Abstract
Campylobacter concisus is an oral bacterium that is associated with intestinal diseases. C. concisus was previously described as a bacterium that requires H2-enriched microaerobic conditions for growth. The level of H2 in the oral cavity is extremely low, suggesting that C. concisus is unlikely to have a microaerobic growth there. In this study, the anaerobic growth of C. concisus was investigated. The growth of fifty-seven oral C. concisus strains and six enteric C. concisus strains under various atmospheric conditions including anaerobic conditions with and without H2 was examined. The atmospheric conditions were generated using commercially available gas-generation systems. C. concisus putative virulence proteins were identified using mass spectrometry analysis. Under anaerobic conditions, 92% of the oral C. concisus strains (52/57) and all six enteric strains grew without the presence of H2 and the presence of H2 greatly increased C. concisus growth. An oral C. concisus strain was found to express a number of putative virulence proteins and the expression levels of these proteins were not affected by H2. The levels of H2 appeared to affect the optimal growth of C. concisus. This study provides useful information in understanding the natural colonization site and pathogenicity of C. concisus.
1. Introduction
Campylobacter concisus is a Gram-negative bacterium that is commonly present in the human oral cavity [1, 2]. In some individuals, C. concisus may colonize the intestinal tract and was found to be associated with inflammatory bowel disease (IBD) due to its significantly higher prevalence in the intestinal tract of patients with IBD as compared to controls [3–6]. IBD is a chronic inflammatory disease of the gastrointestinal tract (GIT) with unknown aetiology. Crohn's disease (CD) and ulcerative colitis (UC) are the two major clinical forms of IBD [7]. In addition to IBD, C. concisus was also often isolated from diarrheal stool samples, suggesting a possible involvement in diarrheal disease [8–10].
In the literature, it was described that C. concisus requires H2-enriched microaerobic conditions for growth [11, 12]. In laboratory cultivations of C. concisus, microaerobic conditions enriched with 5–10% H2 have been used [2, 9, 12, 13]. The primary colonization site of C. concisus is the human oral cavity [1, 2]. The level of H2 in the human oral cavity is extremely low [14]. Given this, it is unlikely that C. concisus is able to grow microaerobically in the human oral cavity. In previous studies, C. concisus was isolated from gingival plaque and saliva, locations where a large number of anaerobes were found, suggesting that C. concisus is more likely to use an anaerobic growth in the human oral cavity [1, 2, 15].
To date, there have been no studies that systematically examined the growth of C. concisus under anaerobic conditions. Furthermore, there is no information available regarding the impact of H2 on C. concisus growth under anaerobic conditions. These issues were investigated in the current study. Furthermore, we examined the expression of putative virulence proteins of an oral C. concisus strain grown under anaerobic conditions.
2. Materials and Methods
2.1. C. concisus Strains Used in This Study
A total of 63 C. concisus strains were examined in this study, including 57 oral C. concisus strains and six enteric strains. Of the 57 oral C. concisus strains, 19 strains were from patients with CD, 14 strains from patients with UC, and 24 strains from healthy individuals. Of the six enteric C. concisus strains, five strains were isolated from patients with IBD including two strains isolated from intestinal biopsies of patients with UC, two strains isolated from fecal samples of patients with UC, and one strain isolated from intestinal biopsies of a patient with CD. Both oral and enteric C. concisus strains were isolated in our previous studies [1, 3, 6]. C. concisus strain 13826, which was isolated from fecal samples of a patient with bloody diarrhea, was purchased from the American Type Culture Collection.
2.2. Examination of C. concisus Growth under Various Atmospheric Conditions
The growth of the above 63 C. concisus strains under the following atmospheric conditions was examined.
2.2.1. Anaerobic Condition without H2 (AnaeroH2−)
AnaeroH2− condition was generated using AN25A gas-generation system as instructed by the manufacturer (Oxoid, Hampshire, UK).
2.2.2. Anaerobic Condition Containing 9% of H2 (AnaeroH2+)
AnaeroH2+ condition was generated using BR38B gas-generation system, which was placed into a 3.5 L jar in the presence of a catalyst following the manufacturer's instruction (Oxoid).
2.2.3. Microaerobic Condition without H2 (MicroH2−)
A MicroH2− condition was generated using two different gas-generation systems, following the manufacturer's instruction (Oxoid). The first gas-generation system was BR56A (MicroH2−a), which was placed into a 3.5 L jar in the presence a catalyst. The second gas-generation system was CN25A (MicroH2−b), which was placed into a 2.5 L jar.
2.2.4. Original Isolation Condition
The C. concisus strains used in this study were isolated in our previous studies [1, 3, 6]. The atmospheric condition used in isolation of these C. concisus strains from clinical samples in our previous studies was to place the BR56A gas-generation system into a 2.5 L jar with a catalyst, which was a modification of the manufacturer's instruction. In this study, we refer to this atmospheric condition as the original isolation condition (Oricon).
Each of the 63 C. concisus strains was streaked onto three horse blood agar (HBA) plates for each atmospheric condition. The HBA plates were prepared using blood agar base number 2 supplemented with 6% (v/v) heat-inactivated defibrinated horse blood and 10 μg/mL of vancomycin (Oxoid). Following incubation at 37°C under AnaeroH2−, AnaeroH2+, MicroH2−a, MicroH2−b, and Oricon conditions, respectively, for 48 hours, plates were examined for the appearance of colonies under a stereo microscope. The morphology of all grown C. concisus strains was examined using a phase-contrast microscope.
2.3. Quantitative Comparison of C. concisus Growth under AnaeroH2− and AnaeroH2+ Conditions
To further quantitatively compare the growth of C. concisus under AnaeroH2− and AnaeroH2+ conditions, the colony forming unit (CFU) of 12 C. concisus strains grown under these two conditions was determined. These 12 strains included six oral strains from patients with CD, three oral strains from UC, two oral strains from healthy controls, and one enteric strain from a patient with UC. These 12 strains were randomly selected from the 63 strains used in the experiments in Section 2.2.
C. concisus strains were first cultured on HBA plates under Oricon condition at 37°C for 48 hours. The bacterial cells were collected and washed once with phosphate buffered saline (PBS). The bacterial pellet of each strain was resuspended in PBS and OD600 was adjusted to 0.05, which was used as the initial inoculum for further assessment of the growth of C. concisus under AnaeroH2− and AnaeroH2+ conditions.
The initial inoculum suspension (50 μL) of each C. concisus strain was inoculated onto six HBA plates using a sterile L-shaped glass rod. Three plates were incubated under AnaeroH2− condition and the remaining three plates were incubated under AnaeroH2+ condition for 48 hours at 37°C.
The bacterial cells of each C. concisus strain collected from the three plates incubated under AnaeroH2− condition and the three plates incubated under AnaeroH2+ condition were pooled, respectively (1 mL of PBS was used for collection of bacterial cells from each plate). From each pooled C. concisus suspension, serial dilutions (1 : 10 to 1 : 108) were prepared. Each of the dilutions (5 μL) was inoculated onto HBA plates in triplicate. The plates were further incubated under Oricon condition for 48 hours at 37°C to determine the CFU numbers.
2.4. Examination of C. concisus Growth under Anaerobic and Microaerobic Conditions in the Presence of Different Concentrations of H2
P3UCO-S1 strain was used for this experiment. P3UCO-S1 strain is an oral strain previously isolated from a patient with UC [6, 16]. In a previous study of analysis of six housekeeping genes, we showed that the six housekeeping genes of P3UCO-S1 strain were identical to the strain isolated from intestinal biopsies of the same patient (P3UCB-S1) suggesting that this oral C. concisus strain was able to colonize the intestinal tract. Given this, we have decided to use P3UCO-S1 strain in this part of the study.
P3UCO-S1 strain was first cultured on a HBA plate under Oricon condition for 48 hours at 37°C. Following this, bacterial cells were collected and suspended into PBS and the OD600 was adjusted to 0.05. The bacterial suspension (30 μL) was inoculated onto 18 HBA plates. The plates were incubated under either anaerobic conditions or microaerobic conditions containing various conditions of H2 (three plates in each condition). Anaerobic and microaerobic conditions were generated using gas-generation system AN25A and gas-generation system CN25A, respectively (Oxoid). Hydrogen gas was supplemented by including 0.021 g, 0.042 g, or 0.083 g of sodium borohydride and 10 mL of H2O in a container placed in a 2.5 L jar, respectively, which generated 2.5%, 5.0%, and 10.0% of H2, respectively. H2 gas was generated by the chemical reaction NaBH4 + 4H2O = 4H2 + NaB(OH)4.
After a period of 48 hours of incubation at 37°C, C. concisus bacterial cells were collected from each plate using 1 mL of PBS. Three plates cultured under each condition were pooled and eight serial dilutions (1 : 10 to 1 : 108) were prepared. Each of the eight dilutions (5 μL) was inoculated onto HBA plates in four replicates. The plates were further incubated under Oricon condition for 48 hours at 37°C to determine the CFU numbers.
2.5. Putative Virulence Proteins Expressed by an Oral C. concisus Strain Cultured under AnaeroH2− and AnaeroH2+ Conditions
Proteins expressed by C. concisus cultured under AnaeroH2− and AnaeroH2+ conditions were analysed using mass spectrometry. C. concisus strain P6CDO-S1 was used in this experiment. P6CDO-S1 is an oral strain previously isolated from saliva of a patient with CD. Our previous studies showed that this oral C. concisus strain was genetically close to a C. concisus strain isolated from the intestinal biopsies of a patient with CD [6]. Therefore, we decided to investigate whether this oral C. concisus strain expresses putative virulence proteins and whether these proteins are expressed differentially when the strain is grown under AnaeroH2− and AnaeroH2+ conditions.
Briefly, C. concisus P6CDO-S1 strain was grown on HBA plates for 48 hours under AnaeroH2− and AnaeroH2+ conditions, respectively. C. concisus bacteria were collected and washed with PBS and then 19 μg whole cell proteins were separated on 12% SDS-PAGE as described previously [16]. The gel lane of each sample was cut into 10 slices. In-gel protein trypsin digestion was performed. The extracted peptides were separated by liquid chromatography and analysed by MS/MS as previously described [16, 17].
Mascot Daemon program (Matrix Science, London, UK) was used for bacterial protein identification against the NCBI database. The spectral counts of the same proteins expressed by P6CDO-S1 under AnaeroH2− and AnaeroH2+ conditions were compared using the Scaffold-3 software (Proteome software, OR, USA) [18]. The experiment was carried out in duplicate and repeated twice.
Mass spectrometry was conducted at the Bioanalytical Mass Spectrometry Facility, University of New South Wales, Australia.
2.6. Statistical Analysis
Unpaired t-test was used for comparison of CFU numbers. Fisher's exact test was used for analysis of the growth rate of C. concisus strains isolated from patients with IBD and controls. GraphPad Prism 5 software was used for statistical analysis (San Diego, CA). P value < 0.05 was considered a significant difference.
3. Results
3.1. The Growth of C. concisus Strains under Different Atmospheric Conditions
Of the 57 oral strains examined, 52 strains (91%) grew under AnaeroH2− conditions. Of the oral C. concisus strains isolated from patients with CD and UC, the positive growth rates under AnaeroH2− condition were 84% (16/19) and 86% (12/14), respectively, which were not statistically different from the positive growth rate of oral C. concisus strains (100%, 24/24) isolated from healthy controls (P > 0.05) (Table 1). All oral C. concisus strains grew under AnaeroH2+ conditions (Table 1).
Table 1.
Positive growth rates of oral C. concisus strains under AnaeroH2− and AnaeroH2+ conditions.
| Strains | AnaeroH2− | AnaeroH2+ |
|---|---|---|
| Strains from CD (n = 19) | 84% | 100% |
| Strains from UC (n = 14) | 86% | 100% |
| Strains from control (n = 24) | 100% | 100% |
|
| ||
| Total strains (n = 57) | 91% | 100% |
AnaeroH2−: anaerobic conditions without H2.
AnaeroH2+: anaerobic conditions with H2.
All strains did not grow under microaerobic conditions without H2 (MicroH2−a and MicroH2−b).
All six enteric C. concisus strains grew under both AnaeroH2− and AnaeroH2+ conditions.
The colonies of C. concisus strains, both the oral and enteric strains, grown under AnaeroH2− conditions appeared much smaller than those grown under AnaeroH2+ conditions. The morphology of C. concisus grown under AnaeroH2− and AnaeroH2+ conditions was not different under phase contrast microscopy.
None of the C. concisus strains grew under microaerobic condition without H2; no bacterial colonies were observed on plates cultured under both MicroH2−a and MicroH2−b conditions. All strains grew under Oricon condition.
3.2. Quantitative Comparison of C. concisus Growth under AnaeroH2− and AnaeroH2+ Conditions
To further compare the growth of C. concisus strains under AnaeroH2+ and AnaeroH2− conditions, the CFUs of 12 C. concisus strains grown under these two atmospheric conditions were determined. All strains had a greatly increased growth under AnaeroH2+ condition in comparison to the AnaeroH2− condition. The CFU numbers of all 12 C. concisus strains grown under AnaeroH2+ condition were significantly higher than those of the respective strains grown under AnaeroH2− condition (P < 0.05) (Table 2).
Table 2.
CFU of C. concisus strains cultured under AnaeroH2+ and AnaeroH2− conditions.
| Strain | AnaeroH2+ CFU | AnaeroH2− CFU |
|---|---|---|
| P1CDO-S1 | 517 ± 23.6 | 0.863 ± 0.061 |
| P1CDO-S2 | 328 ± 25.5 | 0.813 ± 0.0318 |
| P2CDO-S1 | 93.0 ± 8.49 | 1.36 ± 0.212 |
| P4CDO-S1 | 123 ± 4.71 | 0.195 ± 0.0252 |
| P6CDO-S1 | 126 ± 9.55 | 1.21 ± 0.125 |
| P8CDO-S1 | 112 ± 14.4 | 3.15 ± 0.388 |
| P3UCO-S1 | 550 ± 70.7 | 1.43 ± 0.0106 |
| P7UCO-S1 | 311 ± 105 | 9.42 ± 0.118 |
| P13UCO-S3 | 111 ± 2.12 | 6.50 ± 0.707 |
| P3UCB-S1 | 146 ± 36.8 | 1.57 ± 0.330 |
| H3O-S1 | 73.8 ± 15.9 | 9.50 ± 1.91 |
| H5O-S1 | 135 ± 5.30 | 9.50 ± 0.707 |
The values (means ± standard deviation) were from triplicates. CFU: (colony forming unit) × 108/mL. The CFU numbers of all 12 strains grown under AnaeroH2+ conditions were significantly higher than those of the respective strains grown under AnaeroH2− conditions (P < 0.05). Strains P1CDO-S1, P1CDO-S1, P2CDO-S1, P4CDO-S1, P6CDO-S1, and P8CDO-S1 were oral strains isolated from patients with CD. Strains P3UCO-S1, P7UCO-S1, and P13UCO-S3 were oral strains isolated from patients with UC. Strain P3UCB-S1 was an enteric strain isolated from intestinal biopsies of a patient with UC. Strains H3O-S1 and H5O-S1 were oral strains isolated from healthy controls.
3.3. The Growth of C. concisus under Anaerobic and Microaerobic Conditions Containing Different Concentrations of H2
P3UCO-S1 strain was used as a representative strain to evaluate the growth of C. concisus under anaerobic and microaerobic conditions containing different concentrations of H2. Under anaerobic conditions, the CFUs of P3UCO-S1 strain cultured in the presence of 2.5%, 5%, and 10% H2 were (1.10 ± 0.42) × 109/mL, (9.15 ± 0.82) × 109/mL, and (1.90 ± 1.33) × 109/mL, respectively. The CFU numbers of 5% H2 were significantly higher than the CFU number of 2.5% and 10% H2 (both P < 0.0001). The CFU numbers of 10% H2 and 2.5% H2 were not significantly different (P = 0.3) (Figure 1).
Figure 1.
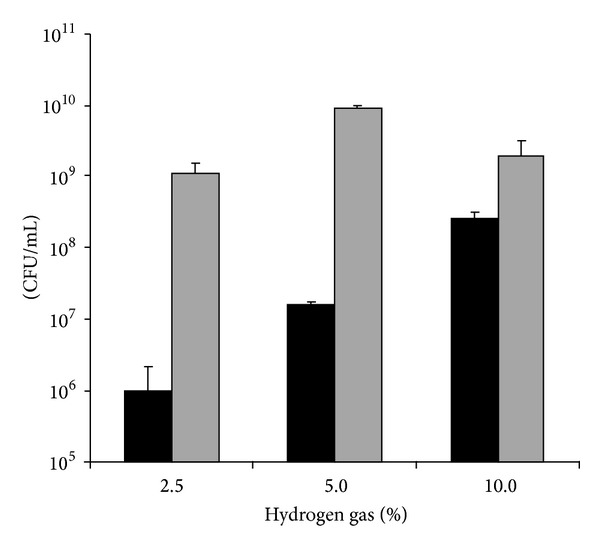
Growth of C. concisus P3UCO-S1 strain under various hydrogen gas concentrations. CFU of C. concisus was measured after incubation under microaerobic (black bar) or anaerobic (grey bar) condition with different hydrogen gas concentrations for 48 hours. The experiment was repeated twice. The error bar indicates the standard deviation. P3UCO-S1 strain is an oral strain isolated from a patient with UC.
Under microaerobic conditions, the CFU numbers of P3UCO-S1 strain cultured in the presence of 2.5%, 5%, and 10% H2 were (1.0 ± 1.15) × 106/mL, (1.60 ± 0.16) × 107/mL, and (2.67 ± 0.5) × 108/mL, respectively. The CFU number of 2.5% H2 was significantly lower than the CFU numbers of 5% and 10% H2 (P < 0.0001 and P < 0.005, resp.). The CFU number of 5% H2 was significantly lower than the CFU number of 10% H2 (P < 0.005) (Figure 1).
3.4. Putative Virulence Proteins Expressed by C. concisus P6CDO-S1 Strain Cultured under AnaeroH2− and AnaeroH2+ Conditions
Proteins expressed by strain P6CDO-S1 under AnaeroH2+ and AnaeroH2− conditions were subjected to mass spectrometry analysis. A number of putative virulence proteins such as fibronectin-binding protein, outer membrane protein (Omp), protease htpx, S-layer-RTX protein, hemagglutinin/hemolysin-related protein, CjaC, and EvpB family type VI secretion protein were identified. The expression levels of these putative virulence proteins, indicated by the spectral counts, were not statistically different when C. concisus strain P6CDO-S1 was grown under AnaeroH2+ and AnaeroH2− conditions (Table 3).
Table 3.
Putative virulence proteins expressed by C. concisus P6CDO-S1 strain cultured under AnaeroH2+ and AnaeroH2− conditions∗.
| Proteins | Locus tag | SC (AnaeroH2+) | SC (AnaeroH2−) |
|---|---|---|---|
| Flagellin B | CCC13826_2297 | 16.9 ± 3.59 | 15.1 ± 3.96 |
| Fibronectin-binding protein | CCC13826_0739 | 12.8 ± 3.43 | 12.8 ± 1.98 |
| Protease htpx | CCC13826_1039 | 8.44 ± 0.990 | 10.5 ± 1.93 |
| Omp18 | CCC13826_0923 | 7.49 ± 4.48 | 7.80 ± 3.51 |
| S-layer-RTX protein | CCC13826_1838 | 8.15 ± 3.74 | 4.00 ± 1.62 |
| CjaC | CCC13826_0963 | 4.93 ± 2.90 | 6.33 ± 0.768 |
| EvpB family type VI secretion protein | CCC13826_1182 | 4.96 ± 0.555 | 5.33 ± 0.722 |
| Hemagglutinin/hemolysin-related protein | CCC13826_0009 | 4.39 ± 1.77 | 2.33 ± 0.667 |
∗Putative virulence proteins were identified using mass spectrometry analysis.
SC: the value of the mean spectral counts from four replicates with standard deviation. The SC values of virulence proteins in C. concisus P6CDO-S1 strain cultured under AnaeroH2+ and AnaeroH2− conditions were not significantly different (P > 0.05).
AnaeroH2+: anaerobic condition with H2.
AnaeroH2−: anaerobic condition without H2.
P6CDO-S1 strain is an oral strain isolated from a patient with CD.
4. Discussion
In this study, the growth of C. concisus strains under different atmospheric conditions was examined. It was previously described that C. concisus is a bacterium which requires H2-enriched microaerobic conditions for growth and some C. concisus strains may grow under anaerobic conditions if fumarate and formate are present in the culture plates [2, 11, 12]. In this study, we found that under anaerobic conditions the majority of oral C. concisus strains (91%, 52/57) grew on HBA plates containing no formate or fumarate without the presence of H2, suggesting that oral C. concisus is an anaerobic bacterium and that H2 gas, formate, and fumarate are not essential requirements for the anaerobic growth of oral C. concisus strains. None of the 57 oral C. concisus strains grew under microaerobic conditions without H2, suggesting that microaerobic growth of C. concisus requires the presence of H2, which is consistent with previous findings [9, 11].
Under anaerobic conditions, the presence of H2 greatly increased the growth of C. concisus, demonstrated by the increased colony sizes observed macroscopically and the increased CFU numbers of the same strain cultured under AnaeroH2+ and AnaeroH2− conditions (Table 2). These results suggest that under anaerobic conditions C. concisus has different metabolic pathways in generating energy for growth and oxidization of H2 is a pathway generating high energy for a rapid growth. The solubility of H2 gas in H2O is extremely low; thus liquid culture methods are not suitable for assessing the impact of H2 gas on C. concisus growth [19]. Given this, in this study, the CFU numbers of C. concisus strains were determined using a plate culture method.
In humans, H2 is produced by anaerobic bacteria predominantly in the colon [20, 21]. H2 generated in the intestine is disposed by H2 consuming bacteria such as methanogenic bacteria, sulfate-reducing bacteria, and acetogens [22]. Some H2 is diffused into blood and this H2 can be measured by breath testing [23]. Dietary factors and the composition of an individual's intestinal microbiota affect intestinal H2 production and consumption [24–26]. The natural host of C. concisus is humans and the primary colonization site is the human oral cavity [1, 2]. The concentration of excreted H2 in the oral cavity is extremely low. The basal level of hydrogen in healthy individuals is usually less than 10 ppm, thus having a H2 level of less than 0.001% (1 ppm = 0.0001%) [14]. In addition to anaerobic bacteria in the intestine, oral anaerobic bacteria may also produce H2 by fermentation of carbohydrate residues from food. However, the level of H2 produced by oral anaerobes is very low. Mastropaolo and Rees showed that, following a solid meal, the H2 produced by oral anaerobes was 25 ppm (0.0025%) and this level was retained for only 73 minutes [27]. Given this, C. concisus colonizing the oral cavity is unlikely to have constantly available H2 for growth. The finding in this study that oral C. concisus strains were able to grow without the presence of H2 under anaerobic conditions helps to explain why C. concisus is able to colonize the human oral cavity.
Despite the fact that H2 dramatically increases the growth of C. concisus and the intestine is the dominant place for H2 production in humans, it is interesting to note that C. concisus has selected the oral cavity, rather than the intestinal environment, as its natural colonization site. This suggests that in healthy individuals there are some factors in the gastrointestinal tract that inhibit C. concisus intestinal colonization. It is likely that such inhibitory factors are low or lacking in patients with IBD, which contributes to the higher intestinal prevalence of C. concisus in these patients. One of such factors may be methanogenic bacteria, the dominant H2 consuming bacteria in the human intestine that produce methane. It is possible that methanogenic bacteria in the intestine compete with C. concisus for use of H2.
A study by McKay et al. examining hydrogen and methane excretion in patients with IBD and controls showed that the prevalence of methane excretion was 13% in patients with CD and 15% in patients with UC, which was significantly lower than that in healthy controls (54%) [28]. This observation was supported by a study from Pimentel et al., which showed that 97% of patients with IBD (75/78), who had predominantly a diarrheal condition, excreted H2 only and no methane [29]. These results suggest that there is a low level of methanogenic bacteria in patients with IBD. Indeed, a study conducted by Scanlan et al. detected a low prevalence of intestinal methanogenic bacteria in patients with IBD in comparison to healthy controls and other disease groups [30]. Methanogenic bacteria play a predominant role in disposing intestinal H2 in humans [31]. The lack of sufficient intestinal methanogenic bacteria in patients with IBD may have generated an intestinal environment that allows C. concisus to use H2 for a rapid growth.
We previously showed that some oral C. concisus strains were able to colonize the intestinal tract and have the potential to cause enteric disease [16, 32]. In this study, we found that P6CDO-S1 strain, an oral C. concisus strain isolated from a patient with CD, expressed a number of putative virulence proteins. These proteins were previously reported to contribute to the virulence of other bacterial species [32–39]. However, their roles in C. concisus virulence remain to be characterized. If indeed these putative proteins play a role in C. concisus virulence, the finding in this study that the expression levels of these proteins remain similar when P6CDO-S1 strain is cultured under anaerobic conditions with and without H2 suggests that the impact of H2 on C. concisus virulence is unlikely through affecting these proteins. It is likely that H2 may affect C. concisus virulence through increasing the growth of C. concisus to a disease-causing threshold.
This study also found that, under anaerobic conditions, P3UCO-S1 strain, an oral strain isolated from a patient with UC, had a significantly higher CFU in the presence of 5% H2, as compared to 2.5% H2 and 10% H2. Under microaerobic conditions, this strain had a significantly higher CFU in the presence of 10% H2 compared to 2.5% and 5% H2. It appeared that the concentrations of H2 supplied in bacterial cultivation affect the optimal growth of C. concisus. This aspect should be further investigated by examining more C. concisus strains using systems that are able to supply fixed concentrations of CO2, N2, and H2, which will provide useful information to clinical laboratories in isolation of C. concisus from clinical samples.
In addition to the 57 oral C. concisus strains, we have included six enteric strains, with five strains being isolated from patients with IBD, into this study. These enteric strains showed an anaerobic growth pattern that was similar to oral C. concisus strains.
In summary, this study found that oral C. concisus strains were able to grow under anaerobic conditions without the presence of H2, formate, or fumarate and that these strains did not grow in microaerobic conditions without H2, suggesting that they are anaerobes. The presence of H2 in the anaerobic conditions greatly increased the growth of oral C. concisus strains. Using mass spectrometry analysis, an oral C. concisus strain isolated from a patient with CD was found to express a number of putative virulence proteins and the expression levels of these proteins under anaerobic conditions with and without H2 remained similar. While the numbers of enteric C. concisus strains included in this study were small, these enteric strains and oral C. concisus had a similar anaerobic growth pattern. This study provides useful information in understanding the natural colonization site and pathogenicity of C. concisus.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Zhang L, Budiman V, Day AS, et al. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. Journal of Clinical Microbiology. 2010;48(8):2965–2967. doi: 10.1128/JCM.02391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner AC, Badger S, Lai CH, Listgarten MA, Visconti RA. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodensfrom humans with periodontal disease. International Journal of Systematic Bacteriology. 1981;31(4):432–445. [Google Scholar]
- 3.Zhang L, Si MM, Day AS, et al. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn's disease. Journal of Clinical Microbiology. 2009;47(2):453–455. doi: 10.1128/JCM.01949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, Mitchell H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn's disease. Inflammatory Bowel Diseases. 2010;16(6):1008–1016. doi: 10.1002/ibd.21157. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM, Hold GL. Detection of Campylobacter concisus and other campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021490.e21490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahendran V, Riordan SM, Grimm MC, et al. Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025417.e25417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nature Clinical Practice Gastroenterology and Hepatology. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 8.Lastovica AJ. Clinical relevance of Campylobacter concisus isolated from pediatric patients. Journal of Clinical Microbiology. 2009;47(7):p. 2360. doi: 10.1128/JCM.00568-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engberg J, On SLW, Harrington CS, Gerner-Smidt P. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. Journal of Clinical Microbiology. 2000;38(1):286–291. doi: 10.1128/jcm.38.1.286-291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen HL, Ejlertsen T, Engberg J. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clinical Microbiology and Infection. 2013;19(5):445–450. doi: 10.1111/j.1469-0691.2012.03852.x. [DOI] [PubMed] [Google Scholar]
- 11.Vandamme P, Dewhirst FE, Paster BJ, On SLW. Genus I. Campylobacter . In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey's Manual of Systematic Bacteriology. 2nd edition. New York, NY, USA: Springer; 2005. pp. 1147–1160. [Google Scholar]
- 12.Lastovica AJ. Emerging Campylobacter spp.: the tip of the iceberg. Clinical Microbiology Newsletter. 2006;28(7):49–56. [Google Scholar]
- 13.Istivan TS, Coloe PJ, Fry BN, Ward P, Smith SC. Characterization of a haemolytic phospholipase A2 activity in clinical isolates of Campylobacter concisus . Journal of Medical Microbiology. 2004;53(6):483–493. doi: 10.1099/jmm.0.45554-0. [DOI] [PubMed] [Google Scholar]
- 14.Simrén M, Stotzer P-O. Use and abuse of hydrogen breath tests. Gut. 2006;55(3):297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamma JJ, Diamanti-Kipioti A, Nakou M, Mitsis FJ. Profile of subgingival microbiota in children with mixed dentition. Oral Microbiology and Immunology. 2000;15(2):103–111. doi: 10.1034/j.1399-302x.2000.150206.x. [DOI] [PubMed] [Google Scholar]
- 16.Ismail Y, Mahendran V, Octavia S, et al. Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0038217.e38217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatlin CL, Kleemann GR, Hays LG, Link AJ, Yates JR., III Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography- microspray and nanospray mass spectrometry. Analytical Biochemistry. 1998;263(1):93–101. doi: 10.1006/abio.1998.2809. [DOI] [PubMed] [Google Scholar]
- 18.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JAM. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Research. 2007;35(2):W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pray HT, Schweickert CE, Minnich BH. Solubility of hydrogen, oxygen, nitrogen, and helium in water. Industrial and Enginnering Chemistry. 1952;44:1146–1151. [Google Scholar]
- 20.Levitt MD. Production and excretion of hydrogen gas in man. The New England Journal of Medicine. 1969;281(3):122–127. doi: 10.1056/NEJM196907172810303. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JH. Fermentation in the human large intestine: evidence and implications for health. The Lancet. 1983;1(8335):1206–1209. doi: 10.1016/s0140-6736(83)92478-9. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annual Review of Food Science Technology. 2010;1:363–395. doi: 10.1146/annurev.food.102308.124101. [DOI] [PubMed] [Google Scholar]
- 23.Levitt MD, Donaldson RM. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. The Journal of Laboratory and Clinical Medicine. 1970;75(6):937–945. [PubMed] [Google Scholar]
- 24.Bond JH, Levitt MD. Effect of dietary fiber on intestinal gas production and small bowel transit time in man. American Journal of Clinical Nutrition. 1978;31(10):S169–S174. doi: 10.1093/ajcn/31.10.S169. [DOI] [PubMed] [Google Scholar]
- 25.Gibson GR, Cummings JH, Macfarlane GT, et al. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31(6):679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strocchi A, Levitt MD. Factors affecting hydrogen production and consumption by human fecal flora: the critical roles of hydrogen tension and methanogenesis. Journal of Clinical Investigation. 1992;89(4):1304–1311. doi: 10.1172/JCI115716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastropaolo G, Rees WDW. Evaluation of the hydrogen breath test in man: definition and elimination of the early hydrogen peak. Gut. 1987;28(6):721–725. doi: 10.1136/gut.28.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay LF, Eastwood MA, Brydon WG. Methane excretion in man—a study of breath, flatus, and faeces. Gut. 1985;26(1):69–74. doi: 10.1136/gut.26.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Digestive Diseases and Sciences. 2003;48(1):86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 30.Scanlan PD, Shanahan F, Marchesi JR. Human methanogen diversity and incidence in healthy and diseased colonic groups using mcrA gene analysis. BMC Microbiology. 2008;8, article no. 79 doi: 10.1186/1471-2180-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strocchi A, Furne JK, Ellis CJ, Levitt MD. Competition for hydrogen by human faecal bacteria: evidence for the predominance of methane producing bacteria. Gut. 1991;32(12):1498–1501. doi: 10.1136/gut.32.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jönsson K, Guo BP, Monstein H, Mekalanos JJ, Kronvall G. Molecular cloning and characterization of two Helicobacter pylori genes coding for plasminogen-binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):1852–1857. doi: 10.1073/pnas.0307329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahteenmaki K, Virkola R, Pouttu R, Kuusela P, Kukkonen M, Korhonen TK. Bacterial plasminogen receptors: in vitro evidence for a role in degradation of the mammalian extracellular matrix. Infection and Immunity. 1995;63(9):3659–3664. doi: 10.1128/iai.63.9.3659-3664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science. 1992;258(5084):1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 35.Lertpiriyapong K, Gamazon ER, Feng Y, et al. Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042842.e42842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Istivan TS, Smith SC, Fry BN, Coloe PJ. Characterization of Campylobacter concisus hemolysins. FEMS Immunology and Medical Microbiology. 2008;54(2):224–235. doi: 10.1111/j.1574-695X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Kalischuk LD, Inglis GD. Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiology. 2011;11, article 53 doi: 10.1186/1471-2180-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. International Journal of Medical Microbiology. 2002;291(8):605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 39.Thompson SA. Campylobacter surface-layers (S-layers) and immune evasion. Annals of Periodontology. 2002;7(1):43–53. doi: 10.1902/annals.2002.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]


