with GraphPad Prism, Version 4.03 (GraphPad software, San Diego, CA, USA). All values were expressed as mean ± standard deviation. Statistical analyses of the data were performed using a one-way analysis of variance (ANOVA). Differences in quantitative cytokine mRNA expression were analyzed using Student’s t- test. In all cases, probability (P) values below 0.05 were considered significant.
Results
Effect of heavy metals on weight of mice
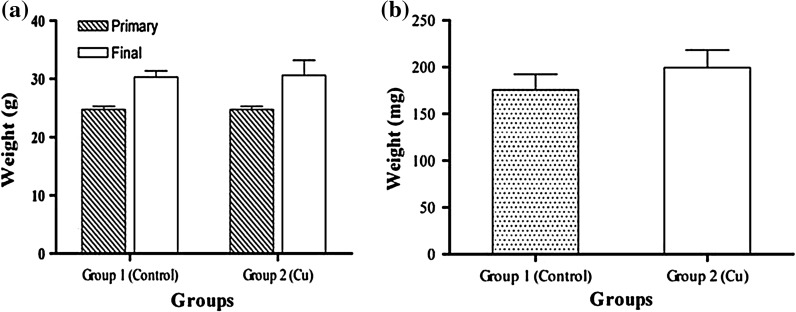
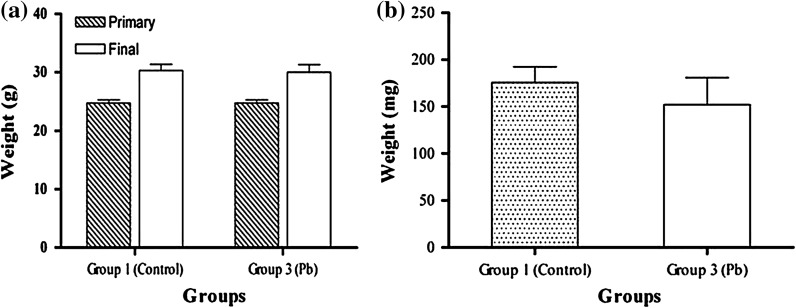
Two groups of BALB/C mice were exposed to drinking-water containing Pb(NO3)2 (group 2) and Cu(NO3)2 (group 3) for 2 weeks. For the non-treated control (group 1), the mice were exposed to distilled water. The results showed that the weight of the mice increased in group 3, which consumed copper ions, however, this change was not considered significant (p < 0.05) (Fig. 1). The weights of spleens between the groups, before and after treatments, were compared and no changes were found (Fig. 2). No difference was found in the volume of water and food uptake in the different mice groups.
Fig. 1.
The comparison of weight averages of mice in group 2 (Cu- treated) and group 1 (Control) before (primary) and after treatment (final) (a) and weight averages of spleens between these groups after treatment (b). The average weight of mice after treatment in group 2 was found higher than in the control group but this difference was not significant (p < 0.05)
Fig. 2.
The comparison of weight averages of mice in group 3 (Pb- treated) and group 1 (Control) before (primary) and after treatment (final) (a) and weight averages of spleens between these groups after treatment (b). The average weight of mice after treatment in group 2 was found higher than in the control group but this difference was not significant (p < 0.05)
Quantification and comparison of mRNA cytokine expression by spleen cells in treated and non-treated mice
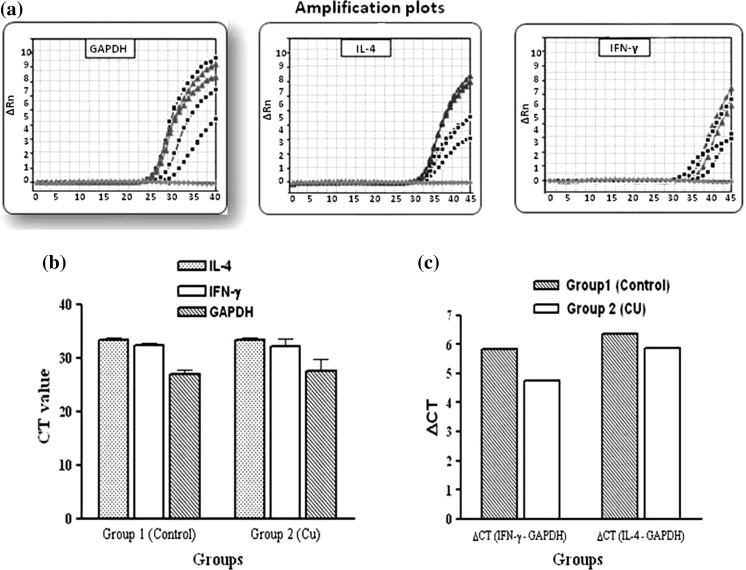
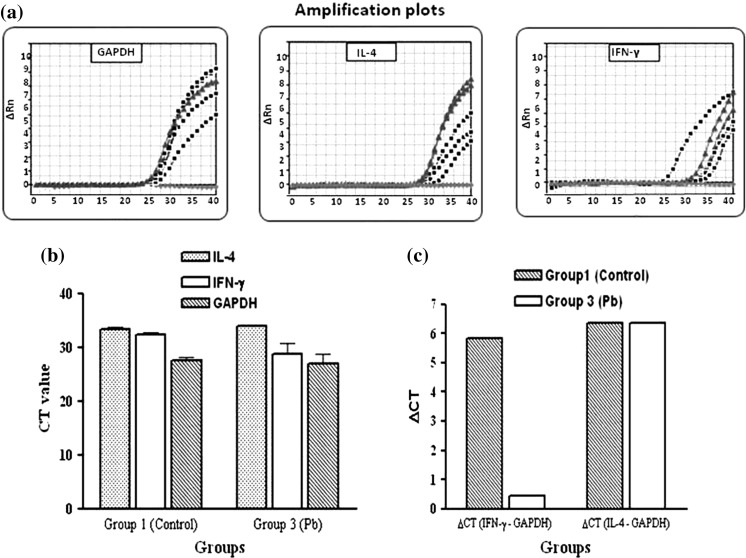
To establish whether spleen cells express IL-4, and IFN-γ in the presence of Pb or Cu heavy metal ions, RNA was isolated from spleen cells of the three groups of mice after 2 weeks of treatment and the relative genes expression of IFN-γ, and IL-4 were determined by real-time PCR and the ΔCT quantification method. Results were presented as fold changes relative to the control.
mRNA expression of cytokines IFN-γ was up-regulated in both treated groups in comparison of group 1 (non treated control). Test/comparator ratios for groups 2 (Cu treated) and 3 (Pb treated) were found 2.11 and 42.51, respectively. In mice exposed to Pb ions, IL-4 mRNA expression was down-regulated, with test/comparator ratios rising with −1.2 fold.
In contrast, in animals of group 2 (Cu), IL-4 mRNA expression was clearly up-regulated, with test/comparator ratios rising about 1.38 (Figs. 3, 4).
Fig. 3.
Real-time RT-PCR results for group 2 (Cu). (a) Amplification plots of GAPDH (left), IL-4 (middle) and IFN-γ (right). (b) Comparison of CT values. (c) Comparison of ΔCT between groups 1 (Control) and 2 (Cu) for IL-4 and IFN-γ. Test/comparator ratios of IFN-γ and IL-4 for groups 2 (Cu) was found 2.11 and 1.38, respectively
Fig. 4.
Real-time RT-PCR results for group 3 (Pb). (a) Amplification plots of GAPDH (left), IL-4 (middle) and IFN-γ (right). (b) Comparison of CT values. (c) Comparison of ΔCT between groups 1 (Control) and 3 (Pb) for IL-4 and IFN-γ. Test/comparator ratios of IFN-γ and IL-4 for groups 3 (Pb) was found 42.51 and -1.2, respectively
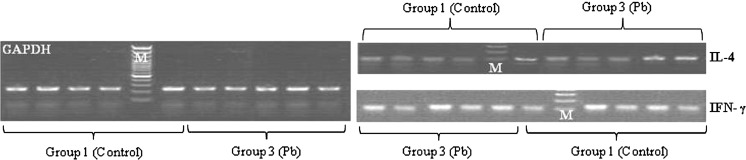
Finally, all PCR products were verified on agarose gel and the integrity and specificity of reaction were confirmed (Figs. 5, 6).
Fig. 5.
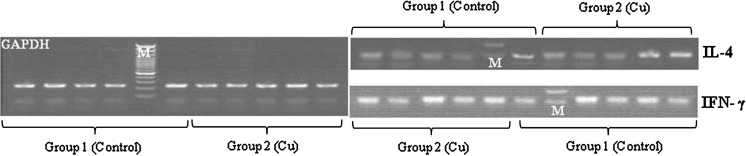
The electrophoresis of real-time RT-PCR products of RNA extracted from group 2 (Cu) on agarose gel (1.5 %) (M: DNA marker, 100 bp Fermentas, from bottom to top: 100, 200, 300, 400, 500, 600, 700, 800, 900, 1,000 bp). Amplified GAPDH, IL-4 and IFN-γ are seen as 236-bp, 95-bp and 92-bp bands, respectively
Fig. 6.
The electrophoresis of real-time PCR products of RNA extracted from group 3 (Pb) on agarose gel (1.5 %) (M: DNA marker, 100 bp Fermentas, from bottom to top: 100, 200, 300, 400, 500, 600, 700, 800, 900, 1,000 bp). Amplified GAPDH, IL-4 and IFN-γ are seen as 236-bp, 95-bp and 92-bp bands, respectively
Discussion
Environmental hazards may refer to any chemical, physical, mechanical, biological or even psychosocial condition that causes a threat to the environment and they can lead to health problems.
Environmental hazards affect living beings in numerous ways. One of the most important issues is how it affects our immune systems and our body’s ability to battle against diseases (Kazbariene et al. 2007).
Water pollution is one important kinds of these hazards. Drinking safe water and removing toxins are important in maintaining health and avoid disorders. Limitations in access to safe drinking-water resulted in describing terms such as “water conflict” for this “blue gold”. Water pollution poses a significant health risk and it occurs when contaminants such as harmful chemicals are discharged directly into the water resources. Contamination of water with heavy metals is one of the greatest concerns, world wide seen (World Health Organization 2011).
Exposure to heavy metals can cause birth defects in the newborn, reproductive failure and immune suppression (Bowring 2005; Nassar et al. 2010). It causes the immune system to break down and makes the person vulnerable to other diseases. The immune system components are coordinated by specific cells. T helper (Th) cells play a central role in this through direct interactions with cells and the release cytokines will coordinate the immune response. Water pollution is one of the main extrinsic factors which may affect the function of the immune system (Matranga et al. 2012a).
Current EPA guidelines allow for acceptable levels of pollutants, such as chlorine, lead, arsenic, and aluminum in our water. Water pollution and its effects on the health of the population is an important issue under study (http://water.epa.gov/drink/contaminants/index.cfm).
Vinodhin et al. showed that some heavy metal ions are potentially tumorigenic. Heavy metals may adsorb into the body by drinking polluted water and accumulate in the body tissues until rise to toxic threshold levels (Vinodhini and Narayanan 2009). In order to dilute toxins, the body tissues retain the water and fat. It is elucidated the heavy metal salts might cause a change in the regulation of the appetite in the central nervous system. The minor weight gain of the treated mice which were exposed to Pb and Cu in comparison to the control mice may be explained by this hypothesis. It is suggested that the overweight of the treated mice reflects the long term effects of drinking water containing Pb and Cu metals (Freundt and Ibrahim 1990).
In this study, the effects of the presence of Cu and Pb ions in drinking-water of BALB/c mice on the expression of IL-4 and IFN-c were assessed by realtime RT-PCR. These cytokines are considered as biomarkers of Th2 and Th1 immune responses and are used for the evaluation of immune responses. The results showed that the expression of IFN-c gene was elevated in both groups who drank water containing these ions compared with the control group. This increase was more significant in the group exposed to Pb ions.
On the other hand, the expression of IL-4 in the group exposed to Pb ions decreased and in the group exposed to Pb ions decreased and in group exposed to Cu increased.
Different studies have been done for identifying the effects of heavy metals on the immune system. It was shown that the long-term exposure to Pb in mice leads to a decrease of T CD4+ and an increase of T CD8+ cells but Cu caused an increase of both kinds of these cells (Matranga et al. 2012b).
Villanueva et al. 2000 confirmed that Pb ions inhibit the activities of Th1 cells but increase activities of Th2 cells.
All these reports indicate the impairment of the immune system. The disagreements of the reports may result from differences in the design of the study (short or long-term), strains of studied animals and cell populations (blood or spleen cells).
Here we showed that even short-term exposure (2 weeks) to drinking-water contaminated with Pb and Cu salts results in disproportion of IL-4 and IFN-γ expression. This results in deviation of cellular and humoral immune responses.
However, the mechanisms and specific immune system components affected by the heavy metals were not clearly defined. Thus further studies are needed in order to assess the protein levels of IL-4 and IFN-γ in relation to effects of long term exposure to Cu and Pb ions. Measurements of other cytokines can help to better understanding of raised questions in this study.
Acknowledgments
The authors do not have any conflict of interest and express their appreciation to Razi Vaccine and Serum Research Institute (RVSRI), Mashhad, Iran and Department of Biology, Faculty of Sciences, Mashhad Branch, Islamic Azad University, Mashhad, Iran for their financial and technical supports.
References
- Bowring J. Heavy metal toxicity and the unborn child. Midwifery Today Int Midwife. 2005;76:48–67. [PubMed] [Google Scholar]
- Chakrabarty S, Sarma HP. Heavy metal contamination of drinking water in Kamrup district, Assam, India. Environ Monit Assess. 2011;179:479–486. doi: 10.1007/s10661-010-1750-7. [DOI] [PubMed] [Google Scholar]
- Ercal N, Neal R, Treeratphan P, Lutz PM, Hammond TC, Dennery PA, Spitz DR. A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed Fisher 344 rats. Arch Environ Contam Toxicol. 2000;39:251–256. doi: 10.1007/s002440010102. [DOI] [PubMed] [Google Scholar]
- Faith RE, Luster MI, Kimmel KA. Effect of chronic developmental lead exposure on cell-mediated immune functions. Clin Exp Immunol. 1979;35:413–420. [PMC free article] [PubMed] [Google Scholar]
- Freundt KJ, Ibrahim HA. Growth of rats during a subchronic intake of the heavy metals Pb, Cd, Zn, Mn, Cu, Hg, and Be. Pol J Occup Med. 1990;3:227–232. [PubMed] [Google Scholar]
- Gillialand F, Mcconnell R, Peters J, Gong HJ. A theoretical basis for investigating ambient air pollution and children’s respiratory health. Environ Health Perspect. 1999;107:403–407. doi: 10.1289/ehp.99107s3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Kazbariene B, Kalibatas J, Krikstaponiene A, Zabulyte D, Monceviciute-Eringiene E. Alterations of human immune system functions in relation to environmental contamination, gender and alcohol consumption intensity. Cent Eur J Public Health. 2007;15:13–17. doi: 10.21101/cejph.a3404. [DOI] [PubMed] [Google Scholar]
- Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62:412–416. doi: 10.1093/ajcn/62.2.412. [DOI] [PubMed] [Google Scholar]
- Kvietkauskaite R, Dringeliene A, Markevicius A, Siaurys A, Acaite J. Effect of low copper exposure on the antioxidant system and some immune parameters. Vet Hum Toxicol. 2004;46:169–172. [PubMed] [Google Scholar]
- Markevièius A, Dringeliene A. Comparison of lead and copper exposure effect on immune cells in mice. Acta Medica Lituanica. 2004;11:14–18. [Google Scholar]
- Marth E, Jelovcani S, Kleinhappl B, Gutschi A, Bart S. The effect of heavy metals on the immune sysytem at low concentrations. Int J Occup Med Environ Health. 2001;14:375–386. [PubMed] [Google Scholar]
- Matranga V, Pinsino A, Randazzo D, Giallongo A, Dubois P. Long-term environmental exposure to metals (Cu, Cd, Pb, Zn) activates the immune cell stress response in the common European sea star (Asterias rubens) Mar Environ Res. 2012;76:122–127. doi: 10.1016/j.marenvres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Matranga V, Pinsino A, Randazzo D, Giallongo A, Dubois P. Long-term environmental exposure to metals (Cu, Cd, Pb, Zn) activates the immune cell stress response in the common European sea star (Asterias rubens) Mar Environ Res. 2012;76:122–127. doi: 10.1016/j.marenvres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- Nassar N, Abeywardana P, Barker A, Bower C. Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med. 2010;67:585–589. doi: 10.1136/oem.2009.048272. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- Peinnequin A, Mouret C, Birot O, Alonso A, Mathieu J, Clarençon D, Agay D, Chancerelle Y, Multon E (2004) Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol 5:3 [DOI] [PMC free article] [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sen G. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Singh D, Nath K, Trivedi SP, Sharma YK. Impact of copper on haematological profile of freshwater fish, Channa punctatus. J Environ Biol. 2008;29:253–257. [PubMed] [Google Scholar]
- Skoczynska A, Poreba R, Sieradzki A, Andrzejak R, Sieradzka U. The impact of lead and cadmium on the immune system. Med Pr. 2002;53:259–264. [PubMed] [Google Scholar]
- Van Oosterhout A, Motta A. Th1/Th2 paradigm: not seeing the forest for the trees? Eur Respir J. 2005;25:591–593. doi: 10.1183/09031936.05.00014105. [DOI] [PubMed] [Google Scholar]
- Villanueva MBG, Koizumi S, Jonai H. Cytokine production by human peripheral blood mononuclear cells after exposure to heavy metals. J Health Sci. 2000;46:358–362. doi: 10.1248/jhs.46.358. [DOI] [Google Scholar]
- Vinodhini R, Narayanan M. The impact of toxic heavy metals on the hematological parameters in common carp (Cyprinus carpio L.) Iranian J Environ Health Sci Eng. 2009;6:23–28. [Google Scholar]
- World Health Organization (2011) Guidelines for drinking-water quality, 4th edn
- Yin JL, Shackel NA, Zekry A, Mcguinness PH, Richards C, Putten KV, Mccaughan GW, Eris JM, Bishop GA. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol. 2001;79:213–221. doi: 10.1046/j.1440-1711.2001.01002.x. [DOI] [PubMed] [Google Scholar]