Abstract
Venom from the sea anemone, Heteractis magnifica, has multiple biological effects including, cytotoxic, cytolytic and hemolytic activities. In this study, cytotoxicity induced by H. magnifica venom was investigated using the crystal violet assay on human breast cancer T47D and MCF7 cell lines and normal human breast 184B5 cell line. Apoptosis was also assayed via Annexin V-flourescein isothiocyanate and propidium iodide (PI) staining followed by flow cytometric analysis. Cell cycle progression and mitochondria membrane potential were studied via flow cytometry following PI and JC-1 staining respectively. H. magnifica venom induced significant reductions in viable cell numbers and increases in apoptosis in T47D and MCF7 in dose-dependent manners. A significant apoptosis-related increase in the sub G1 peak of the cell cycle in both breast cancer cell lines was also observed. Moreover, treatment by venom cleaved caspase-8, caspase-9, and activated caspase-3. Overall, H. magnifica venom was highly cytotoxic to T47D and MCF7 human breast cancer cells, and the phenomenon could be the killing phenomenon via the death receptor-mediated and the mitochondria-mediated apoptotic pathways. Consequently, H. magnifica venom has potential for the development of a breast cancer therapeutic.
Keywords: H. magnifica, 184B5, T47D cells, MCF7 cells, Apoptosis, Caspases
Introduction
Sea anemones belong to the phylum cnidaria and contain small stinging capsules called cnidae (nematocysts). Upon chemical or physical stimulation, the thread tubule folded in the nematocyst is discharged and penetrates the epithelium of the victim (Hutton and Valerie 1996; Marino et al. 2004; Albert 2008; Andreev et al. 2008; Alvarez et al. 2009). Some of the most potent marine toxins known are from sea anemones, and include a rich source of peptide toxins, sodium channel toxins and potassium channel toxins. Some anemone toxins bind to the same site on sodium channels as the scorpin α-toxins, and both types of toxins slow down the process of sodium inactivation (Hutton and Valerie 1996; Kem et al. 1999; Honma and Shiomi 2006). Some anemones also contain smaller peptide toxins that selectively block some potassium channels. A potassium channel toxin, HmK (Mr_31.5 kDa), has been isolated from the sea anemone Heteractis magnifica which inhibits the binding of [125I]-R-dendrotoxin (a ligand for voltage-gated K channels) to rat brain synaptosomal membranes (Gendeh et al. 1997).
In vitro and in vivo studies demonstrated that more than 32 species of sea anemones produce lethal cytolytic peptides and proteins (Anderluh and Macˇek 2002). For example, a 19 kDa cytolysin was purified from the sea anemone H. magnifica using anion exchange chromatography and gel filtration (Karthikayalu et al. 2010). Three lethal and hemolytic toxins were isolated from sea anemone Actinia equine. The pure toxins, Equinatoxins I, II and III (EqT I, II and III), exhibited high lethal potency in mice. EqT I and II killed mice within 5 min, and EqT III acted in a timeframe of a few minutes to 12 h, depending on the dose (Macek and Lebez 1988). Equinatoxin II increased membrane electrical conductance, indicating that the cytotoxic action of equinatoxin II involves an increase in the permeability of membranes to Ca2+ (Zorec et al. 1990).
The cytotoxic and cytolytic properties of extracts from the anemones Stichodactyla mertensii and Stichodactyl haddoni, was measured in vivo using Artemia salina nauplii. Significant cytotoxicity was observed, with LC50s of 0.65 and 0.90 mg/ml, respectively (Veeruraj et al. 2008). Venom extract from Aiptasia mutabilis, induced complete necrosis of a monolayer of renal monkey Vero cells after 1 h of treatment with a ten-fold dilution of the nematocyst constituting 0.17 mg/ml of protein (Marino et al. 2004). Soletti et al. (2008) demonstrated that two sea anemone cytolysins, toxin Bc2 and EqTx-II, induced cytotoxicity against U87 and A172 human glioblastoma cell lines. There have only been a small number of studies on the cytotoxicity of venom from anemone on cancer cells (Soletti et al. 2008; Ramezanpour et al. 2012). However, the mechanisms underlying any cytotoxic effects of sea anemone venom on cancer cells have not been elucidated. In the past decade there has been a dramatic increase in the number of preclinical anticancer lead compounds from marine life entering human clinical trials (Simmons et al. 2005). The current study of the potential cytotoxicity of sea anemone venom against breast cancer cells will contribute to this emerging area. Discovery of agents that target apoptosis is a major goal in the research for novel anticancer therapeutics, therefore, it is important to uncover the mechanism of action of cancer cytotoxic agents from marine sources.
The purpose of this study was to thoroughly investigate the anti-breast cancer effects induced by the anemone venom from H. magnifica, including studying the molecular basis for the action of H. magnifica. In addition, the mechanism of action was investigated to determine whether the venom induced apoptosis in T47D and MCF7 breast cancer cell lines and to determine whether this occurred via caspase cascade and/or mitochondria-mediated pathways.
Materials and methods
Reagents
Crystal violet powder, acetic acid, sodium dodecyl sulfate (SDS, 99 %), bicinchoninic acid solution, copper (II) sulfate pentahydrate solution 4 % (w/v), Propidium iodide, Triton X-100, Sodium azide, trypsin–EDTA and Ribonuclease A were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol was purchased from Merck (64271 Darmstadt, Germany). The FITC Annexine V-apoptosis Detection kit was purchased from BD Biosciences (San Diego, CA, USA). Caspase-3/7, -8 and -9 kits were purchased from Promega (Promega Corporation, Sydney, Australia).
Human cell culture
Human adherent breast cancer cells T47D (ductal carcinoma, endogenously expressing mutant p53), MCF7 (adenocarcinoma, a p53 wild type cell line) and human normal breast 184B5 cell line were obtained from American type culture collection (Manassas, VA, USA). 184B5 cell line was cultured in MEBM (Mammary Epithelial Basal Medium), (Lonza, VIC, AU) and T47D and MCF7 cells were maintained in RPMI medium (Sigma-Aldrich), with both supplemented with 10 % fetal bovine serum (FBS; Trace Biosciences, Castle Hill, Australia) and 1 % penicillin/streptomycin (Thermo Scientific, Melbourne, Australia). Cells were maintained in a fully humidified incubator with 5 % CO2 at 37 °C.
Sea anemone
Three individual H. magnifica sea anemones were collected from the Great Barrier Reef near Cairns Queensland, Australia, and obtained by purchase from Cairns Marine Aquarium Suppliers. They were housed in marine aquaria (tropical sea water) within the Animal House facility at Flinders University. Anemones were fed weekly with prawn, but were fasted for a week prior to venom collection. Crude venom extracts were obtained using a milking technique, which is a variation of the mechanical stimulation method (Sencic and Macek 1990). Anemones were placed in a plastic bag where its tentacles were massaged to facilitate nematocyst discharge and the release of gastrocoelic fluids and mucus. Samples were frozen immediately, freeze-dried using a bench-top lyophilizer (VirTis, Warminster, PA, USA) and ground into a fine powder. Samples were re-solvated in 100 mg/ml (w/v) in sterile water. The concentration of total protein in the crude extracts of venom from H. magnifica was adjusted to 400 μg/ml following quantitation using a BCA assay (Bio-Rad, Gladesville, VIC, Australia) (Walker 1996).
Cell viability test
To assess the cytotoxicity of venom extract, the crystal violet assay was used to evaluate the optical density based on the amount of dye taken up by the adherent monolayer of cells (Kueng et al. 1989; Saotome et al. 1989). Briefly, 1 × 104 cells were seeded in a volume of 100 μl per well of 96-well flat bottom plates (3 × 103 cells/mm2) and allowed to adhere overnight. Cells were then treated with venom extract for 24 h. Media and dead cells were washed away after treatment and the remaining cells were stained with crystal violet. The cells were stained with crystal violet for 10 min (Young et al. 2005). The microplates were rinsed with distilled water and distained with acetic acid. OD was measured at 570 nm. The amount of purple dye retained is proportional to the number of cells remaining adhered to the well (Young et al. 2005).
Cell cycle analysis
Cell cycle distribution was evaluated by flow cytometry (Nicoletti et al. 1991). Cells were established at 1 × 106 cells/ml in a T25 cm2 flask (4 × 103 cells/mm2) and incubated overnight at 37 °C in 5 % CO2 to allow adherence. The cells were treated with the venom extract for 24 h at 37 °C in 5 % CO2. Following treatment, the cells were harvested by trypsinisation then fixed with 3 ml ice-cold 70 % ethanol at −20 °C overnight. The cell pellet was resuspended in 1 ml of mixture solution (20 μg/ml of PI and 200 μg/ml of RNase in 0.1 %Triton X-100 in phosphate buffered saline (PBS)) and incubated at room temperature in the dark for 30 min. Samples were analyzed by Accuri C6 flow cytometry.
Apoptosis assessed by flow cytometry
Treatment of cells for apoptosis assessment was as for the cell cycle analysis. Cells were counted using trypan blue exclusion assay (50 μl cell suspension mixed with equal volume of 0.2 % trypan blue then counted at 40×magnification using a haemocytometer). Then cells were washed twice with cold 0.1 % sodium azide in PBS. The pellets were re-suspended in binding buffer (0.1 M Hepes/NaOH, 1.4 M NaCl, 25 Mm CaCl2) at 106 cells/ml. 100 μl of the solution was transferred to a culture tube, then 5 μl of Annexin V-FITC and 5 μl of PI were added to double stain the cells. After 15 min of incubation in the dark, at room temperature, 200 μl of binding buffer was added to the cells and analyzed by Accuri C6 flow cytometry to determine early and late apoptosis frequency.
Caspase assay
Caspase activities were measured using Caspase-3/7, -8 and -9 assay kits (Promega Corporation). 1 × 104 cells/well (3 × 103 cells/mm2) were seeded in a 96-well luminometer plate (BD Biosciences) and incubated for 24 h at 37 °C in 5 % CO2. Cells were treated with different concentrations of venom extract for 24 h then washed with 1 × PBS. To each series of wells, 50 μl of Caspase-3/7, -8 and -9 reagents were added separately and the luminescence was recorded every 10 min for 1 h at 28 °C in a microplate reader.
Mitochondrial membrane potential
Cell cultures were established, incubated overnight, treated for 24 h harvested and resuspended as described for the cell cycle analysis. Cells were stained with JC-1 (Sigma), and the numbers of cells exhibiting green and red fluorescence were quantified via flow cytometry using an Accuri C6. The data were analyzed with CellQuest software (BD, Oxford, U.K.) (Cossarizza et al. 1993; Tsujimoto and Shimizu 2007).
Statistical analysis
Data are presented as the mean ± SEM. The experiments were replicated at least three independent times. Statistical analysis was carried out using ANOVA, followed by Tukey’s HSD post hoc test using SPSS (version 18). Differences were considered significant when p value ≤0.05. Responses to treatment were compared to the untreated control for a given cell line. In addition the response of each cancer cell line in the crystal violet assay was compared to the non-cancer control cell line.
Results
H. magnifica venom extract induces cell killing of T47D and MCF7 cells
A range of increasing concentrations of H. magnifica venom extract decreased the survival of cancer cells lines, T47D and MCF7. The venom extract significantly reduced the numbers of viable cells in a dose-dependent manner (p < 0.05). There was greater than 90 % reduction in cell numbers of the cancer cell lines after 24 h treatment with 10 μg/ml or greater of venom extract (Fig. 1). In contrast, there was less killing of the control cell line 184B5. Statistical comparison of 184B5 to T47D and MCF7 showed that this difference was significant (p < 0.05, 2 way ANOVA) (Fig. 1). Survival was also estimated for cells treated in flasks as part of the apoptosis assay. As shown in Fig. 2, relative survival decreased in a dose-dependent-manner for T47D and MCF7.
Fig. 1.
Cell viability percentage of T47D, MCF7 and 184B5 cells was estimated by crystal violet assay in 96-well plates following 24 h exposure to H. magnifica a venom extract. Data are shown as relative surviving cell numbers % compared to the untreated control (0 μg/ml) and are presented as the mean ± SEM of three separate trials. Doses of 10 μg/ml or greater, significantly killed T47D and MCF7 compared to 0 μg/ml control (p < 0.05)
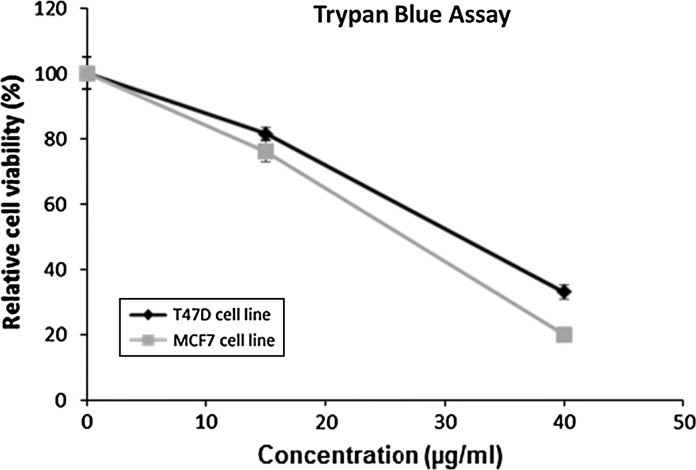
Fig. 2.
Cell viability estimated by the trypan blue assay of T47D and MCF7 cell lines treated for 24 h in flasks for analysis in the apoptosis assay. Data are shown as % relative cell viability compared to the untreated control (0 μg/ml) and are presented as the mean ± SEM of three separate trials
H. magnifica venom extract deregulates cell-cycle control
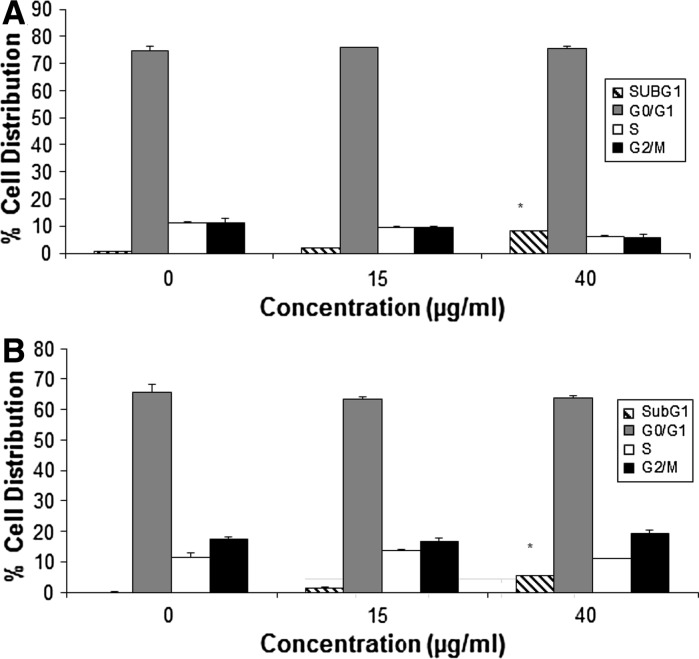
Cell cycle distribution was analyzed by flow cytometry using PI staining. H. magnifica venom treatment of the breast cancer cell lines significantly increased the sub G1 peak with a concomitant decrease in G1 phase, compared to the untreated control of each cancer cell line (Fig. 3). The increase in sub G1 was significant at 40 μg/ml for both cell lines.
Fig. 3.
Effect of H. magnifica venom extract on cell cycle distribution of (a) T47D and (b) MCF7, determined by PI staining and DNA content by flow cytometry. Data were obtained from 20,000 events and are presented as the percentage of cells in the sub G1, G0/G1, S and G2/M phases. The values are shown as mean ± SEM, n = 3. *Significantly different from the untreated control at p < 0.05
H. magnifica venom extract induces apoptosis of T47D and MCF7 cells
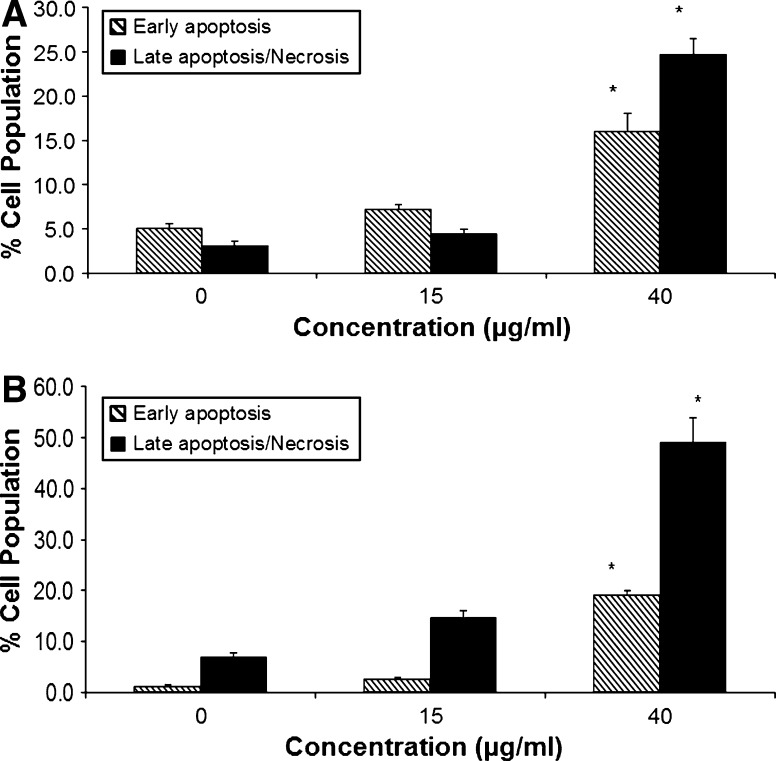
To determine whether the reduction in viability induced by the venom extract involved the induction of apoptosis, the numbers of apoptotic cells were estimated by staining the cells with Annexin V and PI, followed by flow cytometry. H. magnifica venom extract induces apoptosis in both human breast cancer cell lines tested, and the level is significant at 40 μg/ml (p < 0.05). The largest increment of the response to the treatment was in MCF7 lines (Fig. 4).
Fig. 4.
Apoptotic effect of H. magnifica venom extract on (a) T47D and (b) MCF7 as determined by Annexin V-conjugated PI staining through flow cytometry. Data were obtained from 20,000 events with early apoptotic cells (Annexin positive) and late apoptotic cells (Annexin positive/PI positive, including necrotic cells) presented as a % of total cells analyzed. The values are shown as mean ± SEM. *Significantly different from the untreated control at p < 0.05, n = 3
H. magnifica venom extract increase the activation of caspases
Chemotherapeutic agents induce apoptosis in cancer cells via a variety of mechanisms (Cline et al. 1995; Lowe et al. 2004). Caspases perform critically important roles in the induction of apoptosis (Friesen et al. 2008). Treatment of T47D and MCF7 cells with venom extract at 30 and 40 μg/ml for 24 h significantly increased the activation of caspases, via caspases-3, -8, and -9 (Fig. 5).
Fig. 5.
Caspase activities were determined using a luminescent kit for (a) T47D and (b) MCF7 cells after 24 h treatment with H. magnifica venom extract. Data are presented as relative luminescence units (RLU). The values are shown as mean ± SEM of 3 independent experiments. *Significantly different from the untreated control at p < 0.05
H. magnifica venom extract increases mitochondrial membrane permeability
Mitochondrial dysfunction is another key aspect of apoptosis. Increases in Δψ were observed for both cancer cell lines. Treatment of T47D and MCF7 cells with 40 μg/ml of venom significantly increased the percentage of cells positive for JC-1 monomers from 4 % in untreated cells to 53 and 34.5 %, respectively (Fig. 6).
Fig. 6.
Loss of mitochondrial membrane potential as indicated by JC-1 dye using flow cytometry after 24 h treatment with different concentrations of venom extract from H. magnifica. Data were obtained from 20,000 events and presented as the percentage of cells positive for JC-1 monomer. The values are shown as mean ± SEM, n = 3. *Significantly different from the untreated control (0 μg/ml) at p < 0.05
Discussion
Marine organisms are a rich source of biologically active natural products (Simmons et al. 2008; Leal et al. 2012). One of the most ancient marine organisms which possess a variety of peptide and protein toxins for chemical defense is sea anemones. Chemical defense toxins are currently being investigating for biomedical properties and for designing novel drugs for human therapeutics (Honma and Shiomi 2006; Tejuca et al. 2009). The potential cytotoxic effect of H. magnifica on human cancer cells and the underlying mechanism causing cell death have not been elucidated. In this study, the venom from the sea anemone, H. magnifica, was highly effective in killing the breast cancer T47D and MCF7 cell lines in a dose-dependent manner. In the crystal violet assay, the treatment of T47D and MCF7 cell lines with 15 μg/ml of venom for 24 h resulted in less than a 10 % survival. In contrast, an identical concentration of venom exerted less effect on the survival of 184B5 human normal breast cells which exhibited 50 % cell survival. This finding is encouraging because it suggests the potential for therapeutic development using this venom, whereby cancer cells are targeted and killed by a particular dosage which has less impact on non-cancer cells.
Control of the progression of the cell cycle of cancer cells is an effective strategy for cancer therapy because deregulated cell-cycle control is a fundamental aspect of cancer for many common malignancies (Senderowicz 2003). Analysis of cell cycle phase distribution of human breast cell lines T47D and MCF7 treated with venom of H. magnifica revealed that decreased survival involved cell cycle arrest. Cell treatment with H. magnifica venom induced accumulation of a sub-G1 population with a concomitant decrease in the G1 phase, supporting that the induction of apoptosis was occurring. Apoptosis can play a crucial defense role against the propagation of cancer and the induction of this process is one mechanism by which chemotherapeutic agents can kill cancer cells (Campbell et al. 2007). There are at least two broad pathways that lead to apoptosis, the death receptor pathway (extrinsic) and the mitochondrial pathway (intrinsic). The extrinsic pathway is triggered by the binding of death inducing ligands to cell surface receptors, which results in the activation of caspase-8. The intrinsic pathway, in contrast, is triggered by cytotoxic stress, which converge in the mitochondria, leading to the release of several mitochondrial inter-membrane space proteins, such as cytochrome c, which associate with Apaf-1 and procaspase-9 to form the apoptosome (Elmore 2007). T47D and MCF7 cell lines underwent induction of apoptosis as evidenced by significant increases in mitochondrial potential.
A toxin (RTX-A) from a closely related anemone species, H. crispa, has also been found to induce apoptosis in a way similar to the current study in a malignant transformation model of mouse JB6P+CI41 cells (Fedorov et al. 2010). RTX-A toxin (H. crispa), induces P53-independent apoptosis and inhibits the activation of the oncogenic nuclear transcriptional factors AP-1 and NF-KB (Fedorov et al. 2010). The intrinsic and extrinsic apoptosis pathways converge with the activation of caspase-3 and subsequently other executioner caspases and nucleases drive the terminal events of programmed cell death (MacFarlane 2003; Crow et al. 2004; Prunell et al. 2005; Elmore 2007; Pizon et al. 2011). Activated initiator caspases can cleave and activate effector caspases such as caspase-3, which in turn cleaves a variety of cellular substrates (Chandler et al. 1998). H. magnifica venom extract cleaved caspase-8, caspase-9 and activated caspase-3 as detected using luminescence assays. The findings imply the induced release of cytochrome c from the mitochondria into the cytosol. Also observed was enhanced permeability of the outer mitochondrial membranes. Breakdown of Δψ leads to the release of cytochrome c from the mitochondria and activation of caspase cascades that result in cell death (Crompton 2000; Kroemer 2003; Wang et al. 2005; Malhi et al. 2010). Our findings presented here, believed to be the first of their kind for breast cancer cells, demonstrate that H. magnifica venom extract induces apoptosis through the activation of caspases. This could be via both the death receptor-mediated and the mitochondria-mediated apoptotic pathways. Another possibility is that the extrinsic pathway is activating the intrinsic pathway via caspase 8. Caspase 8 is also being activated by caspase 9. Significant activation in the three caspases were observed for MCF7 and T47D, with T47D exhibiting the greater response. The different magnitude in the response of the two cell lines may be due to inherent phenotypic differences due to the different genetic background of each cell line, such as p53 status.
Noncytotoxic concentrations of cytolysins have been shown to enhance cytotoxicity induced by low doses of anticancer agents in vitro using two human glioblastoma cell lines (U87, a p53 wild type cell line, and A172, a p53 mutant GBM cell line)(Soletti et al. 2008). With this in mind, combining H. magnifica venom extract with existing anti-cancer agents may have an even greater potential to enhance their effect.
In summary, this study has found that H. magnifica venom significantly kills breast cancer cells in a dose-dependent manner. This was associated with induced apoptosis in T47D and MCF7 human breast cancer cell lines, and this effect was mediated by the activation of caspases. This study provides the molecular evidence to support using H. magnifica venom as an in vitro apoptosis-inducing agent. These promising results mean that further studies should be conducted in an experimental animal model to evaluate the potential of H. magnifica venom as a breast cancer cytotoxic agent.
Acknowledgments
This study was supported by an FMC Foundation Grant and a Flinders Centre for Innovation in Cancer (FCIC) research grant. The authors are thankful to Mrs. Bailey from the Department of Immunology, Allergy and Arthritis, Flinders Medical Centre, for her Collaboration with Flow Cytometry.
Contributor Information
Mahnaz Ramezanpour, Phone: +61-8-72218564, FAX: 72218555, Email: Rame0010@flinders.edu.au.
Karen Burke da Silva, Phone: +61-8-82012010, FAX: 72218555, Email: Karen.burkedaSilva@flinders.edu.au.
Barbara J. S. Sanderson, Phone: +61-8-72218556, FAX: 72218555, Email: Barbara.Sanderson@flinders.edu.au
References
- Albert B. Cancer as a microevolutionary process. Molecular biology of the cell 5. USA: Garland Science; 2008. pp. 1205–1256. [Google Scholar]
- Alvarez C, Mancheño J, Martínez D, Tejuca M, Pazos F, Lanio M. Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes. Toxicon. 2009;54:1135–1147. doi: 10.1016/j.toxicon.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Macˇek P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/S0041-0101(01)00191-X. [DOI] [PubMed] [Google Scholar]
- Andreev YA, Kozlov SA, Koshelev SG, Ivanova EA, Monastyrnaya MM, Kozlovskaya EP, Grishin EV. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1) J Biol Chem. 2008;283:23914–23921. doi: 10.1074/jbc.M800776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Prince M, Landry GM, Kha V, Kleiner HE. Pro-apoptotic effects of 1′-acetoxychavicol acetate in human breast carcinoma cells. Toxicol Lett. 2007;173:151–160. doi: 10.1016/j.toxlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Cohen GM, MacFarlane M. Different subcellular distribution of Caspase-3 and Caspase-7 following Fas-induced apoptosis in mouse liver. J Biol Chem. 1998;273:10815–10818. doi: 10.1074/jbc.273.18.10815. [DOI] [PubMed] [Google Scholar]
- Cline EI, Wiebe LI, Young JD, Samuel J. Toxic effects of the novel protein UpI from the sea anemone Urticina piscivora. Pharmacol Res. 1995;32:309–314. doi: 10.1016/S1043-6618(05)80020-9. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Crompton M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr Opin Cell Biol. 2000;12:414–419. doi: 10.1016/S0955-0674(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov S, Dyshlovoy S, Monastyrnaya M, Shubina L, Leychenko E, Kozlovskaya E, Jin JO, Kwak JY, Bode AM, Dong Z, Stonik V. The anticancer effects of actinoporin RTX-A from the sea anemone Heteractis crispa (=Radianthus macrodactylus) Toxicon. 2010;55:811–817. doi: 10.1016/j.toxicon.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen C, Uhl M, Pannicke U, Schwarz K, Miltner E, Debatin KM. DNA-ligase IV and DNA-protein kinase play a critical role in deficient caspases activation in apoptosis-resistant cancer cells by using doxorubicin. Mol Biol Cell. 2008;19:3283–3289. doi: 10.1091/mbc.E08-03-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendeh GS, Young LC, de Medeiros CL, Jeyaseelan K, Harvey AL, Chung MC. A new potassium channel toxin from the sea anemone Heteractis magnifica: isolation, cDNA cloning, and functional expression. Biochemistry. 1997;36:11461–11471. doi: 10.1021/bi970253d. [DOI] [PubMed] [Google Scholar]
- Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar Biotechnol (NY) 2006;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton DMC, Valerie SH. Antibacterial properties of isolated amoebocytes from the sea anemone Actinia equina. Biol Bull. 1996;191:441–451. doi: 10.2307/1543017. [DOI] [PubMed] [Google Scholar]
- Karthikayalu S, Rama V, Kirubagaran R, Venkatesan R. Characterization, purification and phylogenetic analysis of a cytolysin from the sea anemone Heteractis magnifica of the Indian Ocean. J Venom Anim Toxins. 2010;16:223–240. [Google Scholar]
- Kem WR, Pennington MW, Norton RS. Sea anemone toxins as templates for the design of immunosuppressant drugs. Perspect Drug Discovery Des. 1999;15–16:111–129. doi: 10.1023/A:1017071131670. [DOI] [Google Scholar]
- Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304:433–435. doi: 10.1016/S0006-291X(03)00614-4. [DOI] [PubMed] [Google Scholar]
- Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- Leal MC, Puga J, Dio JS, Gomes NCM, Calado R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—where and what are we bioprospecting? PLoS ONE. 2012;7:30580. doi: 10.1371/journal.pone.0030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Macek P, Lebez D. Isolation and characterization of three lethal and hemolytic toxins from the sea anemone Actinia equina L. Toxicon. 1988;26:441–451. doi: 10.1016/0041-0101(88)90183-3. [DOI] [PubMed] [Google Scholar]
- MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139:89–97. doi: 10.1016/S0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino A, Valveri V, Muià C, Crupi R, Rizzo G, Musci G, Giuseppa LS. Cytotoxicity of the nematocyst venom from the sea anemone Aiptasia mutabilis. Comp Biochem Physiol C. 2004;139:295–301. doi: 10.1016/j.cca.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani FCR. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- Pizon M, Rampanarivo H, Tauzin S, Chaigne-Delalande B, Daburon S, Castroviejo M, Moreau P, Moreau JF, Legembre P. Actin-independent exclusion of CD95 by PI3 K/AKT signalling: implications for apoptosis. Eur J Immunol. 2011;41:2368–2378. doi: 10.1002/eji.201041078. [DOI] [PubMed] [Google Scholar]
- Prunell GF, Arboleda VA, Troy CM. Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr Drug Targets CNS Neurol Disord. 2005;4:51–61. doi: 10.2174/1568007053005082. [DOI] [PubMed] [Google Scholar]
- Ramezanpour M, Burke da Silva K, Sanderson BJ. Differential susceptibilities of human lung, breast and skin cancer cell lines to killing by five sea anemone venoms. JVAT. 2012;18:157–163. [Google Scholar]
- Saotome K, Morita H, Umeda M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol In Vitro. 1989;3:317–321. doi: 10.1016/0887-2333(89)90039-8. [DOI] [PubMed] [Google Scholar]
- Sencic L, Macek P. New method for isolation of venom from the sea-anemone Actinia-cari—purification and characterization of cytolytic toxins. Comp Biochem Phys B. 1990;97:687–693. [PubMed] [Google Scholar]
- Senderowicz AM. Novel small molecule cyclin-dependent kinases modulators in human clinical trials. Cancer Biol Ther. 2003;2:84–95. doi: 10.4161/cbt.207. [DOI] [PubMed] [Google Scholar]
- Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- Simmons TL, Coates RC, Clark BR, Engene N, Gonzalez D, Esquenazi E, Dorrestein PC, Gerwick WH. Biosynthetic origin of natural products isolated from marine microorganism–invertebrate assemblages. Proc Natl Acad Sci USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soletti RC, de Faria GP, Vernal J, Terenzi H, Anderluh G, Borges HL, Moura-Neto V, Gabilan NH. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-forming proteins in human glioblastoma cells. Anticancer Drugs. 2008;19:517–525. doi: 10.1097/CAD.0b013e3282faa704. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Pérez-Barzaga V, Pazos F, Álvarez C, Lanio ME. Construction of sea anemone cytolysin-based immunotoxins for selective killing of cancer cells. Rev Cub Física. 2009;26:15–22. [Google Scholar]
- Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- Veeruraj A, Arumugam M, Ajithkumar T, Balasubramanian T. Isolation and biological properties of neurotoxin from sea anemone (Stichodactyla mertensii, S. haddoni) Internet J Toxicol. 2008;5:159–167. [Google Scholar]
- Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. In: Walker JM, editor. The protein protocols handbook. NY: Humana Press; 1996. pp. 11–14. [Google Scholar]
- Wang Y, He QY, Sun RW, Che CM, Chiu JF. Gold III porphyrin 1a induced apoptosis by mitochondrial death pathways related to reactive oxygen species. Cancer Res. 2005;65:11553–11564. doi: 10.1158/0008-5472.CAN-05-2867. [DOI] [PubMed] [Google Scholar]
- Young FM, Phungtamdet W, Sanderson BJ. Modification of MTT assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, WIL2NS. Toxicol In Vitro. 2005;19:1051–1059. doi: 10.1016/j.tiv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zorec R, Tester M, Macek P, Mason WT. Cytotoxicity of equinatoxin II from the sea anemone Actinia equina involves ion channel formation and an increase in intracellular calcium activity. J Membr Biol. 1990;118:243–249. doi: 10.1007/BF01868608. [DOI] [PubMed] [Google Scholar]