Abstract
Following respiratory syncytial virus (RSV) challenge, mice immunized with RSV G or with formalin-inactivated RSV (FI-RSV) exhibit severe disease associated with type 2 cytokine production and pulmonary eosinophilia. This has led to the proposal that the presence of RSV G is the factor in FI-RSV that induces disease-enhancing T-cell responses. Therefore, we evaluated the role of RSV G and its immunodominant region in the induction of aberrant immune responses during FI-RSV immunization. BALB/c mice were immunized with FI preparations of wild-type (wt) RSV or recombinant RSV (rRSV) containing deletions of (i) the entire G gene, (ii) the region of the G gene encoding amino acids 187 to 197 of the immunodominant region, or (iii) the entire SH gene. After challenge, illness, RSV titers, cytokine levels, and pulmonary eosinophilia were measured. Peak RSV titers postchallenge were significantly greater in mice immunized with FI preparations of the deletion viruses than in those immunized with FI-rRSV wt, suggesting that the absence of G or SH in FI-RSV reduced its protective efficacy. Deletion of G or its epitope did not reduce illness, cytokine production, or eosinophilia relative to that in mice immunized with FI-rRSV wt. While cytokine levels and eosinophilia were similar, illness was reduced in mice immunized with SH-deleted FI-RSV. These data suggest that G-specific immune responses may be important for vaccine-induced protection and are not solely the basis for FI-RSV vaccine-enhanced illness. These data suggest that the method of RSV antigen delivery, rather than the protein composition, influences the phenotype of the induced immune responses and that RSV G should not necessarily be excluded from potential vaccine strategies.
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family of viruses. The negative-sense single-stranded RNA genome contains 10 genes that encode 11 proteins (15). Three proteins are present on the surface of the virus and of virally infected cells. The fusion (F) glycoprotein mediates fusion of the virus to the host cell during viral entry and, late in infection, between infected cells and their uninfected neighbor to form the characteristic syncytia. RSV G, the putative attachment protein, is naturally expressed as both a membrane-anchored and a secreted form (18, 46). RSV exists as two antigenic subgroups, A and B, with the greatest divergence occurring in RSV G (22, 23, 36). RSV F and G are the only significant neutralization antigens, and previous studies suggest that these are the major protective antigens (6). A third glycoprotein is expressed on the surface of the virus and of infected cells, the small hydrophobic (SH) protein, although a definitive function has not been identified for RSV SH to date. Thus, these three surface RSV proteins, especially the F and G glycoproteins, should be considered for inclusion in any vaccine regimen.
RSV is the major cause of respiratory disease in infants and young children, resulting in >120,000 hospitalizations in the United States each year (50). Most infants infected with RSV are symptomatic, and a significant fraction experience lower respiratory tract disease, but serious disease requiring hospitalization is relatively infrequent and is estimated to have an incidence rate of 0.5% (50). Infants and children who develop lower respiratory tract disease during infection with RSV have an increased incidence of childhood asthma, with frequencies ranging from 23 to 60% compared to 1 to 19% of children with mild RSV disease (34, 35, 51, 68). Although passive antibody prophylaxis is available for selected high-risk groups of infants, its use is limited by expense. Therefore, development of an RSV vaccine is of high priority.
In the early 1960s clinical trials of formalin-inactivated alum-precipitated RSV (FI-RSV) were conducted. However, rather than being protected against infection, children immunized with the FI-RSV preparation experienced a greater incidence and greater severity of disease following subsequent natural exposure to the virus, resulting in hospitalization of 80% of FI-RSV-immunized infants and two deaths compared to 5% hospitalization in children immunized with a similar preparation of parainfluenza virus (27, 30). Subsequent analyses of blood from these children demonstrated significant titers of nonneutralizing serum antibody (30) and heightened lymphoproliferative responses (31). Histopathologic examination of lung tissue from one of the infants who died revealed eosinophilia (14, 30). Animal models of RSV pathogenesis have similarly demonstrated enhanced disease in FI-RSV-immunized animals following challenge with live RSV (8, 12, 38, 45). This vaccine-enhanced disease is typified by pulmonary eosinophilia and the production of type 2 cytokines, especially interleukin-4 (IL-4) and IL-5. Interference with the function of these cytokines decreases disease severity (8, 24, 26, 56-58), underscoring their importance in mediating FI-RSV vaccine-enhanced immunopathogenesis.
Recombinant vaccinia viruses expressing RSV G induce CD4+ T cells that secrete IL-4, IL-5, and IL-13 and result in pulmonary eosinophilia upon RSV challenge of vaccinated mice (1, 16, 25, 40, 53, 54). Mice immunized with vaccinia virus expressing only the secreted form of RSV G (vvGs) exhibit more severe disease after challenge than do mice immunized with vaccinia virus expressing wild-type (vvGwt) or only membrane-anchored (vvGr) RSV G (1, 25). In vitro analyses have shown that T cells from BALB/c mice immunized with RSV G respond to a single peptide spanning amino acids (aa) 184 to 198 (55, 59) or aa 193 to 203 (52) of RSV G and produce both type 1 and type 2 cytokines following peptide stimulation. Immunization of BALB/c mice with this immunodominant epitope of RSV G is sufficient to induce those immune responses that result in enhanced disease following RSV challenge (59). This immunodominant region in RSV G has also been shown to encompass the linear heparin-binding domain (aa 187 to 197) in subgroup A RSV (11). These similar disease patterns of type 2 cytokine production and pulmonary eosinophilia following RSV challenge of FI-RSV- or G-immunized mice led to the proposal that it is the presence of RSV G in FI-RSV that predisposes for the vaccine-enhanced illness observed (17, 44, 67).
One approach to RSV vaccine development has been the generation of attenuated viruses. Cold passage of RSV generated the cp52 virus, which contains a spontaneous deletion of both the RSV G and SH glycoproteins as well as five coding changes in the F and polymerase (L) proteins (9, 29). Characterization of this virus demonstrated that the G and SH proteins were not required for growth in vitro, but the virus grew poorly and was highly attenuated in vivo. Immunization of mice with an FI preparation of this virus resulted in reduced eosinophilia and reduced IL-4 and IL-5 production following challenge (64). Additionally, primary infection of BALB/c mice with cp52 resulted in decreased production of substance P (65) and increased levels of Th1-enhancing chemokines (62) relative to those in mice infected with the wt B1 parental virus. These data may be interpreted as strengthening the suggestion that RSV G was the basis for the vaccine-enhanced disease observed with FI-RSV immunization, although a role for RSV SH could not be excluded. Additionally, the interpretation of these experiments is complicated by the poor growth of subgroup B RSV in mice and the presence of additional mutations in the cp52 virus.
Development of the reverse genetics system for RSV (5) provides a method by which the role of individual RSV proteins can be examined systematically in the context of an RSV infection with the use of a single RSV genetic background. By use of these engineered viruses, it has been shown that RSV G is not required for infection in vitro but is required for efficient RSV growth in vivo (28, 60, 61). Similarly, RSV lacking the SH glycoprotein grows well in vitro, producing syncytia as large as or larger than those of wt virus (3, 21, 28, 60). However, the growth of SH-deleted virus in vivo is attenuated in multiple animal models (3, 21, 69). In this paper we used recombinant RSV (rRSV) lacking RSV G, the immunodominant region of RSV G, or RSV SH to prepare FI immunogens. Immunization of mice with these vaccine products followed by intranasal challenge with live wt RSV allowed us to examine the contribution of each viral component to vaccine-enhanced disease.
MATERIALS AND METHODS
Viruses and vaccine formulations.
Recombinant wt RSV (rRSV wt) was constructed by reverse genetics and is based on the RSV A2 strain (5). rRSVs were previously constructed and characterized that contained a deletion of the G gene (rRSV ΔG) (61), a deletion of the heparin-binding domain of the G gene (aa 187 to 197) contained within the immunodominant region of aa 184 to 198 (rRSV Gep−) (61), or deletion of the SH gene (rRSV ΔSH) (2). These recombinant viruses, in addition to wt RSV A2, were grown in Vero cells (American Type Culture Collection) in Eagle's modified essential medium supplemented with 10% fetal calf serum, glutamine, and antibiotics (10% Eagle's modified essential medium). FI alum-precipitated stocks of each virus were prepared as previously described with final stocks containing no fetal calf serum (12). As a control, clarified supernatant from mock-infected Vero cells was also FI. An RSV challenge stock was grown in HEp-2 cells as previously described (13). All virus stocks were shown to be free of mycoplasma contamination by PCR (American Type Culture Collection).
Immunization and challenge of mice.
Six-week-old pathogen-free BALB/c mice were purchased from Charles River Laboratories. Mice were immunized intramuscularly with 0.1 ml of FI-Vero, FI-rRSV wt, FI-rRSV ΔG, FI-rRSV Gep−, or FI-rRSV ΔSH, diluting the FI stocks 1:10 in 1× phosphate-buffered saline. Each dose contained virus equivalent to 1.6 × 105 to 4.6 × 105 PFU per ml prior to formalin inactivation. Six weeks later the mice were challenged intranasally with 107 PFU of live RSV A2 in 0.1 ml. Following challenge the mice were weighed daily. The data are expressed as the percentages of the base weights at day 0 of challenge.
RSV titers.
Four and seven days postchallenge subsets of mice were euthanized. The lungs were removed into 10% Eagle's modified essential medium and quick-frozen. The lungs were stored at −80°C until assayed, at which point they were quick-thawed and ground with a mortar and pestle, and the supernatant was collected. RSV titers in the supernatants were measured by plaque assay on subconfluent HEp-2 monolayers as previously described (25). The data are expressed as the log10 PFU per gram of lung tissue.
Cytokine protein levels in the lung.
IL-4, IL-5, IL-13, gamma interferon (IFN-γ), eotaxin, macrophage-inactivating protein 1α (MIP-1α), and MIP-1β protein levels were measured by cytokine-specific sandwich enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, Minn.) with the lung supernatants after viral plaque assays were completed. The data are expressed as picograms per milliliter.
Bronchoalveolar lavage (BAL).
Seven days after challenge a subset of mice from each group was euthanized. A tracheotomy was performed, and the large airways were washed with 0.5 ml of phosphate-buffered saline containing 1% bovine serum albumin. The BAL wash was centrifuged, and the supernatant was removed. The BAL pellet was resuspended, total cell counts of the BAL cell pellet were made by trypan blue exclusion, and cytospins were made with the remaining sample. The cytospins were stained with HemaStain, and differential cell counts were determined by counting at least 300 total cells. The data are expressed as the percentages of eosinophils.
Lung histopathology.
Seven days after challenge mice were euthanized. The left lungs were inflated with 10% formalin and removed into formalin. The tissue was paraffin embedded, and thin sections were cut. Sections were stained with hematoxylin and eosin or with Giemsa stain.
Statistical analysis.
Data were maintained in a Paradox database. Comparisons between immunization groups were made with a Kruskal-Wallis test with SAS software. P < 0.05 was defined as a statistically significant difference.
RESULTS
Illness is not reduced in mice immunized with FI-rRSV ΔG or FI-rRSV Gep− following RSV challenge but is reduced in FI-rRSV ΔSH-immunized mice.
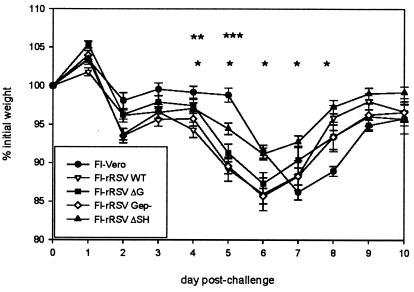
Mice were immunized with FI preparations of rRSV lacking RSV G, lacking the immunodominant region of RSV G, or lacking RSV SH. After challenge with live RSV, the mice were weighed daily. All mice immunized with FI-rRSV preparations had an early onset (day 2) of weight loss relative to control mice immunized with a mock FI preparation made from uninfected cell supernatant (FI-Vero), and the FI-rRSV-immunized mice exhibited statistically greater weight loss than did FI-Vero-primed mice at day 5 postchallenge (Fig. 1, P < 0.05). Relative to mice immunized with FI-rRSV wt, mice immunized with FI-rRSV ΔG or FI-rRSV Gep− had essentially the same severity of illness, except at day 4, when the reduced weight loss was statistically significant in mice immunized with FI-rRSV ΔG (P < 0.05). In contrast, at days 4 to 8 after challenge, mice immunized with FI-rRSV ΔSH had statistically significantly less weight loss than did mice immunized with FI-rRSV wt (Fig. 1, P < 0.05). These data demonstrate that elimination of RSV G or its immunodominant region from FI-RSV preparations did not reduce the severity of illness after live RSV challenge. Thus, immunogenic factors other than the antigenic composition or its mode of administration must be responsible for the generation of these immune responses. Interestingly, these data suggest that the presence of the SH glycoprotein may contribute to disease-enhancing immune responses resulting in severe illness in BALB/c mice. However, while the elimination of SH from the FI-RSV preparation was associated with reduced illness, disease was not prevented nor was recovery accelerated, demonstrating that the presence of RSV SH alone is not responsible for the severe illness observed in FI-RSV-immunized mice.
FIG. 1.
Weight loss in FI-rRSV-immunized mice following challenge with live RSV. Mice were immunized with FI preparations of rRSV and challenged with live RSV 6 weeks later. FI-Vero, consisting of formalin-treated supernatant from mock-infected Vero cells, was used as a negative control. Following RSV challenge mice were weighed daily, and weights were normalized to the base weight at day 0. n = 10 at days 0 to 7 and 5 at days 8 to 10. *, value for FI-rRSV ΔSH-immunized mice statistically less than that for FI-rRSV wt-immunized mice, P < 0.05; **, values for all FI-rRSV-immunized groups statistically greater than that for FI-Vero-immunized mice, P < 0.05; ***, value for FI-rRSV ΔG-immunized mice statistically less than that for FI-rRSV wt-immunized mice, P < 0.05.
Deletion of RSV G, the G epitope, or RSV SH from FI-RSV reduces protection against RSV challenge.
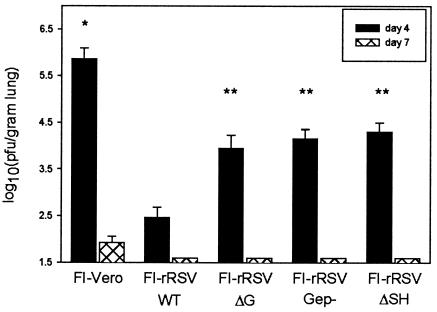
In the BALB/c mouse model of RSV infection, peak viral replication occurs 4 days after primary RSV infection, and virus clearance occurs between days 7 and 9. To assess the level of protection against RSV challenge in FI-rRSV-immunized mice, viral titers in the lung were measured at days 4 and 7 postchallenge. Immunization with any FI-rRSV preparation resulted in statistically significant reductions in peak viral titers at day 4 compared to those for mice immunized with FI-Vero (Fig. 2, P < 0.05). However, mice immunized with FI-rRSV ΔG, FI-rRSV Gep−, or FI-rRSV ΔSH had viral titers that were statistically greater than those in mice immunized with FI-rRSV wt (P < 0.05). At day 7 postchallenge mice immunized with any FI-rRSV preparation had no detectable virus in the lung, whereas virus shedding was still detectable in the lungs of mice immunized with FI-Vero. Therefore, conclusions cannot be made from these studies about the relative abilities of FI-rRSV ΔG, FI-rRSV Gep−, or FI-rRSV ΔSH to accelerate viral clearance relative to wt FI-rRSV. However, these data suggest that the presence of RSV G or SH increases the magnitude of protective antiviral immune responses that are generated during FI-RSV immunization.
FIG. 2.
RSV titers in FI-rRSV-immunized mice following challenge with live RSV. Five mice from each group of FI-rRSV-immunized mice were euthanized on day 4 and on day 7 postchallenge. Viral titers in the lung tissue were measured by plaque assay on subconfluent HEp-2 monolayers. Data represent the means ± standard deviations of the log10 PFU per gram of lung tissue. *, value for FI-Vero-immunized mice statistically greater than those for all FI-rRSV-immunized groups at day 4, P < 0.05; **, value statistically greater than that for FI-rRSV wt-immunized mice at day 4, P < 0.05.
Immunization with FI-RSV lacking RSV G, the immunodominant region of RSV G, or RSV SH does not reduce pulmonary eosinophilia following RSV challenge.
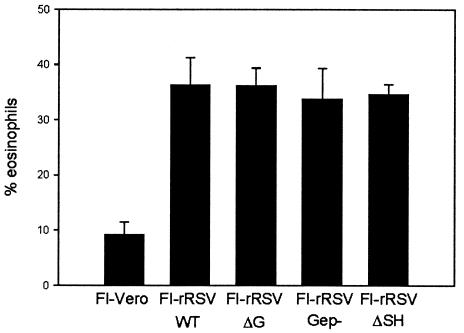
Immunization with FI-RSV (12, 38), with vaccinia virus expressing the entire RSV G glycoprotein (16, 40, 53, 54) or only secreted RSV G (1, 24-26), or with a peptide representing the immunodominant epitope of RSV G (55) results in pulmonary eosinophilia following challenge with live RSV. We sought to determine whether RSV G, the immunodominant region of RSV G, or RSV SH is the factor in FI-RSV that predisposes for eosinophil infiltration. Seven days after challenge of mice immunized with the various FI-rRSV preparations or FI-Vero, mice were euthanized, and BAL was performed. Differential staining of the BAL cells demonstrated profound eosinophilia in mice primed with any FI-rRSV immunogen (Fig. 3, P < 0.0002 compared to FI-Vero-immunized mice). Mice immunized with FI-rRSV lacking RSV G, its immunodominant region, or RSV SH had eosinophil levels similar to those of mice immunized with FI-rRSV wt. These data demonstrate that it is not merely the presence or absence of RSV G or SH during FI-RSV priming that predisposes for severe disease following subsequent RSV exposure.
FIG. 3.
BAL eosinophilia in FI-rRSV-immunized mice following challenge with live RSV. Seven days after RSV challenge FI-rRSV-immunized mice were euthanized, and BAL was performed. Cytospins were prepared with the BAL cells and differentially stained with HemaStain. Data are represented as the means ± standard deviations of the percentages of eosinophils present in at least 300 cells counted. n = 5 mice per group. All FI-rRSV-immunized mice had statistically greater percentages of eosinophils than did FI-Vero-immunized controls (P < 0.05).
Significant levels of type 2 cytokines and chemokines are produced in the lung in FI-rRSV-immunized mice following RSV challenge.
Protein levels of cytokines and chemokines in the lungs were measured 4 and 7 days after challenge of immunized mice by ELISA. Production of IL-4, IL-5, IL-13, IFN-γ, eotaxin, MIP-1α, and MIP-1β was examined in lung supernatants (Tables 1 and 2, respectively). At day 4 postchallenge (Table 1) the levels of IL-4, IL-5, IL-13, IFN-γ, eotaxin, MIP-1α, and MIP-1β detected in lung samples from mice immunized with FI-rRSV wt were statistically greater than those of mice primed with the FI-Vero negative control (P < 0.05). Similarly, these cytokines and chemokines were statistically elevated in mice immunized with FI-rRSV ΔG, FI-rRSV Gep−, and FI-rRSV ΔSH (P < 0.05 compared to FI-Vero). Generally, the levels of cytokines and chemokines produced in these priming groups were not significantly different from the levels in mice immunized with FI-rRSV wt. The only statistically significant differences from mice immunized with FI-rRSV wt were as follows: the levels of eotaxin were statistically lower in FI-rRSV ΔG-immunized mice, and levels of IL-5, eotaxin, and MIP-1β were statistically lower in mice immunized with FI-rRSV Gep−. This may indicate that RSV G or its immunodominant region contributes to the induction of those immune responses resulting in type 2 cytokine production following live virus challenge. However, it should be noted that, while the reductions in these groups compared to those immunized with FI-rRSV wt were statistically significant, the decreases were small, and the cytokine levels remained considerably higher than the levels in FI-Vero-primed mice. While the cytokine and chemokine levels were sufficient to induce similar levels of eosinophil recruitment in all FI-rRSV-immunized mice, statistically significant differences in IL-5 and eotaxin levels may result in functional differences in the eosinophils, since both IL-5 and eotaxin may be involved in eosinophil activation (37, 47). These data suggest that priming for the induction of type 2 cytokines is not solely dependent upon the presence of RSV G or its immunodominant region but is dependent upon other factors.
TABLE 1.
Day 4 cytokine and chemokine levels
| Cytokine or chemokine | Value for mice immunized witha:
|
||||
|---|---|---|---|---|---|
| FI-Vero | FI-rRSV wt | FI-rRSV ΔG | FI-rRSV Gep− | FI-rRSV ΔSH | |
| IL-4 | 19.8 ± 1.8 | 196.8 ± 12.1b (1.0) | 147.5 ± 8.3b (0.75) | 165.0 ± 20.8b (0.84) | 184.6 ± 15.8b (0.94) |
| IL-5 | 50.3 ± 16.2 | 295.8 ± 19.6b (1.0) | 223.3 ± 17.3b (0.75) | 204.8 ± 17.3b,c (0.69) | 245.1 ± 25.8b (0.83) |
| IL-13 | 99.4 ± 8.4 | 762.6 ± 73.2b (1.0) | 602.0 ± 69.0b (0.79) | 490.5 ± 79.0b (0.64) | 685.8 ± 78.8b (0.90) |
| IFN-γ | 93.4 ± 6.6 | 284.4 ± 27.7b (1.0) | 265.8 ± 24.3b (0.93) | 261.5 ± 15.8b (0.92) | 211.8 ± 16.4b (0.74) |
| Eotaxin | 482.9 ± 85.3 | 1,842.2 ± 133.0b (1.0) | 1,328.1 ± 21.3b,c (0.72) | 1,371.9 ± 154.1b,c (0.74) | 1,647.1 ± 83.0b (0.89) |
| MIP-1α | 96.2 ± 18.8 | 518.5 ± 70.0b (1.0) | 459.7 ± 71.3b (0.89) | 468.6 ± 83.3b (0.90) | 373.7 ± 42.1b (0.72) |
| MIP-1β | 289.5 ± 61.9 | 949.3 ± 95.0b (1.0) | 793.8 ± 63.1b (0.84) | 625.9 ± 83.6b,c (0.66) | 739.1 ± 36.6b (0.78) |
Data represent the concentrations of cytokines and chemokines present in the lung supernatants in picograms per milliliter (mean ± standard deviation; n = 5). The values in parentheses are normalized to cytokine levels in FI-rRSV wt-immunized mice.
Statistically greater than cytokine-chemokine level in FI-Vero-immunized mice (P < 0.05).
Statistically less than cytokine-chemokine level in mice immunized with FI-rRSV wt (P < 0.05).
TABLE 2.
Day 7 cytokine and chemokine levels
| Cytokine or chemokine | Value for mice immunized witha:
|
||||
|---|---|---|---|---|---|
| FI-Vero | FI-rRSV wt | FI-rRSV ΔG | FI-rRSV Gep− | FI-rRSV ΔSH | |
| IL-4 | 63.1 ± 13.9 | 53.6 ± 3.9 (1.0) | 70.0 ± 13.0 (1.31) | 42.1 ± 5.1 (0.79) | 50.1 ± 3.5 (0.93) |
| IL-5 | 42.8 ± 2.5 | 118.2 ± 9.9b (1.0) | 106.1 ± 10.3b (0.90) | 100.7 ± 25.0 (0.85) | 94.6 ± 11.4 (0.80) |
| IL-13 | 87.4 ± 6.7 | 154.2 ± 23.8 (1.0) | 173.4 ± 33.2 (1.12) | 114.0 ± 15.2 (0.74) | 168.8 ± 7.5 (1.09) |
| IFN-γ | 2,654.2 ± 778.7 | 179.3 ± 11.9c (1.0) | 256.7 ± 33.3c (1.43) | 200.1 ± 16.1c (1.12) | 264.8 ± 56.3c (1.48) |
| Eotaxin | 899.9 ± 84.9 | 2,691.2 ± 388.0b (1.0) | 1,995.5 ± 161.7b (0.74) | 2,492.2 ± 170.1b (0.93) | 2,555.3 ± 167.8b (0.95) |
| MIP-1α | 864.3 ± 151.1 | 399.9 ± 43.6c (1.0) | 402.2 ± 59.1c (1.01) | 333.6 ± 43.7c (0.83) | 404.9 ± 31.5c (1.01) |
| MIP-1β | 918.3 ± 102.3 | 536.0 ± 34.2c (1.0) | 519.5 ± 58.2c (0.97) | 443.0 ± 30.9c (0.83) | 502.8 ± 42.8c (0.94) |
Data represent the concentrations of cytokines and chemokines present in the lung supernatants in picograms per milliliters (mean ± standard deviation; n = 5). Values in parentheses are normalized to cytokine levels in FI-rRSV wt-immunized mice.
Statistically greater than cytokine-chemokine level in FI-Vero-immunized mice (P < 0.05).
Statistically less than cytokine-chemokine level in FI-Vero-immunized mice (P < 0.05).
At day 7 postchallenge cytokine and chemokine protein levels were also measured (Table 2). The levels of IL-4 in all FI-rRSV-immunized groups were essentially the same as those in the FI-Vero negative control. Each of the FI-rRSV-primed groups had elevated levels of IL-5 compared to those of the FI-Vero control, but this increase was statistically significant only for the groups immunized with FI-rRSV wt or with FI-rRSV ΔG. The levels of IL-13 were higher in each of the FI-rRSV-immunized animals than in the FI-Vero control, although the difference was statistically significant only in the case of FI-rRSV ΔG-immunized mice. Mice immunized with any of the FI-rRSV preparations still had statistically higher levels of eotaxin (P < 0.05 relative to FI-Vero-primed mice). In contrast, all FI-rRSV-immunized mice had statistically lower levels of IFN-γ, MIP-1α, and MIP-1β in lung supernatants at day 7 relative to FI-Vero-immunized mice. Importantly, there were no significant differences detected between FI-rRSV wt-immunized mice and all other FI-rRSV-immunized mice for any cytokine or chemokine measured at day 7. It should be noted that we consider the changes in cytokine and chemokine expression that occurred at day 4 to be of greater functional significance since the cytokine and chemokine signals generated around day 4 are the inflammatory signals that subsequently result in eosinophil recruitment and disease at day 7. However, the data from each day demonstrate that priming for type 2 cytokine production was similar between FI-rRSV wt and the FI-rRSV preparations lacking RSV G, its immunodominant region, or RSV SH.
Deletion of RSV SH, RSV G, or the RSV G immunodominant region does not reduce the severity of lung histopathology.
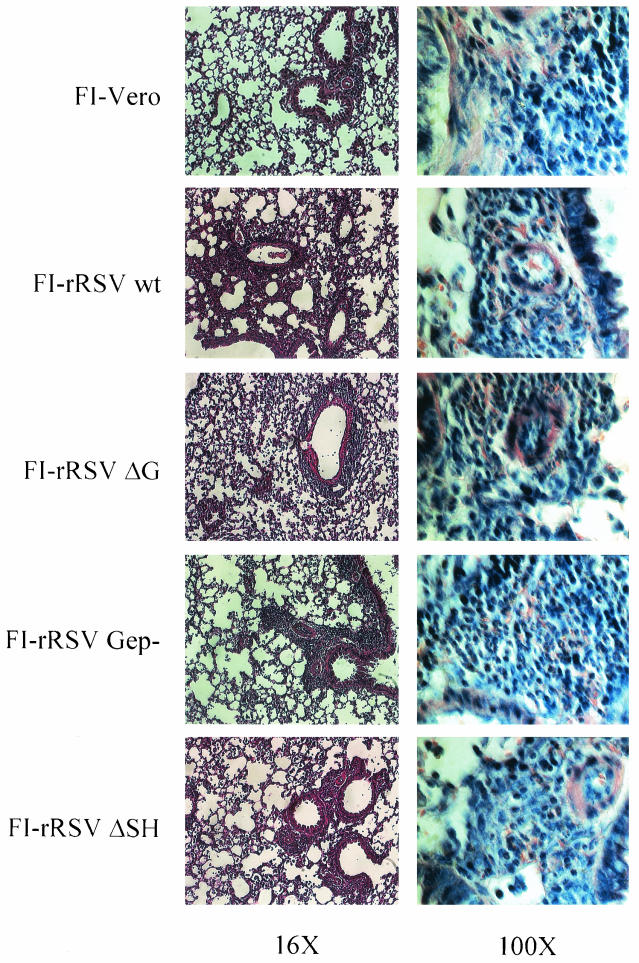
Primary RSV infection of BALB/c mice results in a mild to moderate infiltration of the pulmonary tissue with the infiltrate being composed of mononuclear cells (13). In contrast, extensive inflammation is observed following RSV challenge of FI-RSV- or RSV G-immunized mice, and numerous eosinophils are recruited (8, 24, 25, 45). Seven days after RSV challenge lungs were removed from FI-rRSV-immunized mice for histopathologic analysis. Thin sections stained with hematoxylin and eosin were examined at an ×16 magnification to evaluate the extent of inflammatory infiltration into the lung. Compared to mice immunized with the FI-Vero control preparation, all FI-rRSV-immunized mice had more inflammatory infiltrate (Fig. 4, left panels), particularly around the bronchovascular bundles and in the interstitium. However, there was no clear difference in the extent or location of inflammation when the FI-rRSV groups were compared. To assess the composition of the inflammatory response, Giemsa-stained sections were examined at an ×100 magnification (Fig. 4, right column). In the FI-Vero-immunized RSV-challenged mice, the infiltrating cells were almost exclusively mononuclear cells with no eosinophils present. In marked contrast, numerous eosinophils could clearly be seen in mice immunized with any of the FI-rRSV preparations. Furthermore, no significant difference was evident between the mice immunized with FI-rRSV ΔG, FI-rRSV Gep−, or FI-rRSV ΔSH and the FI-rRSV wt-immunized control mice. (The panels in Fig. 4 have been reduced in size to construct this composite. Full-size photos of the ×100 microscopic fields may be reviewed at http://www.vrc.nih.gov/vrc/johnson.htm.) These data demonstrate that the presence of RSV G, the immunodominant BALB/c region, or RSV SH is not required during FI-RSV immunization to induce those immune responses that result in pulmonary eosinophilia following RSV challenge, suggesting that the pathway of antigen presentation, and not merely the antigenic content, of an RSV immunogen is a primary factor in the induction of those immune responses that predispose for severe inflammation and pulmonary eosinophilia following RSV challenge.
FIG. 4.
Histopathology in FI-rRSV-immunized mice following challenge with live RSV. Seven days after RSV challenge mice were euthanized, and the left lungs were removed and fixed in formalin. Thin sections of paraffin-embedded tissue were cut and stained with hematoxylin and eosin or with Giemsa stain. The degree of inflammation was evaluated in hematoxylin-and-eosin-stained tissue at a ×16 magnification (left panels). The extent of eosinophilia was evaluated in Giemsa-stained tissue at a ×100 magnification (right panels). A representative section (of five per group) is shown at each magnification. Full-size photos of the Giemsa-stained sections with eosinophils indicated can be viewed at http://www.vrc.nih.gov/vrc/johnson.htm.
DISCUSSION
RSV remains the leading viral agent of serious pediatric respiratory tract disease worldwide as well as a major cause of disease in individuals of all ages, and the development of a safe and effective vaccine remains a human health priority. The need to immunize very young infants and the previous experience with FI-RSV vaccine-enhanced illness emphasize the importance of ensuring vaccine safety. The RSV G glycoprotein is one of the two neutralization antigens and one of the major protective antigens and thus would seem an obvious candidate for inclusion in a vaccine. On the other hand, numerous studies have suggested that immunization with RSV G is associated with the induction of aberrant immune responses that are manifest upon subsequent exposure to RSV and result in severe disease. Therefore, the exclusion of RSV G from a vaccine has also been suggested.
In the present study we compared the disease-priming capabilities of FI-RSV vaccines prepared either from rRSV wt or from derivatives lacking RSV G, its immunodominant region, or RSV SH. Deletion of RSV G or its immunodominant region did not diminish the ability of FI-RSV to prime for enhanced disease, whereas deletion of RSV SH resulted in only a modest diminution of the vaccine-enhanced disease induced by FI-RSV priming. These results clearly demonstrate that neither RSV G nor its immunodominant region is essential for disease priming by FI-RSV and indeed they do not make a discernible additive contribution under these conditions. RSV SH appeared to contribute to priming for increased disease but was not essential. In addition, the protective component of the immune response to FI-RSV was diminished by removal of RSV G or, unexpectedly, RSV SH. These results indicate that RSV G in the context of FI-RSV contributes protective epitopes as well as those that may be associated with disease enhancement.
While disease in FI-RSV- and RSV G-immunized animals may appear to be analogous, there are several indications that they have distinct pathogenic mechanisms, leading to a common final pathway. It has been demonstrated that immunization with FI-RSV (8, 12, 38, 45, 58) or with RSV G (1, 16, 25, 40, 53, 54) predisposes for severe RSV-induced disease typified by enhanced illness, pulmonary eosinophilia, and type 2 cytokine production in the BALB/c mouse model. While the end points may be similar, it is apparent that the cytokine requirements for these two immunogens are distinct. For example, while illness and type 2 cytokine production are reduced in RSV-challenged FI-RSV-immunized mice with IL-4 depletion (24, 56), the enhanced disease is unaltered in vvGs-primed mice when IL-4 function is inhibited either by antibody depletion or in IL-4-deficient mice (24). A similar pattern is seen for IL-13. Inhibition of IL-13 alone alters disease in FI-RSV-primed mice but not in vvGs-primed mice (26). Thus, disease associated with FI-RSV can be modulated by blocking IL-4 or IL-13 alone, whereas both IL-4 and IL-13 function must be blocked to modulate disease in mice immunized with RSV G (26).
Vaccine-enhanced disease was observed in 80% of FI-RSV vaccinees in the 1960s trial (27, 30) and occurs in many animal models (4, 8, 25, 26, 43, 45, 58) and thus does not appear to be dependent upon a specific genetic background. In contrast, some elements of the immune responses associated with RSV G-induced illness appear to be genetically restricted, such as pulmonary eosinophilia, which is absent or dramatically reduced in mouse strains other than BALB/c and other H-2d-restricted mouse strains (20, 24, 55). Immunization with peptides from the immunodominant region of RSV G is sufficient to elicit pulmonary eosinophilia and both type 1 and type 2 cytokine production in BALB/c mice (52, 55, 59) and is largely restricted to a subset of CD4+ T cells expressing the Vβ14 T-cell receptor (66). The existence of this immunodominant region that is sufficient by itself to induce those immune responses associated with vaccine-enhanced disease underscores the phenomenon of genetically restricted RSV G immunogenicity. This is very different from the nearly universal induction of vaccine-enhanced illness generated by the FI-RSV vaccine in children less than 6 months of age, where the frequency of 80% indicated a lack of dependence on a specific genetic background (30).
The ability of RSV G to predispose for eosinophilia and type 2 cytokine production is not unique among the RSV proteins. When administered as a purified protein in the context of alum, immunization with RSV F or with an FG chimeric protein also induces immune responses resulting in eosinophilia and IL-4 and IL-5 production following RSV challenge (7, 16, 38). However, in contrast to G-specific responses, F-specific immune responses may be modified by Th1-modulating adjuvants such as monophosphoryl lipid A or QS-21 (16, 38). Thus, the ability to induce disease-enhancing immune responses is not restricted to RSV G but instead may be characteristic of an antigen that is administered parenterally and presented to the immune system as a soluble protein that cannot be processed via the major histocompatibility complex (MHC) class I processing pathway.
These data are the first to suggest a role for RSV SH in disease pathogenesis. Although the functional role(s) of SH remains unknown, its status as a viral surface glycoprotein may influence early events of virus infection as well as host cell integrity later in infection. A second mechanism may be the alteration of membrane permeability. Expression of SH proteins from several viruses including RSV (42), human immunodeficiency virus type 1 (33, 49), and influenza virus (33) has been shown to increase the permeability of the host cell membrane. Our data suggest that the removal of SH from a candidate RSV vaccine may improve safety.
In the present study the RSV G component of FI-RSV clearly functioned as a protective antigen as evidenced by the reduced protective efficacy of FI-RSV ΔG. A similar effect was observed with FI-RSV Gep−, consistent with the idea that aa 187 to 197 represent the major antigenic site within RSV G in the BALB/c mouse model. Unexpectedly, removal of the SH component from FI-RSV also resulted in a reduction in protective efficacy. RSV SH is not generally considered to be a protective antigen because it did not function as an independent protective antigen when expressed in rodents by a recombinant vaccinia virus (6). However, RSV SH has been shown to contain epitopes for B cells and T-helper T lymphocytes in the BALB/c mouse model (39). Thus, RSV SH may function directly as a protective antigen or may contribute indirectly, possibly by contributing T-helper cell epitopes or by maintaining the integrity of RSV G and F.
There is precedence for exacerbated type 2 cytokine production and pulmonary eosinophilia upon aerosol challenge when initial antigen exposure is via the parenteral route. During primary RSV infection mice sensitized to ovalbumin exhibit enhanced airway hyperresponsiveness, increased production of type 2 cytokines, and more severe illness (41). Similarly, initial exposure to antigens by cutaneous routes results in Th2 responses following subsequent inhalation contact with the same substance (10, 19). Additionally, obligate MHC class II processing of particulate antigens can bias immune responses toward a Th2-restricted pattern while the same protein processed by the MHC class I pathway generates more-Th1-like responses (32). These patterns of antigen presentation are similar to those described in this study and may suggest common pathogenic mechanisms.
During final preparation of the manuscript Haynes et al. published data demonstrating reduced cellular infiltration in the BAL compartment of mice immunized with FI preparations of RSV expressing either wt or mutant forms of RSV G after RSV challenge (17) and concluded that FI-RSV-enhanced disease may be reduced by removal of RSV G or by inhibition of the CX3C-CX3CR1 interaction. These data appear to contradict the data presented here. However, there are several points to consider that may account for the apparent discrepancies. Much of the data reported are in mice primed with FI-A2, a wt RSV, and challenged with mutant RSV or with antibody treatment at RSV challenge, thus inhibiting recall of the memory response. This is in contrast to the system used in our experiments in which rRSV was utilized during immunization, affecting induction of immune responses. In contrast to the targeted removal of defined and specific genetic sequences in the rRSV that we used, the mutant viruses used for challenge in the studies by Haynes et al. have multiple mutations, with cp52 having deletions of RSV G and SH and mutations in F and L proteins (9, 29) and with R10C7G having 10 nucleotide changes resulting in six amino acid changes in RSV G (48). While in vitro replication of these viruses appears to be intact, these alterations have been shown to result in reduced in vivo replication (9, 29) or altered electrophoretic mobility suggestive of altered protein structure and, potentially, disruption of RSV G epitopes other than the CX3C fractalkine domain (48). Anti-CX3CR1 antibody treatment was shown to reduce RSV infectivity (63). Thus, an alternative explanation for the reduced BAL cell numbers and eosinophilia observed in FI-A2 anti-CX3CR1-treated mice and in cp52-challenged mice is altered cell trafficking due to reduced viral burden and not merely the absence of RSV G or inhibition of CX3CR1 interaction. Additionally, most of the analyses were conducted at 40 h after challenge, which may not reflect the peak of cellular recruitment, previously shown to be around day 6 postchallenge for BAL cells (40) and 7 to 9 days postinfection for lung tissue (13). This may be of particular importance in comparing mice challenged with wt RSV to mice challenged with mutant viruses producing lower viral burdens where the kinetics of cell recruitment may be different. These combined factors make it difficult to compare the data described by Haynes et al. to the data that we now report.
The data reported here demonstrate that the factor involved in the induction of disease-enhancing immune responses during immunization with FI-RSV is not RSV G or its immunodominant region. Rather, this study suggests that it is the pathway of initial antigen presentation, specifically obligate processing of exogenous antigen through the MHC class II pathway, that is the critical determining factor in the induction of immune responses that result in severe disease upon intranasal challenge with live RSV. This hypothesis is strengthened by previous studies from our lab (24, 25) that demonstrated that mice immunized with vaccinia virus expressing only the membrane-anchored form of RSV G are protected against severe disease following RSV challenge. Thus, the presentation of FI-RSV antigens in the context of alum is sufficient to induce those immune responses that result in type 2 cytokine production and pulmonary eosinophilia following subsequent infection with live RSV. Further studies are required to determine the basic molecular and cellular mechanisms that are involved in the programming for vaccine-enhanced immune responses during immunization with exogenous RSV antigens that require antigen processing and presentation via endocytic pathways. Taken together, these and previous studies from our lab (24, 25) suggest that the basis of RSV vaccine-enhanced disease is not antigenic content but is rather the pathway of antigen processing and, therefore, that RSV G should not necessarily be excluded from potential vaccine products that target endogenous antigen processing and presentation by MHC class I molecules.
REFERENCES
- 1.Bembridge, G. P., R. Garcia-Beato, J. A. Lopez, J. A. Melero, and G. Taylor. 1998. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J. Immunol. 161:2473-2480. [PubMed] [Google Scholar]
- 2.Bukreyev, A., B. R. Murphy, and P. L. Collins. 2000. Respiratory syncytial virus can tolerate an intergenic sequence of at least 160 nucleotides with little effect on transcription or replication in vitro and in vivo. J. Virol. 74:11017-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev, A., S. S. Whitehead, B. R. Murphy, and P. L. Collins. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J. Virol. 71:8973-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon, M. J., P. J. M. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors, M., P. L. Collins, C.-Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors, M., P. L. Collins, C.-Y. Firestone, A. V. Sotnikov, A. Waitze, A. R. Davis, P. P. Hung, R. M. Chanock, and B. R. Murphy. 1992. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine 10:475-484. [DOI] [PubMed] [Google Scholar]
- 8.Connors, M., N. A. Giese, A. B. Kulkarni, C.-Y. Firestone, I. Morse, and B. R. Murphy. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 68:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe, J. E., Jr., P. T. Bui, C.-Y. Firestone, M. Connors, W. R. Elkins, R. M. Chanock, and B. R. Murphy. 1996. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J. Infect. Dis. 173:829-839. [DOI] [PubMed] [Google Scholar]
- 10.Cua, D. J., R. L. Coffman, and S. A. Stohlman. 1996. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J. Immunol. 157:2830-2836. [PubMed] [Google Scholar]
- 11.Feldman, S. A., R. M. Hendry, and J. A. Beeler. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J. Virol. 73:6610-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, B. S., G. S. Henderson, Y.-W. Tang, X. Lu, K. M. Neuzil, and D. G. Colley. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 151:2032-2040. [PubMed] [Google Scholar]
- 13.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 14.Graham, B. S., J. A. Rutigliano, and T. R. Johnson. 2002. Respiratory syncytial virus immunobiology and pathogenesis. Virology 297:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes, L. M., L. P. Jones, A. Barskey, L. J. Anderson, and R. A. Tripp. 2003. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. J. Virol. 77:9831-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks, D. A., K. Baradaran, K. McIntosh, and J. L. Patterson. 1987. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J. Gen. Virol. 68:1705-1714. [DOI] [PubMed] [Google Scholar]
- 19.Herrick, C. A., H. MacLeod, E. Glusac, R. E. Tigelaar, and K. Bottomly. 2000. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J. Clin. Investig. 105:765-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussell, T., A. Georgiou, T. E. Sparer, S. Matthews, P. Pala, and P. J. M. Openshaw. 1998. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J. Immunol. 161:6215-6222. [PubMed] [Google Scholar]
- 21.Jin, H., H. Zhou, X. Cheng, R. Tang, M. Munoz, and N. Nguyen. 2000. Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2-2 genes are attenuated in vitro and in vivo. Virology 273:210-218. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, P. R., R. A. Olmsted, G. A. Prince, B. R. Murphy, D. W. Alling, E. E. Walsh, and P. L. Collins. 1987. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 61:3163-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, P. R., M. K. Spriggs, R. A. Olmsted, and P. L. Collins. 1987. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc. Natl. Acad. Sci. USA 84:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, T. R., R. A. Parker, J. E. Johnson, and B. S. Graham. 2003. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 170:2037-2045. [DOI] [PubMed] [Google Scholar]
- 27.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shvedoff, and C. E. Stewart. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405-421. [DOI] [PubMed] [Google Scholar]
- 28.Karger, A., U. Schmidt, and U. J. Buchholz. 2001. Recombinant bovine respiratory syncytial virus with deletions of the G or SH genes: G and F proteins bind heparin. J. Gen. Virol. 82:631-640. [DOI] [PubMed] [Google Scholar]
- 29.Karron, R. A., D. A. Buonagurio, A. F. Georgiou, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. B. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 31.Kim, H. W., S. L. Leikin, J. Arrobio, C. D. Brandt, R. M. Chanock, and R. H. Parrott. 1976. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr. Res. 10:75-78. [DOI] [PubMed] [Google Scholar]
- 32.Kurup, V. P., H. Choi, P. S. Murali, A. Resnick, J. N. Fink, and R. L. Coffman. 1997. Role of particulate antigens of Aspergillus in murine eosinophilia. Int. Arch. Allergy Appl. Immunol. 112:270-278. [DOI] [PubMed] [Google Scholar]
- 33.Lamb, R. A., and L. H. Pinto. 1997. Do Vpu and Vpr of human immunodeficiency virus type 1 and NB of influenza B virus have ion channel activities in the viral life cycles? Virology 229:1-11. [DOI] [PubMed] [Google Scholar]
- 34.Martinez, F. D., A. L. Wright, L. M. Taissig, C. J. Holberg, M. Halonen, W. J. Morgan, and the Group Health Medical Associates. 1995. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 332:133-138. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh, K. 1976. Bronchiolitis and asthma: possible common pathogenetic pathways. J. Allergy Clin. Immunol. 57:595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan, L. A., E. G. Routledge, M. M. Willcocks, A. C. R. Samson, R. Scott, and G. L. Toms. 1987. Strain variation of respiratory syncytial virus. J. Gen. Virol. 68:2781-2788. [DOI] [PubMed] [Google Scholar]
- 37.Mould, A. W., A. J. Ramsay, K. I. Matthaei, I. G. Young, M. E. Rothenberg, and P. S. Foster. 2000. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J. Immunol. 164:2142-2150. [DOI] [PubMed] [Google Scholar]
- 38.Neuzil, K. M., J. E. Johnson, Y.-W. Tang, J.-P. Prieels, M. Slaoui, N. Gar, and B. S. Graham. 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15:525-532. [DOI] [PubMed] [Google Scholar]
- 39.Nicholas, J. A., M. A. Mitchell, M. E. Levely, K. L. Rubino, J. H. Kinner, N. K. Harn, and C. W. Smith. 1988. Mapping an antibody-binding site and a T-cell-stimulating site on the 1A protein of respiratory syncytial virus. J. Virol. 62:4465-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Openshaw, P. J. M., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4:493-500. [DOI] [PubMed] [Google Scholar]
- 41.Peebles, R. S. J., J. R. Sheller, J. E. Johnson, D. B. Mitchell, and B. S. Graham. 1999. Respiratory syncytial virus infection prolongs methacholine-induced airway hyperresponsiveness in ovalbumin-sensitized mice. J. Med. Virol. 57:186-192. [DOI] [PubMed] [Google Scholar]
- 42.Perez, M., B. Garcia-Barreno, J. A. Melero, L. Carrasco, and R. Guinea. 1997. Membrane permeability changes induced in Escherichia coli by the SH protein of human respiratory syncytial virus. Virology 235:342-351. [DOI] [PubMed] [Google Scholar]
- 43.Piedra, P. A., H. S. Faden, G. Cammussi, D. T. Wong, and P. L. Ogra. 1989. Mechanism of lung injury in cotton rats immunized with formalin-inactivated respiratory syncytial virus. Vaccine 124:34-38. [DOI] [PubMed] [Google Scholar]
- 44.Polack, F. P., M. N. Teng, P. L. Collins, G. A. Prince, M. Exner, H. Regele, D. D. Lirman, R. Rabold, S. J. Hoffman, C. L. Karp, S. R. Kleeberger, M. Wills-Karp, and R. A. Karron. 2002. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prince, G. A., A. B. Jenson, V. G. Hemming, B. R. Murphy, E. E. Walsh, R. L. Horswood, and R. M. Chanock. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 57:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts, S. R., D. L. Lichtenstein, L. A. Ball, and G. W. Wertz. 1994. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J. Virol. 68:4538-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothenberg, M. E., R. Ownbey, P. D. Mehlhop, P. M. Loiselle, M. van de Rijn, J. V. Bonventre, H. C. Oettgen, P. Leder, and A. D. Luster. 1996. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol. Med. 2:334-348. [PMC free article] [PubMed] [Google Scholar]
- 48.Rueda, P., B. Garcia-Barreno, and J. A. Melero. 1994. Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations). Virology 198:653-662. [DOI] [PubMed] [Google Scholar]
- 49.Schubert, U., A. V. Ferrer-Montiel, M. Oblatt-Montal, P. Henklein, K. Strebel, and M. Montal. 1996. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 398:12-18. [DOI] [PubMed] [Google Scholar]
- 50.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 51.Sigurs, N. 2002. Clinical perspectives on the association between respiratory syncytial virus and reactive airway disease. Respir. Res. 3:S8-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparer, T. E., S. Matthews, T. Hussell, A. J. Rae, B. Garcia-Barreno, J. A. Melero, and P. J. M. Openshaw. 1998. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 187:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J. Virol. 71:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific, CD8+ T lymphocytes down regulate Th2 type cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srikiatkhachorn, A., W. Chang, and T. J. Braciale. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, Y.-W., and B. S. Graham. 1994. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J. Clin. Investig. 94:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang, Y.-W., and B. S. Graham. 1997. T cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J. Clin. Investig. 99:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang, Y.-W., K. M. Neuzil, J. E. Fischer, F. W. Robinson, R. A. Parker, and B. S. Graham. 1997. Determinants and kinetics of cytokine expression patterns in lungs of vaccinated mice challenged with respiratory syncytial virus. Vaccine 15:597-602. [DOI] [PubMed] [Google Scholar]
- 59.Tebbey, P. W., M. Hagen, and G. E. Hancock. 1998. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 188:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng, M. N., S. S. Whitehead, and P. L. Collins. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283-296. [DOI] [PubMed] [Google Scholar]
- 62.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2:732-738. [DOI] [PubMed] [Google Scholar]
- 64.Tripp, R. A., D. Moore, L. Jones, W. M. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tripp, R. A., D. Moore, J. Winter, and L. J. Anderson. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 15:637-646. [DOI] [PubMed] [Google Scholar]
- 67.Varga, S. M., E. L. Wissinger, and T. J. Braciale. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487-6495. [DOI] [PubMed] [Google Scholar]
- 68.Welliver, R. C., and L. Duffy. 1993. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing, and pulmonary function at age 7-8 years. Pediatr. Pulmonol. 15:19-27. [DOI] [PubMed] [Google Scholar]
- 69.Whitehead, S. S., A. Bukreyev, M. N. Teng, C.-Y. Firestone, M. St. Claire, W. R. Elkins, P. L. Collins, and B. R. Murphy. 1999. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J. Virol. 73:3438-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]