Abstract
Human induced pluripotent stem (iPS) cells have great value for regenerative medicine, but are facing problems of low efficiency. MicroRNAs are a recently discovered class of 19–25 nt small RNAs that negatively target mRNAs. miR302/367 cluster has been demonstrated to reprogram mouse and human somatic cells to iPS cells without exogenous transcription factors, however, the repetition and differentiation potentiality of miR302/367-induced pluripotent stem (mirPS) cells need to be improved. Here, we showed overexpression of miR302/367 cluster reprogrammed human embryonic kidney 293T cells into mirPS cells in serum-free N2B27-based medium. The mirPS cells had similar morphology with embryonic stem cells, and expressed pluripotent markers including Oct4, Sox2, Klf4, and Nanog. In addition, through formation of embryoid bodies, various cells and tissues from three germ layers could be determined. Moreover, we examined the potential of mirPS cells differentiating into germ cells both in vitro and in vivo. Taken together, these data might provide a new source of cells and technique for the investigation of the mechanisms underlying reprogramming and pluripotency.
Keywords: MicroRNA (miRNA), miR302/367, Induced pluripotent stem cells (iPSCs), Reprogramming, N2B27
Introduction
Previous studies have shown that human somatic cells could be reprogrammed to achieve remarkable characteristics of embryonic stem (ES) cells via the introduction of two small sets of transcription factors, including OCT3/4, Sox2, Klf4 and c-MYC or OCT3/4, Sox2, LIN28 and Nanog (Takahashi and Yamanaka 2006; Takahashi et al. 2007; Yu et al. 2007). The ability to restore pluripotency from somatic cells has created powerful new opportunities for modeling human diseases and offered hopes for personalized regenerative cellular therapies (Robinton and Daley 2012). In spite of improvements of gene-delivery methods/techniques and addition of small molecules (Feng et al. 2009; Jia et al. 2010; Seki et al. 2010), the efficiency of reprogramming human somatic cells to induced pluripotent stem (iPS) cells is still low and hamper the use of iPSC technology.
MicroRNAs (miRNAs) are a recently discovered class of 19–25 nt small RNAs that negatively target mRNAs (Hwang and Mendell 2007). They act in multiple processes of cells, including cell proliferation, death, migration, senescence and development (Hwang and Mendell 2007; Nimmo and Slack 2009; Ambros 2011; Trompeter et al. 2011; Kane et al. 2012). Recent reports have demonstrated that miR302/367, a unique cluster of miRNAs highly expressed in embryonic stem cells (Suh et al. 2004), could directly reprogram mouse and human somatic cells into a pluripotent stem cell state in the absence of any transcription factors (Anokye-Danso et al. 2011; Koide et al. 2012). Most importantly, this miRNA-based reprogramming approach is two magnitudes more efficient than standard methods (10 vs. 0.2–1.0 %) (Anokye-Danso et al. 2011). What’s more, it’s the first time that miR302/367 could reprogram mouse and human somatic cells so efficiently and thoroughly (Lin et al. 2008). However, the mechanism of miR302/367-induced reprogramming remains largely unknown and the availability should be verified in various types of cells.
N2B27 supplements were reported to be the best chemically-defined substitution for knockout serum replacement (KSR) to maintain human ESCs (Liu et al. 2006). Lately, taking advantage of serum-free N2B27 medium, Koide et al. (2012) generated miR302/367-induced pluripotent stem (mirPS) cells from human embryonic kidney (HEK) 293 cells via electroporation of miR302/367 expression vector. However, the characterization of pluripotency and self-renewal ability was not detailed enough in the mirPS cells because there lack evidences to support the differentiality potentiality in vivo (Koide et al. 2012). Generally, differentiation into three germ layer lineages, even germ cells in vivo and in vitro is an important assay to evaluate the potentiality of ESCs or iPSCs (Eguizabal et al. 2011; Nayernia et al. 2006; Niu et al. 2013).
Thus, we used our constructed lentivirus of miR302/367 expression vector to generate mirPS cells from human embryonic kidney (HEK) 293T cells, and further investigated the characterization and differentiation potential into germ cells in vitro and in vivo. The results showed that the mirPS cells were efficiently produced by lentivirus transduction of miR302/367 expression vector, and these cells highly shared characteristics of ES cells, including their morphology, markers and potentiality of differentiation. This study might provide an efficient method to generate human pluripotent stem cells and germ cells derived from human HEK293T cell lines.
Materials and methods
ICR strain mice used in the study were maintained under standard conditions with free access to food and water at the Animal Facilities in our lab. All of the feeding and experimental procedures on animals were in accordance with the guidelines approved by the Northwest A&F University.
Cell culture
Human HEK293T cells were stored in Shaanxi Centre of Stem Cells Engineering and Technology, Northwest A&F University, which were cultured in Dulbecco’s modified Eagle’s medium (DMEM) high-glucose (Invitrogen, Carlsbad, CA, USA, 12800-017) medium containing 10 % fetal bovine serum (FBS, Hyclone, Logan, UT, USA, SH30071.03), 2 mM l-glutamine (Invitrogen, 21051024), 1 % nonessential amino acids (Invitrogen, 11130-051), 0.1 mM β-mercaptoethanol (Sigma, M7154), 100 U/ml/100 mg/ml penicillin/streptomycin at 37 °C under 5 % CO2.
Lentiviral vector construction and viral production
A mouse genomic DNA fragment comprising miR302/367 cluster of miRNA was amplified by PCR using primers listed in Table 1. The amplified fragment was cloned into multiple clone site of pCDH-Promoter-MCS-EF1 Lentivector (CD513B-1, SBI, Mountain View, CA, USA) by emzyme restriction of EcoRI and BamHI, verified by sequencing and resulting in the generation of the vector pCDH-miR302/367. For lentivirus production, HEK293T cells were transfected with pCDH-miR302/367 along with pMD2.G (addgene, a gift from Dr. Du) and psPAX2 (addgene, a gift from Dr. Du) vectors. The virus-containing supernatant was collected at 48 h after transfection, filtered to remove cell debris, and used for infection.
Table 1.
The primer sequences for PCR and QRT-PCR
| Gene | Primer (5′ → 3′) |
|---|---|
| β-Actin | Forward: GCGGCATCCACGAAACTAC |
| Reverse: TGATCTCCTTCTGCATCCTGTC | |
| Oct4 | Forward: GTGTTCAGCCAAAAGACCATCT |
| Reverse: GGCCTGCATGAGGGTTTCT | |
| Sox2 | Forward: CCGAGTGGAAACTTTTGTCG |
| Reverse: GGCAGCGTGTACTTATCCTTCT | |
| c-Myc | Forward: GGACTTGTTGCGGAAACGAC |
| Reverse: ACTCAGCCAAGGTTGTGAGGT | |
| Klf4 | Forward: CCCACATGAAGCGACTTCCC |
| Reverse: CAGGTCCAGGAGATCGTTGAA | |
| Nanog | Forward: AAGGTCCCGGTCAAGAAACAG |
| Reverse: CTTCTGCGTCACACCATTGC | |
| Pax4 | Forward: GGCACTGGAGAAAGAGTTCC |
| Reverse: GGCACTGGAGAAAGAGTTCC | |
| β-III-tubulin | Forward: CTTTTGGCCAGATCTTTAGACC |
| Reverse: CTCGTTGTCAATGCAATAGGTC | |
| Vimentin | Forward: GTCCAAGTTTGCCGACCTCT |
| Reverse: AGCGCATCCACTTCACAGG | |
| NSE | Forward: CTGATGCTGGAGTTGGATGG |
| Reverse: CCATTGATCACGTTGAAGGC | |
| miR302/367 | Forward: GATCTCTAGATAATGTGGGTTTGCTCTTCTGTTT |
| Reverse: GATCGGATCCTAAGATGGGCGAGGAGGTTAT |
PCR polymerase chain reaction
Induction of mirPS cells
To test the role of miR302/367 in cell reprogramming, we chose HEK293T cells as target cells for human mirPS cell induction using our constructed lentivirus vector pCDH-miR302/367 expressing GFP, derived from pCDH-GFP (pCDH-GFP, SBI). HEK293T cells were plated at a density of 1 × 104 cells in a 60 mm dish. After 12 h, HEK293T cells were infected with virus-containing supernatant in the presence of 4 μg/ml polybrene and incubated overnight at 37 °C and 5 % CO2. After 24 h, the medium was discarded and replaced with fresh DMEM medium supplemented with puromycin (40 μg/ml, Sigma, P8833) for selection (3 days). For mirPS cell induction, we used serum-free N2B27-based medium (500 ml scale, DMEM/F12 (240 ml, Invitrogen, 12660-012) mixed with Neurobasal medium (240 ml, Invitrogen, 21103-049), adding N2 supplement (5 ml, Invitrogen, 17502-048), B27 supplement (10 ml, Invitrogen, 17504-044), 1,000 U/ml leukemia inhibitory factor (LIF, Millipore, Billerica, MA, USA, ESG1107), 2 mM l-glutamine (Invitrogen), 1 % nonessential amino acids (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), 5 mg/ml BSA (Sigma, A9647), 0.3 μM PD0325901 (Sigma, PZ0162) and 3 μM CHIR99021 (Stemgent, Cambridge, MA, USA, 04-0004-02) (Koide et al. 2012). The medium was changed every other day until the colonies became large enough to be picked up. The protocol is summarized in Fig. 1a. Vitamin C (Sigma, A4403) and A83-01 (Stemgent, 04-0014) and fibroblast growth factor (bFGF, Sigma, F0291) were used to optimize the culture of mirPS cells. The protocol is illustrated as Fig. 1a. The feeder-primary mouse embryonic fibroblast (MEF) layer were treated with Mitomycin-C (Sigma, 10 μg/ml for 3 h) and directly plated onto gelatin coated 6-well plate for further use.
Fig. 1.
Induction of miR302/367-induced human mirPS cells from HEK293T cells. a Time schedule of mirPS cell generation. b Lentivirus backbone used in the study. c Morphology of induced cells at D10. d Morphology of induced cells at D15. e Morphology of induced cells at D15 before being picked up. f Morphology of human mirPS cells at passage 10. c, d, f ×100; e ×40. The top panels are images of bright field (c–f). The bottom panels are images of immunofluorescence (c–f)
Alkaline phosphatase (AP) staining
Alkaline phosphatase (AP) activity was determined essentially as described by Piedrahita et al. (1998). Briefly, culture plates were rinsed three times in PBS and fixed in 4 % paraformaldehyde (PFA) for 10–15 min at room temperature. Fixed cells were washed three times with PBS and stained with naphthol AS-MX phosphate (200 μg/ml, Sigma, N4875) and Fast Red TR salt (1 mg/ml, Sigma, F8764) in 100 mM Tris buffer, pH 8.2–8.4, for 10–30 min at room temperature, and washed with PBS to terminate staining.
RT-PCR
Total RNA was extracted with Trizol reagent (TaKaRa, Dalian, China, 9109) from miR302/367-induced human mirPS cells at passage 2 and passage 11 or treated EBs. Single strand cDNAs were prepared from 2 μg RNA using a reverse transcription Kit (Fermentas–Fisher Scientific, Pittsburgh PA, USA, K1622) and specific gene expressions were analyzed. The RT-PCR primers used are listed in Table 1, which are markers of stem cells, three germ layers or germ cells (Nayernia et al. 2006; Takahashi et al. 2007; Yu et al. 2007; Nicholas et al. 2009; Eguizabal et al. 2011; Panula et al. 2011; Easley et al. 2012; Hayashi et al. 2012; Niu et al. 2013). PCR conditions were: initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s; the annealing temperature used was in accordance to the primer sequences for 30 s and 72 °C for 30 s with a final extension at 72 °C for 10 min. The PCR products were analysed using 2 % agarose (Invitrogen, 16500100) gel electrophoresis, stained with ethidium bromide (Invitrogen, 15585-011), and visualized under UV illumination.
QRT-PCR
The QRT-PCR reactions were set up in 25 μl reaction mixtures containing 12.5 μl 1× SYBR® PremixExTaq™ (BIOER, Hangzhou, China, BSB03l1), 0.5 μl sense primer, 0.5 μl antisense primer, 11 μl distilled water, and 0.5 μl template. The reaction conditions were as follows: 95 °C for 30 s, followed by 45 cycles of 95 °C for 5 s, and 58 °C for 30 s. All expression levels were normalized to β-actin in each well. Expression was quantified as the ratio of the mRNA levels obtained from untreated HEK293T cells.
Immunocytofluorescence analysis
Samples of cells were fixed in 4 % paraformldehyde (PFA), treated with 0.1 % Triton X-100 for 10 min at room temperature. After blocking with 10 % FBS for 30 min, the cells were incubated with primary antibodies against pluripotent markers: OCT4 (1:500, Chemicon, Temecula, CA, USA, MAB4401), Sox2 (1:400, Chemicon, MAB4343), Klf4 (1:400, Chemicon, AB4138), c-MYC (1:400, Chemicon, AB3252), Nanog (1:400, CST, 3580), differentiation markers: GFAP (1:100, Chemicon, AB5804), ISLET1 (1:1,000, DSHB, Iowa City, IA, USA, 39.3F7), GLUT-2 (1:200, Chemicon, AB1342), and germ cell markers: EMA1 (1:100, DSHB, primordial germ cell surface marker), VASA (1:200, Abcam, Cambridge, UK, ab13840, primordial germ cell marker), PLZF (1:200, Santa Cruz., CA, USA, H-300, a Marker of Spermatogonial Stem Cells), SCP3 (1:300, Abcam, ab15093, meiotic prophase marker) respectively for overnight at 4 °C. After washing three times in PBS, appropriate FITC or FITC- or TRITC-conjugated secondary antibodies (Chemicon, USA) were incubated for 1 h at room temperature in the dark. The nuclei of cells were stained by Hoechst 33342 or DAPI. Images were captured with a Leica fluorescent microscope.
In vitro differentiation of mirPS cells
To determine the differentiation ability of miR302/367-induced human mirPS cells in vitro, the 5th passage mirPS cells were harvested by trypsinization and transferred to bacterial culture dishes in the spontaneous differentiation medium. After 3 days, the aggregated cells (EBs) were plated onto gelatin-coated tissue culture dishes and incubated for another 3–7 days. (Takahashi et al. 2007). After 6 days of culture, the cells aggregated and formed EBs. The resulting EBs were transferred onto a 48-well culture plate (10–15 EBs per well) coated with 0.1 % gelatin. Attached EBs were supplemented with 0.1 μM retinoic acid (RA, Sigma, R2625) or bone morphogenetic protein 4 (BMP4, Peprotech, Rocky Hill, NJ, USA, 120-05) in spontaneous differentiation medium (DMEM supplemented with 15 % FBS, 2 mM L-glutamine, 1 % nonessential amino acids and 0.1 mM B-mercaptoethanol), which was changed every 2 days to avoid its degradation. Simultaneously, some EBs were induced by chondrocytes differentiation medium and analysed by Alcian Blue staining according to manufacturer’s instructure (Cyagen Biological Corporation, HUXMA-90041) (Qiu et al. 2012). Seven days later, the EBs and suspension cells were harvested and analyzed by Alcian Blue staining according to manufacturer’s instructions (Cyagen Biological Corporation, Guangzhou, China, HUXMA-90041).
Teratoma formation
The mirPS cells of passage 5 were cultured and dispersed into single cells by incubation in 0.05 % trypsin for 2–3 min at 37 °C. MirPS cells (2 × 106) were transplanted into the kidney capsules of ICR mice, which were given a single dose of busulfan (40 mg/kg body weight) by intraperitoneal injection to destroy endogenous spermatogenesis (Hua et al. 2011). After 9 weeks of growth, tumor tissue was removed, fixed in 4 % PFA, and processed for paraffin section (Niu et al. 2013). Additionally, human mirPS cells were transplanted into dorsal flanks of kidney in busulfan-treated mice.
Results
miR302/367 induced the generation of human mirPS cells from HEK293T cells
HEK293T cells were transduced with lentivirus pCDH-miR302/367 or pCDH-GFP, and cultured following the protocol (Fig. 1a, b). Approximately 1 week later, a number of HEK293T cells gradually gathered together or proliferated to form granulated colonies (Fig. 1c). At day 15, we observed distinct types of colonies (Fig. 1d), and we picked human ES-like colonies (Fig. 1e). miR302/367-induced human mirPS cells were passaged according to previous reports (Takahashi et al. 2007), maintaining an ESC-like morphology but a weaker GFP fluorescence at passage 10 (Fig. 1f). In order to rule out the possibility of N2B27-based medium gathering HEK293T cells to form ES-like colonies, we transduced HEK293T cells with pCDH-GFP lentivirus and cultured them in the same conditions. HEK293T cells expressing GFP could proliferate on MEFs in N2B27-based medium, but they failed to form compact ES-like colonies (data not shown).
miR302/367-induced human mirPS cells express human ES cell markers
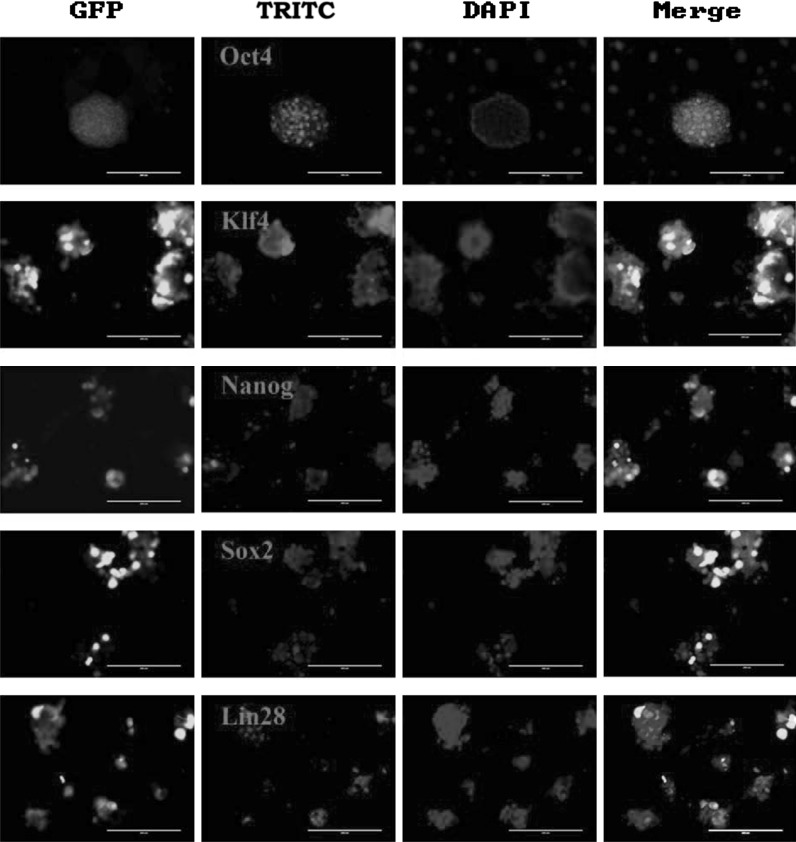
Real-time PCR analysis showed that induction of miR302/367 incresed the expression of Sox2, Klf4, c-Myc and Nanog genes (Fig. 2a). RT-PCR analysis confirmed the results of human mirPS cells expressing several undifferentiated ES cell markers, such as Oct4, Nanog and Klf4 (Fig. 2b). Immunofluorescence analysis also showed miR302/367-induced human mirPS cells were positive for OCT4, Nanog, Sox2, Klf4 and LIN28 (Fig. 3).
Fig. 2.
MiR302/367-induced human mirPS cells expressed pluripotent genes and vitamin C and bFGF improved the morphology and AP activity of mirPS cells. a QRT-PCR analysis of pluripotent genes which were normalized to β-actin. b RT-PCR analysis of pluripotent genes. c The effects of vitamin C, A83-01, and bFGF on the morphology and AP activity of mirPS cells. The top panels are images of bright field c. The bottom panels are images of AP staining (c–f)
Fig. 3.
Immunofluorescent analysis of mirPS cells for expression of OCT4 (red), Klf4 (red), NANOG (red), Sox2 (red), and LIN28 (red). Nuclei were stained with Hoechst 33342 (blue). Bar 200 μm
Additionally, we found two small molecules (vitamin C and A83-01) and bFGF could better shape the morphology of miR302/367-induced human mirPS cells and increased AP-positive colonies (Fig. 2c). Therefore, we added vitamin C and bFGF in the N2B27-based mirPS cell culture medium.
In vitro differentiation of miR-302/367-induced human mirPS cells
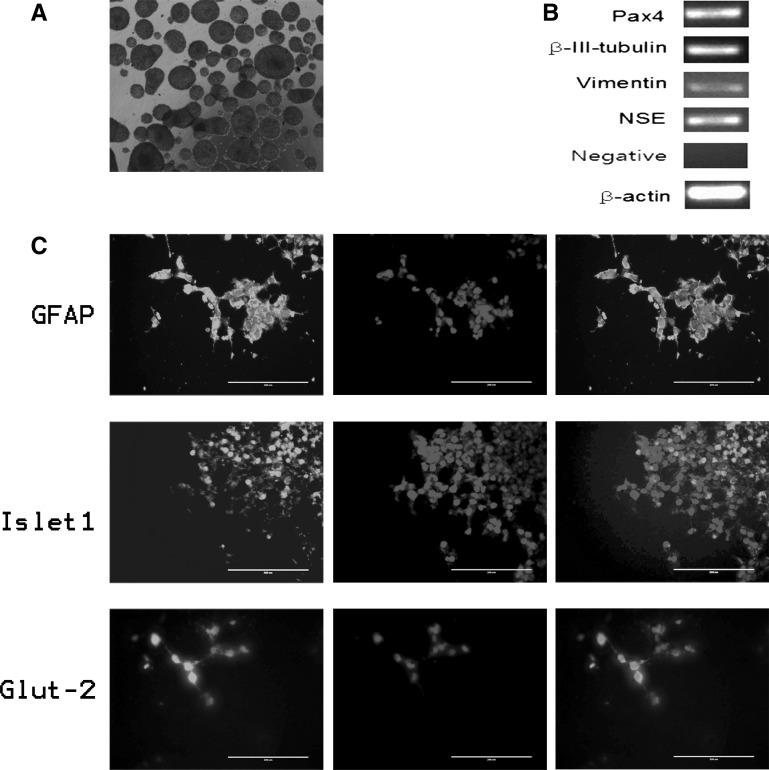
After the cells cultured in suspension for 6 days, miR302/367-induced human mirPS cells formed ball-shaped structures (EBs) (Fig. 4a). By contrast, most of untreated HEK293T cells tiled on the non-adhesive surfaces of Petri dishes, with only a few uncompacted aggregation (data not shown). When the mirPS cell-induced EBs were formed and transferred onto Matrigel-coated plates, these embryoid body-like structures attached to the bottom and initiated spontaneous differentiation. After 7 days culture, the attached differentiated cells were positive for glial fibrillary acidic protein (GFAP, ectoderm), Islet1 (ISL1, mesoderm), and Glucose transporter 2 (GLUT2, endoderm) analyzed by immunofluorescence staining (Fig. 4c). RT-PCR analysis confirmed that these differentiated cells expressed βIII-tubulin (ectoderm), NSE (ectoderm), Vimentin (mesoderm), and PAX4 (endoderm) (Fig. 4b). In addition, we added TGFβII to promote chondrocytes differentiation. After 7 days, the induced cells were positive for Alcian Blue (Fig. 5a).
Fig. 4.
Embryoid body-mediated differentiation of mirPS cells into three germ layer cells. a Floating culture of mirPS EBs at D3. b RT-PCR analysis of various differentiation markers for the three germ layers. c Immunofluorescent analysis of GFAP (ectoderm, red), Islet1 (mesoderm, red) and Glut-2 (entoderm, red) after 7 days of differentiation. Nuclei were stained with Hoechst 33342 (blue). The first column of panels: TRITC-fluorescence, the second column of panels: Hoechst 33342 staining, the third column of panels: Merger of the first and the second columns. Bar 200 μm
Fig. 5.
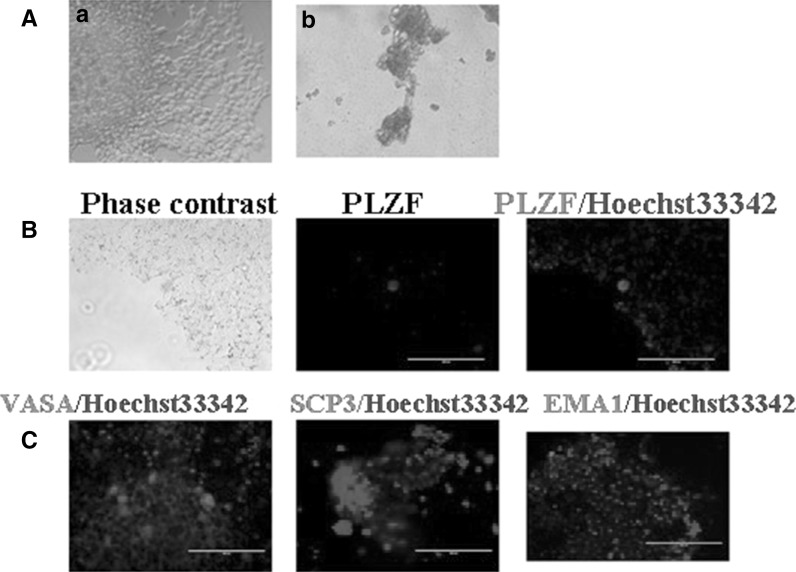
Embryoid body-mediated differentiation of mirPS cells into chondrocytes and germ cells. a After 7 days, the induced cells and some were positive for Alcian Blue (the left column: Phase contrast, the right column: Alcian blue staining). b The differentiated cells resembled germ cells, and expressing PLZF (red). c Immunofluorescent analysis showed differentiated cells expressed germ cell markers EMA1(red), VASA (red), and the SCP3 (red). Nuclei were stained with Hoechst 33342 (blue). Bar 200 μm
Differentiation of miR302/367-induced human mirPS cells into germ-like cells in vitro and in vivo
We then induced the mirPS cells into germ cells by culturing adherent EBs in BMP4 and RA differentiation medium for 7 days. Some large round cells emerged after induction, which resembled germ cells and expressed germ cell marker-PLZF. Immunofluorescence analysis also showed the expression of other germ cell markers, such as EMA1, VASA, and SCP3 (Fig. 5c).
To test the pluripotency in vivo, we transplanted human mirPS cells subcutaneously into dorsal flanks of kidney in busulfan-treated mice. 9 weeks later, we observed the formation of tumor-like structures. Histological examination demonstrated that the tumor contained ectoderm and mesoderm tissues, however, no typical endoderm structures were formed. By immunofluorescence staining, we found that there were EMA1-, PLZF-, and VASA-positive cells in transplanted cells (Fig. 6), indicating a potential differentiation of miR302/367-induced human mirPS cells into germ-like cells in vivo.
Fig. 6.
Germ cell differentiation from mirPS cells in vivo. Immunofluorescent analysis of transplanted mirPS cells were positive for germ cell markers EMA1(red), PLZF (red), and VASA (red). Bar 200 μm
Discussion
Since Takahashi and Yamanaka (2006) discovered that somatic cells could be reprogrammed into iPSCs, the original combination of reprogramming factors (Oct4, Sox2, Klf4, and c-Myc) has been used to generate iPSCs from a wide-range of cell types. Improvements of gene-delivery methodology (Jia et al. 2010; Seki et al. 2010) and addition of small molecules (Feng et al. 2009; Cai et al. 2013) could increase the efficiency to some extent, yet still remaining at very low levels. In addition, the feasibility and safety of using iPS cells for clinical applications require further studies.
Whether transcription factors could be optimized or replaced during reprogramming need further investigation. Recently, evidences from different investigations have suggested an important role of miRNAs in maintaining ES cell biology and reprogramming somatic cells into iPSCs (Lin et al. 2008; Barroso-del Jesus et al. 2009; Liao et al. 2011; Lin et al. 2011; Fareh et al. 2012; Kuo et al. 2012). Judson et al. (2009) found that ESC miRNAs increased the generation efficiency of mouse iPS cells combined with Sox2, Oct4, and Klf4. Among different ESC miRNAs, miR-294 exhibited the greatest effect on reprogramming and increased the efficiency of iPS cell generation from 0.01–0.05 % to 0.4–0.7 % (Ren et al. 2009).
The miR302/367 family (mir-302s) consists of four highly homologous miRNA members mir-302b, mir-302c, mir-302a, mir-302d, and mir-367, which are expressed abundantly in human ES cells and decreased quickly after cell differentiation (Suh et al. 2004; Barroso-delJesus et al. 2008). The miR302/367 cluster, with predicted targets associated with chromatin modification (Chen et al. 2007), was shown to promote reprogramming (Liao et al. 2011; Subramanyam et al. 2011; Kuo et al. 2012; Zhang and Wu 2013). Furthermore, ectopic expression of mir-302 s without transcription factors was demonstrated to be capable of reprogramming differentiated cells into pluripotent state (Lin et al. 2008, 2011; Miyoshi et al. 2011; Lin and Ying 2013). Altering miR302/367 expression disrupts the balance of lung endoderm progenitor proliferation and differentiation, as well as the apical-basal polarity (Tian et al. 2011). It was recently reported that miR302/367-mediated reprogramming is two orders of magnitude more efficient than standard Oct4/Sox2/Klf4/Myc-mediated methods (Anokye-Danso et al. 2011). Since miRNAs generally target scores or hundreds of mRNAs, homologous sequence of mir-302 s can amplify the effects, thus increasing the efficiency.
Very recently, Koide et al. (2012) employed N2B27 medium in place of FBS, successfully generating miR302/367-induced pluripotent stem (mirPS) cells from human embryonic kidney (HEK) 293 cells. However, the detailed characterization including pluripotency and self-renewal ability was omitted (Koide et al. 2012). In our study, we obtained ES-like cells derived from HEK293T cells in serum-free N2B27 medium. Moreover, our results showed that these cells had the potential to differentiate into three germ layers in vitro and in vivo. Furthermore, we induced the cells differentiating into germ-like cells.
Germ cells are responsible for passing genetic information to the next generation. It is of vital importance to establish in vitro model of germ cell differentiation. Recent studies have shown that mouse ESCs and iPSCs could differentiate into primordial germ cells (PGCs), some of which subsequently even develop into gametes (Nayernia et al. 2006; Nicholas et al. 2009; Eguizabal et al. 2011; Panula et al. 2011; Easley et al. 2012; Hayashi et al. 2012; Niu et al. 2013). In our study, we used the previous methods to demonstrate that the mirPS cells derived from HEK293T cells could differentiate into germ-like cells, which were positive for germ cell markers including PLZF, EMA1, VASA, and SCP3 (Hua et al. 2009; Gassei and Orwig 2013). However, the function of the induced cells and the efficiency need to be verified.
Vitamin C was reported to enhance the efficiency of generating human iPS cells (Esteban et al. 2010). Basic fibroblast growth factor (bFGF) could support the maintenance of human ES and iPS cells (Mochiduki and Okita 2012). In our study, we found that addition of Vitamin C and bFGF promoted the induced mirPS cells maintain similar morphology as ES cells and higher percentage of AP-positive cells maintain more compact and AP-positive. HEK293T cells have been transducted with SV40 large T antigen (T), which has been reported to enhance the efficiency of generating iPS cells by 23–70 fold from both human adult and fetal fibroblasts (Mali et al. 2008). Whether miR302/367 and SV40 large T antigen have synergistic effects or they are independent is still worth of further investigation.
In conclusion, we found overexpression of miR302/367 cluster could reprogram human embryonic kidney (HEK) 293T cells into induced pluripotent stem (mirPS)-like cells in serum-free N2B27-based medium. Additionally, we first demonstrated the potential of mirPS cells differentiating into germ cells both in vitro and in vivo. These data suggest that differentiated cell lines could be induced into pluripotent-like cells in an appropriate niche and transcription state.
Acknowledgments
This work was supported by the grants from the Program (31272518) of National Natural Science Foundation of China, Doctoral Fund of Ministry of Education of China (RFDP, 20120204110030), the Fundamental Research Funds for the Central Universities (QN2011012).
Abbreviations
- miRNA
MicroRNA
- iPSCs
Induced pluripotent stem cells
- mirPS
miR302/367-induced pluripotent stem (cell)
- DMEM
Dulbecco’s modified Eagle’s medium
- AP
Alkaline phosphatase
- EBs
Embryoid bodies
- MEF
Mouse embryonic fibroblasts
- RA
Retinoic acid
- BMP4
Bone morphogenetic protein 4
- GFAP
Glial fibrillary acidic protein
- TGFβII
Transforming growth factor II
Footnotes
Long Wang and Haijing Zhu have contributed equally to this work.
References
- Ambros V. MicroRNAs and developmental timing. Curr Opin Genet Dev. 2011;21:511–517. doi: 10.1016/j.gde.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-del Jesus A, Lucena-Aguilar G, Menendez P. The miR-302-367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2009;8:394–398. doi: 10.4161/cc.8.3.7554. [DOI] [PubMed] [Google Scholar]
- Barroso-delJesus A, Romero-López C, Lucena-Aguilar G, Melen GJ, Sanchez L, Ligero G, Berzal-Herranz A, Menendez P. Embryonic stem cell-specific miR302-367 cluster: human gene structure and functional characterization of its core promoter. Mol Cell Biol. 2008;28:6609–6619. doi: 10.1128/MCB.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Wang YD, Zheng PS. The microRNA-302-367 cluster suppresses the proliferation of cervical carcinoma cells through the noveltarget AKT1. RNA. 2013;19:85–95. doi: 10.1261/rna.035295.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ridzon D, Lee CT, Blake J, Sun Y, Strauss WM. Defining embryonic stem cell identity using differentiation-related microRNAs and their potential targets. Mamm Genome. 2007;18:316–327. doi: 10.1007/s00335-007-9032-6. [DOI] [PubMed] [Google Scholar]
- Easley CA, 4th, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua Belmonte JC. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29:1186–1195. doi: 10.1002/stem.672. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;8:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fareh M, Turchi L, Virolle V, Debruyne D, Almairac F, de-la Forest Divonne S, Paquis P, Preynat-Seauve O, Krause KH, Chneiweiss H, Virolle T. The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19:232–244. doi: 10.1038/cdd.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Gassei K, Orwig KE. Sall4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS One. 2013;8:e53976. doi: 10.1371/journal.pone.0053976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- Hua J, Yu H, Dong W, Yang C, Gao Z, Lei A, Sun Y, Pan S, Wu Y, Dou Z. Characterization of mesenchymal stem cells (MSCs) from human fetal lung: potential differentiation of germ cells. Tissue Cell. 2009;41:448–455. doi: 10.1016/j.tice.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Hua J, Zhu H, Pan S, Liu C, Sun J, Ma X, Dong W, Liu W, Li W. Pluripotent male germline stem cells from goat fetal testis and their survival in mouse testis. Cell Reprogram. 2011;13:133–144. doi: 10.1089/cell.2010.0047. [DOI] [PubMed] [Google Scholar]
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2007;96(Suppl):R40–R44. [PubMed] [Google Scholar]
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human mirPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane NM, Howard L, Descamps B, Meloni M, McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, Mountford JC, Milligan G, Emanueli C, Baker AH. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells. 2012;30:643–654. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide N, Yasuda K, Kadomatsu K, Takei Y. Establishment and optimal culture conditions of microrna-induced pluripotent stem cells generated from HEK293 cells via transfection of microrna-302 s expression vector. Nagoya J Med Sci. 2012;74:157–165. [PMC free article] [PubMed] [Google Scholar]
- Kuo CH, Deng JH, Deng Q, Ying SY. A novel role of miR-302/367 in reprogramming. Biochem Biophys Res Commun. 2012;417:11–16. doi: 10.1016/j.bbrc.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, Xue Y, Cai J, Guo X, Qin B, Zhang R, Wu J, Lai L, Teng M, Niu L, Zhang B, Esteban MA, Pei D. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Ying SY. Mechanism and method for generating tumor-free iPS cells using intronic microRNA miR-302 induction. Methods Mol Biol. 2013;936:295–312. doi: 10.1007/978-1-62703-083-0_23. [DOI] [PubMed] [Google Scholar]
- Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu DT, Chen DT, Ying SY. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA. 2008;14:2115–2124. doi: 10.1261/rna.1162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Chang DC, Lin CH, Ying SY, Leu D, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Song Z, Zhao Y, Qin H, Cai J, Zhang H, Yu T, Jiang S, Wang G, Ding M, Deng H. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem Biophys Res Commun. 2006;346:131–139. doi: 10.1016/j.bbrc.2006.05.086. [DOI] [PubMed] [Google Scholar]
- Mali P, Ye Z, Hommond HH, Yu X, Lin J, Chen G, Zou J, Cheng L. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, Saito T, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Mochiduki Y, Okita K. Methods for iPS cell generation for basic research and clinical applications. Biotechnol J. 2012;7:789–797. doi: 10.1002/biot.201100356. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A, Wulf G, Ehrmann IE, Elliott DJ, Okpanyi V, Zechner U, Haaf T, Meinhardt A, Engel W. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RA. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet. 2009;18:4376–4389. doi: 10.1093/hmg/ddp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo RA, Slack FJ. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–418. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z, Hu Y, Chu Z, Yu M, Bai Y, Wang L, Hua J. Germ-like cell differentiation from induced pluripotent stem cells (iPSCs) Cell Biochem Funct. 2013;31:12–19. doi: 10.1002/cbf.2924. [DOI] [PubMed] [Google Scholar]
- Panula S, Medrano JV, Kee K, Bergström R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, Reijo Pera RA. Human germ cell differentiation from fetal-and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita JA, Moore K, Oetama B, Lee CK, Scales N, Ramsoondar J, Bazer FW, Ott T. Generation of transgenic porcine chimeras using primordial germ cell-derived colonies. Biol Reprod. 1998;58:1321–1329. doi: 10.1095/biolreprod58.5.1321. [DOI] [PubMed] [Google Scholar]
- Qiu P, Bai Y, Liu C, He X, Cao H, Li M, Zhu H, Hua J. A dose-dependent function of follicular fluid on the proliferation and differentiation of umbilical cord mesenchymal stem cells (MSCs) of goat. Histochem Cell Biol. 2012;138:593–603. doi: 10.1007/s00418-012-0975-7. [DOI] [PubMed] [Google Scholar]
- Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009;7:20. doi: 10.1186/1479-5876-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N, Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, Cha KY, Chung HM, Yoon HS, Moon SY, Kim VN, Kim KS. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, Morrisey EE. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompeter HI, Abbad H, Iwaniuk KM, Hafner M, Renwick N, Tuschl T, Schira J, Müller HW, Wernet P. MicroRNAs MiR-17, MiR-20a, and MiR-106b act in concert to modulate E2F activity on cell cycle arrest during neuronal lineage differentiation of USSC. PLoS One. 2011;6:e16138. doi: 10.1371/journal.pone.0016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wu WS. Sodium butyrate promotes generation of human induced pluripotent stem cells through induction of the miR302/367 cluster. Stem Cells Dev. 2013;22:2268–2277. doi: 10.1089/scd.2012.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]