Abstract
Cleavage of the mouse hepatitis coronavirus strain A59 spike protein was blocked in a concentration-dependent manner by a peptide furin inhibitor, indicating that furin or a furin-like enzyme is responsible for this process. While cell-cell fusion was clearly affected by preventing spike protein cleavage, virus-cell fusion was not, indicating that these events have different requirements.
The surface glycoproteins of many enveloped viruses are initially synthesized as inactive precursors, proteolytic cleavage of which is often required for maturation and full functional activity. In several virus families, this processing step is carried out by cellular proprotein convertases (21), most commonly furin, a component of the constitutive secretory pathway of many different types of cells (9, 33). Furin is a membrane-bound, calcium-dependent subtilisin-like protease whose primary site of action is the trans-Golgi network (TGN), although cycling of furin between TGN and plasma membrane through the exocytic and endocytic pathways has also been demonstrated (6, 28). The enzyme is also secreted from cells in an active soluble form, which is produced by self-cleavage in the TGN (43, 45).
The mouse hepatitis coronavirus (MHV) spike (S) protein is responsible for attachment to the viral receptor, for virus-cell fusion during viral entry, and for cell-cell fusion during infection. It is a class I fusion protein (5) that is cotranslationally glycosylated to a 150-kDa glycoprotein, which is processed to a 180-kDa form during transport from the endoplasmic reticulum through the Golgi complex. As a late event in maturation, the protein is cleaved into two 90-kDa subunits, S1 and S2 (10, 31, 34). The S proteins of murine coronaviruses are cleaved to different extents, depending on the strain and the cell line used (10). Cleavage of strain MHV-A59 S protein takes place between residues 717 and 718 at the sequence RRAHR↓SVS (26). This sequence resembles the furin consensus sequence motif, RXR/KR (1, 21, 27). We now demonstrate, for the first time, that furin or a furin-like enzyme is indeed the protease responsible for cleavage of the S protein. Moreover, we investigated the consequences of cleavage, or rather of cleavage inhibition, of the S protein on its fusion activity and on the infectivity and cell entry of the virus.
The importance of S protein cleavage for cell-cell fusion has been studied by several groups with inconsistent results. Using a vaccinia virus expression system (11) some investigators found the S proteins from MHV-A59 and MHV-JHM not to require cleavage for the induction of cell-cell fusion but syncytium formation to be delayed in the absence of cleavage (3, 38). Others observed that a mutant MHV-JHM S protein, which was not proteolytically cleaved, induced syncytium formation to the same extent as the wild-type S protein (35). In contrast, transient expression of the uncleaved MHV-2 S protein apparently did not result in cell-cell fusion while a cleavable form of this protein did (47). Strikingly, the S protein from a different MHV-2 strain that was cleaved was not able to induce syncytia (39). Other groups used virions or infected cells to study the role of cleavage for fusion. Sturman et al. (36), for instance, examined the effect of trypsin treatment of MHV-A59 virions on their ability to induce rapid syncytium formation (cell fusion from without), while Frana et al. (10) studied the effect of treating MHV-A59-infected cells with a protease inhibitor. Gombold et al. (16) investigated the fusion behavior of an MHV-A59 mutant impaired in S cleavage. The combined results directly correlated the extent of cleavage of the spike protein with its ability to induce fusion of cells.
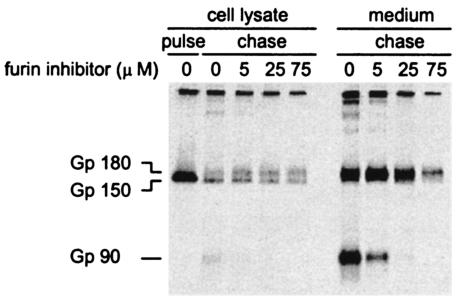
To demonstrate that furin is the host cell protease responsible for cleavage of the S protein, we made use of peptidyl chloromethylketone (dec-RVKR-cmk), which has been shown to inhibit furin cleavage activity in cultured cells (44). Parallel cultures of LR7 cells were infected with MHV-A59. At 1 h postinfection, the culture medium was replaced and the cells were further incubated for 5 h in the presence of different concentrations of the inhibitor, as indicated in Fig. 1. While maintaining the same inhibitor concentrations, the cells were subsequently pulse-labeled with 35S-amino acids and chased for 2 h. The S protein present in the cells and in virions released into the culture supernatant was assayed by immunoprecipitation with the S-specific monoclonal antibody WA3.10 (14). The precipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As shown in Fig. 1, no cleavage was observed in the cell-associated S protein after 15 min of pulse-labeling. After the 2-h chase in the absence of the peptide, a substantial fraction of the S protein was cleaved, although most of it remained uncleaved. However, the presence of the inhibitor resulted in a concentration-dependent decrease in the appearance of the S protein cleavage products, S1/S2. The simultaneous increase of the mature 180-kDa form of the S protein (Gp180) indicates that the furin inhibitor did not appear to affect the oligosaccharide maturation, and thus transport, of the S protein. At a 75 μM concentration, no trace of S1/S2 protein was detectable in the released virus, not even after a very long exposure of the gel (data not shown). The reduction in the incorporation of S protein into virions by the presence of the inhibitor did not appear to be general, as it was not seen in other experiments (data not shown). The results provide strong evidence that furin or a furin-like enzyme is responsible for the cleavage of the S protein in cultured cells.
FIG. 1.
Concentration-dependent inhibition of S protein cleavage by the furin inhibitor dec-RVKR-cmk. Parallel cultures of LR-7 cells (15) were inoculated with MHV-A59 at an MOI of 10 in 35-mm culture dishes. After 1 h, the inoculum was replaced by culture medium, which in some cases additionally contained the furin inhibitor peptidyl chloromethylketone (dec-RVKR-cmk; Calbiochem) at the indicated concentrations. In the latter cultures, the inhibitor was maintained at the same concentrations in the culture medium during all subsequent steps. At 4.5 h postinfection, the cells were starved for 30 min in cysteine- and methionine-free minimal essential medium containing 10 mM HEPES (pH 7.2). The medium was replaced with the same medium containing 100 μCi of 35S in vitro labeling mix (Amersham). After a 15-min pulse, the cells were chased for 2 h in fresh medium supplemented with 2 mM both cold methione and cysteine. The culture supernatants were precleared at 1,500 rpm at 4°C for 5 min, while the cells were washed once with ice-cold phosphate-buffered saline and solubilized in lysis buffer as described before (15). The culture supernatants and the cell lysates were subsequently prepared for and subjected to immunoprecipitation by using the MHV S-specific monoclonal antibody WA3.10, after which the immune complexes were analyzed by electrophoresis in an SDS-PAGE (10% polyacrylamide) gel followed by fluorography as described before (15). The position of the immature S protein precursor Gp150, its mature form, Gp180, and the S1 and S2 cleavage products thereof (Gp90) are indicated on the left side of the gel.
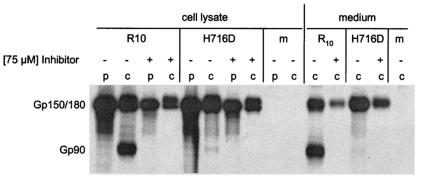
An important issue when studying the significance of S protein cleavage for cell-cell and virus-cell fusion is to exclude any residual cleavage of the S protein. Therefore, we decided to use a recombinant mutant, MHV-SA59H716D (kindly provided by Susan Weiss). In this virus, the basic histidine residue at position −2 relative to the S protein cleavage site has been replaced by aspartic acid. According to the known sequence specificity of furin (25), cleavage of this mutated S protein should be very inefficient, as was indeed demonstrated by Hingley et al. (19). However, some residual cleavage still occurred, and definite conclusions about the importance of cleavage could thus not be made. To achieve complete inhibition of cleavage, the recombinant mutant virus was tested in combination with the furin inhibitor (Fig. 2). Cleavage of the MHV-SA59H716D S protein was compared to that of a reconstructed wild-type virus (MHV-SA59R10, also obtained from Susan Weiss). Parallel cultures of LR-7 cells were infected with MHV-SA59H716D or with MHV-SA59R10, and after 1 h, the culture supernatant was replaced by medium containing 75 μM inhibitor, after which the inhibitor remained present during all subsequent steps. Control cultures without inhibitor were also included in the experiment. At 7 h postinfection, proteins were pulse-labeled for 15 min with 35S-amino acids and chased for 2 h. The S protein present in the cells and in the culture supernatants were analyzed by immunoprecipitation with the monoclonal antibody WA 3.1 followed by SDS-PAGE and autoradiography. After the pulse-labeling, only the 150-kDa form of the S protein was detectable in the cell lysate, the 180-kDa form emerging after the chase period. In the culture supernatant, only the mature spike protein was observed. In agreement with the results of Hingley et al. (19), cleavage of the S protein of MHV-SA59H716D virus was greatly reduced compared to the wild-type virus, indicating that the point mutation indeed affected the susceptibility of the cleavage site for the protease. Importantly, the residual cleavage could be completely blocked by adding the furin inhibitor.
FIG. 2.
Cleavage pattern of wild-type and mutant S proteins in the presence or absence of dec-RVKR-cmk. Parallel cultures of LR-7 cells were infected with MHV-SA59H716D (H716D) or MHV-SA59R10 (R10), treated with the furin inhibitor, labeled, chased, and processed for immunoprecipitation and electrophoresis as described in the legend to Fig. 1, except the cells were starved at 6.5 h postinfection. m, mock-infected cells; p and c, pulse and chase samples, respectively.
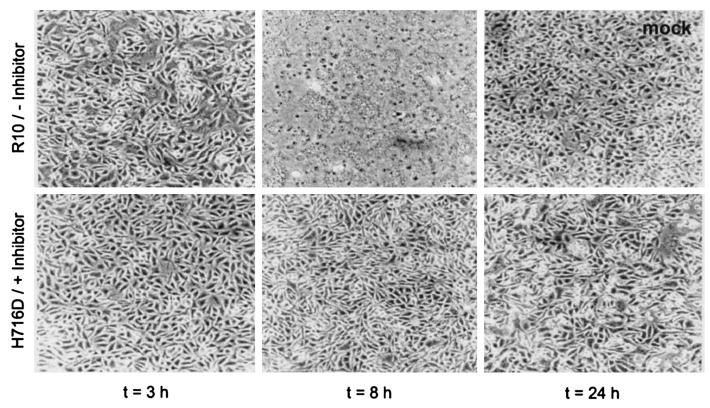
Next, we used the MHV-SA59H716D virus in combination with the furin inhibitor to study the effect of full cleavage inhibition of the S protein on cell-cell fusion. LR-7 cells were infected at a high multiplicity of infection (MOI) with MHV-SA59R10 and MHV-SA59H716D. In the case of MHV-SA59H716D, 75 μM dec-RVKR-cmk was present throughout the experiment. After 1 h, the inoculum was replaced by culture medium and the cells were further incubated for the indicated times. Cells infected with MHV-SA59R10 already started to show syncytium formation around 3 h postinfection: at 8 h postinfection, all cells had fused (Fig. 3). In contrast, for cells infected with MHV-SA59H716D in the presence of the inhibitor, the induction of syncytium formation was dramatically reduced and delayed: at 8 h postinfection, only a few fused cells could be detected and even 24 h after the inoculation most of the cells were still intact. Syncytium formation was not promoted by incubation at acidic pH, indicating that the lack of syncytium formation was not the result of a low pH requirement. MHV-OBLV60 (12) (kindly provided by Micheal Buchmeier) a virus dependent on acidic pH for fusion, was taken along as a positive control (data not shown). Since the inhibitor is not stable in aqueous solutions (half-life of 4 to 8 h) (13), a low level of cleavage having occurred at later times during infection cannot be fully excluded. Therefore, an absolute requirement of MHV-A59 S protein cleavage for cell-cell fusion cannot be concluded from this experiment.
FIG. 3.
Effect of S protein cleavage inhibition on cell-cell fusion. LR-7 cells were infected with MHV-SA59R10 (R10) or MHV-SA59H716D in phosphate-buffered saline-DEAE at an MOI of 10. In the case of MHV-SA59H716D-infected cells, the furin inhibitor was added to the culture medium to a concentration of 75 μM at 1 h postinfection and maintained at this concentration throughout the experiment. Photographs were taken at the indicated time points.
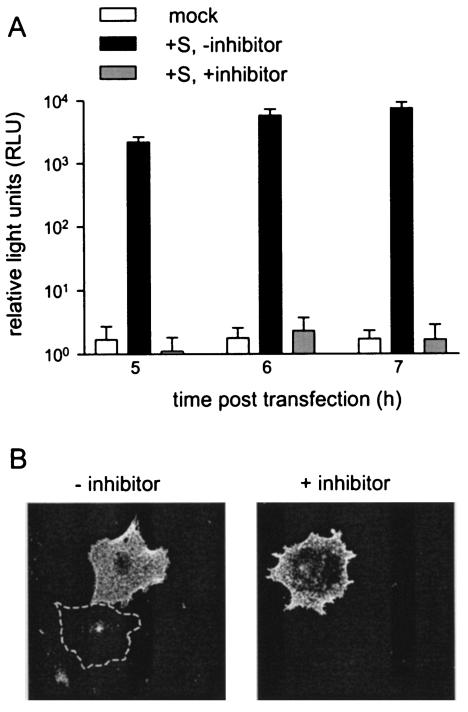
To study the cleavage dependence of cell-cell fusion in more detail, we made use of a very sensitive firefly luciferase fusion assay (5). The MHV-A59 S protein was expressed in BHK-21 effector cells by using the vaccinia virus T7 transient expression system (11). From 1 h posttransfection, the furin inhibitor was present in the culture media at a concentration of 75 μM. At 4, 5, or 6 h posttransfection, target cells (LR7 cells transfected with a plasmid containing the firefly luciferase gene under the control of a T7 promoter) were added. The incubation was continued for 1 h, after which the generated luciferase activity was determined. As shown in Fig. 4A, luciferase activity could easily be detected when no furin inhibitor had been added. In contrast, in the presence of the furin inhibitor, the amount of luciferase activity detected was at least 1,000-fold lower, comparable to that of mock-transfected cells, even when the luciferase activity was determined at 7 h posttransfection. The presence of the inhibitor did not appreciably affect the amount of S protein present at the cell surface as determined by immunofluorescence analysis using S protein-specific antibodies on nonpermeabilized cells (Fig. 4B). The results indicate that within the time span investigated, the uncleaved MHV-A59 S protein was unable to induce any cell-cell fusion.
FIG. 4.
Firefly luciferase fusion assay. (A) LR7 cells, used as target cells, were transfected with plasmid pTN3-luc+, which contains the firefly luciferase gene behind an internal ribosomal entry site under control of a T7 promoter (42). BHK-21 cells, designated as effector cells, were infected with vTF7.3 and transfected with plasmid pTUMS (42), which carries the MHV-A59 spike gene under the control of a T7 promoter. When indicated, 75 μM furin inhibitor was added to the culture medium at 1 h posttransfection and kept present throughout the experiment. At 4, 5, or 6 h posttransfection, target cells were added to the effector cells for 1 h. Subsequently, at 5, 6, or 7 h posttransfection, cells were harvested and luciferase activity was determined (Promega firefly luciferase assay system) according to the manufacturer's instructions with a Turner Designs luminometer (TD-20/20). Standard deviations are indicated. (B) In a parallel experiment, the effector cells were fixed at 7 h posttransfection with 3% paraformaldehyde. Indirect immunofluorescence using a polyclonal MHV-A59 antiserum (K134) (32) and a Cy5-conjugated donkey anti-rabbit serum was performed on nonpermeabilized cells. Confocal images of the cells were taken on a Leica inverted fluorescence microscope, using a ×100 oil immersion objective and identical settings for the two conditions. Cy5 was excited at 568 nm. The dashed line contours a nontransfected cell.
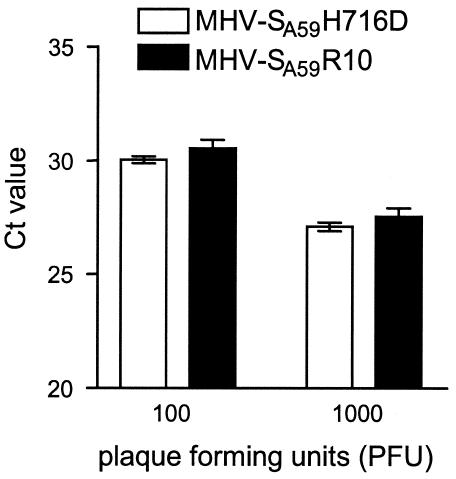
Mechanistically, the process of cell-cell fusion caused by the S protein is supposed to be the same as that of virus-cell fusion. However, only a few studies have actually dealt with the relationship between S protein cleavage and virus infectivity. MHV-2 was demonstrated to have an uncleaved spike protein, implying that cleavage is not obligatory for infectivity of this virus in DBT cells (47). Furthermore, treatment of MHV-A59 virions derived from 17Cl1 cells with trypsin increased the infectivity twofold (10), indicating a correlation between cleavage and infectivity. Using virus-like particles of strain A59, Bos and coworkers (4) demonstrated that cleavage is not required for infectivity. We now studied whether the specific infectivity of viruses with cleaved and uncleaved spikes differs. To this end, LR-7 cells were infected with the recombinant wild-type virus MHV-SA59R10 in the absence of the furin inhibitor and with the mutant virus MHV-SA59H716D in the presence of the inhibitor. After a 10-h incubation, the culture supernatants were harvested and titrated by plaque assay. Subsequently, the amounts of viral RNA present in 100 and 1,000 PFU of each virus were compared by TaqMan single-tube reverse transcription-PCR (RT-PCR) assay (PE Biosystems, Foster City, Calif.). The primers and the probe were selected by using the Primer Express software designed for this purpose (PE Biosystems), amplifying a conserved region in orf1B of MHV-A59 (positions 20248 to 20325). The reactions were performed in triplicate according to the manufacturer's instructions by using the TaqMan RT-PCR kit (PE Biosystems) and an ABI Prism 7700 sequence detector without modifying or moving the samples between the steps. The read-out parameter of this assay is the threshold cycle (CT) value, which in our case is a measure for the amount of genomic RNA. As depicted in Fig. 5, no difference was observed between the CT values of the two viruses. The results were reproducible in independent experiments. We conclude that the specific infectivity of the MHV-A59 is independent of the cleavage state of its S proteins.
FIG. 5.
Comparison of the specific infectivity of MHV with cleaved and uncleaved S proteins as measured by single-tube TaqMan RT-PCR. Virus with cleaved and completely uncleaved S protein was produced by infecting LR-7 cells with MHV-SA59R10 virus or with MHV-SA59H716D—the latter in the presence of the inhibitor at a 75 μM concentration. After a 10-h incubation at 37°C, the culture supernatants were harvested and titrated by a plaque assay on LR7 cells. One hundred and 1,000 PFU of each virus were then used to perform a single-tube TaqMan RT-PCR. The primers Pol1B/F (5′GCGTAAAGACGGTGACGATGT) and Pol1B/R (5′TTACCTTGTGGGCTCCGGTA) and probe Pol1B/P (5′6FAM-ATGGCTCGGTTCAAGGCTCCCTGTA-TAMRA) (6FAM, 6-carboxyfluorescin; TAMRA, N,N,N′,N′-tetramethyl-6-carboxyrhodamine) were selected by using the Primer Express software designed for this purpose (PE Biosystems, Foster City, Calif.). The reactions were performed in triplicate according to the manufacturer's instructions using the TaqMan RT-PCR kit (PE Biosystems). In short, 900 nM each primer and 250 nM probe were used for a 50-μl reaction. The reaction included a 30-min 48°C RT step, followed by 10 min at 95°C and then 45 cycles of amplification using the universal TaqMan standardized conditions: a 15-s 95°C denaturation step followed by a 1-min 60°C annealing/extension step. RT and amplification were carried out in an ABI Prism 7700 sequence detector without modifying or moving the samples between RT and PCR. The read-out parameter of this assay, the CT value, which in our case is a measure for the amount of genomic RNA, is shown for the two viruses. Standard deviations are indicated.
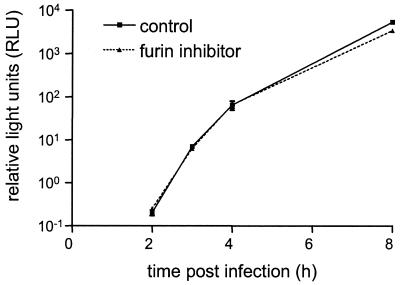
The contrasting impact of S protein cleavage on virus infectivity and cell-cell fusion activity raised the question about its effect on virus entry. If virus-cell fusion would be affected by cleavage similar to cell-cell fusion, the S protein cleavage state of virus would also influence the kinetics of virus entry. To study these kinetics, a recombinant MHV-A59 strain (MHV-ERLM) was used that expresses the Renilla luciferase gene (8). Recombinant virus stocks were grown in either the absence or presence of 100 μM furin inhibitor in order to obtain viruses with cleaved and uncleaved spike proteins, respectively. To prevent unintended cleavage of the latter virus in the course of inoculation and infection by furin activity present in and released by the cells to be infected, LR7 cells were pretreated for 1 h with 75 μM furin inhibitor and the inhibitor was kept present at this concentration throughout the experiment. Infections with the control virus were done in parallel in the absence of the inhibitor. Cells were lysed at different time points postinoculation, and their luciferase activity was determined as a measure of virus replication. Due to the sensitivity of the assay, luciferase activity could already be detected at 2 h postinfection (Fig. 6). No significant difference could be observed in the luciferase activities measured at any of the early time points between the infections by viruses with cleaved and uncleaved spikes. Only at 8 h postinfection, when syncytia could be observed in the absence but not in the presence of the inhibitor, was the luciferase activity significantly higher in the absence of the inhibitor. These results demonstrate that under the conditions of this assay, the kinetics of virus entry are indistinguishable. Obviously, this method would not detect a delay on the order of minutes but would certainly do so when the effect was more comparable to that on cell-cell fusion, which is clearly not the case.
FIG. 6.
Effect of S protein cleavage inhibition on the entry kinetics of the MHV-A59. Stocks were grown in LR7 cells of MHV-ERLM, a recombinant MHV-A59 expressing the Renilla luciferase gene (8), in the absence or presence of 100 μM furin inhibitor (control and furin inhibitor, respectively). These stocks, which were harvested at 8 h postinfection, were used to inoculate parallel cultures of LR-7 cells in Dulbecco's modified Eagle's medium at an MOI of 2. When indicated, cells had been pretreated with the inhibitor for 1 h at a concentration of 75 μM before the inoculation and the inhibitor was subsequently kept present throughout the experiment (furin inhibitor). At the indicated time points, cultures were harvested, cells were lysed, and Renilla luciferase activity was determined (Renilla luciferase assay system; Promega) according to the manufacturer's instructions. Standard deviations are indicated.
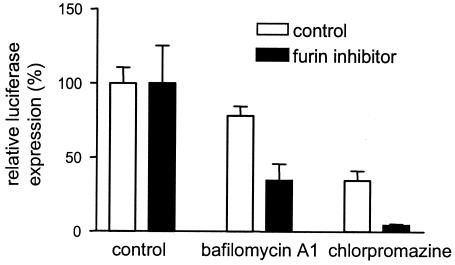
The entry pathway of MHV has not been well defined but appears to depend both on the strain of the virus and on the nature of the cell being infected. Most MHVs cause syncytium formation at neutral pH, and their entry is only mildly affected by lysosomotropic drugs (24, 29); in contrast, the MHV strain OBLV60 appeared to dependent on acidic pH for fusion and entry (12). Our present results show that cell-cell fusion was clearly affected by preventing spike protein cleavage while virus-cell fusion was not. This difference might be explained, at least in part, if virus-cell fusion of virions with uncleaved spike proteins would be enhanced after endocytosis, even though the lack of syncytium formation was not the result of a low pH requirement. This hypothesis was tested by repeating the experiment with MHV-ERLM (Fig. 7) in the absence and presence of bafilomycin A1 and chlorpromazine. Bafilomycin A1, which prevents endosomal acidification by blocking vacuolar proton ATPases, also blocks transport from early to late endosomes (see references 2 and 20 and references therein). Chlorpromazine inhibits clathrin-dependent endocytosis by removing the adapter protein AP-2 from the plasma membrane (37, 46). Cells were pretreated with bafilomycin A1 (50 nM) or chlorpromazine (10 μg/ml) and the furin inhibitor (75 μM) for 1 h, after which the drugs were kept present throughout the experiment. Although the inhibitors of endocytosis also affected the infection of the MHV virions with cleaved spike proteins, the entry of the MHV virions with uncleaved spike proteins was much more affected (Fig. 7). The results indicate a role for the endocytic compartment in the infectious process, which appears to be larger when the spike proteins are not cleaved.
FIG. 7.
Effect of inhibitors of endocytosis on the entry of MHV-A59. The experiment was performed as described in the legend to Fig. 5. When indicated, cells had been pretreated with the furin inhibitor and/or bafilomycin A1 and chlorpromazine for 1 h before the inoculation and the drugs were subsequently kept present throughout the experiment. At 4 h postinfection, the Renilla luciferase activity in the cultures was determined. Standard deviations are indicated.
We recently showed that the coronavirus S protein has many characteristics of a class I fusion protein (5). An important characteristic of all class I virus fusion proteins studied so far is the proteolytical cleavage of the precursor, during its transport through the secretory pathway, into a membrane-distal and a membrane-anchored subunit, an event essential for membrane fusion. As a consequence, the hydrophobic fusion peptide is located at or close to the newly generated amino terminus of the membrane-anchored subunit. However, for coronaviruses, the cleavage requirements remain enigmatic. Many coronaviruses, such as the feline infectious peritonitis virus (41), carry spikes with uncleaved S molecules, while in others, particularly in the group 2 and group 3 coronaviruses, the S proteins are cleaved, often to variable extents, depending, for instance, on the cells in which the viruses have been grown (10). In addition, the MHV S protein does not have a hydrophobic stretch of residues at the distal end of S2 but carries an internal fusion peptide, the location of which has yet to be determined, but which is predicted to occur immediately upstream of the first heptad repeat region (B. J. Bosch and P. J. M. Rottier, unpublished data). It thus appears that cleavage of the coronavirus S protein is not required to expose the internal fusion peptide.
Most research on coronavirus S protein cleavage has focused on its effect on cell-cell fusion, which often showed a strongly positive correlation (for a review, see the study by Cavanagh [7], consistent with our results). Cleavage is not essential for cell-cell fusion, however, nor does it necessarily give rise to it (see references above). Cleavage is also not required for full virus infectivity, as we showed here, or for triggering important conformational changes during interaction of the spike protein with the viral receptor (48). These observations raise the question as to the actual biological relevance of cell fusion. Significantly, studies with MHV-SA59H716D by Hingley et al. (19) indicated that the ability to produce syncytia in vitro is not a predictor of pathogenicity in mice. The cleavage-impaired mutant virus was only slightly attenuated relative to wild-type virus. Still, the conservation of furin cleavage motifs in so many coronaviruses obviously reflects evolutionary advantages, which have yet to be elucidated.
An interesting notion coming from our studies is that virus-cell and cell-cell fusion are not identical processes but have different requirements. Indications for this have been observed as well for other viruses. For example, lateral immobilization of the F or HN protein of Sendai virus was found to result in a strong inhibition of cell-cell fusion but a much weaker inhibition of virus-cell fusion (17). Human immunodeficiency virus type 1-induced syncytium formation and viral infectivity were differentially affected by antibodies (22), hydrophobic peptides (23), or antiviral drugs (30). Also for herpesviruses, different requirements were observed for virus-cell and cell-cell fusion (18). There are a number of possible reasons why in the case of MHV-A59 virus-cell and cell-cell fusion are distinct processes. One is the difference in fusion protein densities between the viral and cellular membranes. This density, which is obviously high in the virion membrane, might need to exceed a certain local level for fusion of membranes to occur. Another feature is the composition of the virion membrane as compared to the infected cell plasma membrane. On the one hand, the lipid composition of the viral envelope is known to reflect that of internal cellular membranes rather than that of the plasma membrane (40). On the other hand, the arrangement of the spikes embedded as they occur in the viral envelope within a dense matrix of M protein molecules is also clearly different from the constellation in the plasma membrane where the spikes find themselves only surrounded by numerous cellular membrane proteins. We can also not exclude a facilitating effect of the interior (e.g., the nucleocapsid) of the virion on the efficiency of spike-mediated fusion. Finally, the virus particle may be endocytosed upon binding to the viral receptor, in which case the conditions for fusion that the spikes encounter in the endosomal compartment will obviously be very different from those existing at cell-cell contact sites. Evidence for endocytosis was provided for some MHV strain JHM variants that apparently depend on low pH for productive cell entry (12, 29). S proteins of these viruses were unable to induce cell-cell fusion at neutral pH. In addition, we observed that inhibitors of endocytosis had a stronger inhibitory effect on MHV-A59 entry when cells were inoculated with viruses carrying uncleaved spikes as compared to viruses with cleaved spikes (Fig. 7).
Acknowledgments
We gratefully acknowledge Susan Weiss, Susan Hingley, and Micheal Buchmeier for providing us with the recombinant viruses MHV-SA59H716D and MHV-SA59R10, and MHV-OBLV60, respectively.
This work was carried out in part with financial support to P.J.M.R. and K.S. from the Commission of the European Community, TMR Network grant ERBFMRXCT98-0225.
REFERENCES
- 1.Barr, P. J. 1991. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell 66:1-3. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, E. C., L. Heijnen, W. Luytjes, and W. J. Spaan. 1995. Mutational analysis of the murine coronavirus spike protein: effect on cell-to-cell fusion. Virology 214:453-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, E. C. W., W. Luytjes, and W. J. Spaan. 1997. The function of the spike protein of mouse hepatitis virus strain A59 can be studied on virus-like particles: cleavage is not required for infectivity. J. Virol. 71:9427-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch, B. J., R. van der Zee, C. A. M. de Haan, and P. J. M. Rottier. 2003. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 77:8801-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosshart, H., J. Humphrey, E. Deignan, J. Davidson, J. Drazba, L. Yuan, V. Oorschot, P. J. Peters, and J. S. Bonifacino. 1994. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J. Cell Biol. 126:1157-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh, D. 1995. The coronavirus surface glycoprotein, p. 73-113. In S. Siddell (ed.), The coronaviridae. Plenum Press, New York, N.Y.
- 8.de Haan, C. A. M., L. van Genne, J. N. Stoop, H. Volders, and P. J. M. Rottier. 2003. Coronaviruses as vectors: position dependence of foreign gene expression. J. Virol. 77:11312-11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denault, J. B., and R. Leduc. 1996. Furin/PACE/SPC1: a convertase involved in exocytic and endocytic processing of precursor proteins. FEBS Lett. 379:113-116. [DOI] [PubMed] [Google Scholar]
- 10.Frana, M. F., J. N. Behnke, L. S. Sturman, and K. V. Holmes. 1985. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J. Virol. 56:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher, T. M., C. Escarmis, and M. J. Buchmeier. 1991. Alteration of the pH dependence of coronavirus-induced cell fusion: effect of mutations in the spike glycoprotein. J. Virol. 65:1916-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garten, W., S. Hallenberger, D. Ortmann, W. Schafer, M. Vey, H. Angliker, E. Shaw, and H. D. Klenk. 1994. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie 76:217-225. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore, W., J. O. Fleming, S. A. Stohlman, and L. P. Weiner. 1987. Characterization of the structural proteins of the murine coronavirus strain A59 using monoclonal antibodies. Proc. Soc. Exp. Biol. Med. 185:177-186. [DOI] [PubMed] [Google Scholar]
- 15.Godeke, G.-J., C. A. M. de Haan, J. W. A. Rossen, H. Vennema, and P. J. M. Rottier. 2000. Assembly of spikes into coronavirus particles is mediated by the carboxy-terminal domain of the spike protein. J. Virol. 74:1566-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gombold, J. L., S. T. Hingley, and S. R. Weiss. 1993. Fusion-defective mutants of mouse hepatitis virus A59 contain a mutation in the spike protein cleavage signal. J. Virol. 67:4504-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henis, Y. I., Y. Herman-Barhom, B. Aroeti, and O. Gutman. 1989. Lateral mobility of both envelope proteins (F and HN) of Sendai virus in the cell membrane is essential for cell-cell fusion. J. Biol. Chem. 264:17119-17125. [PubMed] [Google Scholar]
- 18.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 19.Hingley, S. T., I. Leparc-Goffart, S. H. Seo, J. C. Tsai, and S. R. Weiss. 2002. The virulence of mouse hepatitis virus strain A59 is not dependent on efficient spike protein cleavage and cell-to-cell fusion. J. Neurovirol. 8:400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katen, L. J., M. M. Januszeski, W. F. Anderson, K. J. Hasenkrug, and L. H. Evans. 2001. Infectious entry by amphotropic as well as ecotropic murine leukemia viruses occurs through an endocytic pathway. J. Virol. 75:5018-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klenk, H. D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2:39-43. [DOI] [PubMed] [Google Scholar]
- 22.Konopka, K., E. Pretzer, F. Celada, and N. Duzgunes. 1995. A monoclonal antibody to the gp120-CD4 complex has differential effect on HIV-induced syncytium formation and viral infectivity. J. Gen. Virol. 76:669-679. [DOI] [PubMed] [Google Scholar]
- 23.Konopka, K., E. Pretzer, and N. Duzgunes. 1995. Differential effects of a hydrophobic tripeptide on human immunodeficiency virus type 1 (HIV-1)-induced syncytium formation and viral infectivity. Biochem. Biophys. Res. Commun. 208:75-81. [DOI] [PubMed] [Google Scholar]
- 24.Kooi, C., M. Cervin, and R. Anderson. 1991. Differentiation of acid-pH-dependent and -nondependent entry pathways for mouse hepatitis virus. Virology 180:108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krysan, D. J., N. C. Rockwell, and R. S. Fuller. 1999. Quantitative characterization of furin specificity. Energetics of substrate discrimination using an internally consistent set of hexapeptidyl methylcoumarinamides. J. Biol. Chem. 274:23229-23234. [DOI] [PubMed] [Google Scholar]
- 26.Luytjes, W., L. S. Sturman, P. J. Bredenbeek, J. Charite, B. A. van der Zeijst, M. C. Horzinek, and W. J. Spaan. 1987. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology 161:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molloy, S. S., P. A. Bresnahan, S. H. Leppla, K. R. Klimpel, and G. Thomas. 1992. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 267:16396-16402. [PubMed] [Google Scholar]
- 28.Molloy, S. S., L. Thomas, J. K. VanSlyke, P. E. Stenberg, and G. Thomas. 1994. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 13:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash, T. C., and M. J. Buchmeier. 1997. Entry of mouse hepatitis virus into cells by endosomal and nonendosomal pathways. Virology 233:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Pleskoff, O., M. Seman, and M. Alizon. 1995. Amphotericin B derivative blocks human immunodeficiency virus type 1 entry after CD4 binding: effect on virus-cell fusion but not on cell-cell fusion. J. Virol. 69:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricard, C. S., and L. S. Sturman. 1985. Isolation of the subunits of the coronavirus envelope glycoprotein E2 by hydroxyapatite high-performance liquid chromatography. J. Chromatogr. 326:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rottier, P. J. M., M. C. Horzinek, and B. A. M. van der Zeijst. 1981. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J. Virol. 40:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeekens, S. P. 1993. Processing of protein precursors by a novel family of subtilisin-related mammalian endoproteases. Bio/Technology 11:182-186. [DOI] [PubMed] [Google Scholar]
- 34.Spaan, W., D. Cavanagh, and M. C. Horzinek. 1988. Coronaviruses: structure and genome expression. J. Gen. Virol. 69:2939-2952. [DOI] [PubMed] [Google Scholar]
- 35.Stauber, R., M. Pfleiderera, and S. Siddell. 1993. Proteolytic cleavage of the murine coronavirus surface glycoprotein is not required for fusion activity. J. Gen. Virol. 74:183-191. [DOI] [PubMed] [Google Scholar]
- 36.Sturman, L. S., C. S. Ricard, and K. V. Holmes. 1985. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: activation of cell-fusing activity of virions by trypsin and separation of two different 90K cleavage fragments. J. Virol. 56:904-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subtil, A., A. Hemar, and A. Dautry-Varsat. 1994. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J. Cell Sci. 107:3461-3468. [DOI] [PubMed] [Google Scholar]
- 38.Taguchi, F., T. Ikeda, K. Saeki, H. Kubo, and T. Kikuchi. 1993. Fusogenic properties of uncleaved spike protein of murine coronavirus JHMV. Adv. Exp. Med. Biol. 342:171-175. [DOI] [PubMed] [Google Scholar]
- 39.Tsai, C. W., S. C. Chang, and M. F. Chang. 1999. A 12-amino acid stretch in the hypervariable region of the spike protein S1 subunit is critical for cell fusion activity of mouse hepatitis virus. J. Biol. Chem. 274:26085-26090. [DOI] [PubMed] [Google Scholar]
- 40.van Genderen, I. L., G. J. Godeke, P. J. Rottier, and G. van Meer. 1995. The phospholipid composition of enveloped viruses depends on the intracellular membrane through which they bud. Biochem. Soc. Trans. 23:523-526. [DOI] [PubMed] [Google Scholar]
- 41.Vennema, H., L. Heijnen, A. Zijderveld, M. C. Horzinek, and W. J. M. Spaan. 1990. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J. Virol. 64:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vennema, H., R. Rijnbrand, L. Heijnen, M. C. Horzinek, and W. J. Spaan. 1991. Enhancement of the vaccinia virus/phage T7 RNA polymerase expression system using encephalomyocarditis virus 5′-untranslated region sequences. Gene 108:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vey, M., W. Schafer, S. Berghofer, H. D. Klenk, and W. Garten. 1994. Maturation of the trans-Golgi network protease furin: compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. J. Cell Biol. 127:1829-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vey, M., W. Schafer, B. Reis, R. Ohuchi, W. Britt, W. Garten, H. D. Klenk, and K. Radsak. 1995. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology 206:746-749. [DOI] [PubMed] [Google Scholar]
- 45.Vidricaire, G., J. B. Denault, and R. Leduc. 1993. Characterization of a secreted form of human furin endoprotease. Biochem. Biophys. Res. Commun. 195:1011-1018. [DOI] [PubMed] [Google Scholar]
- 46.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada, Y. K., K. Takimoto, M. Yabe, and F. Taguchi. 1997. Acquired fusion activity of a murine coronavirus MHV-2 variant with mutations in the proteolytic cleavage site and the signal sequence of the S protein. Virology 227:215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelus, B. D., J. H. Schickli, D. M. Blau, S. R. Weiss, and K. V. Holmes. 2003. Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37°C either by soluble murine CEACAM1 receptors or by pH 8. J. Virol. 77:830-840. [DOI] [PMC free article] [PubMed] [Google Scholar]