Abstract
Ifosfamide (IFO) is an alkylating nitrogen mustard, administrated as an antineoplasmic agent. It is characterized by its intense urotoxic action, leading to hemorrhagic cystitis. This side effect of IFO raises the requirement for the co-administration with sodium 2-sulfanylethanesulfonate (Mesna) aiming to avoid or minimize this effect. IFO and Mesna were administrated separately on rabbit’s lymphocytes in vivo, which were later developed in vitro. Cytogenetic markers for sister chromatid exchanges (SCEs), proliferation rate index (PRI) and Mitotic Index were recorded. Mesna’s action, in conjunction with IFO reduces the frequency of SCEs, in comparison with the SCEs recordings obtained when IFO is administered alone. In addition to this, when high concentrations of Mesna were administered alone significant reductions of the PRI were noted, than with IFO acting at the same concentration on the lymphocytes. Mesna significantly reduces IFO’s genotoxicity, while when administered in high concentrations it acts in an inhibitory fashion on the cytostatic action of the drug.
Keywords: Ifosfamide, Mesna, Sister chromatid exchanges, Genotoxicity, Peripheral rabbit lymphocytes
Introduction
Ifosfamide (IFO), is a synthetic analogue of Cyclofosfamide (CP), that belongs to the family of nitrogen mustard alkylating oxafosfamides. IFO is an alkylating agent acting in a powerful antineoplasmic fashion against a broad range of tumours, and it is widely administered as an anticancer drug to both adults and children (Broadhead et al. 1998; Pazdur et al. 2008). Its alkylating action is due to the interaction between its alkylating metabolites and DNA. This alkylating action induces the formation of intra and interstrand crosslinks at the DNA, leading possibly to cytotoxicity and cell death (Lokiec 2006).
The prodrome form of IFO in vitro is inactive. In order to express its alkylating action on DNA, IFO is metabolically reactivated with the help of enzymes of the P-45 cytochrome (Johnstone et al. 2000). IFO is either converted to chloroacetaldehyde, presenting with severe nephrotoxicity (Lameire et al. 2011), or is hydroxylated to C-4 of its oxynitrophosphoric ring in order to form the metabolites 4-hydroxy-ifosfamide and isoaldofosfamide (Storme et al. 2009). Isoaldofosfamide, through its spontaneous dissociation gives rise to akrolein and its alkylating metabolite, the isofosfamide mustard (Broadhead et al. 1998; Johnstone et al. 2000). The derivatives, hydroxyl-ifosfamide and chloroacetaldehyde reduce the intracellular levels of glutathione, leading to the reinforcement of cytotoxicity of other cytostatic factors, such as doxorubicine (Malik et al. 1997). Administration of IFO is related to the emergence of haemorrhagic cystitis through the action of akroleinis (Lawson et al. 2008; Glezerman 2009).
Mesna is the sulfur salt of 2-mercapto-ethanol-sulphunic acid. Originally developed to act as a protective agent for controlling the danger of haemorrhagic cystitis following the administration of IFO (Anderson 2010). Mesna is rapidly oxidized towards the form of its main metabolite; disulphide mesna (dimesna). Dimesna remains in the intravascular compartment and is rapidly transported to the kidneys (Wilmer et al. 1986a; Zaki et al. 2003). In the epithelium of the renal tubules, the biggest part of dimesna is reduced to the free thiol group of mesna, which reacts chemically with the metabolites of urotoxic IFO, towards forming stable non-urotoxic molecules, causing their “neutralization”. More particularly, mesna inhibits the disruption of the 4-hydroxy-oxynitrophosphoric metabolites and the formation of the extremely toxic akroleinis, producing complexes of a non-urotoxic thioether (Zaki et al. 2003). In fact, along with the binding of 4-OH-IFO, reduction of intracellular glutathione is also prevented (Souid et al. 2001). Mesna does not present to have any antineoplasmic properties however its cytoprotective role in ifosfamide/mesna combination treatments of cancer must be acknowledged (Dechant et al. 1991; Hogle 2007; Ogura et al. 2013).
The present study focuses on the cytogenetic evaluation of the degree of protection supplied to healthy cells by mesna, in contrast to the potent antineoplasmic IFO. The method used was the study of Sister Chromatid Exchanges (SCEs). IFO is a powerful and effective drug performing exceptionally in anticancer therapy, on the other hand it induces chromatid fragility and genotoxicity even in healthy tissue cells (Chen et al. 2007; Hansen et al. 2007; Lialiaris et al. 2010a). As previously stated, Mesna can help to avoid haemorrhagic cystitis induced following administration of IFO. The contingency that Mesna could act in a protective or even therapeutic fashion against IFO induced genotoxicity, was tested in the present study employing three cytogenetic markers:
(1) The frequency of emergence of SCEs.
(2) The Proliferation Rate Index (PRI).
(3) The mitotic index (MI) on cultured human lymphocytes.
The SCEs method is a sensitive, simple and fast method in detecting DNA damage induced by various environmental and pharmaceutical factors. Employing this method, contributes appreciably in testing efficiently and improving chemotherapeutic techniques. The SCEs method is a notably more sensitive method than the one based on chromosomal aberrations, as chromosome damage is more accurate to detect even in low concentrations of the genotoxic factor (Yilmaz et al. 2009, Lialiaris et al. 2009a, Lialiaris et al. 2010b, Mamur et al. 2012, Istifli and Topakt 2012). With respect to the other two cytogenetic markers used, namely PRI and MI, they are both valuable markers of the cytostatic and cytotoxic action of various factors, including the chemotherapeutic ones (Das 1988; Maskaleris et al. 1998; Fousteris et al. 2006; Lialiaris et al. 2009a; Mourelatos et al. 2012).
Materials and methods
Chemicals
Ifosfamide (Fig. 1 top) (CAS No. 3778-73-2) was provided from Bristoll–Myers Squibb (New York, NY, USA) and Mesna (Fig. 1 bottom) (CAS No. 19767-45-4) from Baxter (Deerfield, IL, USA). The chemicals used for the SCEs assay 5-Bromo-2′-deoxyuridine (CAS No. 59-14-3), bis-benzamide (CAS No. 23491-45-4) and colcemide (CAS No. 64-86-8) were obtained from Applichem (Gatersleben, Germany). All of the chemicals were dissolved in bidistilled water and when lower concentrations were needed they were prepared by serial dilutions just before treatment. All chemicals used in the SCEs assay were of the best grade commercially available.
Fig. 1.
Structural forumla of IFO (top) and Mesna (bottom)
Cytogenetic testing
Thirty two healthy New Zealand rabbits were used for this study, and were divided into 4 groups. The test control group included 5 rabbits, while all other groups were comprised of 9 rabbits each. The three groups that were exposed to the substances tested, were subdivided into 3 subgroups consisting of 3 laboratory animals, respectively. Increasing concentrations of the substances to be tested for each group, were added to the subgroups according to protocol. The substances to be tested were administered intravenously in every experimental animal in one dosage. The control group of rabbits of group 1 was injected with physiological normal saline of equal volume as the substance solutions that were injected to all the other experimental rabbits of all other subgroups. The experimental animals of the second group i.e. subgroup 2, 3 and 4 were injected with increasing concentrations of IFO 30, 45 and 60 mg/kg, respectively. The experimental animals of the third group i.e. subgroup 5, 6 and 7 were injected with increasing concentrations of mesna at 6, 9 and 12 mg/kg, respectively. In the forth group of rabbits, namely subgroups 8, 9 and 10 a combination of the above mentioned increasing concentrations for both IFO and mesna were administered, respectively, i.e.: subgroup 8:30 mg/kg IFO + 6 mg/kg Mesna, subgroup 9: 45 mg/kg IFO + 9 mg/kg Mesna and subgroup 10: 60 mg/kg IFO + 12 mg/kg Mesna.
The rabbits of the subgroups 5–10 were re-injected with mesna of the same concentrations as above mentioned following 4 h after the first injection. This was done due to the limited bioavailability of mesna in comparison with that of IFO. In order to asses the cytogenetic effect of the drug agents employed on the animal’s lymphocytes, blood was drawn from the peripheral auricular vein of the experimental animals. This was performed 7 days following administration of the substances tested.
A 72 h standard synchronised culture method was used to produce extended chromosome preparations from the peripheral lymphocytes of each laboratory animal separately. The presence of 5-Bromo-2′-deoxyuridine (BrdU) ensured the visibility of SCEs in the microscope. All handling and incubation of the cultures were performed in the dark in order to avoid photolysis of BrdU. The cultures were accordingly harvested following the completion of the incubation period. Chromosome preparations were performed using a slightly modified method of fluorescence plus Giemsa (FPG) (Goto et al. 1978; Lialiaris et al. 2008).
The SCEs frequency was assessed, in Giemsa stained slides. Cultures were collected by standard methods of that technique. For each culture 20 to 35 well spread second division metaphases were counted. For establishing the proliferation rate indices (PRIs), 200–300 cells of each culture were counted, according to the formula: PRI = (1M1 + 2M2 + 3M3+)/(M1 + M2 + M3+), where M1 is the percentage of cells in the first division, M2 in the second and M3+ in the third and subsequent divisions. Mitotic indices (MIs) were determined by counting 1,500 to 2,000 activated lymphocytes for each culture.
Statistical analysis
To ensure proper statistical analysis of the drug effects on the chromosomes of lymphocytes, the latter were logarithmically transformed. Statistical analysis of the SCEs was performed using the ANOVA test, and the comparison for the pairs of results was possible employing the Duncan test. Statistical analysis of PRI and MI was based on the χ2 test. Correlation of the three markers SCEs, PRI and MI, as well as Pearson’s correlation coefficient were examined.
Results
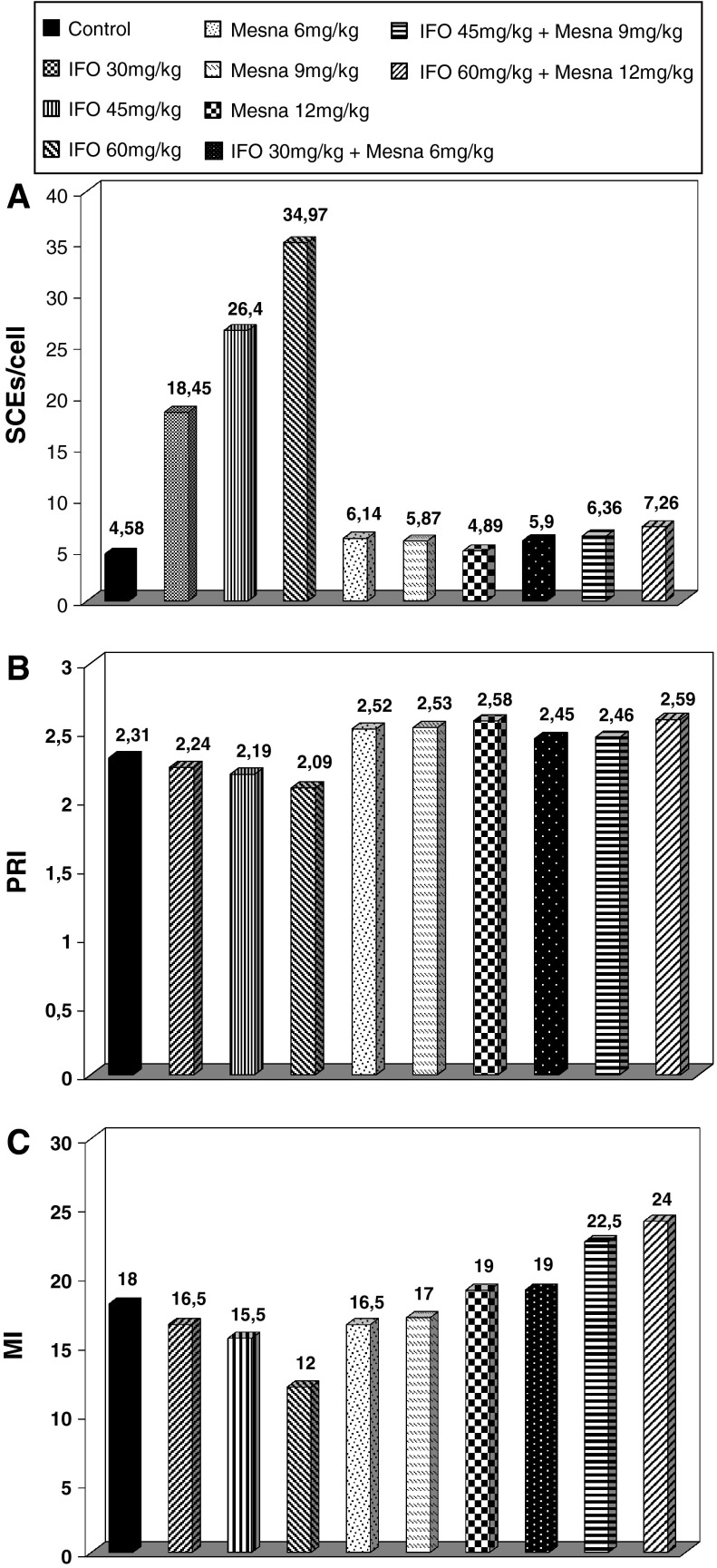
The results of the action of IFO, mesna and their combinations are summarized on Table 1. The count of sister chromatid exchanges on metaphase chromosomes of the second division (SCEs/metaphase), as a genotoxicity marker for the control group, was on average 4.58 exchanges per cell. Subgroup 2 that was exposed to IFO at a concentration of 30 mg/kg, showed 18.45 SCEs/metaphase, a statistically (Ρ < 0.01) significant increase of the SCEs levels, in comparison with the control group. Even higher and statistically significant is the increase of the SCEs marker to 26.40 SCEs/metaphase notified in subgroup 3, where IFO was injected at a concentration of 45 mg/kg. The highest increase of the genotoxicity marker was noted when IFO was injected at a concentration of 60 mg/kg, with the SCEs marker increasing to 34.97/metaphase, also statistically significantly (Ρ < 0.01) in comparison with the control group, and subgroup 2. The genotoxic action of IFO appears to be dose-dependent, as the markers’ value for the subgroups that were exposed to higher concentrations of IFO is statistically (Ρ < 0.01) significantly higher than the values related to the subgroups exposed to lower concentrations of IFO.
Table 1.
Calculating the values of the three cytogenetic markers in vitro; SCEs/metaphase, proliferation rate index (PRI) and mitotic index (MI), on the rabbit lymphocytes exposed to IFO and mesna in vivo
| Group | Treatment | SCEs ± SEM (range) | PRI | MI |
|---|---|---|---|---|
| 1 | Control | 4.58 ± 0.32 (2–6) | 2.31 | 18 ‰ |
| 2 | IFO 30 mg/kg | 18.45a ± 2.54 (14–22) | 2.24 | 16.5 ‰ |
| 3 | IFO 45 mg/kg | 26.40b ± 3.72 (23–31) | 2.19 | 15.5 ‰ |
| 4 | IFO 60 mg/kg | 34.97c ± 4.05 (30–41) | 2.09 | 12 ‰ |
| 5 | Mesna 6 mg/kg | 6.14 ± 0.45 (3–10) | 2.52g | 16.5 ‰ |
| 6 | Mesna 9 mg/kg | 5.87 ± 0.38 (3–10) | 2.53g | 17.0 ‰ |
| 7 | Mesna 12 mg/kg | 4.89 ± 0.35 (2–8) | 2.58g | 19.0 ‰ |
| 8 | IFO 30 mg/kg + Mesna 6 mg/kg | 5.90d ± 0.42 (3–10) EV = 20.01 | 2.45h | 19.0 ‰ |
| 9 | IFO 45 mg/kg + Mesna 9 mg/kg | 6.36e ± 0.51 (4–12) EV = 27.69 | 2.46e | 22.5 ‰ |
| 10 | IFO 60 mg/kg + Mesna 12 mg/kg | 7.26f ± 0.63 (4–14) EV = 35.28 | 2.59f | 24.0 ‰f |
The SCEs frequency was based on 20–35 s division metaphases. For the Proliferation Rate Index (PRI) 200–300 cells were counted. MI was estimated by counting 1,500–2,000 activated lymphocytes. The results were based on four experiments with the same culture protocol. For SCE comparisons, logarithmic transformation of the data was performed using the one-way analysis of variance (ANOVA) and the Tukey test. PRI and MI comparisons were assessed employing the χ2-test
a Ρ < 0.01 in comparison to group 1
b Ρ < 0.01 in comparison to groups 1 and 2
c Ρ < 0.01 in comparison to groups 1, 2 and 3
d P < 0.01 in comparison to group 2
e P < 0.01 in comparison to group 3
f P < 0.01 in comparison to group 4
g Ρ < 0.05 in comparison to group 1
h Ρ < 0,05 in comparison to group 2
The administration of mesna in the 5th subgroup at a concentration of 6 mg/kg slightly increased at a non statistically significant level (Ρ > 0.05), the frequency of appearance of SCEs in comparison with the marker value for the control culture. As the administered concentration of mesna increased to 9 mg/ml for the 6th subgroup, the increase caused by the substance was actually reduced in comparison with the increase caused when mesna was injected at a lower concentration than in subgroup 5. Similarly to the 5th subgroup, the increase of SCEs caused by mesna administration in subgroup 6, was not statistically significant (Ρ > 0.05) in comparison with the control group. When mesna was injected at a higher concentration of 12 mg/ml in subgroup 7, the increase of the SCEs was even lower in comparison with the other subgroups, where mesna was administered at a lower concentration. The SCEs frequency appearance for group 7 is 4.89, a value very close to the control group (subgroups 5 and 6). The increase of the SCEs marker brought on by mesna for subgroup 7, is statistically of no significance (Ρ > 0.05).
When both mesna and IFO were administered in subgroup 8, at 6 and 30 mg/ml, respectively, no statistically important increase (Ρ > 0.05) of the level of SCEs/cell was recorded, in comparison with the value for the control group, as the value for this subgroup was 5.90 SCEs/metaphase. However, comparing the levels of SCEs/cell between subgroup 8 and subgroup 2 where only IFO was added at the same concentration, a significant decrease was noted (Ρ < 0.01). The value of SCEs for subgroup 9 where IFO acted at a concentration of 45 mg/kg, and mesna at a concentration of 9 mg/kg, was 6.36 and much lower (Ρ < 0.01), when compared with the respective markers’ value for subgroup 3, where only IFO was added at the same concentration. Mesna’s action at a concentration of 12 mg/kg combined with IFO concentration at 60 mg/kg, led to a decrease of the genotoxic effect brought on by IFO, and caused a decrease of the value of the SCEs in the lymphocytes of rabbits of subgroup 10 (7.26 SCEs/metaphase). This value is statistically significantly (Ρ < 0.01) lower than the marker for subgroup 4, where only IFO was added at the same concentration. It is evident that increasing mesna concentration strengthens considerably its genoprotective effect (Table 1). The effects of the action of both agents on the lymphocytes of rabbits with respect to the SCEs markers, are illustrated in Fig. 2a.
Fig. 2.
SCEs (a), PRIs (b) and MIs (c) levels of the rabbit lymphocytes in vitro, after administration of increasing concentrations of IFO, mesna and their combinations in vivo
In vivo action of IFO on lymphocytes of subgroup 2 added at a concentration of 30 mg/kg, led to a slight but not statistically significant increase of the PRI (Ρ > 0.05). A non statistically significant (Ρ > 0.05) decrease of the PRI was caused also by the action of IFO when administered to the rabbits of subgroups 3 and 4 in concentrations of 45 and 60 mg/kg, respectively, (Fig. 2b).
In contrast to the effect of IFO on the cell cycle life of the lymphocytes, mesna significantly increased (Ρ < 0.05) the PRI value for all three subgroups (subgroups 5, 6, and 7) when administered, at concentrations of 6, 9 and 12 mg/kg, while the marker was valued at 2.52, 2.53 and 2.58 for every subgroup, respectively. In the cases where both agents IFO and mesna were administered, the action of the latter revoked the cytostatic effect of IFO and led to the increase of PRI for the lymphocytes of the laboratory animals. The value of PRI recorded for subgroup 10, where both IFO and mesna were administered at concentrations of 60 and 12 mg/kg, respectively, was 2.59 and this was the highest recording of this marker in the whole protocol. This value was appreciably higher (Ρ < 0.01) in comparison with the value of the control group and even higher than the value obtained from subgroup 4, where only IFO was added at the same concentration. The PRI values for all subgroups are graphically illustrated in Fig. 2b.
Administration of IFO caused a decrease in Mitotic Index regarding subgroups 2, 3 and 4. However, the decrease of the marker does not appear to be statistically important (Ρ > 0.05). Contrary to the action of IFO, mesna increased the MI in a non statistically significant manner (Ρ > 0.05), when administered in subgroups 5, 6, and 7. Co-administration of both agents proved that the action of mesna reduces the cytotoxic action of IFO (Mitotic Index). The above observation is reinforced by the increase of the MI on the lymphocytes cultures of subgroups where both agents were added (IFO and Mesna), when compared with the subgroups where only IFO was added at the same concentrations. Regarding subgroup 10 where mesna and IFO were co-administered at concentrations of 12 and 60 mg/kg, respectively, there was a significant increase in Mitotic index (24.0 ‰) compared with subgroup 4 (16.5 ‰) where IFO acted at the same concentration alone. It is this observation that proves the strongly protective nature of mesna. The MI values for all lymphocytes of all subgroups of experimental animals are illustrated in Fig. 2c.
Table 1 shows the results for the three cytogenetic indices: SCEs, PRI and MI.
Discussion
IFO is considered to be one of the most effective chemotherapeutic agents in the management of numerous and various neoplasms. However, in spite of its successful anticancer action, the increased toxicity presented in healthy tissues and particularly in the renal and the bladder epithelium, led to its restricted usage and administration. This pressing issue was overcome with the development of mesna acting in an antioxidant fashion, was proven to successfully protect from the running risk of emergence of hemorrhagic cystitis as well as from other side effects of IFO (Anderson 2010; Ichiki et al. 2003; Olver et al. 2005). IFO is a well known and widely applied anticancer substance but it also has cytotoxic effects. It is this property that classifies IFO among agents that should only be administered with great attention and precision with respect to the dosage, and it is for this reason that special software programs have been developed for its administration (Okayasu et al. 2009). IFO and its derivatives, along with other alkylating factors are well known for inducing SCEs (Ciesielska et al. 1993; Fousteris et al. 2007; Karapidaki et al. 2009; Digkas et al. 2010; Mourelatos et al. 2012a).
Mesna is extensively applied during chemotherapy, along with IFO and in combination with other chemotherapeutic factors, in order to decrease the toxic action of the drugs and eliminate or minimize certain side effects (Olver et al. 2005; Hogle 2007; Okayasu et al. 2009; Anderson 2010). Moreover, mesna shields human cultured lymphocytes from the cytotoxic action of cyclofosfamide and its derivatives (Wilmer et al. 1986b; Mourelatos et al. 2012b).
The cytogenetic study of the above mentioned agents, in evaluating and assessing the genetic damage caused on healthy cells by IFO alone, and the degree of protection offered by mesna are the aim of this study.
This study is focused on the in vitro analysis of the SCEs levels of peripheral rabbit lymphocytes, following in vivo administration of IFO, mesna and their combinations in increasing concentrations. Sister chromatid exchanges are indicators of a pre-existing DNA damage, remaining unrepaired during the duration of both cell cycles required for the incorporation of BrdU in vitro. Consequently, SCEs assessment is a sensitive and efficient method for evaluating the mutagenic action of various substances (Mpountoukas et al. 2008; Lialiaris et al. 2009b).
The results support that IFO administration increases appreciably the emergence of SCEs in a dosage-dependent manner, which is in agreement with Ciesielska et al. (1993). Even though IFO acts in a cytostatic manner, our results didn’t reveal strong cytostaticity. This observation could be attributed to the fact that our study was an in vitro examination of the in vivo action of IFO. Consequently, lack of the presence of the agents could potentially result in the cell reverting to its original proliferation pace. Nevertheless, the genetic damage induced on DNA still remains at a high level, possibly due to the inadequacy of the DNA repair mechanisms to deal with the load of breakages caused by the alkylating action of the metabolites of IFO.
The action of mesna on lymphocytes does not appear to drastically affect levels of SCE when acting alone on in vivo lymphocytes. This mesna related finding is in agreement with Becher et al. (1983). Therefore, the action of mesna when administered alone in experimental animals can be considered to be non-genotoxic. On the other hand the action of mesna, appreciably improved the ability of the to lymphocytes advance their cell cycle, a finding that is also evident from the statistically important PRI increase in comparison with the marker’s value for the control. The Mitotic Index values were not considerably altered from the action of mesna.
The combined administration of both agents in experimental animals offers the possibility of controlling the cytogenetic result of the simultaneous action of both agents on the cells. The presence of mesna leads to a dramatic decrease of SCEs levels, confirming the powerful protective effect of this agent on the cells, fighting the genotoxicity caused by IFO. As illustrated by the results, the simultaneous action of mesna and IFO leads to the reduction of the genotoxicity of IFO. This is clearly demonstrated by the statistically significant lower SCE’s levels of rabbit lymphocytes, in subgroups where both factors were administered (IFO and mesna), compared with the respective concentrations of subgroups where IFO was administered alone. Moreover, mesna helps cells to accelerate their cell cycle. This is clearly demonstrated by studying the PRI of the lymphocytes, an index that is statistically significantly higher when mesna is added, than when IFO is acting alone at the same concentration. Furthermore, it is the subgroup of lymphocytes (10) that was exposed to the combination of the highest concentrations for both, IFO and mesna, that presented a statistically drastic increase of the MI.
The administration of mesna requires great attention especially when it is combined with IFO. The reason is that the intense reduction of the genotoxicity caused could countermand the genotoxic and cytostatic effect of IFO, particularly when the latter is administered at a high dosage. On the other hand, the reduction of genotoxicity of IFO that is achieved through mesna administration, acts in a protective fashion towards the cells that are not the target of the IFO therapy. Therefore, mesna contributes to the general survival and wellbeing of the patient. In conclusion, cytogenetic methods provide important and reliable indications for the safety and efficacy of pharmaceutical agents, their derivatives and/or combinations, contributing substantially to the design and development of novel, safer and efficient therapeutic approaches to the battle against cancer.
Conflict of interest
None
References
- Anderson P. Continuously improving ifosfamide/mesna: a winning combination. Pediatr Blood Cancer. 2010;55:599–600. doi: 10.1002/pbc.22652. [DOI] [PubMed] [Google Scholar]
- Becher R, Kakati S, Gibas Z, Sandberg AA. Cytogenetic testing of mutagenic and radioprotective effects of mesna. Oncology. 1983;40:287–289. doi: 10.1159/000225745. [DOI] [PubMed] [Google Scholar]
- Broadhead CL, Walker D, Skinner R, Simmons NL. Differential cytotoxicity of ifosfamide and its metabolites in renal epithelial cell cultures. Toxicol In Vitro. 1998;12:209–217. doi: 10.1016/S0887-2333(97)00113-6. [DOI] [PubMed] [Google Scholar]
- Chen N, Aleksa K, Woodland C, Rieder M, Koren G (2007) The effects of N-acetylcysteine on ifosfamide-induced nephrotoxicity: in vitro studies in renal tubular cells. Transl Res 150:51–57 [DOI] [PubMed]
- Ciesielska E, Mordalska A, Dzwonkowska A, Kinas R, Szmigiero L. Sister chromatid exchanges induced in vitro in human lymphocytes by N-substituted phosphodiaminic acids. Acta Biochim Pol. 1993;40:77–79. [PubMed] [Google Scholar]
- Das BC. Factors that influence formation of sister chromatid exchanges in human blood lymphocytes. Crit Rev Toxicol. 1988;19:43–86. doi: 10.3109/10408448809040817. [DOI] [PubMed] [Google Scholar]
- Dechant KL, Brogden RN, Pilkington T, Faulds D (1991) Ifosfamide/mesna. A review of its antineoplastic activity, pharmacokinetic properties and therapeutic efficacy in cancer. Drugs 42:428–467 [DOI] [PubMed]
- Digkas EN, Chrisafi S, Passadaki T, Tsalkidis A, Hatzimichail A, Vargemezis V, Lialiaris TS (2010) The effect of recombinant human erythropoietin on the frequency of sister chromatid exchanges (SCEs) alone or in combination with MMC. Chemotherapy 56:239–247 [DOI] [PubMed]
- Fousteris MA, Koutsourea AI, Arsenou ES, Papageorgiou A, Mourelatos D, Nikolaropoulos SS (2006) Structure-anti-leukemic activity relationship study of B- and D-ring modified and non-modified steroidal esters of chlorambucil. Anticancer Drugs 17:511–519 [DOI] [PubMed]
- Fousteris MA, Papageorgiou A, Arsenou ES. Antileukemic and cytogenetic activity by triple administration of three modified steroidal derivatives of nitrogen mustards. Chemotherapy. 2007;53:118–126. doi: 10.1159/000099983. [DOI] [PubMed] [Google Scholar]
- Glezerman IG. Successful treatment of ifosfamide-induced hyponatremia with AVP receptor antagonist without interruption of hydration for prevention of hemorrhagic cystitis. Ann Oncol. 2009;20:1283–1285. doi: 10.1093/annonc/mdp312. [DOI] [PubMed] [Google Scholar]
- Goto K, Maeda S, Kano Y, Sugiyama T (1978) Factors involved in differential Giemsa staining of sister chromatids. Chromosoma (Berl) 66:351–359 [DOI] [PubMed]
- Hansen RJ, Ludeman SM, Paikoff SJ. Role of MGMT in protecting against cyclophosphamide-induced toxicity in cells and animals. DNA Repair (Amst) 2007;6:1145–1154. doi: 10.1016/j.dnarep.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle W. Cytoprotective agents used in the treatment of patients with cancer Semin Oncol. Nursing. 2007;23:213–224. doi: 10.1016/j.soncn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ichiki M, Gohara R, Rikimaru T, Kitajima T, Fujiki R, Shimada A, Aizawa H (2003) Combination chemotherapy with irinotecan and ifosfamide as second-line treatment of refractory or sensitive relapsed small cell lung cancer: a phase II study. Chemotherapy 49:200–205 [DOI] [PubMed]
- Istifli ES, Topakt M (2012) Genotoxicity of pemetrexed in human peripheral blood lymphocytes. Cytotechnology 65:621–628 [DOI] [PMC free article] [PubMed]
- Johnstone CE, Lind JM, Griffin JM, Boddy VA. Ifosfamide metabolism and DNA damage in tumour an peripheral blood lymphocytes of breast cancer patients. Cancer Chemother Pharmacol. 2000;46:433–441. doi: 10.1007/s002800000185. [DOI] [PubMed] [Google Scholar]
- Karapidaki I, Bakopoulou A, Papageorgiou A, Iakovidou Z, Mioglou E, Nikolaropoulos S, Mourelatos D, Lialiaris T (2009) Genotoxic, cytostatic, antineoplastic and apoptotic effects of newly synthesized antitumour steroidal esters. Mutat Res 675:51–59 [DOI] [PubMed]
- Lameire N, Kruse V, Rottey S. Nephrotoxicity of anticancer drugs—an underestimated problem? Acta Clin Belg. 2011;66:337–345. doi: 10.1179/acb.2011.001. [DOI] [PubMed] [Google Scholar]
- Lawson M, Vasilaras A, De Vries A, Mactaggart P, Nicol D (2008) Urological implications of cyclophosphamide and ifosfamide. Scand J Urol Nephrol 42:309–317 [DOI] [PubMed]
- Lialiaris T, Lyratzopoulos E, Papachristou F, Simopoulou M, Mourelatos C, Nikolettos N (2008) Supplementation of melatonin protects human lymphocytes in vitro from the genotoxic activity of melphalan. Mutagenesis 23:347–354 [DOI] [PubMed]
- Lialiaris TS, Papachristou F, Mourelatos C, Simopoulou M (2009a) Antineoplastic and cytogenetic effects of chlorpromazine on human lymphocytes in vitro and on Ehrlich ascites tumor cells in vivo. Anticancer Drugs 20:746–751 [DOI] [PubMed]
- Lialiaris T, Polyzou A, Mpountoukas P, Tsiggene A, Kouskoukis A, Pouliliou S, Paraskakis E, Tentes I, Trypsianis G, Chatzimichail A (2009b) Chromosome instability on children with asthma. J Asthma 46:841–844 [PubMed]
- Lialiaris T, Mavromatidou P, Digkas E, Passadaki T, Mpountoukas P, Panagoutsos S, Vargemezis V (2010a) Chromosome instability in patients with chronic renal failure. Genet Test Mol Biomarkers 14:37–41 [DOI] [PubMed]
- Lialiaris T, Mavromatidou P, Dogkas E. Chromosome instability in patients with chronic renal failure. Genet Test Mol Biomarkers. 2010;14:37–41. doi: 10.1089/gtmb.2009.0109. [DOI] [PubMed] [Google Scholar]
- Lokiec F. Ifosfamide: pharmacokinetic properties for central nervous system metastasis prevention. Ann Oncol. 2006;17:iv33–iv36. doi: 10.1093/annonc/mdj997. [DOI] [PubMed] [Google Scholar]
- Malik AI, Mehboobali N, Iqbal MP. Effect of ifosfamide on intracellular glutathione levels in peripheral blood lymphocytes and its correlation with therapeutic response in patients with advanced ovarian cancer. Cancer Chemother Pharmacol. 1997;39:561–565. doi: 10.1007/s002800050616. [DOI] [PubMed] [Google Scholar]
- Mamur S, Yuzbasioglu D, Unal F, Aksoy H. Genotoxicity of food preservative sodium sorbate in human lymphocytes in vitro. Cytotechnology. 2012;64:553–562. doi: 10.1007/s10616-012-9434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskaleris T, Lialiaris T, Triantaphyllidis C. Induction of cytogenetic damage in human lymphocytes in vitro and of antineoplastic effects in Ehrlich ascites tumor cells in vivo treated by methotrexate, hyperthermia and/or caffeine. Mutat Res. 1998;422:229–236. doi: 10.1016/S0027-5107(98)00198-5. [DOI] [PubMed] [Google Scholar]
- Mourelatos C, Nikolaropoulos S, Fousteris M, Pairas G, Argyraki M, Kareli D, Dafa E, Mourelatos D, Lialiaris T (2012a) Synergistic cytogenetic and antineoplastic effects by the combined action of esteric steroidal derivatives of nitrogen mustards. Genet Test Mol Biomarkers 16:558–562 [DOI] [PubMed]
- Mourelatos C, Kareli D, Dafa E, Argyraki M, Koutsourea A, Papakonstantinou I, Fousteris M, Pairas G, Nikolaropoulos S, Lialiaris TS (2012b) Cytogenetic and antineoplastic effects by newly synthesized steroidal alkylators in lymphocytic leukemia P388 cells in vivo. Mutat Res 746:1–6 [DOI] [PubMed]
- Mpountoukas P, Vantarakis A, Sivridis E, Lialiaris T. Cytogenetic study in cultured human lymphocytes treated with three commonly used preservatives. Food Chem Toxicol. 2008;46:2390–2393. doi: 10.1016/j.fct.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Ogura K, Goto T, Imanishi J, Shinoda Y, Okuma T, Tsuda Y, Kobayashi H, Akiyama T, Hirata M, Yamamoto A, Kawano H (2013) Neoadjuvant and adjuvant chemotherapy with modified mesna, adriamycin, ifosfamide and dacarbazine (MAID) regimen for adult high-grade non small round cell soft tissue sarcomas. Int J Clin Oncol 18:170–176 [DOI] [PubMed]
- Okayasu S, Nakamura M, Sugiyama T, Chigusa K, Sakurai K, Matsuura K, Yamamoto M, Kinosada Y, Itoh Y (2009) Development of computer-assisted biohazard safety cabinet for preparation and verification of injectable anticancer agents. Chemotherapy 55:234–240 [DOI] [PubMed]
- Olver I, Keefe D, Myers M, Caruso D (2005) A phase I study of prolonged ambulatory infusion of ifosfamide with oral mesna. Chemotherapy 51:142–146 [DOI] [PubMed]
- Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (2008) Cancer management: a multidisciplinary approach. Medical Surgical and radiation oncology 11th edition: CMP medica; Available from http://www.cancernetwork.com/cancer-management-12/display/article/10165/1546278
- Storme T, Deroussent A, Mercier L, Prost E, Re M, Munier F, Martens T, Bourget P, Vassal G, Royer J, Paci A (2009) New ifosfamide analogs designed for lower associated neurotoxicity and nephrotoxicity with modified alkylating kinetics leading to enhanced in vitro anticancer activity. J Pharmacol Exp Ther 328:598–609 [DOI] [PubMed]
- Souid AK, Fahey RC, Aktas MK, Sayin OA, Karjoo S, Newton GL, Sadowitz PD, Dubowy RL, Bernstein ML (2001) Blood thiols following amifostine and mesna infusions, a pediatric oncology group study. Drug Metab Dispos 29:1460–1466 [PubMed]
- Wilmer JL, Erexson GL, Kligerman AD. Attenuation of cytogenetic damage by 2-mercaptoethanesulfonate in cultured human lymphocytes exposed to cyclophosphamide and its reactive metabolites. Cancer Res. 1986;46:203–210. [PubMed] [Google Scholar]
- Wilmer JL, Erexson GL, Kligerman AD. Attenuation of cytogenetic damage by 2-mercaptoethasulfonate in cultured human lymphocytes exposed to cyclophosphamide and its reactive metabolites. Cancer Res. 1986;46:203–210. [PubMed] [Google Scholar]
- Yilmaz S, Unal F, Yuzbasioglu D. The in vitro genotoxicity of benzoic acid in human peripheral blood lymphocytes. Cytotechnology. 2009;60:55–61. doi: 10.1007/s10616-009-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki EL, Springate JE, Taub M. Comparative toxicity of ifosfamide metabolites and protective effect of mesna and amifostine in cultured renal tubule cells. Toxicol In Vitro. 2003;17:397–402. doi: 10.1016/S0887-2333(03)00044-4. [DOI] [PubMed] [Google Scholar]