Abstract
Complex organisms may coordinate molecular responses to hypoxia by specialized avenues of communication across multiple tissues, but these mechanisms are poorly understood. Plasma-based, extracellular microRNAs have been described, yet, their regulation and biological functions in hypoxia remain enigmatic. We found a unique pattern of release of the hypoxia-inducible microRNA-210 (miR-210) from hypoxic and reoxygenated cells. This microRNA is also elevated in human plasma in physiologic and pathologic conditions of altered oxygen demand and delivery. Released miR-210 can be delivered to recipient cells, and its direct suppression of its direct target ISCU and mitochondrial metabolism is primarily evident in hypoxia. To regulate these hypoxia-specific actions, prolyl-hydroxylation of Argonaute 2 acts as a molecular switch that reciprocally modulates miR-210 release and intracellular activity in source cells as well as regulates intracellular activity in recipient cells after miR-210 delivery. Therefore, Argonaute 2-dependent control of released miR-210 represents a unique communication system that integrates the hypoxic response across anatomically distinct cells, preventing unnecessary activity of delivered miR-210 in normoxia while still preparing recipient tissues for incipient hypoxic stress and accelerating adaptation.
Keywords: endothelial, circulating microRNA, hypoxia, hypoxamir, mitochondrial metabolism
Introduction
Hypoxic-ischemic and reoxygenation injury is associated with a variety of complex human diseases and is a leading cause of morbidity and mortality, affecting every major organ system. Although poorly understood, hypoxic adaptation in a complex mammal may depend upon close communication among anatomically distant tissues in order to protect vital organs from incipient hypoxic stress. For example, remote ischemic preconditioning (remote IPC) is a powerful innate mechanism which prevents lethal ischemic injury by prior sub-lethal ischemia at an anatomically distant site [1] and has been exploited in a number of clinical settings [2]. Remote IPC and its associated applications may rely upon release of endocrine factors from ischemic cells that initiate adaptive gene programs in recipient tissue [3], and a number of these factors may be directly regulated by the master transcription factor of hypoxia, HIF-α [4–6]. However, the full complement of such HIF-dependent molecular effectors remains undefined.
Previously, we reported that the hypoxia-inducible microRNA-210 (miR-210) controls such adaptive responses to hypoxia at the intracellular level [7]. To do so, HIF-1α up-regulates expression of miR-210, which then represses its direct target, the iron-sulfur cluster assembly protein, ISCU. Consequently, deficient ISCU expression reduces iron-sulfur cluster integrity and thus represses key metabolic processes including mitochondrial electron transport and the tricyclic acid (TCA) cycle in favor of glycolysis. Additionally, miR-210 has been found to down-regulate other related mitochondrial targets [8, 9], thereby producing a combinatorial effect on inhibiting electron transport and mitochondrial respiration. As a result, this metabolic shift, known as the “Pasteur effect,” improves cell survival and function in acute hypoxia while avoiding excessive generation of toxic reactive oxygen species (ROS) [10] and thus preserving overall mitochondrial integrity and function after the ischemic threat has ceased.
Recently, it has been discovered that microRNAs (miRNAs) are stably secreted into the bloodstream at rest [11] and in response to tissue injury and other pathological conditions [12–14]. Increases of plasma-based expression of miR-210 have been reported in the context of various cancers, such as pancreatic [15], renal [16, 17], and breast [18, 19] cancer, as well as other systemic diseases [20–23]. Plasma-based, “circulating” miRNAs may be protected from degradation by several complementary mechanisms including packaging in phospholipid bilayer-encapsulated microvesicles or exosomes [24] and/or via the formation of miRNA-protein complexes, such as those bound to the Argonaute 2 protein (AGO2) [25] or high density lipoprotein (HDL) [26]. In the setting of microvesicle release, it has been reported that some miRNAs are also complexed with AGO2, can be directly taken up by recipient cells, and have been implicated in cellular functions important in certain diseases [26–30] such as modulation of atherosclerotic lesions [31] and tumor metastasis [32] in mice, to name a few examples. Yet, the molecular regulation of the release and activity of extracellular miRNAs, especially in hypoxia, has been poorly characterized to date. Furthermore, beyond its potential role as a biomarker, a specific function of miR-210 as a circulating factor in hypoxia to communicate among distinct tissues has not been fully explored.
In this report, we define released miR-210 as a highly regulated effector communicating between hypoxic source tissue and recipient cells. Moreover, we describe prolyl-hydroxylation of AGO2 as a pivotal molecular switch that controls miR-210 release and miR-210 activity upon uptake into recipient cells. These results augment our fundamental understanding of how distant tissues may communicate via extracellular miRNAs in order to maintain precise control of hypoxic adaptation across anatomically distinct cells.
Materials and Methods
Cells, animals, and human reagents
Primary human pulmonary arterial endothelial cells (HPAECs), human pulmonary arterial smooth muscle cells (HPASMCs), and normal human lung fibroblasts (NHLF) were commercially purchased (Lonza); experiments were performed at passages 3–9. Human embryonic kidney 293 cells (HEK293) and HT-29 colon adenocarcinoma cells were obtained from ATCC. The generation of mmu-miR-210 −/− mice was previously described [33], and these mice were a generous gift from Dr. A. Bradley and Dr. H. Prosser (Wellcome Trust, U.K.). The Harvard Center for Comparative Medicine approved the use of animals in these experiments.
Human Participants
Written informed consent was obtained from all participants; this conformed to the standards set by the latest revision of the Declaration of Helsinki. Before study initiation, the Partners Human Research Committee approved all protocols for human plasma study and collection. A case:control strategy was used in all comparisons. In the first case, as chosen from a larger, previously described cohort [34, 35], women of Andean descent at 36 week gestation with healthy pregnancies were compared -- those living chronically at low altitude (400 m, Santa Cruz, Bolivia) and those living at neighboring high altitude (3,600m–4,100m, La Paz or El Alto, Bolivia). In the second case, age-matched human subjects were chosen from the vascular medicine research laboratory at the Brigham and Women’s Hospital and stratified by the presence or absence of leg claudication and peripheral artery disease based on ankle:brachial index <0.90.
Exposure of cultured mammalian cells to conditions of hypoxia and reoxygenation
Cells were cultured in standard non-hypoxic cell culture conditions (20% O2, 5% CO2, with N2 balance at 37°C). Alternatively, hypoxic conditions (0.2%–2% O2, 5% CO2, with N2 balance at 37°C) were generated in a modular hypoxia chamber. Cells were exposed to either normoxia (20% O2) or hypoxia (0.2% O2) for 24 and 48 hours (e.g., HEK293 cells in DMEM media supplemented with 10% fetal calf serum, and HPAECs in EGM-2 media with 5% fetal calf serum [Lonza]). To simulate reoxygenation, cells were exposed to hypoxia (either 24 or 48 hours) followed by aspiration of culture media. Cells were then washed with PBS before adding fresh media followed by incubation for an additional 24 or 48 hours in normoxia (20% O2).
MiRNA/AGO2 co-immunoprecipitation of conditioned media
HEK293 cells or HPAECs were exposed to growth media (as above) for 48 hours in 0.2% O2. In order to remove cellular debris, conditioned media were centrifuged at 3000 × g for 10 minutes at 4°C, and then syringe filtered (Nalgene, 0.2 μm). Media were then concentrated ~ 100-fold via centrifugation through a 100 kDa membrane filter, per the manufacturer’s instructions (Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-100 membrane, Millipore). Concentrated conditioned media were then exposed to Protein A/G magnetic beads conjugated to anti-AGO2 or isotype IgG control mouse monoclonal antibody (Millipore) for 4 hours at 4°C, per the manufacturers’ instructions (Magna RIP RNA-Binding Protein Immunoprecipitation Kit, Millipore). After serial washing of beads, bound protein-miRNA complexes were removed from beads. Eluant was subjected to immunoblotting to confirm proper immunoprecipitation. Separately, miRNA expression in the eluant was assessed by RNA extraction and RT-qPCR, as described in Supplementary Data.
Exposure of cultured recipient cells to conditioned media
To generate high levels of released miR-210, donor HEK293 cells were transfected with an expression vector encoding miR-210, plenti-210 (versus plenti-Cont) and exposed to DMEM media supplemented with 10% fetal calf serum for 48 hours in 0.2% O2. Separately, to enrich conditioned media with endogenously expressed miR-210, non-transfected HT-29 cells were exposed to hypoxia (0.2% O2) for 48 hours followed by a 6 hour period of reoxygenation (20% O2). Conditioned media and donor cells were then harvested. Following centrifugation and syringe filtering of conditioned media as above to remove cellular debris as above, an equal volume of fresh growth media was added to conditioned media in order to replenish growth factors and nutrients after initial conditioning. Immediately afterwards, naive recipient miR-210 −/− MEF cells cultured in advance in 6-well plates, were then exposed to these conditioned media (2 ml/well) for 48 hours in hypoxia (0.2% O2) (or continued normoxia as a control). The cells were harvested for RT-qPCR analysis, standard immunoblotting, or Complex I enzyme activity quantification, as described below and in Supplementary Data.
Production of HPAEC conditioned media for microvesicle analysis
HPAECs were cultured in 10mm dishes in 1% FBS containing cell culture medium in order to minimize serum protein interference with microvesicle analysis. To ensure that all microvesicles contained in the conditioned media were derived from source cells, fetal bovine serum was subjected to ultracentrifugation (100,000 × g for two hours) prior to addition to basal cell culture media. Cells were exposed to either 48 hours of normoxia (20% O2), 48 hours of hypoxia (0.2% O2), or 48 hours of hypoxia (0.2% O2) followed by PBS wash, media replacement, and an additional 48 hours of reoxygenation (20% O2). Resultant conditioned media was harvested for microvesicle analysis.
Separation of microvesicles in culture media for subsequent exposure to recipient cells
Cells and debris were removed from conditioned media produced as described above by differential centrifugation at 400 × g for 10 min and at 12,000 × g for 30 min. Five ml of clarified conditioned medium were then subjected to ultracentrifugation at 120,000 × g for 2 hours in order to deplete the sample of microvesicles. To expose recipient cells to these fractionated samples and determine delivery of extracellular miRNAs, the microvesicle-enriched sediments (pellets) were resuspended in a volume of media equivalent to that of the supernatant. An equal volume of fresh growth media was added to each sample in order to replenish growth factors and nutrients after initial conditioning. Media were then used to culture naïve miR-210 −/− MEFs for 48 hours.
MiRNA and protein analysis of fractionated conditioned media
Conditioned media from HPAECs were produced and subjected to ultracentrifugation at 120,000 × g for 2 hours, as described above. To analyze the separation of microvesicles by ultracentrifugation, equivalent volumes of non-ultracentrifuged (non-processed) conditioned media and ultracentrifuged supernatant were treated with 8.3% polyethylene glycol (Sigma, 89782; PEG4000, ~50% in H2O), 250 mM NaCl, 50 mM HEPES (pH7.2) at 4°C, for 18hrs) to precipitate microvesicles. Immunoblotting for microvesicle markers was then performed in order to compare microvesicles present in PEG precipitants with the ultracentrifuged sediments (pellets). Antibodies used to detect microvesicle markers included: Alix (3A9 clone), Flotillin-1, CD63 (H-193 clone) (Santa Cruz Biotechnology). Fibronectin was used as an unrelated control (Abcam). This method was previously described in detail [36]. Separately, to measure miRNAs present in each compartment, equivalent volumes of non-processed conditioned media and ultracentrifuged supernatant were subsequently concentrated via ultrafiltration using a 100kDa cutoff filter (Amicon Ulltra, Millipore). RNA was then extracted from both the concentrates and ultracentrifuged sediments for RT-qPCR analysis.
Detection of AGO2 complexes in the culture medium
HEK293 cells were co-transfected with plenti-210 along with either AGO2 WT FLAG or AGO2 P(700)A FLAG constructs. Transfected cells were incubated for 15 hours in normoxia (20% O2) under serum-free conditions. From each condition, 1 ml of media was centrifuged to pellet cellular debris (12,000 × g, 10 minutes) and then incubated overnight with 250 uL of Trichloroacetic Acid, 6.1 N (Sigma, T0699) at 4°C to precipitate the soluble protein components. To detect AGO2 FLAG-tagged transgenes, the pelleted precipitates were then immunoblotted with the M2-Flag mouse monoclonal antibody (Sigma-Aldrich). Intracellular levels of AGO2 FLAG-tagged constructs were compared in parallel by standard harvesting and immunoblotting with the M2-Flag mouse monoclonal antibody. Immunoblot for actin (Sigma-Aldrich) was used as a loading control for cellular components; Ponceau S stain (Sigma-Aldrich) was performed for extracellular components.
P4HA1 knockdown in HPAECs, HEK293 cells, and miR-210 −/− MEFs
HEK293 cells or HPAECs were transfected in 6-well plates with either siRNA specific for human P4HA1 (20 nM, Santa Cruz Biotechnology) or si-Control (20 nM, Santa Cruz Biotechnology). Cells were then exposed to 48 hours of hypoxia (0.2% O2), and cells and media were harvested for RT-qPCR analysis. Separately, recipient MEFs were transfected similarly with siRNA specific for mouse P4HA1 (20 nM, Santa Cruz Biotechnology) or si-Control and were exposed for 48 hours of hypoxia (0.2% O2) to filtered, conditioned media (as described above) derived from HEK293 cells transfected with plenti-210. Recipient MEFs were then harvested for RT-qPCR analysis and standard immunoblotting.
Detection of AGO2 FLAG in recipient miR-210 −/− MEF cells
miR-210 −/− MEF cells were exposed for 48 hours to filtered, conditioned media (prepared as above) derived from HEK293 cells transfected with either AGO2 WT FLAG or AGO2 P(700)A FLAG constructs and conditioned in hypoxia (0.2% O2) for 48 hours. Subsequently, MEF cells were lysed with 1% detergent in PBS (lauryl maltoside, Sigma-Aldrich), and lysates were exposed to Protein A/G magnetic beads conjugated to Anti-M2 FLAG mouse monoclonal antibody (Sigma-Aldrich) for 12 hours at 4°C, as per the manufacturer’s instructions (Magna RIP RNA-Binding Protein Immunoprecipitation Kit, Millipore). After serial washing of the beads, presence of FLAG-tagged AGO2 was determined by immunoblotting of the eluant using the M2 FLAG antibody.
RNA Interference Transactivation Assays
miR-210 −/− MEFs were transfected with either psicheck-ISCU or psicheck-cont (2 μg/well) in 6-well plates. After 24 hours, transfected cells were exposed to miR-210-enriched conditioned media versus miR-210-deficient media, as derived from HEK293 donor cells described above. Cells were then exposed to normoxia or hypoxia for 48 hours. Subsequently, Renilla luciferase activity and firefly luciferase activity were quantified by the Dual Luciferase Assay System (Promega). For each well, Renilla luciferase activity was normalized to firefly luciferase activity.
Complex I Activity Assay
Cultured cells were lysed in 1% detergent (lauryl maltoside, Sigma-Aldrich) in PBS for membrane protein extraction. As previously described [7], Complex I activity was measured by immunocapture and spectrophotometry (A340nm) reflecting oxidation of NADH to NAD+, per the manufacturer’s instructions (Mitosciences).
Statistical analysis
Unless otherwise indicated, all numerical quantifications represent mean ± standard error of the mean for three or more independent experiments, each performed at least in triplicate (N=number of independent experimental repetitions). Images are representative of experiments that have been repeated at least three times. Paired samples were compared by Student’s t test. Comparison of multiple samples was performed by one-way ANOVA followed by Student Newman-Keuls post hoc tests to calculate p-values. In the peripheral arterial disease cohort, a Wilcoxon Rank Sum test was used to analyze non-normally distributed continuous variables (HDL and triglycerides in patient samples); a chi-squared test was used to analyze categorical variables. Error bars reflect SEM; * p≤0.05, ** p≤ 0.01, *** p≤0.001, NS signifies p>0.05.
Additional information
See Supplementary Data where previously published procedures are described for RNA extraction from plasma, conditioned media, and cells; RT-qPCR, immunoblotting, plasmids, antibodies, and standard methods of transfection of cultured cells, immunoblotting, isolation of MEFs, and human plasma sampling.
Results
MiR-210 is released from mammalian cells in culture and in vivo during hypoxia and reoxygenation
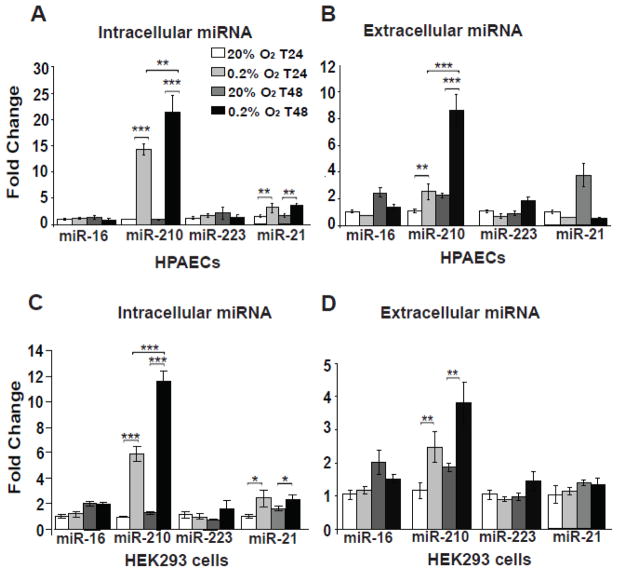
We first wanted to determine whether a direct causative link exists between hypoxia, reoxygenation, and the release of miR-210 into the extracellular space. To do so, based on our prior observations of the robust actions of miR-210 in primary cultured human pulmonary arterial endothelial cells (HPAECs) and transformed human embryonic kidney 293 cells (HEK293) [7], these cell types were first exposed to normoxia (20% O2) versus hypoxia (0.2% O2) for 24 hours and normoxia versus hypoxia for 48 hours. For comparison, miR-16 was chosen for analysis, given its high levels of reported release [25] but stability of expression in hypoxia. miR-223 was also studied, given its reported transport in HDL-containing complexes [26]. miR-21 was chosen, given its known regulation by hypoxia, especially in HPAECs [37]. Unlike miR-16 or miR-223, intracellular levels of miR-210, and to a lesser extent intracellular miR-21, were progressively up-regulated by hypoxia at both time points and in both HPAECs (Fig. 1A) and HEK293 cells (Fig. 1C). Mirroring these intracellular levels, extracellular miR-210 levels were also progressively up-regulated under these conditions (Fig. 1B, 1D). Importantly, however, released levels of miR-16, miR-223, and miR-21 were not substantially altered in hypoxia, indicating a specificity of miR-210 release in hypoxia.
Figure 1. MiR-210 is released during hypoxia from human PAECs and HEK293 cells.
In contrast to miR-16 and miR-223 which were not hypoxia-dependent, intracellular miR-210, and to a lesser extent miR-21, in human PAECs (A) and HEK293 cells (C) increased after 24 hours of hypoxia (0.2% O2) and continued to increase after 48 hours of hypoxia. Importantly, contrasting extracellular miR-16, miR-223, and miR-21 levels, extracellular miR-210 from HPAECs (B) and HEK293 cells (D) was modestly increased after 24 hours of hypoxia and further increased after 48 hours of hypoxia. In all panels, for each miRNA tested, miRNA expression after 24 hours of normoxia was assigned a fold change of 1, to which other conditions were compared. Error bars reflect SEM; * p≤0.05, ** p≤ 0.01, *** p≤0.001, NS signifies p>0.05.
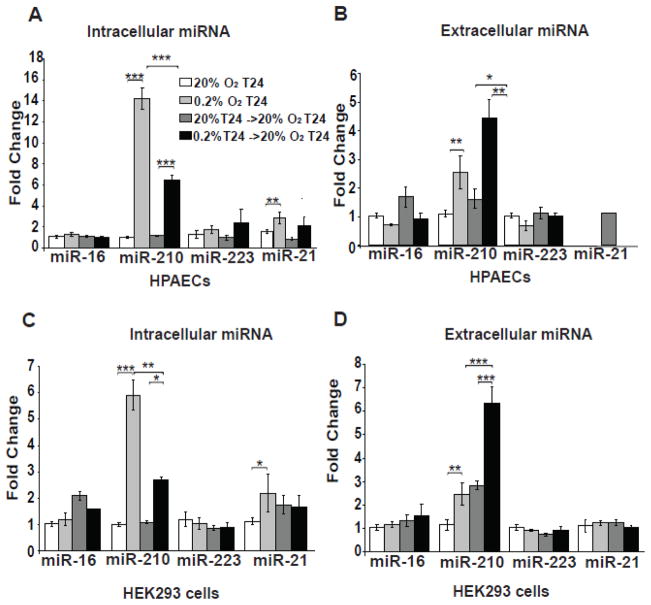
To determine whether such a linear relationship of intracellular and extracellular miR-210 exists during reoxygenation, both cell types were exposed to normoxia (20% O2) or hypoxia (0.2% O2) for 24 hours followed by media exchange and exposure to normoxia for an additional 24 hours. Intracellular miR-210 was up-regulated by hypoxia but then down-regulated by reoxygenation in both cell types (Fig. 2A, 2C). Extracellular miR-210 levels were also up-regulated during hypoxia in both cell types (Fig. 2B, 2D), but notably, released miR-210 expression remained elevated during reoxygenation, in contrast to the down-regulation of intracellular miR-210 in that time frame. In contrast, extracellular patterns of expression for miR-16, miR-223, and miR-21 were not similar to miR-210, again reflecting the specificity of these release patterns. Similar results of miR-210 release in reoxygenation were found in many, but not all (e.g., pulmonary arterial smooth muscle cells), primary and transformed cell types, with a particularly high level of release from HT-29 adenocarcinoma cells (Supplemental Fig. 1). Thus, although there is some heterogeneity of release depending upon cell context, specific mechanisms across multiple cell types exist that similarly regulate the release of miR-210 in hypoxia and reoxygenated states and do not solely reflect changes in intracellular miRNA or generalized miRNA release.
Figure 2. MiR-210 is released during reoxygenation from human PAECs and HEK293 cells.
Unlike miR-16, miR-223, or miR-21, intracellular miR-210 in human PAECs (A) and HEK293 cells (C) increased after 24 hours of hypoxia (0.2% O2) but then decreased after 24 hours of hypoxia followed by 24 hours of re-oxygenation. Extracellular miR-210 from human PAECs (A) and HEK293 cells (C) also increased after 24 hours of hypoxia but was released in greater quantity during reoxygenation. Neither hypoxia nor reoxygenation increased extracellular release of miR-16 or miR-223. In all panels, for each miRNA tested, miRNA expression after 24 hours of normoxia was assigned a fold change of 1, to which other conditions were compared. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
To determine whether extracellular miR-210 is preferentially released into the circulating blood stream in vivo, two models of physiologic and pathophysiologic exposure to hypoxia and ischemia were characterized in humans. In a cohort of Andean women of 36 weeks gestation chronically living at altitude (3,600–4,100 m, La Paz or El Alto, Bolivia), plasma levels of miR-210 were increased as compared with matched pregnant Andean controls living at sea level (400 m, Santa Cruz, Bolivia) [Fig. 3A; subject demographics previously described in [34, 35] with additional parameters summarized in Table S1]. Alternatively, circulating levels of miR-210 were increased in the plasma of humans with intermittent claudication secondary to peripheral artery disease as compared with age/gender matched healthy controls (Fig. 3C; subject demographics summarized in Table S2). In contrast, the brain-enriched miR-134, which is not hypoxia-responsive, displayed negligible alterations among these matched cohorts (Fig. 3B, 3D). As a result, by analysis of conditioned cell culture media and human plasma, we have determined that exposure to hypoxia or alterations in oxygen demand/delivery in both physiologic and disease contexts increases the release of miR-210, possibly reflecting a fundamental action of this extracellular miRNA.
Figure 3. Extracellular miR-210 is increased in human plasma during conditions of increased oxygen demand or deficient oxygen delivery.
Circulating levels of miR-210 (A) but not miR-134 (B) were increased in plasma of Andean women at 36 weeks gestation chronically living at altitude as compared with Andean women at 36 weeks gestation chronically living at sea level. Similarly, circulating miR-210 (C) but not miR-134 (D) was increased in the plasma of subjects suffering from active claudication secondary to peripheral arterial disease as compared with healthy controls. In all panels, control miRNA levels (low altitude subjects in A–B; healthy controls in C–D) were assigned a fold change of 1, to which other conditions were compared. Data are presented as box and whisker plots signifying fold changes in c-miRNA levels, where horizontal lines denote median, boxes denote 25% and 75% percentile confidence intervals, and error bars reflect maximum and minimum values. * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
The majority of miR-210 found in the extracellular space is not contained within microvesicles
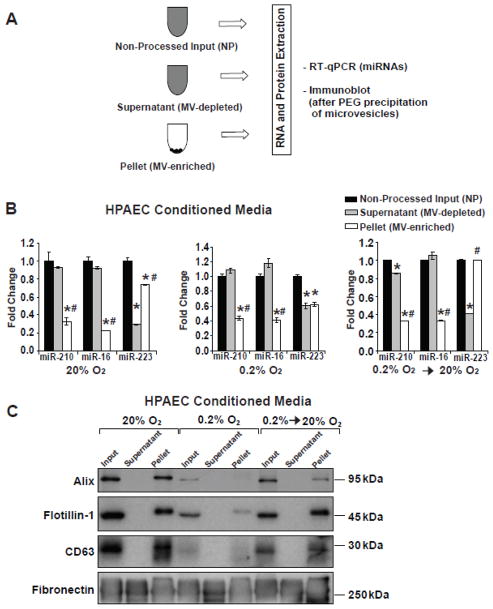
Previously, c-miR-210 (circulating or extracellular miR-210) has been reported within [38–40] and outside of microvesicles [25]. To determine the relative levels of endogenous packaging of released miR-210 after hypoxia or reoxygenation, HPAECs cultured in media containing 1% FBS (previously depleted of microvesicles) were subjected to 48 hours of normoxia; 48 hours of hypoxia; or 48 hours of hypoxia followed by media replacement and 48 additional hours of reoxygenation. In each context, conditioned media was subjected to ultracentrifugation (120,000 × g for 2h) (Fig. 4A). By immunoblot, microvesicle markers were found in the ultracentrifuged pellet and precipitated contents of the non-ultracentrifuged (non-processed) conditioned media but not supernatant (Fig. 4C), thus confirming successful separation by ultracentrifugation. In those samples, a higher proportion of miR-223 content was found in microvesicle-enriched fractions in normoxia and reoxygenation, while equivalent levels were observed in both fractions during hypoxic exposure (Fig. 4B). In contrast, for both miR-16 and miR-210, a majority of the total miRNA content was present in microvesicle-depleted fractions in normoxia, hypoxia, and reoxygenation (Fig. 4B). Thus, while all of these miRNAs were found in both fractions, a specificity in the patterns of miRNA release was evident, suggesting the existence of a regulatory system controlling miRNA packaging during exposures to variable oxygen conditions.
Figure 4. Distinct patterns of packaging miRNAs for release are evident during normoxia, hypoxia, and re-oxygenation.
Conditioned media derived from HPAECs were generated as described in Figure 2 and processed according to the schema in (A) to generate microvesicle-enriched (pellet) and microvesicle-depleted (supernatant) samples. (B) The relative amounts of specific miRNAs were quantified as contained in supernatant and pellet fractions of conditioned media after exposure to normoxia (20% O2), hypoxia (0.2% O2), and reoxygenation (0.2% O2 -> 20% O2). For each miRNA tested, miRNA expression in non-processed input was assigned a fold change of 1, to which other conditions were compared. Error bars reflect SEM; * denotes p≤0.05 when comparing with non-processed input; # denotes p≤0.05 when comparing with supernatant fraction. (C) Immunoblot analysis revealed the presence of microvesicle markers Alix, Flotillin-1, and CD63 in pellet but not supernatant fractions. Fibronectin was analyzed as an unrelated protein control. Immunoblots are representative of experiments performed at least in triplicate.
Hypoxia induces release of miR-210 associated with AGO2
Certain circulating miRNAs (c-miRNAs) have been described to bind AGO2 both within and outside of microvesicles [25, 41], but a specific association between AGO2 and circulating miR-210 has not been described. Therefore, we analyzed conditioned media from either HEK293 cells or HPAECs after exposure to hypoxia for 48 hours followed by centrifugal concentration of media using a 100 kDa filter. Concentrated media was then treated with detergent to release microvesicle contents, followed by co-immunoprecipitation using an α-AGO2 antibody that was confirmed to pull down AGO2 effectively (Fig. 5A). Released miR-210, but not released miR-223, co-immunoprecipitated specifically in the presence of α-AGO2 as compared with isotype control IgG (Fig. 5B–C). Thus, from both hypoxic HPAECs and HEK293 cells, miR-210 is released in association with AGO2.
Figure 5. Hypoxia induces release of miR-210 associated with AGO2.

From HPAEC protein lysates, immunoblot (A) demonstrated effective immunoprecipitation of AGO2 protein using an α-AGO2 antibody as compared with isotype IgG control. Using this α-AGO2 antibody for co-immunoprecipitation from concentrated conditioned media derived from hypoxic HEK293 cells (B) and hypoxic HPAECs (C), released miR-210, but not released miR-223, was found to associate specifically with AGO2. For RT-qPCR experiments, control miR-210 levels that were non-specifically immunoprecipitated by isotype IgG were assigned a fold change of 1, to which other conditions were compared. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
Intracellular hypoxic activation and extracellular release of miR-210 are conversely regulated by prolyl-hydroxylation of AGO2
Previously, hydroxylation of proline residue 700 of AGO2 was reported to increase the intracellular activity of miRNA for target gene repression [42]. Furthermore, hypoxia specifically induces such AGO2 prolyl-hydroxylation by induction of the C-P4H(I) prolyl-hydroxylase [43]. Given the association of miR-210 with AGO2 during extracellular release, we wanted to determine whether such prolyl-hydroxylation also influences miR-210 activity and release in hypoxia. Correlating with prior observations [43], we observed transcriptional up-regulation of the alpha subunit of C-P4H(I) (P4HA1) after 24 and 50 hours of hypoxia in HEK293 cells and HPAECs, accompanied by down-regulation of P4HA1 with reoxygenation (Supplemental Fig. 2A–B). siRNA-mediated knockdown of P4HA1 was also performed (Supplemental Fig. 2C–E) in order to verify the role of AGO2 prolyl-hydroxylation in miR-210 release. In HEK293 cells, while intracellular expression of miR-210 was not altered by siRNA delivery (Fig. 6A), expression of extracellular miR-210 was increased by P4HA1 knockdown (Fig. 6B) – a result that was especially evident after comparison to 50 hours of hypoxia and endogenous P4HA1 up-regulation with control siRNA. Similarly, knockdown of P4HA1 increased extracellular miR-210 derived from hypoxic HPAECs, but this was also accompanied by an additional increase of intracellular miR-210 (Fig. 6C–D). Importantly, similar results were obtained in HPAECs exposed to less severe hypoxia (2% O2, Supplemental Fig. 3). Thus, under a range of hypoxic conditions, P4HA1 controls the expression of extracellular miR-210.
Figure 6. Knockdown of P4HA1 augments the expression of released miR-210 in hypoxia.
In HEK293 cells exposed to either normoxia (20% O2) or hypoxia (0.2% O2) for 50 hours, while siRNA-mediated knockdown of P4HA1 did not alter intracellular expression of miR-210 (A), such knockdown of P4HA1 increased extracellular miR-210 expression (B). Similarly, in HPAECs exposed to either normoxia (20% O2) or hypoxia (0.2% O2) for 50 hours, knockdown of P4HA1 increased extracellular miR-210 expression (D) which in this case was accompanied by increased intracellular miR-210 expression (C). In all panels, miR-210 levels in normoxic cells treated with si-Control were assigned a fold change of 1, to which other conditions were compared. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
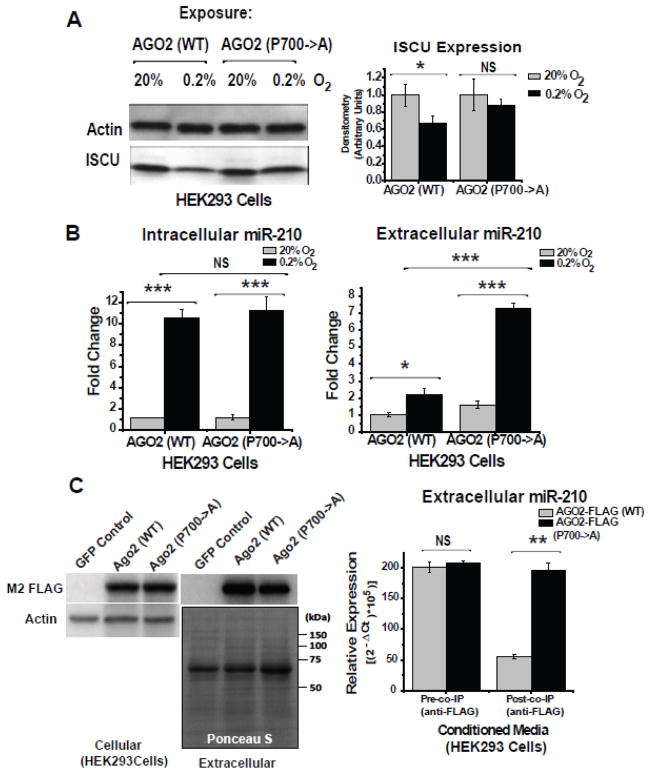
We then wanted to determine whether prolyl-hydroxylation of AGO2 specifically regulates the release of miR-210 rather than merely miR-210 expression. To do so, in HEK293 cells, two types of FLAG-tagged AGO2 transgenes were overexpressed: wildtype AGO2 and prolyl(700)-to-alanine hydroxylation mutant AGO2 [42]. In contrast to the wildtype AGO2 transgene that enabled miR-210-dependent repression of its direct target ISCU in hypoxia, overexpression of the non-hydroxylated proline-to-alanine (700) mutant AGO2 reduced the repressive activity of miR-210, allowing ISCU protein levels to increase in hypoxia (Fig. 7A). Thus, prolyl(700)-hydroxylation enhances intracellular miR-210 activity for target gene repression. Conversely, while hypoxic induction of intracellular miR-210 expression was comparable in the presence of either AGO2 transgene, release of miR-210 to the extracellular space was substantially increased by non-hydroxylated mutant AGO2 expression, particularly evident after 48 hours of hypoxia (Fig. 7B). Importantly, neither AGO2 transgene was expressed at substantially different levels in the intracellular or extracellular space (Fig. 7C). Nonetheless, demonstrated by co-immunoprecipitation of the extracellular AGO2 transgene products using an α-FLAG antibody, a higher proportion of released miR-210 was associated with the non-hydroxylated mutant as compared with wildtype AGO2 (Fig. 7C). Thus, the regulation of miR-210 release by AGO2 hydroxylation state does not rely solely upon a more abundant expression or release of the non-hydroxylated AGO2 itself. Rather, it may influence the propensity of miR-210 to associate with AGO2 under various oxygen-dependent exposures. Thus, considering together these convergent results, prolyl-hydroxylation appears to act as a molecular switch controlling cellular release of miR-210. In hypoxia, prolyl-hydroxylation maximizes the intracellular activity of miR-210 while blunting, but not entirely abrogating, its release. In normoxia and reoxygenation, prolyl-hydroxylation of AGO2 is inhibited, thus decreasing miR-210 intracellular activity and expediting the transport of excess miR-210 to the extracellular space.
Figure 7. Hypoxic activation and release of miR-210 are reciprocally regulated by state of prolyl-hydroxylation of AGO2.
(A) In HEK293 cells, direct repression of ISCU expression by intracellular miR-210 in hypoxia was decreased by a hydroxylation-deficient proline-to-alanine (700) mutant AGO2 as compared to wildtype AGO2. Immunoblots are representative of experiments performed at least in triplicate; gel densitometry was normalized to actin levels and compared as arbitrary units. (B) In contrast to intracellular miR-210 which was up-regulated to equivalent levels in HEK293 cells exposed to 0.2% O2 for 24 hours in the presence of either AGO2 transgene (left graph), release of miR-210 from HEK293 cells was increased by the hydroxylation-deficient mutant AGO2, especially in hypoxia (right graph). miR-210 expression from normoxic samples transfected with AGO2 (WT) transgene was assigned a fold change of 1, to which other conditions are compared. (C) By immunoblot, equivalent expression of AGO2 (WT) and AGO2 P700->A transgenes was observed both in the intracellular and extracellular compartments after transfection of HEK293 cells (left blots, HEK293 cells transfected with a non-FLAG tagged GFP transgene were used as a control). However, when comparing equivalent levels of released miR-210 from HEK293 cells expressing either AGO2 transgene (pre-co-IP), an increased level of released miR-210 co-immunoprecipitated with the non-hydroxylated mutant AGO2 as compared with wildtype AGO2 (post-co-IP) (right graph). Immunoblots are representative of experiments performed at least in triplicate. Ponceau S stain is shown as a loading control for extracellular protein components. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
Released miR-210 is transported into recipient cells, but its metabolic activity is augmented during hypoxia
To determine definitively whether extracellular miR-210 can be transported into recipient cells and to rule out the possibility of up-regulation of endogenous miR-210 encoded by recipient cells, miR-210 −/− murine embryonic fibroblasts (MEFs) were used as recipient cells and confirmed to express negligible levels of miR-210 (Fig. 8A). For these experiments, conditioned media replete with released miR-210 were prepared from HEK293 cells transfected with an expression plasmid for miR-210 and exposed to hypoxia for 48 hours. Upon exposure to miR-210 −/− MEFs either under normoxic or hypoxic conditions, intracellular miR-210 levels increased (Fig. 8A), thus demonstrating direct miRNA uptake.
Figure 8. Released miR-210 is delivered into recipient cells but its activity in regulating ISCU and mitochondrial metabolic activity is augmented in hypoxia.
(A) During normoxia (20% O2) and hypoxia (0.2% O2), intracellular miR-210 levels increased in miR-210 −/− MEF cells when exposed for 48 hours to conditioned media derived from HEK293 cells overexpressing miR-210. (B) As derived from conditioned media of HEK293 cells overexpressing miR-210, a higher proportion of extracellular miR-210 was observed in microvesicle-depleted (supernatant) media fractions as compared with microvesicle-enriched (pellet) fractions. (C) In contrast to conditioned media derived from miR-210 −/− MEFs (without detectable miR-210 denoted by #), both microvesicle-enriched (pellet) and microvesicle-depleted (supernatant) media fractions derived from HEK293 cells overexpressing miR-210 were sufficient to allow for delivery of miR-210 into recipient miR-210 −/− MEFs. Notably, a greater level of miR-210 delivery was observed with supernatant exposure which correlated with the differences in released miR-210 expression present in those media fractions (as shown in B). (D) During hypoxia, ISCU expression was down-regulated in miR-210 −/− MEF cells after exposure to conditioned media replete with miR-210. (E) During hypoxia, normalized luciferase reporter expression carrying the ISCU 3′ UTR binding site for miR-210 was down-regulated in miR-210 −/− MEF cells after exposure to conditioned media replete with miR-210. Normalized luciferase activity in MEF cells exposed to control media was assigned a fold change of 1, to which other conditions were compared. (F) During hypoxia, mitochondrial Complex I activity was down-regulated in miR-210 −/− MEF cells after exposure to conditioned media replete with miR-210. Immunoblots are representative of experiments performed at least in triplicate; gel densitometry was normalized to actin levels and compared as arbitrary units. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
We then wanted to determine whether packaging of released miR-210 influences delivery to recipient cells. Conditioned media replete with released miR-210 from HEK293 cells were ultracentrifuged in order generate microvesicle-depleted supernatant and microvesicle-enriched pellets (as in Fig. 4C). Similar to the findings of hypoxic conditioned media from HPAECs (Fig. 4B), a majority of miR-210 was detected in the microvesicle-depleted supernatant (Fig. 8B). Importantly, exposure of naive miR-210 −/− MEFs to either supernatant or resuspended pellet led to delivery of miR-210 (Fig. 8C), thus indicating that released miR-210 can enter recipient cells despite being packaged outside of microvesicles.
To ascertain whether exogenously delivered miR-210 is active in recipient cells, ISCU expression and mitochondrial respiratory complex activity were assessed, as a primary example of a robust target by which activity of delivered miR-210 can be quantified and consistent with the predominant functions of this miRNA [7]. During hypoxia (Fig. 8D) but less so during normoxia (Supplemental Fig. 4A), ISCU expression was down-regulated in miR-210 −/− MEF cells after delivery of miR-210. Correspondingly, primarily evident during hypoxia (Fig. 8E) and less so during normoxia (Supplemental Fig. 4B), did delivered miR-210 repress luciferase reporter gene expression carrying the ISCU-specific miR-210 binding site in its 3′ untranslated region. Finally, augmented during hypoxia (Fig. 8F), but not normoxia (Supplemental Fig. 4C), mitochondrial Complex I activity was down-regulated in MEF cells after exposure to extracellular miR-210. Thus, extracellular miR-210 can be transported into recipient cells under both normoxic and hypoxic conditions; however, its metabolic activity is particularly augmented during hypoxic exposure.
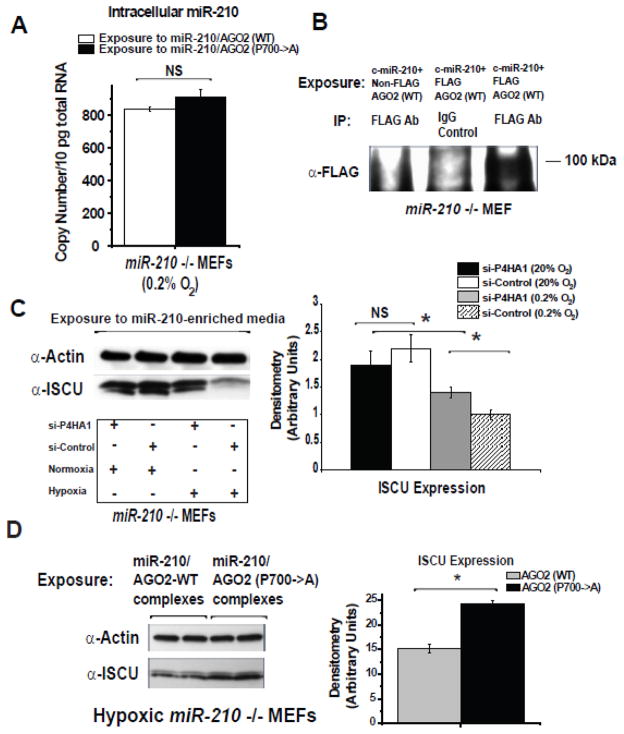
Given the importance of AGO2 prolyl-hydroxylation in the activity and release of miR-210 in hypoxic “source” cells, we wanted to determine whether this switch also regulates the actions of delivered miR-210 in recipient cells. To do so, conditioned media were generated from HEK293 cells carrying released miR-210 complexed with either wildtype or (P700A) hydroxylation mutant AGO2. After normalizing for similar levels of released miR-210, a comparable degree of miR-210 delivery was achieved in miR-210 −/− MEF recipient cells in the presence of wildtype AGO2 or hydroxylation mutant AGO2 (Fig. 9A). Importantly, as demonstrated by immunoblotting of α-FLAG immunoprecipitant from recipient cell lysates, exogenously derived FLAG-tagged AGO2 (~100 kDa) was also detectable in the intracellular space after delivery of miR-210 (Fig. 9B), indicating that both miR-210 and AGO2 are transported into recipient cells. Yet, while delivery itself was not affected, we postulated that activity of delivered miR-210 is dependent upon AGO2 prolyl-hydroxylation. Consistent with such a theory, siRNA-mediated knockdown of P4HA1 in miR210 −/− MEFs during exposure to miR-210-enriched media blunted the miR-210-dependent down-regulation of ISCU in hypoxia (Fig. 9C). Similarly, the repressive effects on ISCU expression by delivered miR-210 in hypoxia were evident primarily in the presence of delivered wildtype AGO2 but not the hydroxylation mutant AGO2 (Fig. 9D). Thus, while not directly affecting uptake of miR-210, AGO2 prolyl-hydroxylation exerts precise control over delivered miR-210 in recipient tissue by specifically accelerating its activity in hypoxia.
Figure 9. Status of AGO2 prolyl-hydroxylation determines the activity of delivered miR-210 in recipient cells.
(A) Delivery of miR-210 into miR-210 −/− MEF cells was similar after exposure of released miR-210 derived from cells expressing either AGO2 (WT) or hydroxylation-deficient mutant AGO2. (B) As shown by immunoblot of anti-AGO2 immunoprecipitant from recipient cell lysate, FLAG-tagged AGO2 (WT) (~100 kDa) was detectable in recipient miR-210 −/− MEF cells after exposure to miR-210-enriched media. (C) siRNA-mediated knockdown of P4HA1 in miR210 −/− MEFs during exposure to miR-210-enriched media blunted the down-regulation of ISCU in hypoxia but had a negligible effect on ISCU expression in normoxia. (D) In miR-210 −/− MEFs, the hypoxic repressive effects on ISCU by delivered miR-210 were diminished when miR-210 was released from cells expressing the hydroxylation-deficient mutant AGO2. Immunoblots are representative of experiments performed at least in triplicate; gel densitometry was normalized to actin levels and compared as arbitrary units. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
Finally, we aimed to determine whether endogenously released miR-210 is released in sufficient quantities for active uptake by recipient cells. To do so, HT-29 adenocarcinoma cells, which release relatively high levels of miR-210 (Supplemental Fig. 1), were used to produce conditioned media enriched for released miR-210 by exposure to a protocol of hypoxia-reoxygenation (Fig. 10A). For comparison, conditioned media without any released miR-210 were harvested from miR-210 −/− MEFs, and conditioned media enriched for miR-210 were harvested from HEK293 cells expressing a miR-210 transgene. While miR-210 was undetectable in media derived from miR-210 −/− MEFs, substantial but approximately 10-fold lower levels of extracellular miR-210 were observed in media derived from HT-29 cells as compared with media from transfected HEK293 cells (Fig. 10A). To determine whether these lower concentrations of endogenously produced miR-210 could be delivered to recipient cells, naive miR-210 −/− MEFs were then exposed to HT-29 conditioned media and were subsequently exposed either to normoxia (20% O2) or hypoxia (0.2% O2) for 48 hours. Importantly, within recipient cells exposed to HT-29-conditioned media but not miR-210 −/− MEF-conditioned media, miR-210 was detected (Fig. 10B). Similar to exposures using conditioned media from transfected HEK293 cells (Fig. 8), such miR-210 delivery was accompanied by a decrease of ISCU as well as an additional miR-210 target, E2F3 [44], particularly during hypoxic, but not normoxic, exposure of recipient cells (Fig. 10C–D). Thus, even at lower concentrations, endogenously released miR-210 is indeed sufficient for uptake and suppression of miR-210 gene targets in recipient cells.
Figure 10. Endogenously released miR-210 is sufficient for uptake and suppression of miR-210 targets ISCU and E2F3.
(A) HT-29 adenocarcinoma cells were used to produce conditioned media (CM) enriched for released miR-210 by exposure to hypoxia-reoxygenation. Conditioned media without any miR-210 were harvested from miR-210 −/− MEFs exposed to the same conditions. For comparison, HEK293 cells expressing a miR-210 transgene were used to generate conditioned media. While miR-210 was undetectable in media derived from miR-210 −/− MEFs (denoted by #), substantial but 10-fold lower levels of extracellular miR-210 were observed in media derived from HT-29 cells versus transfected HEK293 cells. (B) In both normoxia (20% O2) and hypoxia (0.2% O2), miR-210 was detected in recipient naive miR-210 −/− MEFs exposed to HT-29-conditioned media but not to miR-210 −/− MEF-conditioned media (undetectable levels denoted by # and &). By immunoblot analysis and gel densitometry, such miR-210 delivery was accompanied by a decrease of the miR-210 target genes ISCU (C) and E2F3 (D) particularly during hypoxic exposure of recipient cells. Immunoblots are representative of experiments performed at least in triplicate; gel densitometry was normalized to actin levels and compared as arbitrary units. Error bars reflect SEM; * p≤0.05, ** p≤0.01, *** p≤0.001, NS signifies p>0.05.
Discussion
Here, we define a unique molecular communication system whereby miR-210 is released by hypoxic cells and taken up by recipient cells to regulate adaptive programs in mitochondrial metabolism and potentially key features related to remote IPC (Fig. 11). In particular, we introduce a highly specific regulatory system by which AGO2 prolyl-hydroxylation not only controls miR-210 activity but also, in a reciprocal fashion, modulates the release of miR-210. In doing so, our data suggest the presence of a precisely controlled molecular messaging and depot system -- one that regulates the release, transport, storage, and activation of miR-210 in recipient tissue, thus preventing adverse miR-210 activity in normoxia while still accelerating adaptation in hypoxia.
Figure 11. Model of extracellular miR-210 as a communicable effector of hypoxic adaptation and controlled by AGO2 prolyl-hydroxylation.
A molecular model is presented whereby the release and activity of extracellular miR-210 are specifically controlled by hypoxia-dependent AGO2 prolyl-hydroxylation. (A) During hypoxia, intracellular miR-210 is transcriptionally up-regulated and drives a measured release of miR-210 bound to AGO2. However, simultaneously, AGO2 hydroxylation increases in hypoxia, which acts as a homeostatic brake on miR-210 release, thus maximizing intracellular retention and activity of this miRNA. In contrast, during normoxia/reoxygenated states when AGO2 hydroxylation is down-regulated, intracellular miR-210 activity is decreased, and release of miR-210 is expedited, again preferentially bound to AGO2. (B) Released miR-210 can be delivered to recipient tissue. However, in normoxia, reduction of AGO2 prolyl-hydroxylation reduces potentially adverse activity of delivered miR-210 in recipient cells. Conversely, only when recipient tissue is directly exposed to hypoxia does AGO2 prolyl-hydroxylation augment activity of delivered miR-210, thus accelerating metabolic and hypoxic adaptation. Thus, our data suggest the presence of a highly specific, molecular depot system to effectively store miR-210 in recipient tissue, moderating adverse actions of miR-210 in normoxia while still preparing that tissue for incipient hypoxia and accelerating adaptation upon low oxygen exposure.
Post-translational modifications of AGO2 in general may represent a major regulatory mechanism by which the cell can rapidly control specific circulating miRNAs such as miR-210 under dynamically changing conditions. For instance, EGF-dependent phosphorylation of AGO2 has been reported as a key switch controlling microRNA activity in hypoxia [45]. The additional regulation of released miR-210 by prolyl-hydroxylation of AGO2 offers substantial teleological advantages by which mammals may prepare for impending hypoxic injury. Similar to HIF-1α itself, we have found that miR-210 assumes an adaptive role during hypoxia but a maladaptive role in normoxia [7]. Thus, of paramount importance to tissue homeostasis and survival is to ensure that miR-210 is activated primarily during hypoxia. In part, this is possible via the direct regulation of miR-210 expression by HIF-1α binding to the miR-210 promoter and up-regulation of transcription in hypoxic tissue [46]. Our current findings reveal that such precise control can also be achieved via expediting removal of miR-210 from the intracellular space in normoxia. Moreover, when miR-210 is delivered to recipient tissue that has yet to experience hypoxic stress, reduced prolyl-hydroxylation of AGO2 prevents unnecessary and potentially adverse activity of this miRNA. Notably, even in the absence of hypoxia, delivered miR-210 can still carry biological activity, as reported recently [39, 40]. However, especially evident when miR-210 is delivered in modest amounts, our data demonstrate that hypoxic induction of AGO2 prolyl-hydroxylation can immediately maximize the adaptive functions of this miRNA. Thus, mirroring the rapid on-off switch of HIF-1α accumulation and degradation, we now propose an equally intricate system for maximizing the control of miR-210 activity in both source and recipient tissue in order to anticipate and to respond to hypoxic stimuli.
The inducible properties of miR-210 also allow for an adept function of shuttling between source and recipient tissue in order to guide optimal adaptation. Notably, the transport of extracellular forms of other miRNA has been reported in non-hypoxic contexts, yet enthusiasm regarding the biological importance of extracellular miRNA in general has been tempered by the relative inefficiency of miRNA import into recipient cells. Specifically, in many cases, the repressive functions of miRNA require higher levels of expression than delivery of extracellular miRNA can achieve [47]. Furthermore, recipient tissues often already express substantial levels of endogenous miRNA prior to delivery, thus decreasing the relevance of slight increases in total intracellular expression after miRNA uptake. In contrast, the biology of miR-210 circumvents those issues. MiR-210 is expressed only at relatively low levels in normoxic contexts [48], and its ability to engage its direct targets such as ISCU may require as little as 1.5–3-fold up-regulation during hypoxia [7]. Thus, unlike a number of other miRNA that appear to necessitate much higher absolute levels of expression to engage their target genes effectively [i.e., miR-21 [37]], delivery of miR-210, even in modest quantities, is especially suited to prepare normoxic tissue for incipient hypoxic stress when relatively little endogenous miR-210 is available.
Beyond these specific implications of our understanding of hypoxic adaptation, this study substantially clarifies aspects of the regulation and function of released miRNA in hypoxia and provides a necessary foundation for further exploration. Notably, our data delineate the specificity by which release of miR-210 is regulated by hypoxia-dependent AGO2-prolyl hydroxylation, but other miRNAs such as miR-16, miR-223, or miR-21, are not released in the same patterns. MiRNA sequence could constitute a determinant of such specificity, as has recently been demonstrated for exosomal packaging of miRNAs in T-cells by sumoylation of hnRNPA2B1 [49]. However, it is equally plausible that the robust transcriptional induction of miR-210 in hypoxia and its robust down-regulation in reoxygenation may allow the hydroxylation state of AGO2 to exert greater control over this miRNA. Such a model could be envisioned whereby actively generated or degraded miRNAs are preferentially transported to subcellular organelles or pathways that make them more dependent upon association with AGO2 and shuttling either to target gene engagement or packaging for release. This latter explanation resonates well with our findings that AGO2 hydroxylation state can influence the association of miR-210 with extracellular AGO2 (Fig. 7C). Clarification of these possibilities warrants future investigation. Moreover, while much attention has been placed on miRNA delivery via microvesicle transfer, we now report that some miRNAs, including miR-210, are primarily released independent of microvesicles during exposures to various oxygen contents, and these forms are sufficient for delivery in recipient cells. While some molecular pathways that drive exosomal [28] and non-exosomal [26] release have been characterized, given our results here of the control of the level of miR-210 release by AGO2 prolyl-hydroxylation, it remains an intriguing possibility that other aspects of transport, delivery, and degradation of extracellular miR-210 may also be influenced by such a mechanism. Finally, it is possible that other proteins important in regulating miRNA activity, such as those in the RNA induced silencing complex (RISC), may also be associated in the secreted complex. Consequently, RISC modulation in general may serve as a primary determinant of both subcellular and extracellular localization of RNA, and its actions may extend far beyond controlling gene silencing activity alone.
In addition to our findings in cultured cells, our data expand the variety of both physiologic and pathophysiologic conditions in which circulating levels of miR-210 are induced and maintained. Specifically, circulating miR-210 has been reported as up-regulated in both plasma and urine in clinical conditions where hypoxia and/or HIF expression predominate – these include cardiovascular diseases such as chronic heart failure [50, 51] and atherosclerotic disease [52, 53], a variety of human solid tumors [15–19], and transplant rejection [54]. When considered with the intricate control of miR-210 release and activity by AGO2 prolyl-hydroxylation, these observations suggest that such conditions in vivo may exploit the actions of delivered miR-210. If so, mirroring prior findings in remote IPC where long-term mitochondrial function is protected [55–57], effective therapeutic options can be envisioned, whereby delivered miR-210 inhibits mitochondrial respiration during acute phases of hypoxia in order to preserve mitochondrial integrity and respiratory potential once the ischemic threat ceases. To date, the most promising approaches in manipulating miRNA function in vivo entail the use of modified antisense oligonucleotides or plasmids to inhibit specific endogenous miRNA (i.e., antagomirs, anti-miRs, and miRNA sponges) [as reviewed by [58]]. Yet, the ability to force expression of specific miRNA in vivo is less well developed. Exploration of an efficient approach to load miR-210 artificially onto AGO2 complexes for in vivo delivery could improve the protocols for remote IPC and optimize hypoxic adaptation in a variety of physiologic and disease-oriented contexts. Such an approach may also synergize well with other rapidly progressing miRNA delivery technologies, such as nanoparticles or artificial microvesicles.
In summary, we describe the AGO2-dependent release and uptake of miR-210 as a unique messaging system designed to facilitate hypoxic communication across anatomically distinct cells. It may be a key finding in understanding the physiological integration of how complex organisms detect and respond to hypoxic stress. These data should serve as a foundation for studies designed to explore further other hypoxia-induced extracellular miRNAs and how these molecules may synergize with the actions of miR-210. Additionally, these results offer valuable insights for therapeutic development of extracellular miRNAs for accelerating adaptation to hypoxia and ischemia in a wide variety of human diseases.
Supplementary Material
Highlights.
Patterns of extracellular release of miR-210 are unique in hypoxia/reoxygenation.
Released miR-210 complexes with AGO2 in and outside of microvesicle fractions.
Released miR-210 can be delivered to recipient cells for target gene repression.
Activity and release of miR-210 are regulated by AGO2 prolyl-hydroxylation.
The AGO2-miR-210 switch facilitates hypoxic communication across distinct cells.
Acknowledgments
We thank S.K. Chan and J.W. Snow (critical advice and reading of manuscript); S. Tribuna (administrative assistance); Drs. A. Bradley and H. Prosser (generous provision of mmu-miR-210 −/− mice); and Drs. H. Qi and Y. Shi (generous provision of the wildtype AGO2 and AGO2 (P700A) expression constructs).
This work was supported by the NIH (KO8 grant), the Lerner, Harris, and Watkins Funds, Gilead Research Scholars Fund, and the Pulmonary Hypertension Association (S.Y.C.). The collection of the Bolivian plasma samples was supported by NIH HL RO1 079647 (L.G.M.). S.K. and S.A.M. were supported by NIH RO1 HL0055454 and HL085446 grants.
Footnotes
Author Contributions
S.Y.C. conceived and designed the research, performed the experiments, and wrote the manuscript. A.H., C.L., S.A., P-K.M., and S.J.H. aided in the design of the research, performance of the experiments, and manuscript revision. C.L., S.A.M, and S.K. designed and performed the experiments to assess the packaging of released miR-210 and revised the manuscript. R.P., M.A.G., C.G.J., and L.G.M. recruited and provided human specimens and aided in experimental design and manuscript revisions.
Conflict of Interest Statement
The authors confirm that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008;150:304–30. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]
- 2.Kloner RA. Clinical application of remote ischemic preconditioning. Circulation. 2009;119:776–8. doi: 10.1161/CIRCULATIONAHA.108.832832. [DOI] [PubMed] [Google Scholar]
- 3.Bolte CS, Liao S, Gross GJ, Schultz Jel J. Remote preconditioning-endocrine factors in organ protection against ischemic injury. Endocr Metab Immune Disord Drug Targets. 2007;7:167–75. doi: 10.2174/187153007781662585. [DOI] [PubMed] [Google Scholar]
- 4.Kant R, Diwan V, Jaggi AS, Singh N, Singh D. Remote renal preconditioning-induced cardioprotection: a key role of hypoxia inducible factor-prolyl 4-hydroxylases. Mol Cell Biochem. 2008;312:25–31. doi: 10.1007/s11010-008-9717-5. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA. 2013;110:17462–7. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314. doi: 10.1007/s00395-012-0314-0. [DOI] [PubMed] [Google Scholar]
- 7.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–8. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 9.Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2010;18:465–78. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza G. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–83. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 13.Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10:543–50. doi: 10.1016/j.coph.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 15.Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, et al. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol. 2010;3:109–13. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wulfken LM, Moritz R, Ohlmann C, Holdenrieder S, Jung V, Becker F, et al. MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PLoS One. 2011;6:e25787. doi: 10.1371/journal.pone.0025787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao A, Li G, Peoc’h M, Genin C, Gigante M. Serum miR-210 as a novel biomarker for molecular diagnosis of clear cell renal cell carcinoma. Exp Mol Pathol. 2012 doi: 10.1016/j.yexmp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Jung EJ, Santarpia L, Kim J, Esteva FJ, Moretti E, Buzdar AU, et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118:2603–14. doi: 10.1002/cncr.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madhavan D, Zucknick M, Wallwiener M, Cuk K, Modugno C, Scharpff M, et al. Circulating miRNAs as Surrogate Markers for Circulating Tumor Cells and Prognostic Markers in Metastatic Breast Cancer. Clin Cancer Res. 2012;18:5972–82. doi: 10.1158/1078-0432.CCR-12-1407. [DOI] [PubMed] [Google Scholar]
- 20.Gunel T, Zeybek YG, Akcakaya P, Kalelioglu I, Benian A, Ermis H, et al. Serum microRNA expression in pregnancies with preeclampsia. Genet Mol Res. 2011;10:4034–40. doi: 10.4238/2011.November.8.5. [DOI] [PubMed] [Google Scholar]
- 21.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011;3:1265–72. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 22.Zeng L, Liu J, Wang Y, Wang L, Weng S, Chen S, et al. Cocktail Blood Biomarkers: Prediction of Clinical Outcomes in Patients with Acute Ischemic Stroke. Eur Neurol. 2012;69:68–75. doi: 10.1159/000342896. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Kandic I, Faughnan ME, Kutryk MJ. Elevated circulating microRNA-210 levels in patients with hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations: a potential new biomarker. Biomarkers. 2013;18:23–9. doi: 10.3109/1354750X.2012.728624. [DOI] [PubMed] [Google Scholar]
- 24.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mok Y, Schwierzeck V, Thomas DC, et al. MiR-210 is induced by Oct-2, regulates B cells, and inhibits autoantibody production. J Immunol. 2013;191:3037–48. doi: 10.4049/jimmunol.1301289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, et al. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1313–24. doi: 10.1152/ajpregu.00806.2006. [DOI] [PubMed] [Google Scholar]
- 35.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1564–75. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, et al. MicroRNA-21 Integrates Pathogenic Signaling to Control Pulmonary Hypertension: Results of a Network Bioinformatics Approach. Circulation. 2012;125:1520–32. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–59. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–51. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–61. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 42.Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, et al. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature. 2008;455:421–4. doi: 10.1038/nature07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. 2011;31:4760–74. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–64. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–7. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulshreshtha R, Ferracin M, Wojcik S, Garzon R, Alder H, Agosto-Perez F, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, et al. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol. 2007;25:1457–67. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- 48.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–83. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao DS, Chen Y, Jiang H, Lu JP, Zhang G, Geng J, et al. Serum miR-210 and miR-30a expressions tend to revert to fetal levels in Chinese adult patients with chronic heart failure. Cardiovasc Pathol. 2013;22:444–50. doi: 10.1016/j.carpath.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Endo K, Naito Y, Ji X, Nakanishi M, Noguchi T, Goto Y, et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol Pharm Bull. 2013;36:48–54. doi: 10.1248/bpb.b12-00578. [DOI] [PubMed] [Google Scholar]
- 52.D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–73. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, et al. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011;412:66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, et al. Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant. 2011;11:2221–7. doi: 10.1111/j.1600-6143.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Oka N, Tropak M, Callahan J, Lee J, Wilson G, et al. Remote ischemic preconditioning elaborates a transferable blood-borne effector that protects mitochondrial structure and function and preserves myocardial performance after neonatal cardioplegic arrest. J Thorac Casrdiovasc Surg. 2008;136:335–42. doi: 10.1016/j.jtcvs.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 56.Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, et al. Remote Cardioprotection by Transfer of Coronary Effluent from Ischemic Preconditioned Rabbit Heart Preserves Mitochondrial Integrity and Function via Adenosine Receptor Activation. Cardiovasc Drugs Ther. 2014;28:7–17. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- 57.Slagsvold KH, Rognmo O, Hoydal MA, Wisloff U, Wahba A. Remote Ischemic Preconditioning Preserves Mitochondrial Function and Influences Myocardial MicroRNA Expression in Atrial Myocardium During Coronary Bypass Surgery. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.114.302751. [DOI] [PubMed] [Google Scholar]
- 58.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.