Abstract
LP-BM5 retrovirus-infected C57BL/6 mice develop splenomegaly, lymphadenopathy, hypergammaglobulinemia, and immunodeficiency; thus, this disease has been named mouse AIDS. In this syndrome, CD154/CD40 interactions are required for but do not mediate disease by upregulation of CD80 or CD86. We report here that there is nonetheless a necessity for CD40 signaling competence, specifically an intact tumor necrosis factor receptor-associated factor 6 (TRAF 6) binding site.
A complex immunodeficiency disease syndrome develops in certain inbred strains of mice, such as the highly susceptible C57BL/6 (B6) strain, after infection with the LP-BM5 murine leukemia retrovirus isolate. Replication-competent ecotropic (BM5eco), and possibly recombinant mink cell cytopathic focus-inducing, helper viruses and replication-negative/defective viruses (BM5def), which serve as the proximal agent causing the syndrome, are the essential components of this retrovirus mixture (2, 8, 17, 19, 31). Many features of LP-BM5 induced disease are similar to those seen in human immunodeficiency virus-infected individuals. Disease similarities include activation-related parameters, such as hypergammaglobulinemia (hyper-Ig), splenomegaly, and lymphadenopathy, and immunodeficiency-related consequences, including severely dampened T- and B-cell responses and increased susceptibility to disease progression and death when animals were exposed to environmental pathogens that normally caused limited infections and the development of terminal B-cell lymphomas (4, 7, 23, 24, 27, 29, 30, 32, 38). Thus, this syndrome has been designated murine AIDS (MAIDS).
B6 mice infected by LP-BM5 after in vivo depletion of either CD4 T cells or B cells do not develop MAIDS (7, 38, 39). Consistent with these cellular requirements, we have previously demonstrated that CD154/CD40 interactions are necessary for both the induction and progression of MAIDS. By in vivo treatment with anti-CD154 (CD40 ligand) monoclonal antibody—either at the initiation of, or 3 to 4 weeks after, infection—splenomegaly, hyper-Ig, and immunodeficiency were efficiently inhibited in LP-BM5-infected B6 mice (13, 15). As confirmation of this requisite molecular interaction for MAIDS pathogenesis, we and others have also reported that B6 CD154 (14) and CD40 (14, 40) knockout (k.o.) mice are essentially resistant to LP-BM5-induced MAIDS. Importantly, CD40 k.o. mice do express relatively high levels of BM5def (and especially BM5eco), compared to B6 mice, indicating that the block to viral pathogenesis is not at the level of initial infection and virus spread (9, 14, 40). In fact, LP-BM5-infected CD40 k.o. mice do show some hyper-Ig, at least in terms of immunoglobulin G (IgG) subclasses and IgE (14, 40).
Regarding the cellular basis for this CD154/CD40 molecular requirement, we have shown that CD4 T cells and B cells are necessary for CD154 and CD40 expression, respectively (14). Although ligation of CD40 on professional antigen-presenting cells, including B cells, induces a number of overlapping signal transduction pathways, a central paradigm is that many of the ultimate functional consequences are determined by the known downstream upregulation of the costimulatory ligands for CD28 and CTLA-4: B7-1 (CD80) and B7-2 (CD86) (6, 21, 35-37). However, we have recently reported that the requirement for CD40 signaling in MAIDS pathogenesis does not depend on this classic upregulation of CD80 and/or CD86. Indeed, B6 CD80/CD86 double-k.o. mice were susceptible to LP-BM5-induced MAIDS (16). While not absolutely required, evidence has been reported suggesting that CD80 and/or CD86 may play some role in MAIDS pathogenesis. Thus, soluble CTLA4Ig or transgenic CTLA4Ig overexpression can partially inhibit disease induction by a helper-free, related but distinct, virus preparation, Du5H (10, 11). In addition, other factors and experimental differences may account for these apparently conflicting data, as we have discussed in some detail (16).
Here we explore the upstream events more proximal to CD40 ligation as an alternative approach to define the signal transduction pathways necessary for MAIDS pathogenesis. Recent studies have shown that CD154 oligomerization of CD40 results in recruitment of the cytoplasmic tumor necrosis factor receptor-associated factors (TRAFs). Specifically, the TRAF 1, 2, 3, and 5 proteins, as opposed to the TRAF 6 protein, bind to the classic shared 2,3/5 site, as opposed to the unique 6 site, on the CD40 cytoplasmic tail domain (26, 33, 34). This binding of these TRAFs to their analogous sites on CD40 leads to activation of Ser and Thr kinases, which then propagate the signal from the CD40 receptor complex to downstream mitogen-activated protein kinase and IκB kinase cascades.
Our approach here was to utilize a series of mice transgenic for chimeric hu/mouse CD40 (originally generated by the NICHD Transgenic Mouse Development Facility, University of Alabama, Birmingham), which were used after backcrossing to CD40 k.o.'s, as detailed in a previous study (1). Available for study were a CD40 transgenic strain with unaltered (wild-type [wt]) CD40 sequences and transgenic strains that expressed selectively mutagenized amino acid sequences in the CD40 cytoplasmic tail to alter the various TRAF binding sites so as to inhibit TRAF association with CD40. These same “Δ-TRAF site” constructs were instrumental in a previous in vitro study that found that cellular responses to CD40 stimulation in a mouse B-cell line may be TRAF dependent or independent (28) and in an in vivo setting in which it was determined that TRAF 6 is necessary for regulation of antibody affinity maturation and the generation of long-lived plasma cells (1). As in this previous in vivo study, all CD40 transgenic mice used in the present report were backcrossed to the MAIDS-resistant CD40 k.o. background (22) so that the only CD40 molecule in the system was the chimeric transgene-encoded species. Although the levels of transgene expression, as measured by flow cytometry, varied somewhat, the unaltered wt chimeric transgenic strain (which supports LP-BM5-induced MAIDS—see below) had the lowest expression, while the TRAF 2,3/5−, 6− strain had the highest (1).
MAIDS susceptibility of the transgenic strains, in comparison to prototypic susceptible B6, was determined after standard inoculation with LP-BM5. Mice were evaluated for three or more of the following disease readouts, which we and others have routinely used, to evaluate disease susceptibility (2, 4, 7, 8, 13-15, 19, 21, 25, 31): spleen size; B- and T-cell immunodeficiency as measured by lipopolysaccharide (LPS), concanavalin A (ConA), and allospecific cytolytic T-lymphocyte (allo-CTL) responsiveness and by spleen cell marker phenotypic changes. Disease parameters were assessed at somewhat extended times post-LP-BM5 infection, 11 to 14 weeks, to make sure that we were not misled by merely a shift in disease kinetics.
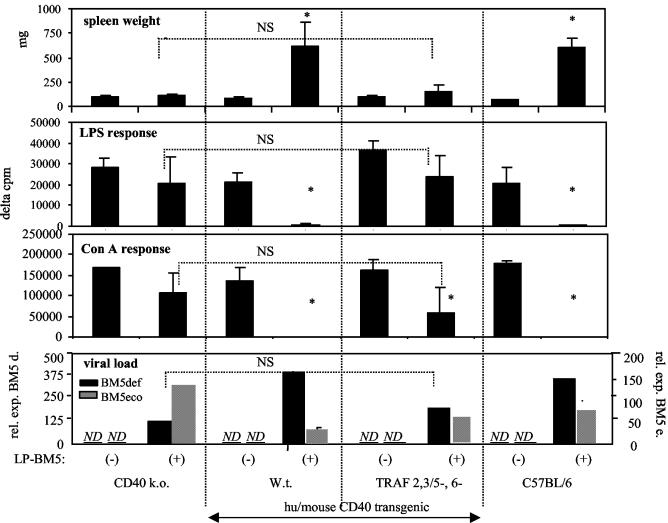
To determine if this transgenic system is amenable to investigate specific CD40 signaling requirements, we first tested the MAIDS susceptibility of transgenic mice with wt chimeric CD40 (TRAF 2,3/5+, 6+), versus chimeric CD40 with both classic TRAF binding sites disrupted (TRAF 2,3/5−, 6−). As shown in Fig. 1, after LP-BM5 infection, wt chimeric CD40 transgenic mice were susceptible to MAIDS-associated splenomegaly, with spleens weighing approximately sixfold more than that for the same strain, uninfected. This level of disease was similiar to that seen for infected B6 mice. Spleen cells from infected wt CD40 transgenic mice were also almost completely nonresponsive to ConA and LPS stimulation, as were cells from infected B6 mice (Fig. 1), and were unable to generate allo-CTL in response to H-2d in vitro stimulation (data not shown).
FIG. 1.
wt chimeric hu/mouse CD40 transgenic mice (TRAF 2,3/5+, 6+) develop MAIDS-associated disease after LP-BM5 infection (3 × 104 PFU of B ecotropic helper virus). In contrast, infected transgenic mice with CD40 cytoplasmic tail mutations that inhibit TRAF protein binding to the TRAF 2,3/5, 6 sites (TRAF 2,3/5−, 6−) do not develop MAIDS. B- or T-cell mitogen responsiveness was determined, respectively, by a 72-h in vitro stimulation with either 10 μg of LPS/ml or 4 μg of ConA/ml, followed by a terminal 6-h pulse with [3H]thymidine. These transgenic mice were all on the CD40 k.o. background. LP-BM5-defective viral load (bottom panel) for spleen tissue was determined by quantitative real-time reverse transcriptase PCR, as members of our laboratory previously reported (9). Briefly, total RNA was isolated and was DNase I treated. One microgram of DNA-free RNA was reverse transcribed to cDNA by using random hexamer priming. Reverse transcriptase PCR was performed with BM5-defective gag (9, 12), ecotropic gag (9), and β-actin (9) primers by amplifying resulting cDNA in the presence of SYBR Green stain on an iCycler iQ instrument (Bio-Rad) by using previously reported temperatures, cycle numbers, and normalization to β-actin expression (9). By this assay, levels of the BM5-defective and BM5 ecotropic viruses were not detectable (ND) in uninfected mice. This experiment is representative of two additional experiments for the TRAF 2,3/5−, 6− transgenic mice, including one in which there was no significant loss of ConA response for the infected and uninfected mice of this group and one additional experiment for the wt transgenic mice. Mean values and standard deviations were derived from four mice per group. *, P < 0.05 by the Student t test, compared to uninfected control mice of the same strain. NS, not significant; rel. exp., relative expression.
In contrast, infected CD40 transgenic mice with disrupted TRAF 2,3/5, and 6 binding sites (TRAF 2,3/5−, 6−) were essentially MAIDS insusceptible. In comparison to uninfected mice, these mice had a similar (not statistically different) average spleen weight and spleen cell response to LPS, with a ConA and allo-CTL response that was only somewhat dampened. However, the ConA (Fig. 1) and allo-CTL (data not shown) responses of TRAF 2,3/5−, 6− transgenic mice were not statistically significantly different (P = 0.2993 and P = 0.1911, respectively) compared to those of the control infected CD40 k.o. strain, which provides the background for all the transgenic strains. This observed slight “susceptibility” of the CD40 k.o. background (strain) is consistent with recent reports by our lab and others that LP-BM5 infection minimally diminishes mitogen responsiveness in CD40 k.o. mice (14, 40). This pattern of results was reproduced in two other experiments involving CD40 wt and TRAF 2,3/5−, 6− transgenic strains. However, in the third experiment, there was no significant difference for the ConA and allo-CTL responses of LP-BM5-infected versus uninfected TRAF 2,3/5−, 6− transgenic mice, underscoring that in B6 mice LP-BM5-induced immunodeficiency largely, if not entirely, depends on intact classical CD40 TRAF binding sites. These findings corroborate our earlier studies, demonstrating the requirement for B-cell-associated CD40 for MAIDS susceptibility (14) and strongly suggest that LP-BM5-induced MAIDS is dependent on activation through CD40, specifically CD40/TRAF signaling.
Determination of viral load by quantitative reverse transcriptase PCR assays (Fig. 1) for both the defective and ecotropic helper viral components of LP-BM5, assessed at termination several weeks postinfection, indicated generally equivalent high expression for the infected control B6 and wt chimeric CD40 transgenic mouse groups. For infected TRAF 2,3/5−, 6− mice, there was also clear evidence of successful infection and early spread. However, the defective viral load for TRAF 2,3/5−, 6− mice was perhaps twofold lower than that of the MAIDS-susceptible strains, while not statistically different from that for their background strain, CD40 k.o. Thus, the requirement of classical CD40 TRAF binding sites for viral pathogenesis was not mediated by an abolition of infection (see discussion below).
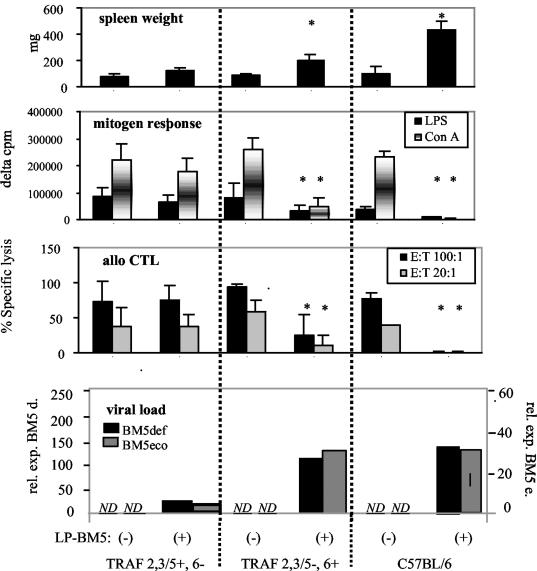
To determine which specific TRAF binding sites are required for MAIDS-associated CD40 signaling, we next infected two CD40 transgenic strains bearing sequence mutations that reciprocally alter the binding of TRAF adaptor molecules to the CD40 cytoplasmic tail (Fig. 2). Thus, either the classic TRAF 2,3/5 common site or the TRAF 6 site was mutated (TRAF 2,3/5−, 6+ or TRAF 2,3/5+, 6− transgenic strains). As previously determined by flow cytometry analysis, the level of CD40 transgene expression in these two strains is essentially identical (1). LP-BM5 infection of the TRAF 2,3/5−, 6+ transgenic mice resulted in MAIDS by a variety of criteria: e.g., splenomegaly, with the average spleen weight approximately threefold higher than that for the uninfected counterparts. In sharp contrast, spleen weights for the infected and uninfected TRAF 2,3/5+, 6− transgenic strains were similar (Fig. 2). Similarly, comparing LP-BM5-infected and uninfected mice, it is clear that infection severely impaired the mitogenic responses to LPS and ConA and inhibited allo-CTL generation, by the TRAF 2,3/5−, 6+ transgenic mice, indicating profound immunodeficiency. On the other hand, these three immunodeficiency readouts were nearly unchanged in the infected versus uninfected TRAF 2,3/5+, 6− transgenic mice. Indeed, in one of the two experiments conducted for Fig. 2, TRAF 2,3/5−, 6− mice were also available for comparison (not shown). The transgenic mice with only the TRAF 6 site mutated versus both TRAF sites mutated behaved very similarly upon infection—there was no evidence that the presence of an intact TRAF 2,3/5 site by itself led to an increase in disease susceptibility.
FIG. 2.
LP-BM5-infected (+) CD40 transgenic mice with mutated TRAF 2,3/5 and intact TRAF 6 binding sites (TRAF 2,3/5−, 6+) are MAIDS susceptible, whereas TRAF 2,3/5+, 6− transgenic mice are relatively insusceptible as assessed by readouts for spleen size and B- and T-cell immunodeficiency. Allo-CTL responses were generated after stimulation with major histocompatibility complex-mismatched irradiated stimulator cells, followed by a standard 51Cr release assay (13, 14). Mitogen responses and viral load assessment were performed as indicated in the legend to Fig. 1. Mean values and standard deviations represent a composite of two experiments of four to seven mice per group. *, P < 0.05 by the Student t test compared to uninfected control mice of the same strain. E:T, effector to target cell.
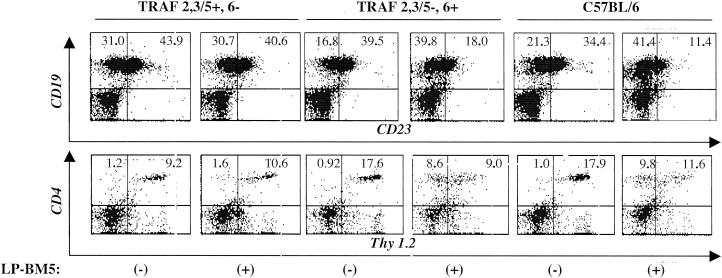
This pattern of results was confirmed by flow cytometry analysis for the characteristic conversion to a MAIDS-associated spleen cell phenotype, as described previously (18, 25). Infected TRAF 2,3/5−, 6+ transgenic mice exhibited the unique changes also seen for infected B6 control mice and at nearly the same magnitude, i.e., expanded populations of CD19+ CD23− and CD4+ Thy1.2− spleen cells (Fig. 3 and Table 1). In contrast, infected CD40 TRAF 2,3/5+, 6− transgenic mice showed either essentially no change (CD19+ CD23−) or only limited increase (CD4+ Thy1.2−) in these disease-associated, novel cell subsets.
FIG. 3.
MAIDS-associated spleen cell phenotypic changes are clearly evident in LP-BM5-infected CD40 transgenic TRAF 2,3/5−, 6+ and B6 wt mice. Increased numbers of spleen cells that are CD19+ CD23− and CD4+ Thy1.2− have been reported as a consequence of MAIDS pathogenesis (25). Histograms are for spleen cell populations from uninfected (−) or LP-BM5-infected (+) CD40 transgenic and B6 control mice. Cells were doubly stained with either the appropriate isotype controls or with either fluorescein isothiocyanate (x axis)- or phycoerythrin (y axis)-labeled αCD23 and αCD19 or αThy1.2 and αCD4 monoclonal antibodies (BD Pharmingen, San Diego, Calif.). The percentages of double-positive cells are found in the upper right quadrant and those of single-positive cells are in the upper left. Cells were analyzed on a FACScan, and histograms are for one representative mouse per group. See Table 1 for the entire data set.
TABLE 1.
Summary of flow cytometry analysis of cell surface phenotypic changes indicative of MAIDSa
| Cell surface markers | Values for:
|
||
|---|---|---|---|
| TRAF 2,3/5+, 6− | TRAF 2,3/5−, 6+ | C57BL/6 | |
| CD4+ Thy 1.2− | 2.9b | 10 | 16.8 |
| CD19+ CD23− | 1.2 | 1.9 | 2.1 |
LP-BM5-infected TRAF 2,3/5−, 6+ CD40 transgenic mice, like B6 mice, express high levels of CD4+ Thy1.2− and CD19+ CD23− spleen cells, compared to uninfected control mice of the same strains.
Values represent the increases (n-fold) of the percent positive cells which express the listed cell surface markers for LP-BM5-infected transgenic and B6 control mice versus uninfected mice. See Fig. 3 legend for flow cytometry details. There were four LP-BM5-infected mice in each group.
Collectively, these findings—clear splenomegaly, profound B- and T-cell immunodeficiency, and substantial spleen cell phenotypic changes occurring as a consequence of LP-BM5 infection in CD40 TRAF 2,3/5−, 6+ but not in TRAF 2,3/5+, 6− transgenic mice—provide strong evidence that the CD40-associated TRAF 6 site is required for MAIDS pathogenesis. The magnitude of various disease parameters was arguably somewhat less dramatic for infected TRAF 2,3/5−, 6+ CD40 transgenic mice than for infected prototypic B6 controls, however, suggesting that there may be CD40-associated aspects other than the TRAF 6 binding site that also contribute in a minor way to MAIDS induction.
As shown in the bottom panel of Fig. 2, viral load for the causative defective genome and the ecotropic helper virus was assessed by quantitative reverse transcriptase PCR by using primers previously described (see legend to Fig. 1). All LP-BM5-infected transgenic mice expressed substantial levels of both the defective and ecotropic viruses, whereas the levels in uninfected mice were not detectable. When comparing experimental transgenic strain groups to controls in a given experiment, defective viral load was within about a two- to threefold difference for those mouse groups that developed MAIDS, versus those that were insusceptible, following LP-BM5 infection—e.g., data from Fig. 1. In the case of comparison of the two transgenic strains with reciprocal mutations in the classical TRAF binding sites, the differences were somewhat more pronounced (Fig. 2). TRAF 2,3/5−, 6+ mice had BM5-defective and ecotropic RNA expression nearly equivalent to that of control B6 mice, whereas TRAF 2,3/5+, 6− mice exhibited BM5def and BM5eco levels that were only about 20% that of the two MAIDS-susceptible strains (Fig. 2). However, based on our experience with virus titration experiments and the study of several MAIDS-resistant versus -susceptible k.o. strains (13, 15, 16), this difference does not seem nearly sufficient to explain the lack of classic disease in the TRAF 2,3/5+, 6− transgenic strain. Rather, it apparently has to do with the fact that MAIDS pathogenesis is synonymous with lymphoproliferation, which in turn is a major contributor to viral load, irrespective of free virus spread per se. In addition, comparison of defective viral loads across experiments (e.g., Fig. 1 and 2) demonstrated similar two- to fourfold variations in defective virus expression among mouse groups that did develop MAIDS. Precise quantitative ecotropic helper virus expression levels correlate even less well among infected mouse strains that do, versus those that do not, develop MAIDS—e.g., infected CD40 k.o. mice exhibit relatively high BM5 ecotropic viral load (Fig. 1), as members of our laboratory previously reported (9). Thus, it is apparent that the viral loads detected for the MAIDS-insusceptible CD40-TRAF binding site mutant strains are likely to be well above any minimum threshold required for disease. Therefore, the observed differential requirement for TRAF site involvement does not appear to operate at the levels of virus infectivity or spread.
Recent reports by other investigators have described a unique role for TRAF 6 in certain CD40-independent and -dependent signaling contexts: for example, as a signal transducer for interleukin-1 (IL-1) (5) and as a critical mediator for CD40-induced IL-6 production, Ig secretion, and upregulation of CD80 (3, 20). As an extension of the unique role of TRAF 6 in B-cell signaling, we provide strong evidence here that the TRAF 6 binding site of CD40 is necessary for in vivo pathogenesis in a physiologically relevant model of murine immunodeficiency that is CD154/CD40 dependent and includes the development of terminal B-cell lymphomas. Collectively, our results further suggest that CD154-induced, TRAF 6-mediated, CD40 signaling is required for LP-BM5-initiated MAIDS. Alternatively, it is possible, for example, that the mutation strategy for the TRAF 6 binding site resulted instead in an overall conformational change in the CD40 cytoplasmic domain such that there was a generalized lack of proper folding. While possible, in vitro binding studies have confirmed the selective and undiminished binding of the reciprocal TRAFs to their respective unmutated binding sites in these constructs (e.g., reference 34). In addition, numerous functional assays, both in vitro and in vivo, with mutant CD40-TRAF 2,3/5 or -TRAF 6 binding sites have demonstrated the continued signaling competence of the unmutated reciprocal TRAF binding site (e.g., references 1 and 28).
Based on these latter results, our favored interpretation of the present data is that TRAF 6 is required for signal propagation for MAIDS induction. Assuming that this conclusion is correct, further studies will obviously be necessary to define the downstream events that are engaged after TRAF 6-mediated CD40 signaling in MAIDS. It is also important to point out that CD40 signaling via the TRAF 6 binding site has to be viewed in the context of other signaling pathways that are involved in MAIDS pathogenesis. For example, a recent study has provided evidence for a role by the CD19/CD21 receptor complex of B cells and its associated Vav-1 signal-transducing factor (25). Whether this is an important independent pathway for MAIDS or whether it relates to the known intersection of the CD19/CD21 pathway with major histocompatibility complex class II-dependent signaling, which in turn potentiates B-cell responsiveness to CD40 activation after ligation by CD154 (see reference 25), remains to be determined. In addition or as an alternative, it is possible that certain specific alterations known to occur in CD40-TRAF 2,3/5+, TRAF 6− transgenic mice—perhaps most notably the relative lack of conventional long-lived plasma cells (1)—may hold the key to explaining the MAIDS insusceptibility of these mice. Similarly, CD40-TRAF 2,3/5+, 6− transgenic strains have a dramatically reduced ability to produce high-affinity IgG1 antibody responses in vivo to a model antigen (1).
Acknowledgments
We thank Bob Rich, On Ho, Wen Li, and Arti Gaur for many helpful discussions concerning this study.
This work was supported in part by U.S. Public Health Service grant CA50157. The flow cytometer was the generous gift of the Fannie E. Rippel Foundation and is partially supported by the core grant of the Norris Cotton Cancer Center (CA23108).
REFERENCES
- 1.Ahonen, C. L., E. M. Manning, L. D. Erickson, B. P. O'Connor, E. F. Lind, S. S. Pullen, M. R. Kehry, and R. J. Noelle. 2002. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat. Immunol. 3:451-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz, D. C., Z. Hanna, and P. Jolicoeur. 1989. Severe immunodeficiency disease induced by a defective murine leukemia virus. Nature (London) 338:505-508. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, G. A., and B. S. Hostager. 2001. Signaling by CD40 and its mimics in B cell activation. Immunol. Res. 24:97-109. [DOI] [PubMed] [Google Scholar]
- 4.Buller, R. M. L., R. A. Yetter, T. N. Fredrickson, and H. C. Morse III. 1987. Abrogation of resistance to severe mousepox in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. J. Virol. 61:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D. V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature 383:443-446. [DOI] [PubMed] [Google Scholar]
- 6.Carreno, B. M., and M. Collins. 2002. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 20:29-53. [DOI] [PubMed] [Google Scholar]
- 7.Cerny, A. A., W. Hugin, R. R. Hardy, K. Hayakawa, R. M. Zinkernagel, M. Makino, and H. C. Morse III. 1990. B cells are required for induction of T cell abnormalities in a murine retrovirus-induced immunodeficiency syndrome. J. Exp. Med. 171:315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattopadhyay, S. K., H. C. Morse III, M. Makino, S. K. Ruscetti, and J. W. Hartley. 1989. A defective virus is associated with induction of a murine retrovirus-induced immunodeficiency syndrome, MAIDS. Proc. Natl. Acad. Sci. USA 86:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, W. J., K. A. Green, J. J. Obar, and W. R. Green. 2003. Quantitative analysis of LP-BM5 murine leukemia retrovirus RNA using real-time RT-PCR. J. Virol. Methods 108:49-58. [DOI] [PubMed] [Google Scholar]
- 10.De Leval, L., S. Colombi, S. Debrus, M. A. Demoitie, R. Greimers, P. Linsley, M. Moutschen, and J. Boniver. 1998. CD28-B7 costimulatory blockade by CTLA4-Ig delays the development of retrovirus-induced murine AIDS. J. Virol. 72:5285-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Leval, L., S. Debrus, P. Plane, J. Boniver, and M. Mountschen. 1999. Mice transgenic for a soluble form of murine cytotoxic T lymphocyte antigen 4 are refractory to murine acquired immune deficiency syndrome development. Immunology 98:630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giese, N. A., T. Giese, and H. C. Morse III. 1994. Murine AIDS is an antigen-driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J. Virol. 68:5819-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, K. A., K. M. Crassi, J. D. Laman, A. Schoneveld, R. R. Strawbridge, T. M. Foy, R. J. Noelle, and W. R. Green. 1996. Antibody to the ligand for CD40 (gp39)inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J. Virol. 70:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, K. A., R. J. Noelle, B. G. Durell, and W. R. Green. 2001. Characterization of the CD154-positive and CD40-positive cellular subsets required for pathogenesis in retrovirus-induced murine immunodeficiency. J. Virol. 75:3581-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, K. A., R. J. Noelle, and W. R. Green. 1998. Evidence for a continued requirement for CD40/CD40ligand (CD154) interactions in the progression of LP-BM5 retrovirus-induced murine AIDS. Virology 241:260-268. [DOI] [PubMed] [Google Scholar]
- 16.Green, K. A., W. J. Cook, A. H. Sharp, and W. R. Green. 2002. The CD154/CD40 interaction required for retrovirus-induced murine immunodeficiency syndrome is not mediated by upregulation of the CD80/CD86 costimulatory molecules. J. Virol. 76:13106-13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley, J. W., T. N. Fredrickson, R. A. Yetter, M. Makino, and H. C. Morse III. 1989. Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J. Virol. 63:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes, K. L., H. C. Morse III, M. Makino, R. R. Hardy, and K. Hayakawa. 1990. A unique subset of normal murine CD4+ T cells lacking Thy-1 is expanded in a murine retrovirus-induced immunodeficiency syndrome, MAIDS. J. Immunol. 20:2783-2787. [DOI] [PubMed] [Google Scholar]
- 19.Huang, M., C. Simard, and P. Jolicoeur. 1989. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science 246:1614-1617. [DOI] [PubMed] [Google Scholar]
- 20.Jalukar, S. V., B. S. Hostager, and G. A. Bishop. 2000. Characterization of the roles of TNF receptor-associated factor 6 in CD40-mediated B lymphocyte effector functions. J. Immunol. 164:623-630. [DOI] [PubMed] [Google Scholar]
- 21.Jones, K. W., and C. J. Hackett. 1996. Activated T hybridomas induce upregulation of B7-1 on bystander B lymphoma cells by a contact-dependent interaction utilizing CD40 ligand. Cell. Immunol. 174:42-53. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe, T., T. Naka, K. Yoshida, T. Tanaka, H. Fujiwara, S. Suematsu, N. Yoshida, T. Kishimoto, and H. Kikutani. 1994. The immune responses in CD40 deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 3:167-178. [DOI] [PubMed] [Google Scholar]
- 23.Klinken, S. P., T. N. Fredrickson, J. W. Hartley, R. A. Yetter, and H. C. Morse III. 1988. Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J. Immunol. 140:1123-1131. [PubMed] [Google Scholar]
- 24.Klinman, D. M., and H. C. Morse III. 1989. Characteristics of B cell proliferation and activation in murine AIDS. J. Immunol. 142:1144-1149. [PubMed] [Google Scholar]
- 25.Knoetig, S. M., T. A. Torrey, Z. Naghashfar, T. McCarty, and H. C. Morse III. 2002. CD19 signaling pathways play a major role for murine AIDS induction and progression. J. Immunol. 169:5607-5614. [DOI] [PubMed] [Google Scholar]
- 26.Kuhne, M. R., M. Robbins, J. E. Hambor, M. F. Mackey, Y. Kosaka, T. Nishimura, J. P. Gigley, R. J. Noelle, and D. M. Calderhead. 1997. Assembly and regulation of the CD40 receptor complex in human B cells. J. Exp. Med. 186:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand, E., R. Daculsi, and J. F. Duplan. 1981. Characteristics of the cell populations involved in extrathymic lymphosarcoma induced in C57BL/6 mice by RadLV-RS. Leuk. Res. 5:223-233. [DOI] [PubMed] [Google Scholar]
- 28.Manning, E. M., S. S. Pullen, D. J. Souza, M. R. Kehry, and R. J. Noelle. 2002. Cellular responses to murine CD40 in a mouse B cell line may be TRAF dependent or independent. Eur. J. Immunol. 32:39-49. [DOI] [PubMed] [Google Scholar]
- 29.Morse, H. C., III, R. A. Yetter, C. S. Via, R. R. Hardy, A. Cerny, K. Hayakawa, A. W. Hugin, M. W. Miller, K. L. Holmes, and G. M. Shearer. 1989. Functional and phenotypic alterations in T cell subsets during the course of MAIDS, a murine retrovirus-induced immunodeficiency syndrome. J. Immunol. 143:844-850. [PubMed] [Google Scholar]
- 30.Mosier, D. E., R. A. Yetter, and H. C. Morse III. 1987. Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS). J. Exp. Med. 165:1732-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosier, D. E., R. A. Yetter, and H. C. Morse III. 1985. Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J. Exp. Med. 161:766-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattengale, P. K., C. R. Taylor, P. Twomey, S. Hill, J. Jonasson, T. Beardsley, and M. Haas. 1982. Immunopathology of B cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV). Am. J. Pathol. 107:362-377. [PMC free article] [PubMed] [Google Scholar]
- 33.Pullen, S. S., H. G. Miller, D. S. Everdeen, T. T. Dang, J. J. Crute, and M. R. Kehry. 1998. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry 37:11836-11845. [DOI] [PubMed] [Google Scholar]
- 34.Pullen, S. S., T. T. Dang, J. J. Crute, and M. R. Kehry. 1999. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 274:14246-14254. [DOI] [PubMed] [Google Scholar]
- 35.Ranheim, E. A., and T. J. Kipps. 1993. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 177:925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranheim, E. A., and T. J. Kipps. 1995. Tumor necrosis factor-a facilitates induction of CD80 (B7-1) and CD54 on human B cells by activated T cells: complex regulation by IL-4, IL-10 and CD40L. Cell. Immunol. 161:226-235. [DOI] [PubMed] [Google Scholar]
- 37.Roy, M., A. Aruffo, J. Ledbetter, P. Linsley, M. Kehry, and R. Noelle. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596-603. [DOI] [PubMed] [Google Scholar]
- 38.Simard, C., S. J. Klein, T. Mak, and P. Jolicoeur. 1997. Studies of the susceptibility of nude, CD4 knockout, and SCID mutant mice to the disease induced by the murine AIDS defective virus. J. Virol. 71:3013-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yetter, R. A., R. M. L. Buller, J. S. Lee, K. L. Elkins, D. E. Mosier, T. N. Fredrickson, and H. C. Morse III. 1988. CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J. Exp. Med. 168:623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, P., R. A. Morawetz, S. Chattopadhyay, M. Makino, T. Kishimoto, and H. Kikutani. 1999. CD40-deficient mice infected with the defective murine leukemia virus LP-BM5def don't develop murine AIDS but produce IgE and IgG1 in vivo. Eur. J. Immunol. 29:615-625. [DOI] [PubMed] [Google Scholar]