Abstract
Snakehead rhabdovirus (SHRV) affects warm-water fish in Southeast Asia and belongs to the genus Novirhabdovirus by virtue of its “nonvirion” (NV) gene. To examine the function of the NV gene, we used a recently developed reverse genetic system to produce a viable recombinant SHRV carrying an NV gene deletion. The recombinant virus was produced at the same rate and same final concentrations as the wild-type virus in cultured fish cells in spite of the NV gene deletion. The role of the NV protein in fish pathogenesis was also investigated. Zebra fish (Danio rerio) were infected with the NV deletion mutant or with a recombinant virus containing a copy of the SHRV genome, and similar mortality rates as well as final mortalities were recorded, suggesting no apparent role for the NV protein in fish pathogenesis. Interestingly, the unsuccessful rescue of fully viable recombinants with genomes containing deletions in the G/NV gene junction suggested a role for the gene junction in virus transcription and replication. Finally, we demonstrated that the SHRV glycoprotein can be replaced by the glycoprotein of infectious hematopoietic necrosis virus (IHNV) or by a hybrid protein composed of SHRV and IHNV sequences.
Snakehead rhabdovirus (SHRV), a rhabdovirus of warm-water fish, was isolated from a diseased snakehead fish (Ophicephalus striatus) during an epizootic outbreak in Thailand (12). The disease was characterized by a severe ulcerative dermal necrosis and was seen in a wide variety of freshwater and estuarine fishes, in the wild and in pond culture, throughout Southeast Asia. Although the disease affected several species, the cultured snakehead fish, the walking catfish (Clarius batrachus), and the sand goby (Oxyeleotris marmoratus) were the most noticeably affected (10). SHRV is a nonsegmented negative-strand RNA virus from the Rhabdoviridae family, genus Novirhabdovirus. The genome of SHRV contains six open reading frames (ORF) in the order 3′-N-P-M-G-NV-L-5′ (13). At the junction between two genes, the stop/polyadenylation signal and the following start signal are separated by an intergenic region composed of several nucleotides that are not present in the mRNAs. The major distinguishing feature of the Novirhabdoviruses is the presence of a nonvirion (NV) gene between the viral glycoprotein (G) and the polymerase (L) genes (13). The NV gene contains a single ORF, which is 335 nucleotides long. The NV protein has been detected in infectious hematopoietic necrosis virus (IHNV)-infected tissue culture cells by autoradiography (14) and immunofluorescence techniques (21) and in tissues of IHNV-infected fish by confocal microscopy (6). While the functions of most IHNV proteins have been determined, the function of the NV protein remains unknown.
In 2000, we reported the recovery of recombinant snakehead rhabdovirus (SHRV) from a full-length cDNA clone of the viral genome (11). The full-length cDNA was assembled from clones of each of the five SHRV genes and their intergenic regions and inserted into a transcription plasmid between the T7 promoter and the hepatitis delta virus ribozyme. The generated plasmid was transfected into epithelioma papulosum cyprini (EPC) cells that expressed the T7 RNA polymerase from recombinant vaccinia virus vTF7-3 (9) together with separate plasmids that encoded the viral nucleocapsid protein (N), polymerase protein (L), and phosphoprotein (P), respectively, under the control of the T7 RNA polymerase promoter. The expression of a positive-strand RNA copy of the genome along with the viral proteins necessary for in vitro viral replication resulted in the generation of recombinant viruses. To examine the function of the NV gene, we constructed an SHRV genomic cDNA clone with two nucleotide changes in the NV ORF that resulted in a stop codon (11). The virus produced from this template appeared to be no different from the wild-type virus in tissue culture infectivity assays. However, only one stop codon was introduced in this NV knockout mutant virus, and readthrough of this single stop codon might account for the apparent lack of phenotypic change in the mutant.
In the present paper, the function of the NV protein was examined further by generating a recombinant SHRV carrying a complete deletion of the NV gene. The successful recovery of a viable deletion mutant confirmed that the NV protein is not essential for virus replication in cell culture. Growth curves revealed similar virus production for the deletion mutant and the wild-type virus, indicating that NV is not necessary for virus growth in cell culture. The NV protein doesn't appear to play a significant role in virus pathogenesis in vivo either. When zebra fish were similarly infected with either the deletion mutant or the recombinant virus containing the entire SHRV genome, no significant differences in mortality were observed. To examine more closely the function of the NV protein, we generated additional SHRV cDNAs lacking 37 or 55 bp of the G/NV gene junction. The failure of these mutant cDNAs to rescue infectivity suggested that the G/NV gene junction might be essential for viral transcription and replication. In addition, we constructed novel recombinant viruses that contained the IHNV G gene or a hybrid IHNV G/SHRV G gene in place of the SHRV G gene.
MATERIALS AND METHODS
Cell and viruses.
EPC cells (8) were used for virus propagation. The cells were grown at 29°C in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and 2 mM l-glutamine. The SHRV was obtained from John Fryer, Oregon State University. For preparation of virus stocks, confluent EPC cells were infected with the wild-type SHRV at a multiplicity of infection (MOI) of 0.001 in MEM with 2% fetal bovine serum. After 1 h of adsorption, the inoculum was removed, and the cells were incubated at 29°C until extensive cytopathic effect (CPE) was observed.
Oligonucleotide primers.
The oligonucleotide primers used in this study are listed in Table 1. The location of each primer within the SHRV genome is indicated by the numbers of the nucleotide positions.
TABLE 1.
Primers used in this study
| Primer code | Sequencec (5′-3′) | Position (nt)a of underlined sequence |
|---|---|---|
| 632b | GCGGCCGCGAGAAACTAGATGTCCCATCTTCTT | 5265-5289d |
| 634b | GCTAGCGGCAGTTTCGTTCTTCTGTTCC | 4872-4893d |
| 635b | CTCGTGATTGGGCTTGAGTCTCT | 4794-4816d |
| 636b | CGGTTCTTTGATTGTGCACTATA | 4834-4857d |
| 653 | GAGTAGAGATCTGATGCGGTC | 4729-4749d |
| 580 | CGTTGTGGGTTGATGGAGGGCCTAGT | 5424-5450d |
| 531 | ATATATGAAGACAACGACTCCTGGTAGA | 5239-5266d |
| 554 | CAAATATCTCGTGGACTCGTG | 7279-7290d |
| 719b | GTCCGGCTAGCTTTGGTTTCATGTTTGGGAG | 3208-3227d |
| 720b | ATTGTGCGGCCGCAACATGGGTGTAAATTGGACTC | 4576-4597d |
| 721b | ATTGTGCGGCCGCTCAGGCCCCATCACAGTT | 4750-4567d |
| 722 | GGGAATGGCAATCGGTGTCA | 2954-2973d |
| 744 | TCTGTTCCTGGGTGTGCTTG | 48594877d |
| 749 | GGATTGTCCTGTTTCTGGTGTTC | 3231-3253d |
| 750 | TGGGTATCTTCTGGTTTGAGGTC | 3473-3495d |
| 738 | GCGCGCGCGCTAGCAACGCAACTCGCAGAGAC | 18-37e |
| 740 | GCGCGCGCGCGGCCGCGTCTGGTGGGGAGGAAGTGAA | 1596-1607e |
| 739 | GCGCGCGCGCGGCCGCGTCTTGTACTGGGCGACGTATTT | 1274-1296e |
| 450 | AACGCAACTCGCAGAGACC | 17-36e |
| 451 | GTCTGGTGGGGAGGAAGTGAA | 1586-1607e |
Plasmid construction and mutagenesis.
A full-length cDNA clone of SHRV RNA (pMJ-SHRV) was assembled from clones of each of the SHRV genes and their intergenic junctions by standard cloning techniques (11). In that study, an SHRV cDNA expression vector was engineered to contain a truncated NV ORF. The vector, pMJ-SHRV-(NV single stop), was created with a two-nucleotide alteration in the NV ORF that resulted in a stop codon 22 nt into the NV ORF (Fig. 1). To construct mutants with deletions in the SHRV genome, region-specific ExSite (Stratagene, La Jolla, Calif.) PCR-based site-directed mutagenesis was performed. This PCR-based mutagenesis strategy uses increased template concentration and reduced cycling number to decrease potential second-site mutations during the PCR amplification. The following pMJ-SHRV cDNA clones with mutations in the NV gene were created: pMJ-SHRV-(NV-ORF−) is missing the NV ORF from nucleotide 4893 to 5265, pMJ-SHRV-(NV-ORF− +37) is missing the NV ORF as well as having an additional 37-bp deletion of the G/NV junction region from nucleotide 4834 to 5265, and pMJ-SHRV-(NV-ORF− +55) is missing the NV mRNA region from nucleotide 4816 to 5265. The structures of the engineered mutants are shown in Fig. 1A. Gene manipulations were carried out in the subclone pMCS-NV, containing the HpaI/SacI 1.3-kb fragment of pMJ-SHRV spanning the C-terminal region of the G gene, G/NV gene junction, NV gene, and part of the NV/L gene border sequence. For the mutagenesis experiments, 0.5 pmol of pMCS-NV was added to a 25 μl of a PCR mixture containing the following reagents: 2.5 μl of 10× mutagenesis buffer; 15 pmol of sense and antisense primers, where the sense primer was 5′ phosphorylated; 1 μl of 25 mM deoxynucleoside triphosphate (dNTP) mixture; and 5 U of ExSite DNA polymerase blend. To create pMCS-NV-(ORF− +55), primers 632 and 635 were added to the PCR mixture. To construct pMCS-NV-(ORF−) and pMCS-NV-(ORF− +37), the primer pairs 632-634 and 632-636 were added to the mutagenesis reaction mixture, respectively. The sequences of the primers used in the mutagenesis experiments are shown in Table 1. All the PCR amplifications were performed on a PTC-200 Peltier thermal cycler with the same cycling parameters: 1 cycle of 30 s at 94°C, followed by 10 cycles of 30 s at 94°C, 30 s at 55°C, and 5 min at 68°C. Following completion of the PCR, the plasmid population was subsequently digested with 10 U of the DpnI restriction enzyme, which is specific for methylated and hemimethylated DNA. The nonmutated parental plasmids, which were methylated during growth in vivo, were digested by the DpnI restriction enzyme. PCR-generated mutagenesis primer-incorporating linear plasmids remain undigested. The undigested linear DNA was then blunt ended with 1.25 U of cloned Pfu DNA polymerase to remove extended bases placed on the 3′ ends of the PCR products by the ExSite DNA polymerase blend. The Pfu DNA polymerase-treated DNA was then intramolecularly ligated at 37°C for 1 h with 4 U of T4 DNA ligase. The ligated DNA was used to transform Escherichia coli XL1-Blue supercompetent cells. Plasmid DNA was purified using the Qiaprep Spin Miniprep kit by following the manufacturer's conditions (Qiagen, Valencia, Calif.). Direct sequence analysis using primers 653 and 580 was used to identify the site-specific mutations in pMCS-NV-(ORF−), pMCS-NV-(ORF− +37), and pMCS-NV-(ORF− +55). Finally, the plasmids were digested with HpaI/SacI, and the fragments containing the altered NV gene were cloned back into pMJ-SHRV to create the final clones pMJ-SHRV-(NV ORF−), pMJ-SHRV-(NV ORF− +37), and pMJ-SHRV-(NV ORF− +55), respectively. The sequences of these clones were determined by sequence analysis using primers 653 and 580.
FIG. 1.
Diagram of the recombinant SHRV genomes. The orders of the genes in the wild-type SHRV and recombinant viruses are shown. The genes are transcribed from left to right from the negative-strand RNA genome, which is shown in the 3′-to-5′ orientation. Numbers indicate the nucleotide positions in the antigenomic sequence of SHRV. Big boxes, coding regions; small boxes, noncoding regions. On the left we show the identification codes of the wild-type (wt) and recombinant viruses. Open arrowheads and vertical boxes, transcriptional start and stop/polyadenylation signals, respectively. Recovery (+) or nonrecovery (−) of the recombinant virus from the corresponding cDNA genomic clone is also indicated. (A) The organizations of full-length SHRV cDNA and NV mutants are compared. (B) Comparison of the structures of the pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) mutants. Gc, cytoplasmic domain of the glycoprotein.
Mutants with modifications in the SHRV glycoprotein gene were also created. The genomic structures of the engineered mutants are shown in Fig. 1B. The complete SHRV G gene was replaced by the IHNV G gene in the pMJ-SHRV-(IHNV G) cDNA expression vector. Mutant pMJ-SHRV-(IHNV G/SHRV G hybrid) was engineered to encode a hybrid SHRV-IHNV glycoprotein containing the extracellular and transmembrane domains of IHNV envelope protein fused to the cytoplasmic domain of SHRV G. To construct both mutants, a 4.5-kb SplI/SacII fragment of pMJ-SHRV containing the G gene was cloned into pMCS, where ExSite PCR-based site-directed mutagenesis was performed by following the manufacturer's instructions. Primer pairs 719-721 and 719-720 were added to the mutagenesis reaction mixture to generate pMCS-(IHNV G) and pMCS-(IHNV G/SHRV G hybrid), respectively. The PCR conditions were as follows: 1 cycle of 30 s at 94°C, followed by 11 cycles of 30 s at 94°C, 30 s at 58°C, and 5 min at 68°C. The coding sequence of the IHNV G gene or of the portion the G gene encoding the amino-terminal domain of the G protein was amplified by PCR from pcDNA3-G (1) with primer pair 738-740 or 738-739, respectively. Primer 738 is located in the ectodomain-coding region, primer 739 overlapped the junction between the transmembrane and cytoplasmic tail coding regions, and primer 740 is located at the coding sequence for the C-terminal end of the IHNV G protein. Primer 738 contained an NheI site, while primers 740 and 739 had NotI restriction sites. The PCR products were cloned in PCR 2.1 with a TA cloning kit (Invitrogen, Carlsbad, Calif.) and verified by sequence analysis. Subsequently, the PCR products were digested with NheI and NotI and inserted at the site of the G deletions in pMCS-(IHNV G) and pMCS-(IHNV G/SHRV G hybrid), respectively. The resulting plasmids were digested with SplI/SacII, and the fragments obtained were cloned back in pMJ-SHRV, which was previously digested with SplI/SacII. The resulting full-length cDNA plasmids were designated pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid). Primers 722 and 744, flanking the SHRV G gene, were used to verify both constructs.
DNA sequence analysis.
All DNA sequence analyses were performed by the Oregon State University Center for Gene Research and Biotechnology Central Services Facility on a 3730 DNA sequencer (Dupont Applied Biosystems, Boston, Mass.).
Generation of a stably transfected EPC cell line expressing T7 RNA polymerase.
EPC cells were transfected with pcDNA3-T7, a plasmid capable of expressing the T7 RNA polymerase under the control of the cytomegalovirus immediate early promoter, with Lipofectamine Plus reagent (Invitrogen). Plasmid pcDNA3-T7 contains a neomycin phosphotransferase gene under the control of the simian virus 40 promoter to confer neomycin resistance. Stably transfected cells were selected by culturing in the presence of neomycin G-418 (800 μg/ml). After a period of growth, a cloned neomycin-resistant population of cells was isolated after limiting dilution passage of the cells in a 96-well plate. Individual colonies were again grown in the presence of neomycin G-418 and subsequently passed to larger culture dishes. The NeoR T7pol cells were tested for T7 polymerase activity as described by Johnson et al. (11). Luciferase expression driven by a T7 polymerase promoter in the NeoR T7pol cells was comparable to or better than that in cells transfected with pCMV-T7pol.
Transfection experiments and recovery of SHRV recombinants from cloned cDNAs.
Recoveries were carried out as described previously (11). Approximately 106 EPC cells overexpressing the T7 RNA polymerase were grown overnight in six-well plates and then transfected by using Lipofectamine Plus reagent (Invitrogen) with 0.5 μg of full-length cDNA constructs encoding either wild-type or mutant SHRV genomes, together with 0.25, 0.5, and 0.2 μg of support plasmids encoding N, L, and P proteins, respectively. Transfected cells were incubated at 29°C until CPE was evident. Cells culture supernatants were then subjected to three cycles of freezing and thawing, clarified by centrifugation at 1,000 × g in a microcentrifuge (Micromase; Thermo IEC), and then used to inoculate fresh cell monolayers.
RNA purification.
Total RNA was extracted from infected EPC cells with RNAzol according to the supplier's instructions (Invitrogen).
Confirmation of virus rescue from cloned cDNA by RT-PCR.
RNA from the first passage of a presumed recombinant SHRV was collected as described above and analyzed by reverse transcriptase PCR (RT-PCR) to demonstrate the presence of the targeted mutations. RT-PCR was performed with the First Strand cDNA synthesis kit by following the manufacturer's recommended protocol (Fermentas, Hanover, Md.). Briefly, a 20-μl reverse transcription reaction mixture containing 5 μg of total RNA, 0.2 μg of random hexamers, 2 μl of 10 mM dNTP mixture, 20 U of RNase inhibitor, 4 μl of 5× Moloney murine leukemia virus (M-MuLV) RT buffer (250 mM Tris-HCl [pH 8.3], 250 mM KCl, 20 mM MgCl2, 50 mM dithiothreitol), and 40 U of M-MuLV RT was incubated for 10 min at 25°C, 60 min at 37°C, and 10 min at 70°C. Following reverse transcription, 1 μl of cDNA was used as template for each of the subsequent PCR amplifications. The PCR mixture contained 5 μl of 10× PCR buffer, 50 pmol of specific sense and antisense primers, 10 mM dNTP, 3 μl of 25 mM MgCl2, and 2.5 U of Taq DNA polymerase in a total volume of 50 μl. Sequences of the primers used in the RT-PCR assays are shown in Table 1. All PCR amplifications were performed with the same parameters: 30 cycles of PCR consisting of 10 s at 95°C, 1 min at 55°C, and 3 min at 72°C. Extension times varied depending on the PCR mixture size. The PCR products were analyzed in agarose gels and visualized with ethidium bromide.
Plaque assays.
Plaque assays were used to determine the titers of wild-type or recombinant viruses in accordance with the method of Burke and Mulcahy (4). After 48 h of incubation to allow SHRV plaque formation, the monolayers were fixed, stained with crystal violet, and photographed.
Virus purification and electron microscopy.
The NV deletion mutant pMJ-SHRV-(NV ORF−) and the wild-type SHRV were grown in EPC cells at a MOI of 0.01 until complete destruction of the respective monolayers. After low-speed centrifugation (1,000 × g) at 4°C, virus in the clarified culture fluid was purified by sucrose gradient centrifugation as described by Engelking and Leong (7). Purified virus was resuspended in TNE buffer (0.1 M Tris-HCl [pH 7.6], 0.1 M NaCl, 0.001 M EDTA) and layered onto an electron microscopy grid. The grids were stained with phosphotungstic acid for 1 min and examined with a transmission electron microscope (Philips).
Growth curves in tissue culture.
EPC cells grown in 25-cm2-high flasks were infected with the recombinant virus pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) or with the wild-type SHRV at a MOI of 0.001. After 1 h of adsorption at 30°C, cells were washed to remove unadsorbed virus. At 2-h intervals for 14 h and at 24 h, two flasks were removed and the titers of non-cell-associated and total virus were assayed. Cell-free virus titer was determined from the supernatant of one flask following low-speed centrifugation. Total virus was determined from both supernatant and homogenized cells after centrifugation at 1,000 × g in a microcentrifuge (Micromase; Thermo IEC). The cells were homogenized in three cycles of freezing and thawing. Samples were frozen at −70°C, and at the end of the 24-h experiment the viral titer was determined by tissue culture end point dilution assay (50% tissue culture infective dose [TCID50]/ml) (17).
Fish challenge.
Adult Ab strain zebra fish (Danio rerio) were intraperitoneally injected with the recombinant virus pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) or with the wild-type SHRV. Triplicate sets of 20 fish were anesthetized by immersion in 50 μg of 3-aminobenzoic acid ethyl ester (MS-222; Sigma, St. Louis, Mo.)/ml for 1 to 2 min and immediately injected with 10 μl of each virus stock (105 PFU/ml). Control fish were treated identically except that they were injected with an equivalent volume of Dulbecco's phosphate-buffered saline instead of infectious virus. The fish were kept in 2.5-liter tanks in a flowthrough system supplied with 28°C of nonchlorinated pathogen-free water throughout the experiment. Mortalities were recorded daily for 21 days postchallenge. Contingency tables of fish groups and replicate tanks were estimated by chi-square analysis. For all analyses, differences were considered statistically significant when the correlation coefficient value (P) was less than 0.05. In Fig. 5, the standard deviation is not included because there was very little variation in the replicates.
FIG. 5.
CPM of zebra fish injected with wild-type SHRV (wt) or pMJ-SHRV, pMJ-SHRV-(NV single stop), and pMJ-SHRV-(NV ORF−) recovered viruses. Triplicate sets of 20 adult zebra fish were infected intraperitoneally with 105 PFU of the recombinant virus pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) or with the wild-type SHRV/ml. Mortalities were recorded daily for 21 days postchallenge.
Nucleotide sequence accession number.
The full-length cDNA clone of SHRV RNA (pMJ-SHRV) has been assigned GenBank accession no. AF147498.
RESULTS
Construction and rescue of a SHRV deletion mutant lacking the NV gene.
We previously described the recovery of recombinant viruses from a full-length cDNA copy of the SHRV genome (pMJ-SHRV) (11). In that study, cells were infected with the recombinant vaccinia virus (vTF7-3) expressing T7 RNA polymerase and then transfected with T7-driven expression plasmids containing a positive-sense copy of the SHRV genome and the three genes encoding the N, L, and P proteins. In the present study, we used a stably transfected helper cell line constitutively expressing T7 RNA polymerase to drive expression of the T7 promoter plasmids. This approach permitted us to recover recombinant virus without contaminating vaccinia virus, and the CPE observed in the transfected cells was likely the result of virus rescue.
For viral rescue, T7 RNA polymerase-expressing EPC cells were cotransfected with plasmid DNA encoding either wild-type or mutant SHRV cDNAs and with plasmids pcDNA3-N, pcDNA3-L, and pcDNA3-M1. Viable recombinant viruses were recovered reproducibly from cells transfected with an unmodified cDNA copy of the SHRV genome (pMJ-SHRV), a cDNA copy of the SHRV genome with an stop codon in the NV gene [pMJ-SHRV-(NV single stop)], or a cDNA clone with the whole NV gene deleted [pMJ-SHRV-(NV ORF−)]. CPE was observed 48 h after the transfection, while the nontransfected cells were still in complete monolayers. The supernatant fluids were collected at this time, diluted 1:100 in MEM with 10% fetal bovine serum, and placed on a fresh monolayer. Within 48 h, cells overlaid with diluted supernatant from the initial experiment underwent cytopathic changes characteristic of an SHRV lytic infection. No CPE was observed in cells transfected with pMJ-SHRV-(NV ORF− +37) or pMJ-SHRV-(NV ORF− +55) clones, which contained deletions in the G/NV gene junction together with the NV gene. Transfection experiments with these two constructs were done in triplicate, and, for each replicate, no recombinant infectious viruses were recovered. This result suggested that the G/NV junction region is required for the virus replication.
Identification by RT-PCR of recombinant virus lacking the NV gene ORF.
Although CPE was observed, it was necessary to demonstrate that the recovered viruses pMJ-SHRV, pMJ-SHRV-(NV single stop), and pMJ-SHRV-(NV ORF−) were in fact recombinants. The RNA from the recombinant viruses was amplified by RT-PCR with primers flanking the introduced mutations, and the sequence of each product was confirmed by direct sequencing. Results of the RT-PCR assays are shown in Fig. 2. When primers flanking the NV coding sequence (653 to 580) were used in the RT-PCR, a band of 727 bp was obtained from RNA of cells infected with the wild-type virus or with pMJ-SHRV-(NV single stop) or MJ-SHRV-(NV ORF−) recombinant virus (Fig. 2A). The RNA from cells infected with pMJ-SHRV-(NV ORF−) yielded a DNA fragment of only 356 bp, the size expected for this deletion mutant. A second RT-PCR assay using the NV gene ORF primer 531 and the L gene ORF primer 554 was performed. With the wild-type, pMJ-SHRV, or pMJ-SHRV-(NV single stop) mRNAs the RT-PCR produced fragments of 2,060 bp. In contrast, no amplification was detected for the NV deletion mutant (Fig. 2B). Sequencing the PCR products confirmed that the original engineered cDNA sequences were rescued in the genomes of pMJ-SHRV, pMJ-SHRV-(NV single stop), and pMJ-SHRV-(NV ORF−) recombinant viruses.
FIG. 2.
RT-PCR analysis of pMJ-SHRV, pMJ-SHRV-(NV single stop), and pMJ-SHRV-(NV ORF−) recombinant viruses. Total RNA from EPC cells infected with wild-type SHRV (lane 1) or with the recombinant viruses pMJ-SHRV (lane 2), pMJ-SHRV-(NV single stop) (lane 3), and pMJ-SHRV-(NV ORF−) (lane 4) was isolated at 2 days p.i. and used for RT-PCR amplifications of the NV region. Amplification products were separated on a 1% agarose gel. Two pairs of primers were used in the PCRs: primer pairs 653-580 (A) and 554-531 (B). Lane M, DNA size markers. Note that the faint band to the right of lane 4 is an artifact.
Deletion of the NV gene ORF did not affect the replication of the recombinant virus in tissue culture cells.
We determined whether deletion of the NV gene would affect the growth rate of SHRV by comparing mutant and wild-type viruses in growth experiments. In these studies, the quantities of both free and cell-associated virus were determined because some rhabdoviruses bud into intracytoplasmic vacuoles during maturation. EPC cells were infected with wild-type or recombinant viruses at a MOI of 0.001. After unabsorbed virus was removed, virus growth was monitored by end point dilution assay. The growth curves are shown in Fig. 3. The wild-type and the recombinant viruses replicated with the same efficiency, reaching similar infectivity titers of about 108 TCID50/ml at 24 h postinfection (p.i.). For all the viruses tested, the concentration of cell-free virus was always below that of total virus during the first 14- to 16-h period but reached a plateau similar to that for total virus concentration at 24 h. The infectivity titers of the recombinant viruses were also determined by plaque assay. The recombinant viruses pMJ-SHRV, pMJ-SHRV-(NV single stop), and pMJ-SHRV-(NV ORF−) produced infectivity titers of 4.2 × 108, 6.8 × 108, and 3.6 × 108 PFU/ml, respectively. The wild-type virus yielded a similar final infectivity titer (7.2 × 108 PFU/ml).
FIG. 3.
Growth curves of the wild-type SHRV (wt) and pMJ-SHRV, pMJ-SHRV-(NV single stop), pMJ-SHRV-(NV ORF−) recombinant viruses. EPC cells were infected with wt SHRV or with the recombinant viruses indicated. (A) The infectivity titer of the cell-free virus was determined from the supernatant of the infected cells at intervals from 0 to 14 h and at 24 h after infection. (B) At the indicated times p.i., the infectivity titer of the total virus was determined for cells infected with the wild-type or recombinant virus.
Deletion of the NV gene ORF did not affect the plaque and virus morphology.
EPC cells were infected with the recombinant virus pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) or with the wild-type SHRV and incubated for 48 h. The cells were then stained with crystal violet, and the plaque sizes and morphologies were compared (Fig. 4). The plaques from all the four viruses had approximately the same size and appearance, indicating that the biological activity of recombinant viruses in tissue culture cells was not substantially altered. To assess whether the alterations in the genome of SHRV had any effect on viral morphology, wild-type and deletion mutant virus particles were purified, negatively stained, and examined by electron microscopy. No obvious morphological differences between the NV deletion mutant, pMJ-SHRV-(NV ORF−), and the wild-type virus were observed (data not shown).
FIG. 4.
Viral plaques of the wild-type SHRV (A) and pMJ-SHRV (B), pMJ-SHRV-(NV single stop) (C), and pMJ-SHRV-(NV ORF−) (D) recovered viruses. Plaque assays were used to determine the titers of wild-type and recombinant viruses. EPC cells were infected with the wild-type SHRV (A) or with the pMJ-SHRV (B), pMJ-SHRV-(NV single stop) (C), and pMJ-SHRV-(NV ORF−) (D) recovered viruses. After 48 h of incubation at 30°C to allow SHRV plaque formation, the monolayers were fixed, stained with crystal violet, and photographed.
NV gene ORF deletion has no role in the pathogenesis of SHRV in zebra fish.
Zebra fish were infected with the recombinant viruses or wild-type SHRV. The challenges were performed in triplicate on groups of 20 fish by intraperitoneal injection with 105 PFU of the recombinant virus pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) or wild-type SHRV/ml. Cumulative percent mortalities (CPM) are shown in Fig. 5. The data represent the three replicate groups and the combined averages from two independent experiments. Animals injected with pMJ-SHRV, pMJ-SHRV-(NV single stop), or pMJ-SHRV-(NV ORF−) recombinant virus showed similar CPM (55%) at 21 days postchallenge. Fish injected with the wild-type SHRV exhibited CPM of 66.4%. Statistical analysis indicated that there was no difference in virulence between wild-type SHRV and any of the recombinant SHRV strains.
Recovery of SHRV recombinant viruses expressing the IHNV glycoprotein or a hybrid protein composed of SHRV and IHNV sequences.
To analyze whether heterologous or chimeric Novirhabdovirus G proteins could assemble and function in SHRV-like particles, two SHRV cDNA clones were constructed. The plasmid pMJ-SHRV-(IHNV G) expresses the complete IHNV G protein instead of the SHRV glycoprotein. The recombinant plasmid pMJ-SHRV-(IHNV G/SHRV G hybrid) was engineered to encode a hybrid IHNV-SHRV glycoprotein containing the extracellular and transmembrane domains of the IHNV envelope protein fused to the cytoplasmic domain of SHRV G. By reverse genetics, recombinant viruses were rescued from both cDNA clones. The genomic structures of the recombinant viruses are shown in Fig. 1B. The IHNV glycoprotein was able to replace SHRV G in all steps of the virus life cycle, as demonstrated by the successful rescue of infectious viral particles. The finding of autonomously replicating virus from pMJ-SHRV-(IHNV G) also indicated that the cytoplasmic domain of the SHRV G protein is not required for the expression of foreign proteins. The identity of the recombinant viruses pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) was further verified by RT-PCR analyses and by sequencing of the PCR products. Results of the RT-PCR assays are shown in Fig. 6. When primer pair 450-742 spanning the extracellular and transmembrane domains of the IHNV glycoprotein was used in the RT-PCRs, DNA fragments of 1,592 bp were obtained from RNA of cells infected with the wild-type IHNV or recombinant viruses pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) (Fig. 6A). In contrast, no amplification was obtained from RNA of cells infected with the parental SHRV, demonstrating the specificity of primer pair 450-742 for the IHNV glycoprotein. Primer pair 450-451, spanning the entire coding region of the IHNV G gene, was used in another RT-PCR amplification. A band of 1,706 bp was just amplified from cells infected with wild-type IHNV or recombinant virus pMJ-SHRV-(IHNV G), confirming the presence of a different cytoplasmic domain in the glycoprotein of pMJ-SHRV-(IHNV G/SHRV G hybrid) (Fig. 6B). Finally, a specific primer pair for the SHRV G gene, 749-750, was used in another RT-PCR assay. Amplification with this primer pair was obtained only from cells infected with the wild-type SHRV, indicating the presence of a foreign G gene in the genomes of pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant viruses (Fig. 6C). Sequence analysis of the rescued virus confirmed that the genomes contained the alterations introduced into the respective cDNA clones. The CPE observed in cells infected with pMJ-SHRV-(IHNV G) or pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant virus 24 h after the infection is shown in Fig. 7. Complete destruction of the cell monolayer was achieved 24 h after infection with wild-type SHRV, whereas cells infected with pMJ-SHRV-(IHNV G) or pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant virus developed less-extensive CPE.
FIG. 6.
Confirmation of pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant viruses rescue from cloned cDNA by RT-PCR. RT-PCR was performed as described in Materials and Methods with primer pair 450-742 (A), 450-451 (B), or 749-750 (C). Amplification products were separated on a 1% agarose gel. Lanes: M, DNA molecular weight markers; lane 1, wild-type IHNV; lane 2, wild-type SHRV; lane 3, pMJ-SHRV-(IHNV G) recombinant virus; lane 4, pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant virus; lane 5, plasmid controls pCMV-G (A and B) and pMJ-SHRV (C). RT-PCR amplifications were carried out on 5 μg of total infected-cell RNA.
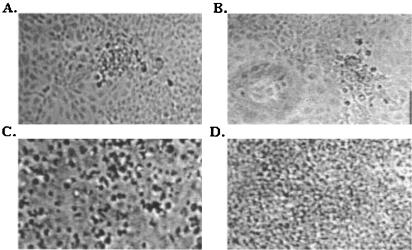
FIG. 7.
CPE observed 24 h after infection of EPC cells with pMJ-SHRV-(IHNV G) (A), pMJ-SHRV-(IHNV G/SHRV G hybrid) (B), and wild-type SHRV (C) viruses. (D) Uninfected EPC cells. Levels of growth of the wild-type SHRV and pMJ-SHRV-(IHNV G) and pMJ-SHRV-(IHNV G/SHRV G hybrid) recombinant viruses in cultured EPC cells were compared after infection at 30°C at a MOI of 0.1. CPE was observed at 24 h after infection.
DISCUSSION
The recent development of systems that allow directed genetic manipulation (reverse genetics) of fish rhabdovirus and the ability to recover full-length virus or minivirus particles entirely from cloned cDNAs have facilitated the molecular genetic analyses of viral proteins and cis regulatory elements (2, 3, 11). The NV gene is present in all known novirhabdoviruses, but its function is still unknown. We report here our continuing efforts to determine whether NV has a function in the pathogenesis of SHRV in fish cells and in fish. It is clear that recombinant virus containing deletions in the NV gene coding region behave no differently from parental virus in tissue culture and in zebra fish. Perhaps these studies will require comparing pathogenesis profiles in snakehead fish, the normal host for the virus, but, until these tests can be conducted, we conclude that an NV protein has no apparent function in vitro and in vivo.
Because rhabdoviruses have relatively small genomes, generally around 12 kb, little of the genome coding capacity is wasted. For this reason, it is reasonable to assume that the NV gene retained in this genus must have a critical function. To analyze whether the G/NV gene junction might have a significant role in the transcription process, we constructed two SHRV cDNA clones with alterations in the size of the NV mRNA [pMJ-SHRV-(NV ORF− +37) and pMJ-SHRV-(ORF− +55)]. The first of these clones presented a deletion of the NV ORF along with a 37-bp deletion of the G/NV gene junction including the NV transcription initiation signal. A complete deletion of the G/NV gene junction along with the NV gene was engineered in pMJ-SHRV-(NV ORF− +55). When EPC cells overexpressing the T7 RNA polymerase were transfected with pMJ-SHRV-(NV ORF− +37) or pMJ-SHRV-(NV ORF− +55), no recombinants were recovered. These results illustrate the importance of regulatory sequences present in the nontranslated regions of the genome for the recognition of viral RNA by viral polymerases. While a complete deletion of the NV gene can be tolerated, deletions in the G/NV junction may change the abundance of the upstream and downstream mRNAs and thus may render such a virus completely defective. We speculate that the NV gene might have a spacing function regulating transcription without translation, but additional support from Northern blot analyses will be required to test this hypothesis.
The reverse genetics system allowed us to determine whether other viral genes may be replaced in the SHRV genome. The SHRV glycoprotein gene was replaced with the IHNV glycoprotein gene in pMJ-SHRV-(IHNV G) and with an IHNV/SHRV hybrid glycoprotein gene in pMJ-SHRV-(IHNV G/SHRV G hybrid). The hybrid construct encoded the ectodomain of the IHNV glycoprotein fused to the SHRV cytoplasmic domain. The successful recovery of SHRV recombinant viruses from both plasmids demonstrates that the SHRV cytoplasmic domain is not required for incorporation of foreign proteins. Although the generation of infectious particles containing the foreign glycoproteins was achieved, recombinant viruses containing hybrid or IHNV glycoprotein genes replicated in cell cultures less efficiently than the wild-type SHRV. At 30°C, the infectivity titer of the wild-type SHRV was 108 TCID50/ml at 3 days p.i., and, at 20°C, the same infectivity titer was reached by wild-type IHNV at 15 days p.i. In contrast, the infectivity titers of the recombinant viruses were 103 TCID50/ml at 15 days p.i. at 30°C. The difference in optimal growth temperatures for the two viruses might result from conformational instability of the IHNV glycoprotein at 30°C as reported by Cain et al. (5).
The requirements for successful assembly of viral glycoproteins into a membrane envelope have turned out to be different for rhabdoviruses. For some viruses, assembly is thought to require specific interactions between the cytoplasmic tail of the viral glycoprotein and internal viral components gathered on the cytoplasmic side of the plasma membrane. For example, insertion of foreign glycoproteins requires conservation of the rabies virus glycoprotein cytoplasmic domain (15, 16). In contrast, the rescue of recombinant vesicular stomatitis virus (VSV) mutants lacking the genetic information for either the cytoplasmic domain or the entire G protein suggested that the cytoplasmic domain of the VSV envelope protein is not required for assembly into virus particles (18, 20). Substitutions of the transmembrane and cytoplasmic domains of the VSV glycoprotein did not affect the incorporation of CD4 into the VSV envelope (19), whereas the CD4 protein was efficiently incorporated only when the rabies virus G cytoplasmic domain was present (15). Recently, a recombinant IHNV with the hemorrhagic septicemia virus envelope was produced (3). Our results confirm their results that the cytoplasmic domain of the SHRV glycoprotein is not required either for the assembly of foreign proteins in the Novirhabdovirus envelopes.
The incorporation of foreign viral spike glycoproteins into the envelope of recombinant SHRV provides a way to develop new vaccines against fish viral diseases. Also, critical residues of the glycoprotein ectodomain required for cell infection might be identified by this approach with mutated SHRV glycoproteins.
Acknowledgments
We acknowledge the support provided by grants R/BT-28-NSI and 04-058-04 from Oregon Sea Grant and grant 3610006041 (Animal Health and Disease Research) from the United States Department of Agriculture to Jo-Ann Leong. Marta Alonso was a recipient of a fellowship from the Spanish Government (Comision Interministerial de Ciencia y Tecnologia).
We thank Estela Thomann for excellent technical assistance.
REFERENCES
- 1.Anderson, E. D., D. V. Mourich, S. C. Fahrenkrug, S. LaPatra, J. Shepherd, and J. A. Leong. 1996. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol. Mar. Biol. Biotechnol. 5:114-122. [PubMed] [Google Scholar]
- 2.Biacchesi, S., M. I. Thoulouze, M. Bearzotti, Y. X. Yu, and M. Bremont. 2000. Recovery of NV knockout infectious hematopoietic necrosis virus expressing foreign genes. J. Virol. 74:11247-11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacchesi, S., M. Bearzotti, E. Bouguyon, and M. Bremont. 2002. Heterologous exchanges of the glycoprotein and the matrix protein in a Novirhabdovirus. J. Virol. 76:2881-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke, J. A., and D. Mulcahy. 1980. Plaquing procedure for infectious hematopoietic necrosis virus. Appl. Environ. Microbiol. 39:872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain, K. D., S. E. LaPatra, B. Shewmaker, J. Jones, K. M. Byrne, and S. S. Ristow. 1999. Immunogenicity of a recombinant infectious hematopoietic necrosis virus glycoprotein produced in insect cells. Dis. Aquat. Org. 36:67-72. [DOI] [PubMed] [Google Scholar]
- 6.Chiou, P. P., C. H. Kim, P. Ormonde, and J. A. Leong. 2000. Infectious hematopoietic necrosis virus matrix protein inhibits host-directed gene expression and induces morphological changes of apoptosis in cell cultures. J. Virol. 74:7619-7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelking, H. M., and J. C. Leong. 1989. The glycoprotein of infectious hematopoietic necrosis virus elicits neutralizing antibody and protective responses. Virus Res. 13:213-230. [DOI] [PubMed] [Google Scholar]
- 8.Fijan, N. 1983. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from common carp Cyprinus carpio. Ann. Virol. 134E:207-220. [Google Scholar]
- 9.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedrick, R. P., W. D. Eaton, J. L. Fryer, W. G. Groberg, and S. Boonyaratpalin. 1986. Characteristics of a birnavirus isolated from cultured sand goby Oxyeleotris marmoratus. Dis. Aquat. Org. 1:219-225. [Google Scholar]
- 11.Johnson, M. C., B. E. Simon, C. H. Kim, and J. A. Leong. 2000. Production of recombinant snakehead rhabdovirus: the NV protein is not required for viral replication. J. Virol. 74:2343-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasornchandra, J., H. M. Engelking, C. N. Lannan, J. S. Rohovec, and J. L. Fryer. 1992. Characteristics of three rhabdoviruses from snakehead fish Ophicephalus striatus. Dis. Aquat. Org. 13:89-94. [Google Scholar]
- 13.Kurath, G., K. G. Ahern, G. D. Pearson, and J. C. Leong. 1985. Molecular cloning of the six mRNA species of infectious hematopoietic necrosis virus, a fish rhabdovirus, and gene order determination by R-loop mapping. J. Virol. 53:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurath, G., and J. C. Leong. 1985. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J. Virol. 53:462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mebatsion, T., and K. K. Conzelmann. 1996. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc. Natl. Acad. Sci. USA 93:11366-11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mebatsion, T., M. J. Schnell, J. H. Cox, S. Finke, and K. K. Conzelmann. 1996. Highly stable expression of a foreign gene from rabies virus vectors. Proc. Natl. Acad. Sci. USA 93:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 18.Schnell, M. J., L. Buonocore, E. Kretzschmar, E., Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnell, M. J., J. E. Johnson, L. Buonocore, and J. K. Rose. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849-857. [DOI] [PubMed] [Google Scholar]
- 20.Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K. Rose. 1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 17:1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schutze, H., P. J. Enzmann, E. Mundt, and T. C. Mettenleiter. 1996. Identification of the non-virion (NV) protein of fish rhabdoviruses, viral haemorrhagic septicaemia virus and infectious haematopoietic necrosis virus. J. Gen. Virol. 77:1259-1263. [DOI] [PubMed] [Google Scholar]