Abstract
Our aim was to design a simple compression system and investigate the influence of mechanical stress on skin-like structures. Many mechanical compression studies have employed intricate culture systems, so the relationship between extracellular matrix material and the response of skin cells to mechanical stress remains unknown. Our approach uses only glass vials, 6-well plates and standard laboratory equipment. We examined the influence of mechanical stress on human skin fibroblasts embedded within a collagen sponge. The results show that mechanical compression increases MMP-1 and MMP-2 release by the cells into the the cell culture. Our results suggest that pressure on the skin may affect extracellular matrix degradation through some as yet unidentified pathways and that IL-6 mRNA expression may be involved in this effect. Using our approach, the effects of static mechanical stress on protein expression by cells in the culture medium and in sponges can be easily examined, and therefore this system will be useful for further analyses of skin responses to mechanical stress.
Keywords: Skin model, Collagen sponge, Fibroblast, Mechanical compression
Introduction
Mechanical stress regulates cell behavior. In vivo, it has been proposed that mechanical stimulation of skin, for example by massage, affects arterial blood pressure (Kimura et al. 1995), the autonomic nervous system (Holey et al. 2011), and wound healing (Timmenga et al. 1991). However, mechanical stimulation of skin is rarely conducted in vitro. Little is known about the relationship between the mechanical stimulation of skin cells and the mechanical stress transmitted to cells via the extracellular matrix. Many mechanical stimulation studies have employed intricate culture systems, so the relationship between extracellular matrix material and the response of skin cells to mechanical stress remains unknown. In this study we designed a static compression system using readily available glass vials and 6-well plates in order to investigate the influence of mechanical stress on skin-like structures.
Materials and methods
Cell culture and mechanical compression
Normal human dermal fibroblasts (NB1RGB cells: Riken, Tsukuba, Japan, population doublings: 17.6, obtained on 2010/10/05) were seeded at a density of 5.0 × 105/sponge in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO, USA) with 10 % fetal bovine serum (Sigma-Aldrich, Lot No. 106K0366) onto a Type 1 collagen sponge (CSM-50: Koken, Tokyo, Japan). The cell-containing collagen sponges were set into 96-well plates one sponge per well and the plates were centrifuged for 5 min at 2,000 rpm. Next, the collagen sponges were incubated with 100 μl Dulbecco’s Modified Eagle’s Medium with 10 % fetal bovine serum for 72 h. The collagen sponges were then subjected to mechanical compression using vials as follows. The collagen sponges were placed individually into the wells of 6-well plates (Multidish 6-well Nunclondelta SI: Thermo Fisher Scientific, Waltham, MA, USA). These sponges were then subjected to mechanical compression using 50 ml vial weights (9-852-09: AS ONE, Osaka, Japan). Compression strength was controlled by adding water to the glass vials. Static compression was applied to the collagen sponges at 0 kPa (non-compression), 20 kPa (0 ml water in the vial), or 40 kPa (32 ml water in the vial) for 1 h. The tops of the vial weights were held in place by the top of the 6-well plate. After stimulation, the sponges were incubated with 100 μl medium with 10 % fetal bovine serum for 24h. After incubation, in order to accurately sample the entire sponge culture, the sponge culture was homogenized using a tissue homogenizer and mixed with culture medium. All experiments were conducted at 37 °C in a 5 % CO2 incubator. The nuclei of the dermal fibroblasts in the collagen sponges were labeled with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI). After compression, the sponges were rinsed with phosphate-buffered saline (PBS) and fixed in 1 % formaldehyde in PBS. The sponges were then washed twice for 15 min in PBS. The sponges were incubated with 2.86 μM DAPI for 10 min. Labeled sponges were visualized using a fluorescence microscope (Biozero: Keyence, Osaka, Japan). All labeling steps were conducted at room temperature.
Quantitative RT-PCR
After stimulation, the total RNA was extracted from the embedded cells as follows. Individual fibroblast-embedded collagen sponges were digested with TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) for 40 min. The total RNA was isolated from the digest using the phenol/chloroform method. Gene expression was quantified using real-time RT-PCR. The sequences of the respective forward and reverse primers used were: glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-GCACCGTCAAGGCTGAGAAC-3′, and 5′-TGGTGAAGACGCCAGTGGA-3; interleukin-6 (IL-6): 5′-AATTCGGTACATCCTCGACGG-3′, and 5′-TTGGAAGGT TCAGGTTGTTTTCT-3′; matrix metalloproteinase-1 (MMP-1): 5′-ATTCTACTGATATCGGGGCTTTGA-3′, and 5′-ATGTCCTTGGGGTATCCGTGTAG-3′; matrix metalloproteinase-2 (MMP-2): 5′-TACAGGATCATTGGCTACACACC-3′, and 5′-GGTCACATCGCTCCAGACT-3′; tissue inhibitor of metalloproteinase-1 (TIMP-1): 5′-AGAGTGTCTGCGGATACTTCC-3′, and 5′-CCAACAGTGTAGGTCTTGGTG-3′. All RNA levels were normalized to those of GAPDH.
Western blot and gelatin zymography
The concentration of protein in the sponges and culture medium was assayed by Western blotting and gelatin zymography. The sponges and medium were homogenized using a tissue homogenizer (Biomasher®: Nippi, Tokyo, Japan) and mixed with E–Z sample buffer (Atto, Tokyo, Japan). The mixtures were then loaded onto 5–20 % SDS-PAGE gels; after electrophoresis, the bands were transferred onto PVDF membranes (Atto). The membranes were treated with a blocking reagent (N101: NOF, Tokyo, Japan) to prevent nonspecific adsorption. Primary antibody reactions were performed with MMP-1 (1:500 dilution, Daiichi Fine Chemical, Toyama, Japan) and GAPDH (1:1,000 dilution, EnoGene Biotech, Nanjing, China). The bound antibodies were detected by goat anti-mouse horseradish peroxidase-conjugated IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) to MMP-1 and goat anti-rabbit horseradish peroxidase-conjugated IgG secondary antibody (Jackson ImmunoResearch Laboratories) to GAPDH. Signals were detected using ECL Western blotting detection substrate (GE Healthcare UK, Buckinghamshire, England) and visualized with ChemiDoc™ XRS + (Bio-Rad, Hercules, CA, USA). The levels of MMP-2 and MMP-9 in the mixtures were assayed using a gelatin zymography kit (AK-45: Primary Cell, Hokkaido, Japan) according to the manufacturer’s instructions. All samples were normalized by protein concentration.
All experiments were done independently at least twice; similar results were obtained each time. Data are reported as mean ± standard deviation followed by testing of statistical significance using Dunnett’s multiple-comparison tests.
Results and discussion
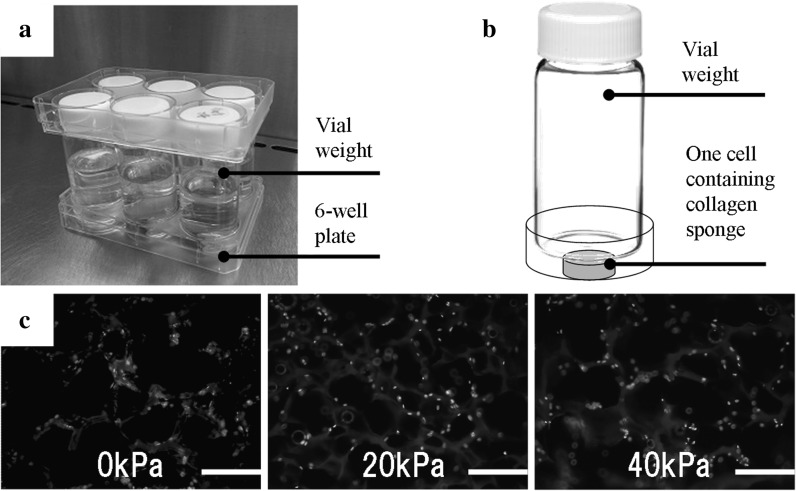
Human skin is mechanically stressed as a part of daily life; skin cells are subjected to compression, stretching, and shear forces. Kessler et al. (2001) reported that skin fibroblasts respond to mechanical stress and have mechanical sensors in their membranes. Several studies have also provided evidence for the mechanism by which skin is protected from breakdown following mechanical stress (Sanders et al. 1995). Ogawa (2008) hypothesized that fibro proliferative diseases such as keloid are mechanosensor or mechanosensitive disorders related to the mechanosensitive channels and cell adhesion molecules in fibroblasts.Ando and Yamamoto reported that shear stress and cyclic strain affect embryonic fibroblast differentiation (Ando and Yamamoto 2009). In this study we designated static compression with 20 kPa as relatively low stress, and 40 kPa as relatively high stress, using readily available glass vials and 6-well plates (Fig. 1a). We selected 40 kPa for artificial compression because other researchers had demonstrated that 40 kPa did not deform their models (Akamine et al. 2012). The diameter of the wells in the 6-well plates was 34.96 mm and the diameter of the vials was 34.90 mm, so the glass vials fitted snugly into the wells (Fig. 1b). Before and after compression, the collagen sponge diameters had not changed. However during compression, the collagen sponge diameters widened (data not shown). These borosilicate glass vials could be sterilized and reused easily. Compression strength was controlled by adding water to the vials. The collagen sponges (CSM-50; 4.4 mm diameter and 3.0 mm thick) were already sterilized and cultured with dermal fibroblasts. After compressing the sponges for 1 h, the nuclei of the dermal fibroblasts within the collagen sponges were labeled with DAPI and the middle sections of the sponges were viewed under a fluorescence microscope. Even though the cells had been exposed to mechanical compression, the labeled cells adhered to the collagen sponges (Fig. 1c). The amount of DNA extracted from cells treated with 40 kPa compression was lower than the amount extracted from non-compressed control cells (data not shown). This result shows that mechanical compression affect cell number.
Fig. 1.
Sponge compression system. a A glass vial in each well of a 6-well plate statically compresses each cell in a three-dimensional scaffold cultured in a CO2 incubator. b The weight of the vial compresses the collagen sponge. Compression of the three-dimensional cultured collagen sponge by the vial weight is controlled by adding water to the vial. c After compression, the nuclei of the dermal fibroblasts within the collagen sponges are labeled with DAPI and the middle sections are viewed under a fluorescence microscope. The labeled cells adhered to the collagen sponges under all conditions tested. Bar, 200 μm
Recently, cell stretch systems have been created to study the relationship between mechanical stress on cells and stretching (Russell et al. 2004; Shelton et al. 2003). Since these studies stretched cells growing in a monolayer, the extracellular matrix that surrounds skin cells was not taken into account. This is important as the responses of skin cells in three-dimensional cultures are different from those of skin cells in monolayer cultures (Pedersen and Swartz 2005). Compression systems for three-dimensional cultures have been created, for example by using an air compressor (FX-4000C: Flexcell International, McKee-sport, PA, USA) or piston weights (Cyclic Load Stimulator: Technoview, Osaka, Japan). These culture systems compress three-dimensional scaffolds statically or cyclically. Akamine et al. (2012) showed that compression of human temporomandibular joint synovium-derived cells appears to modulate inflammatory cytokines and matrix metalloproteinases. Many mechanical compression studies have employed intricate culture systems, so the relationship between extracellular matrix material and the response of skin cells to mechanical stress remains unknown. Our approach uses only glass vials, 6-well plates and standard laboratory equipment. Our results demonstrate that this is an effective set of tools to test the influence of mechanical stress on fibroblasts in three-dimensional scaffolds.
Following 1 h of compression and subsequent incubation for 24 h, the protein concentrations in the mixture of the cells and their culture medium were assayed. The level of metalloproteinase-1 (MMP-1) was assayed by western blotting. The level of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) were assayed by gelatin zymography. Matrix metalloproteinases are a major group of enzymes that regulate extraceller matrix composition. The concentrations of glycosylate-Pro-MMP-1 (57 kDa) and Pro-MMP-1 (52 kDa) in the 40 kPa compressed group were higher than in the non-compressed control, as measured by western blotting. The concentration of glyceraldehyde-3-phosphate dehydrogenase (GAPDH: 37 kDa) did not change in any of the groups. GAPDH was used to normalize for equal loading (Fig. 2a). The level of Pro-MMP-2 in the stimulated group was higher in the 40 kPa compressed group than in the non-compressed control group, as measured by gelatin zymography. The level of MMP-2 (62 kDa) in the 20 kPa compressed group was higher than in the non-compressed control group (Fig. 2b). No band corresponding to Pro-MMP-9 (92 kDa) or MMP-9 (83 kDa) was detected. The result with a proteasome inhibitor (1 or 5 μM MG132) showed no change in the protein level of MMP-1 (data not shown). These results indicate that mechanical stimulation of cells may stimulate MMPs synthesis, independent of mRNA expression. In order to accurately sample the entire sponge culture, the sponge culture was homogenized using a tissue homogenizer and mixed with sample buffer.
Fig. 2.
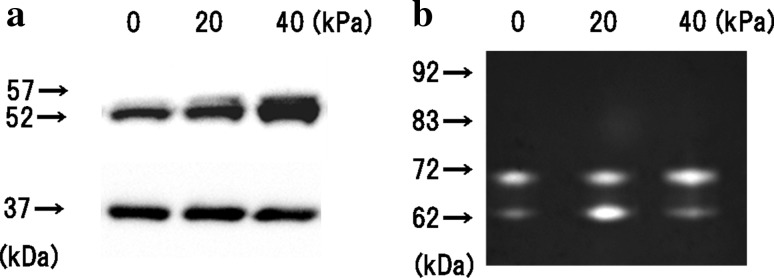
Protein concentrations in the mixture of the cells and their culture medium. The collagen sponges were subjected to mechanical compression using vial static compression applied at 0 (noncompression), 20, or 40 kPa for 1 h and subsequent incubation for 24 h. The concentration of protein in the sponges and culture medium was assayed. a The concentration of MMP-1 was assayed using Western blotting. The concentrations of glycosylate-Pro-MMP-1 (57 kDa) and Pro-MMP-1 (52 kDa) in the 40 kPa compressed group were higher than in the non-compressed control. The concentration of GAPDH (37 kDa) did not change in any of the groups. b The levels of MMP-2 and MMP-9 were assayed using gelatin zymography. No band corresponding to Pro-MMP-9 (92 kDa) or MMP-9 (83 kDa) was detected. The level of Pro-MMP-2 (72 kDa) was higher in the 40 kPa compressed group than in the non-compressed control group. The level of MMP-2 (62 kDa) in the 20 kPa compressed group was higher than in the non-compressed control group. All samples were normalized by protein concentration
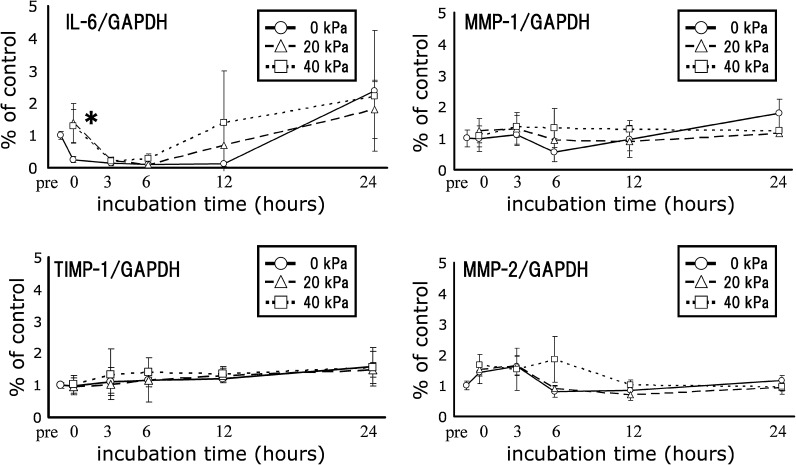
MMP-1, MMP-2, and MMP-9 are related to extracellular matrix degradation and are common MMPs. Also fibroblasts express MMP-1 when they were subjected to stretching (Kessler et al. 2001; Muroi et al. 2007). Gene expression of IL-6, MMP-1, TIMP-1 and MMP-2 in the sponge-cultured cells were quantified using real-time RT-PCR (Fig. 3). Following 1 h of compression, there was a significant difference in IL-6 gene expression in the 20 kPa compressed sample compared to the non-compressed control group immediately following compression. IL-6 is known to enhance MMP-2 expression (Luckett and Gallucci 2007). Further studies are needed to elucidate the transcriptional regulation of IL-6 and MMPs.
Fig. 3.
Temporal changes in mRNA expression. The collagen sponges were subjected to mechanical compression using vial static compression applied at 0 (non-compression), 20, or 40 kPa for 1 h. After stimulation, the total RNA was extracted from the embedded cells and gene expression was quantified using real-time RT-PCR. Real-time RT-PCR was performed prior to compression (pre), right after compression (t = 0), and 3, 6, 12 and 24 h after compression of the sponges. Data are expressed as mean ± standard deviation (N = 3). IL-6 gene expression in the 20 kPa compression group was significantly different from the non-compressed control group right after compression (t = 0). Statistical differences were determined using the Dunnett’s multiple-comparison tests. (*P < 0.05)
We investigated the influence of mechanical stress on human skin fibroblasts embedded within a collagen sponge using a simple static compression system. The results show that mechanical compression increases MMP-1 and MMP-2 secretion in the mixture of the cells and their culture medium. IL-6 mRNA expression in the compressed group was higher than in the non-compressed control group. This study therefore demonstrates that mechanical stress of human fibroblasts embedded in a collagen sponge affects the concentration of some MMP proteins. Our results suggest that pressure on the skin may affect extracellular matrix degradation through some as yet unidentified pathway and that IL-6 mRNA expression may be involved in this effect. This is an innovative idea that will facilitate experiments in mechanical stresses on skin.
In summary, these data show that, under the described experimental conditions, the effects of static mechanical stress on protein expression by cells in the culture medium and in sponges can be easily examined, and therefore this system will be useful for further analyses of skin responses to mechanical stress.
References
- Akamine Y, Kakudo K, Kondo M, Ota K, Muroi Y, Yoshikawa H, Nakata K. Prolonged matrix metalloproteinase-3 high expression after cyclic compressive load on human synovial cells in three-dimensional cultured tissue. Int J Oral Maxillofac Surg. 2012;41:874–881. doi: 10.1016/j.ijom.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983–1992. doi: 10.1253/circj.CJ-09-0583. [DOI] [PubMed] [Google Scholar]
- Holey LA, Dixon J, Selfe J. An exploratory thermographic investigation of the effects of connective tissue massage on autonomic function. J Manip Physiol Ther. 2011;34:457–462. doi: 10.1016/j.jmpt.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ohsawa H, Sato A, Sato Y. Somatocardiovascular reflexes in anesthetized rats with the central nervous system intact or acutely spinalized at the cervical level. Neurosci Res. 1995;22:297–305. doi: 10.1016/0168-0102(95)00907-B. [DOI] [PubMed] [Google Scholar]
- Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol. 2007;156:1163–1171. doi: 10.1111/j.1365-2133.2007.07867.x. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Kakudo K, Nakata K (2007) Effects of compressive loading on human synovium-derived cells. J Dent Res 86:786-791 [DOI] [PubMed]
- Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493–500. doi: 10.1016/j.mehy.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- Russell D, Andrews PD, James J, Lane EB. Mechanical stress induces profound remodelling of keratin filaments and cell junctions in epidermolysis bullosa simplex keratinocytes. J Cell Sci. 2004;117:5233–5243. doi: 10.1242/jcs.01407. [DOI] [PubMed] [Google Scholar]
- Sanders JE, Goldstein BS, Leotta DF. Skin response to mechanical stress: adaptation rather than breakdown. J Rehabil Res Dev. 1995;32:214–226. [PubMed] [Google Scholar]
- Shelton JC, Bader DL, Lee DA. Mechanical conditioning influences the metabolic response of cell-seeded constructs. Cells Tissues Organs. 2003;175:140–150. doi: 10.1159/000074630. [DOI] [PubMed] [Google Scholar]
- Timmenga EJ, Andreassen TT, Houthoff HJ, Klopper PJ. The effect of mechanical stress on healing skin wounds: an experimental study in rabbits using tissue expansion. Br J Plast Surg. 1991;44:514–519. doi: 10.1016/0007-1226(91)90008-8. [DOI] [PubMed] [Google Scholar]