Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is the most important infectious disease agent of pigs worldwide, causing reproductive failure in pregnant sows and respiratory problems in nursing and growing pigs. PRRSV infection is characterized by a prolonged viremia of 30 or more days and an extended persistent infection of lymphoid tissues. To better understand the immunological basis for prolonged acute and persistent PRRSV infection, we have examined the cell-mediated immune (CMI) response throughout the course of infection and compared the results to the local distribution and abundance of PRRSV in infected tissues. PRRSV-specific T cells, enumerated by gamma interferon enzyme-linked immunospot assay, did not appear until 2 weeks after PRRSV inoculation, and their abundance exhibited substantial variation over time and among animals. In all cases the T-cell response was transient. High levels of viral RNA were present in lymphoid tissues of all animals in the acute phase of infection. Viral loads were decreased 1,000-fold or more in persistent infections, with the primary sites of persistence being tonsil, sternal lymph node, and inguinal lymph node. The abundance of virus-specific T cells in either acutely or persistently infected animals was highly variable and showed no correlation to the level of virus in lymphoid tissues. No significant difference in antigen-specific T-cell abundance was observed in secondary lymphoid tissues in either acute or persistent infection except for tonsil, in which the number of responding cells was extremely low. CD4+- and CD8+-T-cell frequencies did not change after PRRSV infection, though a decrease in γδ T cells was observed. Macrophages, the permissive cell type for PRRSV, were present in various levels in all tissue preparations and were not in proportion to local virus load. These findings indicate that a weak CMI response contributes to prolonged PRRSV infection and suggests that PRRSV suppresses T-cell recognition of infected macrophages. Thus, the slow but eventual resolution of PRRSV infection may be dependent on limiting permissive macrophages and on innate immune factors.
Porcine reproductive and respiratory syndrome virus (PRRSV), which causes the most important disease of swine worldwide, is a single-stranded, positive-sense RNA virus belonging to the Arteriviridae family (11, 17, 41, 64). The pathogenesis of PRRSV in pigs consists of an acute infection characterized by viremia lasting for approximately 1 month, followed by a nonviremic persistent infection of secondary lymphoid tissues that lasts for approximately 3 to 4 months (43, 44, 52), although virus can persist for longer periods in some animals (68). Antibodies to PRRSV appear about 10 days after infection, but the cell-mediated immune (CMI) response is reported to appear transiently at 4 to 8 weeks after infection (5, 37) or to become more pronounced months later (40). The CMI response to PRRSV has been examined only in peripheral blood mononuclear cells (PBMC). However, peripheral blood contains only 2% of the total T cells of the body (66) and is the site of neither viral replication nor antigen presentation. Therefore, it is possible that the principal T-cell-dependent response to PRRSV might be occurring in lymphoid tissues, analogous to T-cell responses to simian immunodeficiency virus and human immunodeficiency virus (HIV). In HIV and simian immunodeficiency virus infection, CD4+-T-cell counts are reduced in peripheral blood but increased in spleen and lymph nodes (LN) or remain stable in nonlymphoid organs during asymptomatic infection (59), and the decrease of viral load in tissues in comparison to that in plasma is delayed (33). Similarly, in swine influenza virus infection in pigs, antigen-specific T cells are predominantly localized to the spleen and tracheobronchial LN and are infrequent or absent in blood (35).
The extended period of acute and persistent PRRSV infection indicates that the virus subverts immune defenses. Alternatively, the eventual resolution of viral infection indicates that an effective immune response develops over time. To address these opposing possibilities, we examined the association between virus-specific T-cell immune responses and viral load in secondary lymphoid tissues to test the hypothesis that a prolonged viremia and viral persistence are the results of an ineffective T-cell response.
MATERIALS AND METHODS
Animals and housing.
Two-week-old early weaned, mixed-sex pigs from a herd free of PRRSV were maintained in isolation at the Livestock Infectious Disease Isolation Facility, Iowa State University, Ames. Feed and water were provided ad libitum. Animals received 1 ml per day of ceftiofur (Excenel; 50 mg/ml) intramuscularly for 3 days following arrival. Pigs were assigned to treatment groups at random and observed daily, and rectal temperatures and clinical scores were recorded every other day. Whole blood in EDTA tubes and serum were collected weekly by venipuncture. At necropsy samples of sternal LN, mesenteric LN, inguinal LN, tonsil, femur, and spleen were collected and stored on ice for cell isolation.
Four-month-old female Landrace by Large White cross-bred gilts were obtained from a PRRSV-free herd (Genetiporc USA, LLC, Alexandria, Minn.) and maintained in the University of Minnesota Swine Disease Eradication Center research farm, Holloway Minn., or in animal facilities of the College of Veterinary Medicine at the University of Minnesota. Animals were assigned to treatment groups at random and provided with feed and water ad libitum. Animals were observed daily and bled by venipuncture for whole blood and serum. Tissues, including lung, tracheobronchial LN, skin, kidney, and liver, in addition to those described above, were collected at necropsy.
Viruses and inoculations.
The following PRRSV strains and field isolates were used: the cell culture-adapted vaccine Ingelvac PRRS ATP (Boehringer Ingelheim Vetmedica, Inc., St. Joseph, Mo.), the North American prototype strain VR2332 (17), strain MN30100 isolated from a persistently infected sow (7a), and three isolates from field cases in the state of Minnesota (10221-4, 25616-32, and 34222) provided by the Minnesota Veterinary Diagnostic Laboratory. Nucleotide sequence similarities in the open reading frame 5 (ORF5) gene encoding the envelope glycoprotein are shown in Table 1. The ORF5 sequence is highly variable in PRRSV and is widely used to differentiate field isolates diagnostically (31). Vaccine virus was used according to the manufacturer's recommendation. Other strains and isolates were grown to approximately 80% cytopathic effect on MA-104 cells, released from cells by three freeze-thaw cycles, and clarified by centrifugation. The titers of the viruses were determined, and viruses were frozen in aliquots at −80°C. Pigs were inoculated intranasally with 102.5 of the 50% tissue culture infective dose (TCID50)/2 ml.
TABLE 1.
ORF5 nucleotide sequence similarities of the PRRSV strains and field isolates used in the studya
| Strain on isolate | % Similarity
|
||||
|---|---|---|---|---|---|
| 25616-32 | MN-30100 | IngelvacATP | VR2332 | 34222 | |
| 10221-4 | 92.7 | 95.2 | 89.5 | 88.3 | 85.7 |
| 25616-32 | 94.2 | 90.3 | 89.5 | 85.0 | |
| MN-30100 | 90.7 | 89.7 | 86.0 | ||
| IngelvacATP | 90.5 | 86.5 | |||
| VR2332 | 86.7 | ||||
Nucleotide sequences of ORF5 (600 bp) were aligned in Clustal W (Megalign, Lasergene; DNASTAR, Madison Wis.).
Sample collection.
Blood was collected by superior vena cava venipuncture in EDTA or heparin Vacutainer tubes (Sherwood Medical, St. Louis, Mo.). For tissue isolation, pigs were anesthetized with intramuscular tiletamine-zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, Iowa) and euthanized with Beuthanasia-D solution (Schering Plough Animal Health, Union, N.J.). Pigs were weighed and tissues, including sternal LN, mesenteric LN, inguinal LN, tracheobronchial LN, tonsil, femur, spleen, and lung, were removed as indicated in the Results section and weighed. Approximately 0.5- to 1-g portions of each tissue were weighed and transferred to RNALater (Ambion, Austin, Tex.) for virus quantification. The remaining tissues were weighed and placed in RPMI 1640 medium (Mediatech, Herndon, Va.) containing 10 μg of gentamicin (Invitrogen, Grand Island, N.Y.) per ml for cell isolation.
Cell isolation and culture.
Tissue and cell culture reagents were from Mediatech except as noted. Tissues were diced with scalpel blades, manually disaggregated in sterile phosphate-buffered saline (PBS), and filtered through 70-μm-pore-size cell strainer membranes (BD Falcon, Bedford, Mass.). Femurs were flushed with RPMI 1640 culture media containing 10 μg of gentamicin/ml. Cell preparations from tissue sources, femurs, and peripheral blood were diluted 1:2 with PBS and layered on lymphocyte separation medium (ICN Biomedicals, Aurora, Ohio) and centrifuged for 30 min at 400 × g. Mononuclear cell layers were recovered, and erythrocytes were removed by water lysis for 30 s; the resulting mononuclear cell preparations were washed two times with PBS by centrifugation, followed by resuspension in RPMI 1640 medium. Cells were counted with a hemacytometer. Viability was tested by trypan blue exclusion and was found to be >95%. Cells were cultured in RPMI 1640 medium with 5% fetal bovine serum, 10 μg of gentamicin/ml, 1× minimal essential medium nonessential amino acids, 4 mM l-glutamine, and 250 μM 2-mercaptoethanol (Invitrogen).
One-step TaqMan reverse transcription-PCR.
Tissue samples were removed from RNALater and 250-mg portions were homogenized with a Polytron in 2 ml of RLT buffer (QIAGEN, Valencia, Calif.). Homogenates were centrifuged at 13,000 × g for 5 min, and 200 μl of the supernatant was used for RNA extraction. Total RNA was isolated by using the serum protocol of the QIAamp 96 DNA blood kit (QIAGEN). RNA was eluted in 20 μl of water, and 5 μl was used in a 50-μl PCR. The reverse transcription-PCR was performed essentially as described by Mahlum et al. (39) by the University of Minnesota Diagnostic Lab with universal primers for ORF6 of PRRSV. To quantify viral RNA copies, the titer of a stock of strain VR2332 from media supernatants of BHK cells transfected with purified genomic RNA was determined on MA-104 cells by plaque assay. RNA was extracted from the titered stock, and a series of 10-fold dilutions corresponding to a concentration of 1 to 100,000 PFU/ml were amplified by PCR as described. The resulting standard curve, run in each experiment, was used to express data as PFU per gram of tissue.
IFN-γ ELISPOT.
Briefly, 5 × 105 cells in 50 μl of RPMI 1640 complete medium were added to duplicate wells of enzyme-linked immunospot (ELISPOT)plates (Millipore, Bedford, Mass.) coated with anti-porcine gamma interferon (IFN-γ) monoclonal antibody P2G10 (BD Pharmingen, San Diego, Calif.). Cells were stimulated in 50 μl of complete media with PRRSV strain MN30100 (2 × 105 TCID50) and 2.5 × 107 latex microspheres coated with approximately 25 ng of recombinant nucleocapsid from strain VR2332. Control wells received an equal amount of bovine serum albumin (BSA)-coated beads or 2.5 μg of phytohemagglutinin/ml as negative and positive controls, respectively. Cells were cultured at 37°C in 5% CO2 for 24 h. Plates were washed three times with PBS and four times with PBS containing 0.05% Tween 20 (Fisher Scientific, Pittsburgh, Pa.). Captured IFN-γ was detected with biotinylated anti-porcine IFN-γ antibody P2C11 (BD Pharmingen) and streptavidin-horseradish peroxidase (Prozyme, San Leandro, Calif.). Color was developed with 3-amino-9-ethylcarbazole (Sigma Chemical, St. Louis, Mo.) and read on an Immunospot image analyzer (Cellular Technology, Cleveland, Ohio).
Immunostaining.
Single-cell suspensions (2 × 106 cells) were plated in six-well plates (Corning, Corning, N.Y.) with coverslips for approximately 3 h. Adherent cells were fixed with 3% formaldehyde in PBS for 10 min and permeabilized by treatment with 0.1% Triton-100 (Sigma) in PBS with 0.1% BSA. Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody SDOW17 against PRRSV nucleocapsid (gift from Eric Nelson, South Dakota State University, Brookings) was diluted 100-fold and applied to the coverslips for 1 h in 1% BSA. Coverslips were washed three times in PBS, mounted on microscope slides in 50% glycerol, and observed on a Nikon E8000 epifluorescence microscope.
FACS staining.
Single-cell suspensions (5 × 105 cells in 100 μl of PBS) were incubated with specific antibodies for 30 min at 4°C, washed twice with PBS, incubated with fluorescently labeled second antibody for 30 min if needed, washed twice, and examined in a BD Biosciences FACSCalibur (fluorescence-activated cell sorter [FACS]; San Jose, Calif.). Antibodies included phycoerythrin (PE)-conjugated anti-porcine macrophage monoclonal antibody 74-22-15 (Southern Biotechnology Associates, Birmingham, Ala.), anti-pig CD4 (74-12-4) and CD8 (76-2-11) conjugated to FITC or PE (BD Pharmingen), and anti-pig gamma delta monoclonal antibody PGBL22A (VMRD, Inc., Pullman, Wash.).
Statistical analysis.
One-way analysis of variance (ANOVA), two-tailed t tests, and correlation analysis were used to analyze the data. Graphs were constructed in GraphPad Prism 3.0 software (San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
The distribution of PRRSV-specific T cells in peripheral blood shows substantial variation over time and among animals.
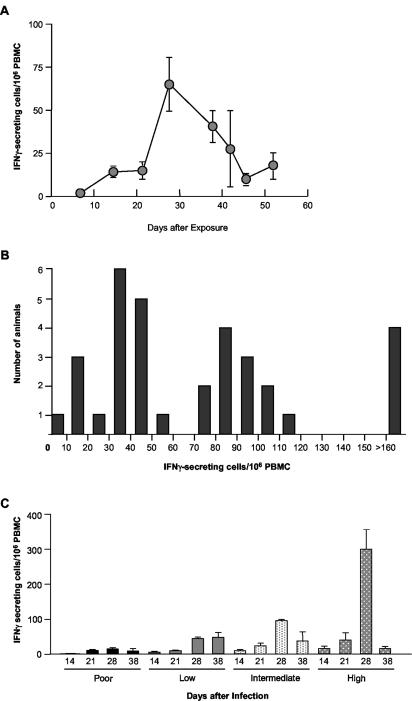
To better understand the immunological basis for prolonged PRRSV infection in pigs, we examined the kinetics of PRRSV-specific T-cell responses in peripheral blood during acute infection. PRRSV-specific (IFN-γ-secreting) T cells were detected in PBMC at day 14 postinfection (p.i.) in a group of 33 pigs exposed at 2 weeks of age. Between days 21 to 28 after infection, the frequency of virus-specific T cells increased substantially, peaking at 28 days p.i. and declining thereafter until the end of the experiment (Fig. 1A). IFN-γ secretion was dependent on the presence of PRRSV, since no responding cells were observed in wells stimulated with beads coated with BSA. Surprisingly, the majority of the mean peak response was due to strong responses in only a few pigs. At peak responsiveness, on day 28, 4 of 33 animals (12%) showed almost no T-cell response (≤20 IFN-γ-secreting cells/106 PBMC) (Fig. 1B and C). A low-responding group (21 to 60 PRRSV-specific T cells/106 PBMC) consisted of 13 of 33 pigs (39%), in which 11 of 13 pigs had 32 to 50 responding cells/106 PBMC. Another 12 of 33 (36%) pigs were intermediate responders (61 to 114 IFN-γ-secreting cells/106 PBMC), and 4 pigs (12%) were high responders (>115 cells/106 PBMC), with the highest animal at 436 PRRSV-specific IFN-γ-secreting cells per 106 PBMC. The differences in T-cell responsiveness to PRRSV at 28 days after infection were maintained in all groups with a few notable exceptions. As shown in Fig. 1B and C, poorly responding animals had few virus-specific T cells at all times over the 38-day period of observation. Low- and intermediate-responding animals showed no significant difference between days 14 and 21 but showed a significant increase at day 28 (P < 0.0001), with numbers in the intermediate group significantly reduced again at day 38 (P < 0.0001). The highly responding animals also showed no significant change in PRRSV-responsive lymphocytes at day 21, a dramatic increase at days 28 to 299 ± 56 (mean ± standard error), and a marked reduction to less than 25 responding cells per 106 PBMC 10 days later (Fig. 1C). These findings stimulated a more thorough examination of the T-cell response to PRRSV during acute and persistent infection, since it was possible that the dramatic decrease in PRRSV-specific lymphocytes at day 38 was due to a redistribution of the cells from peripheral circulation to LN in response to the persistence of PRRSV in lymphoid tissue.
FIG. 1.
Kinetics of IFN-γ ELISPOT response to PRRSV in swine. Thirty-three 2-week-old pigs were inoculated intramuscularly with 2 ml of the modified live vaccine Ingelvac ATP according to the manufacturer's recommendations. IFN-γ ELISPOT was performed on PBMC collected at the indicated times. (A) Time course of responding cells. Data are means and standard errors of 33 pigs per time point through 38 days and 3 pigs per time point at days 42, 45, and 52. (B) Variation in PRRSV-specific IFN-γ ELISPOT results among pigs at 28 days after exposure. (C) Kinetics of PRRSV-specific IFN-γ response in pigs having poor, low, intermediate, and high responses. Sample sizes are n = 4 (poor), n = 13 (low), n = 12 (intermediate), and n = 4 (high).
PRRSV is distributed unevenly in secondary lymphoid tissues in both acute and persistent infection.
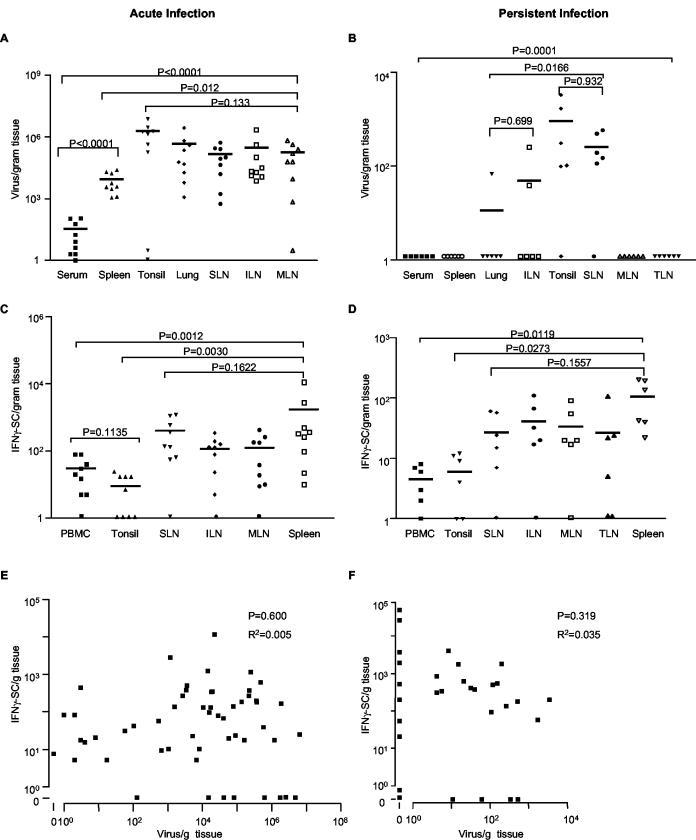
PRRSV primarily infects macrophages in lung and lymphoid tissues (36, 52) and has been reported in serum, thymus, brain, and semen (46, 52, 57). However, the extent of PRRSV in secondary lymphoid tissues has not been examined quantitatively. Therefore, PRRSV load was examined by real-time PCR in serum, spleen, tonsil, lung, sternal LN, inguinal LN, and mesenteric LN at 19 days (acute infection) and 67 days (persistent infection) after inoculation of 15 pigs with PRRSV field isolates 10221-4 (n = 3), 25616-32 (n = 3), MN30100 (n = 3), 34222 (n = 3), and VR2332 (n = 3). Animals inoculated with isolates 10221-4, 25616-32, and MN30100 were sacrificed at 19 days, and animals inoculated with isolates VR2332 and 34222 were sacrificed at 67 days. Although the virus isolates varied between 4.8 to 15.0% in pairwise nucleotide sequence comparisons (Table 1), PRRSV-specific T-cell responses were indistinguishable among strains (data not shown). Therefore, the results were pooled for analysis. At 19 days, PRRSV on average was equally abundant in tonsil, sternal LN, inguinal LN, and mesenteric LN as in lung, the primary site of replication in acute infection (Fig. 2A). The amount of virus was significantly lower in spleen, and the lowest levels were observed in serum, most likely reflecting that blood is not a site of viral replication (19). Within any given tissue, there was substantial variation in viral load among animals, ranging from approximately 100-fold in spleen to more than 107-fold in tonsil, in which the average viral load was highest, and 106-fold in mesenteric LN (Fig. 2A). Likewise, the amount of virus was not consistently high or low in different tissues of the same animal. In persistent infection, at 67 days after inoculation, PRRSV RNA was detected in every animal. However, the viral load was greatly reduced in all tissues, and no viral RNA was found in serum, spleen, mesenteric LN, tracheobronchial LN, skin, kidney, or liver (Fig. 2B). PRRSV RNA was detected in inguinal LN of only two pigs and in lung from one of six pigs, and the viral loads were approximately 10,000-fold lower than in acute infection (Fig. 2A and B). Tonsil and sternal LN were the primary sites of viral persistence, although the levels were about 10,000-fold lower than those in acute infection, and one animal was negative for each tissue (Fig. 2B).
FIG. 2.
Comparison of PRRSV load and PRRSV-specific T-cell frequencies among lung and lymphoid tissues. Fifteen pigs were inoculated intranasally with 5 ml of cell culture fluid containing 102.4 TCID50 PRRSV field isolates. In the acute infection, nine pigs were inoculated with isolates 10221-4, 25616-32, and MN30100 and sacrificed at 19 days after PRRSV infection. In the persistent infection, six pigs were inoculated with isolates VR2332 and 34222 and sacrificed at 67 days after infection. Virus load (A and B) was quantified by real-time PCR and expressed as numbers of virus/gram of tissue. PRRSV-specific T-cell frequencies (C and D) were determined by IFN-γ ELISPOT. Statistical analysis was performed with nonparametric one-way ANOVA in GraphPad Prism software. Each symbol represents an individual sample from one pig. The horizontal bar is the mean of the group. The P value on the top of each frame in panels A to D is the outcome from all the groups within the frame. Panels E and F show the correlation between virus load and antigen-specific T-cell frequency in lung and lymphoid tissues in acute and persistent infection, respectively. Points represent the viral load and PRRSV-specific T-cell values of all tissues examined in panels A to D. Correlation coefficients and P values were calculated by linear regression analysis in GraphPad Prism. SLN, sternal LN; ILN, inguinal LN; MLN, mesenteric LN; TLN, tracheobronchial LN; SC, secreting cells.
Distribution of PRRSV-specific T cells in lymphoid compartments in acute and persistent infection.
The distribution of PRRSV-specific T cells was examined to determine if local immune responses accounted for the differences in virus load that were observed in both acute and persistent infection. In acute infection, PRRSV-specific T lymphocytes were present at approximately 100 to 1,000 cells/g of tissue in sternal, inguinal and mesenteric LN, and spleen (Fig. 2C). Significantly fewer virus-specific T cells were found in tonsil and blood. The wide range in PRRSV-specific T-cell frequencies previously observed in PBMC was also evident in secondary lymphoid tissues. Except for spleen, every tissue was negative in one or more animals, and in spleen there was a 1,000-fold range of variation. The distribution of PRRSV-specific cells followed the same trend in persistent infection (Fig. 2D). As in acute infection, in persistent infection the frequency of responding lymphocytes was significantly lower in tonsil and blood, substantial variation among animals was present, and one or more animals were negative in every tissue except spleen. Tonsil, which had the highest viral load in acute and persistent infection, was noteworthy, since no responding T cells were observed in four of nine animals in acute infection and in two of six animals in persistent infection.
No correlation was present between viral load and antigen-specific T cells.
A correlation analysis was performed to determine if the viral load in lymphoid tissues was associated positively or negatively with the frequency of responding T cells. An extensive range of differences in both viral load and antigen-specific T-cell numbers was observed in all sites of infection; thus, there was no correlation between viral load and the frequency of responding T cells in the same tissue sample in either acute or persistent infection. The correlation coefficients were 0.005 and 0.035 for acute and persistent infection, respectively. The scatter plots in Fig. 2E and F contain the comparative data for viral load and responding T cells in every sample of all tissues shown in panels A through D and demonstrate the absence of correlation during both acute and persistent infection.
T-cell phenotypes at sites of infection.
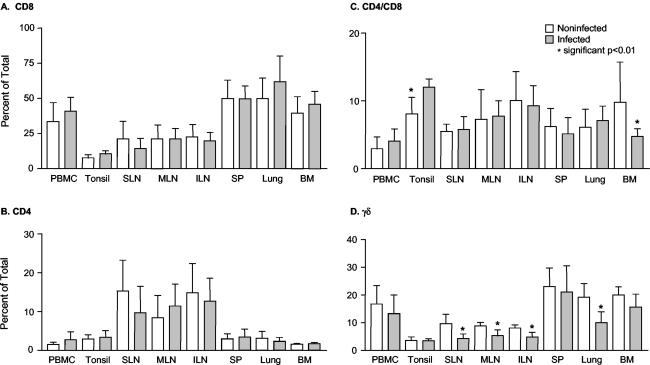
Since the antigen-specific responding T-cell distribution did not account for differences in viral load in infected tissues, the overall distribution of T-cell subsets was examined in acute infection to determine if particular subsets not elucidated by IFN-γ ELISPOT were recruited to sites of infection. Single-cell suspensions were prepared from PBMC, lung, tonsil, sternal LN, inguinal LN, mesenteric LN, bone marrow, and spleen and stained for CD4 and CD8 single-positive T cells, CD4 and CD8 double-positive T cells, and γδ T cells. PRRSV infection did not change the CD4+ or CD8+ population in any tissue; and CD4+ CD8+-T-cell ratios were consistent between control and infected pigs except in tonsil, in which there was a significant increase, and in bone marrow, in which there was a significant decrease. By contrast, γδ-positive T cells were significantly decreased in lung and all LN and reduced, though not significantly, in every other tissue examined (Fig. 3).
FIG. 3.
Comparison of T-cell phenotypes in PRRSV-permissive tissues of noninfected and PRRSV-infected pigs. Single-cell suspensions in 100 μl were stained with anti-porcine CD4-FITC, anti-porcine CD8-PE, or anti-porcine γδ T-cell monoclonal antibodies. Data are means and standard deviations for 5 noninfected pigs and 10 pigs infected for 19 days. SLN, sternal LN; ILN, inguinal LN; SP, spleen; BM, bone marrow.
PRRSV is present in lymphoid tissues of persistently infected pigs.
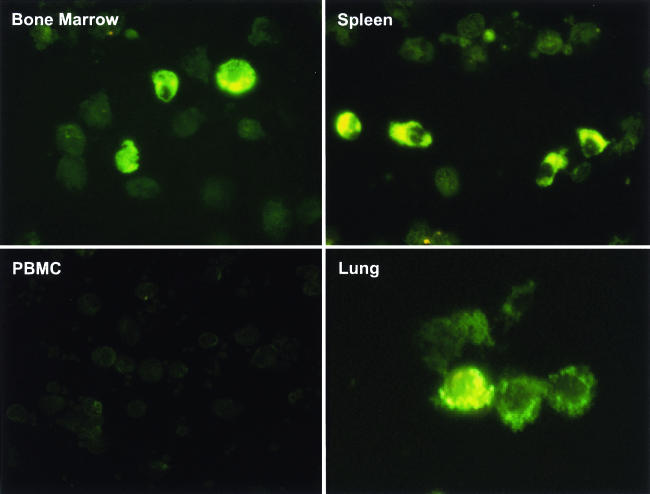
The unexpected absence of a correlation between PRRSV-responding T cells and viral load, which was determined by quantifying viral RNA, raised the possibility that viral antigens were not present in target cells. Thus, the presence of PRRSV in lymphoid tissues in the persistent phase of infection was evaluated by immunostaining of adherent macrophages with antinucleocapsid antibody SDOW17. Single-cell suspensions were prepared from lymphoid tissues and cultured for 3 h. Nonadherent cells were removed, and the remaining adherent cells were greater than 90% macrophages, as determined by staining with monoclonal antibody 74-22-15 (data not shown). Intense SDOW17 cytoplasmic staining was observed in macrophages in lung, spleen, tonsil, mesenteric LN, inguinal LN, and sternal LN. Representative examples are shown in Fig. 4 for lung, spleen, and bone marrow. Based on counting cells on slides from all of the animals, approximately 5% of adherent cells stained positively in spleen and bone marrow, and about 3% were positive in alveolar macrophages (Table 2). About 0.1% of cells were positive in tonsil and in regional lymphoid tissues. No positive cells were detected in PBMC. However, real-time PCR gave negative results for PRRSV viral RNA in all spleen samples, all bone marrow cell samples, and all secondary lymphoid tissues except tonsil, sternal LN, some inguinal LN, and some lung samples (Table 2). These results suggested that viral antigen was present in lung and lymphoid tissues.
FIG. 4.
Immunostaining of macrophages isolated from different tissues in the persistent phase of PRRSV infection. Single-cell suspensions were produced by the mechanical disruption of tissues from PRRSV-infected pigs 67 days after infection, and cells were plated on glass coverslips for 3 h. Adherent cells were stained with FITC-conjugated anti-PRRSV nucleocapsid monoclonal antibody SDOW17. Images are representative macrophage staining results in lung, spleen, bone marrow, and PBMC.
TABLE 2.
PRRSV immunopositive macrophages in persistently infected pig tissuesa
| Tissue | Viral RNA statusb | PRRSV-positive macrophage frequencyc |
|---|---|---|
| PBMC | 0 | − |
| Spleen | 0 | +++ |
| Tonsil | 5/6 | + |
| Lung | 1/6 | +++ |
| Sternal LN | 5/6 | + |
| Inguinal LN | 2/6 | + |
| Mesenteric LN | 0 | + |
| Bone marrow | 0 | +++ |
Macrophages isolated from various tissues 67 days after PRRSV infection were stained by FITC-anti PRRSV nucleocapsid monoclonal antibody.
Numbers represent the PRRSV RNA-positive tissues/total number of tissues tested by real-time PCR.
Three epifluorescent microscopic fields were examined for each tissue in each animal. Each field contained 300 to 400 cells visible by bisbenzimide- stained nuclei. Results were scored as no positive cells (−), 1 to 5 positive cells in at least one field (+), 1 to 5 positive cells in every field (++), >5 cells in every field (+++).
Comparison of viral load and macrophage abundance in acute and persistent infection.
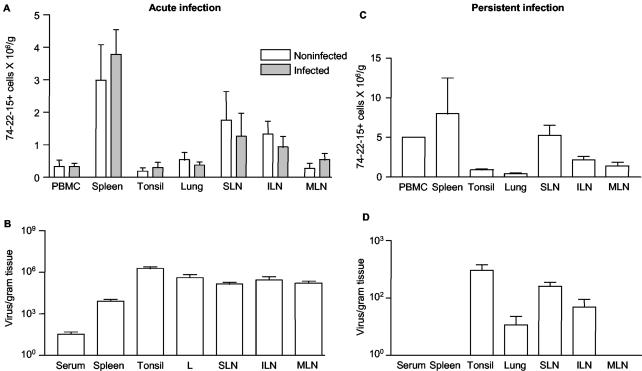
To address the possibility that differences in macrophage abundance might account for the substantial variation in viral load in target tissues during acute infection, the proportion of macrophages was determined by FACS analysis and compared to viral load in 10 gilts infected at 4 to 5 months of age. The number of macrophages per unit mass of tissue was lowest in tonsil and highest in spleen (Fig. 5A). Furthermore, PRRSV acute infection did not change macrophage abundance in any of the tissues, including lung and secondary lymphoid tissues, compared to results from five age-matched noninfected control gilts (Fig. 5A). By this measure of abundance, macrophage frequency did not account for the distribution or level of PRRSV in the same tissues (Fig. 5B). The results also show that tonsil and PBMC, which have relatively low levels of PRRSV-specific IFN-γ-secreting lymphocytes, have sufficient numbers of macrophages or monocytes, respectively, for antigen presentation in the ELISPOT assay, as shown in Fig. 2. In persistent infection, at 67 days, the relative proportions of macrophages in lung, blood, spleen, and peripheral LN are similar to or higher than those observed in acute infection (Fig. 5C). Nevertheless, the tissue richest in macrophages, the spleen, contained no viral RNA in any of 10 animals, while tonsil, with the second lowest proportion of macrophages, had the highest level of PRRSV (Fig. 5D). The comparison of macrophage distribution and viral loads in Fig. 5C and D show that there is no relationship between macrophage availability and the level of persistent infection.
FIG. 5.
Comparison of macrophage density and virus load in permissive tissues in acute and persistent infection. (A) Effect of acute PRRSV infection on macrophage concentrations in various tissues. Single-cell suspensions were stained with anti-porcine macrophage monoclonal antibody 74-22-15. Data are expressed as means and standard deviations of five noninfected control gilts or 10 infected gilts. There are no significant differences between infected and noninfected tissues. (B) Viral load in the tissues at 19 days of acute PRRSV infection. Virus load was determined by real-time PCR. (C) Macrophage density in lung and lymphoid tissues of persistently infected pigs at 67 days after infection. (D) Viral load in tissues in persistent PRRSV infection at 67 days after infection. The data in panels B and D are redrawn from the data in Fig. 2A and B to facilitate comparison with panels A and C, respectively. SLN, sternal LN; ILN, inguinal LN; MLN, mesenteric LN; L, lung.
DISCUSSION
The standard model for CMI response to viral infection involves antigen-specific activation and expansion of CD4+ and CD8+ T cells in draining LN, followed by trafficking of specific cytotoxic lymphocytes back to the sites of infection, where infected cells are destroyed (30, 65). In PRRSV infection of pigs, alveolar macrophages of the lung are the primary target in acute infection, but the virus is found in macrophages throughout the body, including secondary lymphoid tissues (36). Hence, secondary lymphoid tissues, including LN, are sites for viral infection as well as for PRRSV antigen presentation and T-cell activation and expansion. Thus, the presence of antigen-specific T cells in lymphoid tissues of PRRSV-infected pigs should reflect an ongoing local CMI response in acute and persistent infection similar, for example, to the kinetics of antigen-specific T-cell responses in persistent lymphocyte choriomeningitis virus infection, in which the response to four different viral epitopes was closely related to viral load in spleen (67).
PRRSV, however, did not elicit an organized and consistent T-cell response. The antigen-specific T-cell response at sites of infection was highly variable, weak, and independent of the local viral load. The recruitment of T cells to sites of infection did not occur, and the frequency of γδ T cells, which may be involved in innate immunity, was unchanged or decreased in all sites of acute infection. The disparities between viral loads in lymphoid tissues and corresponding antigen-specific T-cell responses was striking. The viral load in tonsil was 1,000 times greater than that in spleen in acute infection, whereas the antigen-specific T-cell frequency in spleen was almost 1,000 times greater than that in tonsil. Furthermore, the outcomes of viral persistence were completely different in LN that had similar antigen-specific T-cell frequencies. For example, virus persisted in sternal LN and some inguinal LN but not in mesenteric LN and tracheobronchial LN. In addition to the absence of a correlation between viral load and responding T cells in sites of infection, there was no apparent effect of PRRSV on local T-cell populations. The proportions of CD4+, CD8+, and CD4+ CD8+ T cells were not altered by PRRSV infection in lung, blood, or lymphoid tissues. The numbers of macrophages in lung and lymphoid tissues were not affected by PRRSV infection, yet macrophage abundance was not associated with viral load in acute or persistent infection.
Surprisingly, there was an apparent decline of γδ T-cell populations in all of the tissues, especially in lung and lymph nodes. The biological functions of γδ T cells are primarily regulatory (9). In mouse influenza virus A infection, γδ T cells show two waves of response, at 10 days and 13 days p.i. (8). The regulatory function of γδ T cells may be carried out by production of proinflammatory cytokines, including tumor necrosis factor alpha (9), to link the initial immune response with the later development of an adaptive response. Van Reeth and colleagues found that production of tumor necrosis factor, interleukin-1, and IFN-α were low or absent in the first 3 days of PRRSV infection, especially compared to levels in influenza virus infection (62). The reduction in the number of γδ T cells in lung may result from the low production of proinflammatory cytokines (62) and may contribute to the impaired cellular immune response to PRRSV (44).
These disparate observations are reminiscent of lactate dehydrogenase-elevating virus (LDV) infection in mice. LDV is the closest relative to PRRSV in the Arteriviridae family (11). LDV infects macrophages and causes a life-long viremia after acute infection (13) that coexists with an active LDV-specific immune response, including cytotoxic and helper T cells (61). Disruption of acquired humoral or cellular immunity did not affect LDV levels in plasma. Moreover, immune-deficient mutant mice had the same level of viremia as that of wild-type mice, suggesting that anti-LDV immune responses do not play a critical role in controlling LDV replication (49). The transient nature of the PRRSV-specific T-cell response reported here was observed previously (5, 37) and also was observed in LDV infection, in which cytotoxic T cells were elicited in acute LDV infection but disappeared in the chronic phase of infection (21).
The association between an antigen-specific cellular response and viral load in plasma has been addressed in HIV (1, 47, 48). An inverse correlation was observed between virus load in plasma and cytotoxic T-lymphocyte responses to Gag, which is consistent with the function of cytotoxic T lymphocytes (20, 45, 48). However, no correlation was found in other studies either between viral load and total or Gag-specific CD4+- or CD8+-T-cell responses (18, 26, 42). Furthermore, in some studies, a positive correlation was identified between the viral load in plasma and the total HIV-, Env-, and Nef-specific CD8+-T-cell frequencies (7, 38). These results indicate that the antigen-specific T-cell response in blood is not directly related to viral load and suggests that the nature of the virus-host interaction at sites of infection are complex.
Real-time PCR quantitation revealed that PRRSV is abundant in lymphoid tissues as well as in lung in acute infection, which is consistent with macrophages' being the main target for PRRSV infection (36). Serum, which is the standard sample for the diagnosis of acute infection, contains unexpectedly low levels of virus compared to all other tissues, as was observed previously (19). The relative differences in viral load between serum and lymphoid tissues depend in part on the time of sampling; PRRSV titers in blood peak on day 5 after infection and wane thereafter, so that in our study the titer values on day 19 would be less than at the peak (58). However, since the real-time PCR assay was based on the ORF6 sequence, the serum results would correspond to genomic RNA, but the tissue results would include both genomic and subgenomic viral RNA. Lymphoid tissues, except for spleen, appear to be sites of viral replication in acute infection, since there were no significant differences from lung in viral load. In the persistent phase, however, PRRSV is restricted primarily to tonsil and sternal LN, which is partially consistent with previous studies (2, 52, 69). Interestingly, the tracheal LN, which is directly attached to and drains lymph from the lung, was negative, whereas the sternal LN and some inguinal LN were positive. Moreover, the lung, the major PRRSV replication site in acute infection, was of minor importance as a site of viral persistence. Overall, PRRSV persisted primarily in tonsil and sternal LN.
The contrast between the presence of PRRSV antigen and the absence of antigen-specific T cells in both acute and persistent infection in tonsil was notable. Tonsil contains lymphoid structures, such as nodules and germinal centers, similar to those of spleen, but the general architecture is less developed than in LN, in which antigen presentation and T-cell activation are optimal (22, 32). The minimal T-cell immune response to PRRSV antigens in tonsil may result from a combination of weak antigen presentation and T-cell activation, lack of PRRSV-specific T-cell homing back to tonsil, and lack of innate cellular sensing of viral infection (44). Antigen-specific T-cell trafficking is related to the original inflammatory environment (30, 65). In acute PRRSV infection, inflammation was observed more often and to a greater degree in tonsil than in spleen (data not shown). This finding suggests that tonsil is a privileged site in PRRSV infection, possibly due to weak activation of intracellular IFN pathways. As noted earlier, PRRSV infection is characterized by a lack of pulmonary IFN-α in acute infection (62).
Persistent infections with hepatitis C virus, another positive-sense single-stranded RNA virus, are facilitated by virally encoded protease inhibition of IFN signaling (56). Although PRRSV encodes at least three proteases (70), a viral protease may not inhibit IFN signaling in infected macrophages. At least 19 genes, including the IFN-inducible gene Mx1 (29), are increased in macrophages by PRRSV infection (60), suggesting that PRRSV elicits an IFN-mediated response but may utilize other mechanisms for evasion of cellular immunity, such as down-regulation of major histocompatibility complex class I (25) or costimulatory molecule expression (27). The absence of an IFN response in lungs of acutely infected pigs (62) suggests that the IFN response observed in macrophages infected in vitro does not occur in vivo.
It has been reported that the CMI response to PRRSV is weak in the first 12 weeks after infection and then develops progressively for more than 20 weeks, during which it is at least partially responsible for the elimination of viral persistence (40). However, we found that the virus load in lung and all lymphoid tissues in acute infection declined 1,000 times or more from acute infection at 19 days to persistent infection at 67 days, which is consistent with previous observations (19). An increase in PRRSV-specific T cells that begins after the major decline in viral load cannot be the causative factor for the eventual resolution of viral persistence.
The weak T-cell response to PRRSV is a feature of this host-virus interaction. PRRSV did not alter the large majority of T-cell subpopulations in blood or lymphoid tissues. An excess of CD8+ to CD4+ T cells in blood is characteristic of swine (50), as is a preponderance of CD4+ T cells in lymphoid tissues and a paucity of both CD4+ and CD8+ T cells in tonsil (55). Moreover, swine are unusual in possessing substantial populations of functionally mature CD4+ CD8+ T cells and CD4− CD8− γδ T cells in all T-cell compartments (54). The most consistent observation was a decrease in γδ T cells, which was statistically significant in lung and in sternal, inguinal, and mesenteric LN. However, the meaning of this observation is not apparent at this time.
IFN-γ secretion is widely used for the assessment of antigen-specific T-cell responses in swine (24, 35, 40, 63, 71). IFN-γ secretion by activated T lymphocytes, both in helper and cytotoxic T-cell subsets, is a hallmark of antiviral immunity. It is critical in host defense against a variety of viral infections, including measles, influenza virus, and HIV (14). IFN-γ can regulate antiviral responses via Mx1, 2′,5′-oligoadenylate synthetase, double-stranded RNA-inducible protein kinase (PKR), RNase L, and nitric oxide synthase (4, 10, 16, 29). IFN-γ also plays a role in PRRSV immunity. IFN-γ mRNA was detected in PBMC, lung, and LN from PRRSV-infected pigs (37, 53), and PRRSV-specific IFN-γ-secreting lymphocytes are present in PBMC of pigs infected with PRRSV (34, 40). In addition, IFN-γ has been demonstrated to inhibit PRRSV replication in macrophages (6) and PRRSV RNA synthesis via PKR (53). In this study, IFN-γ secretion was observed only following antigen stimulation, suggesting that activated T cells responding to PRRSV are rare in blood and at sites of infection and that PRRSV-specific cells are of the memory phenotype (72). A variety of swine T-cell subpopulations, including CD4+ CD8+ memory cells, CD8+ effector cells, CD4+ CD45RA− memory cells, and γδ T cells, secrete IFN-γ upon activation (51). The phenotype of T cells secreting IFN-γ in response to PRRSV appears to consist primarily of CD4+ CD8+ memory cells and CD4+ helper cells (37, 40 and Z. Xiao and M. P. Murtaugh, unpublished data). The paucity of CD8+ anti-PRRSV effector T cells, despite the presence of antigen-positive macrophages, may contribute importantly to the persistence of PRRSV in lymphoid tissues.
The absence of an association between viral load and PRRSV-specific T cells at sites of infection is not due to a loss of host macrophages or lack of viral antigen expression. Macrophages are abundant in spleen and bone marrow, while persistent infection is located primarily in tonsil and LN. Macrophages in affected tissues contained large amounts of viral protein, even when the tissues were negative for PRRSV RNA. Antigen-positive but viral RNA-negative macrophages in spleen and bone marrow may represent infected cells that degraded viral nucleic acids but failed to process viral proteins. Alternatively, antigen-positive cells may have phagocytosed viral proteins from dead cells or may have migrated from other sites of infection. In either case, the absence of this correlation suggests that other mechanisms could be used to resolve viral infection in the absence of a cytotoxic T-lymphoctye response, such as the noncytolytic mechanisms reported in the resolution of hepatitis B virus infection (28). In addition, both cytolytic and noncytolytic T cells are involved in the control of feline immunodeficiency virus infection (15, 23). Interestingly, natural killer (NK) cell cytotoxic activity increased from 15 to 35% of specific lysis in T cells cultured from PRRSV-infected pigs and restimulated with PRRSV for 3 weeks (37). This cellular immune response may play a relevant role in the clearance of PRRSV and the resolution of persistent infection.
If the adaptive cellular response is not effective, one might predict that viral load would be proportional to the amount of local target macrophages. Macrophage numbers vary substantially among lung and lymphoid tissues. Nevertheless, PRRSV load is highest in tonsil and lung and about 1,000 times lower in spleen even though spleen has a significantly higher proportion of macrophages than tonsil and lung. Similarly, spleen was not the major source of LDV released into circulation during LDV infection (12). PRRSV infection did not cause a significant decline of local macrophage frequency in any tissue, including lung. This phenomenon might be due to the rapid replacement of destroyed macrophages, or a subset of permissive macrophages might be distributed unevenly in different tissues, with more in tonsil, lung, and other secondary lymphoid tissues and fewer in spleen. Only a minor population of macrophages appears to be permissive to LDV infection (3), and in pigs less than 2% of lung macrophages were found to be PRRSV positive in acute infection (19). Thus, tissue-specific factors may contribute to macrophage infectivity in vivo.
Since the large majority of macrophages may not be permissive to PRRSV infection in vivo (19), the elimination of permissive cells might limit the extent of infection. Similarly, permissive-cell destruction happens quickly in LDV infection in mice, and newly generated permissive macrophages may be responsible for the lifelong viremia and restriction of infection to certain tissues, such as LN, spleen, and skin (3). In the case of PRRSV, we propose that the depletion of permissive macrophages accounts for the decline in viral load in lung and lymphoid tissues and facilitates the eventual abolishment of virus persistence. If permissive macrophages are depleted by cytolytic infection and only a subpopulation of replacement cells is permissive, the total number of newly permissive cells would decline markedly and result in a self-limiting infection. The complete elimination of viral infection could then be effected by neutralizing antibodies, the weak PRRSV-specific T-cell response, and, possibly, by increased levels of NK cells. Interestingly, NK cell activity was increased in T cells restimulated with PRRSV and cultured in vitro (37). In addition, this model would explain the observed lack of response in PRRSV homologous challenge, since introduced viruses would not find permissive host cells (24). Rechallenge with heterologous PRRSV strains would result in a limited infection based on slight differences in viral genotypes, giving rise to a small number of permissive targets.
Acknowledgments
We greatly appreciated the assistance of Carlos Trincado, Martha Fuentes, and Simone Olivera in managing animals and collecting samples and of Linda Foster, Kendra Hyland, Chad Ramler, Juliana Machado, Gongpin Liu, and Elaine Eggleston in tissue processing and cell isolation. Eric Nelson, South Dakota State University, Brookings, generously provided fluorescently labeled monoclonal antibody SDOW17. ELISPOT readers were provided by Bianca Conti-Fine and Bernard Hering at the University of Minnesota. M. Kariuki Njenga, University of Minnesota, offered many excellent suggestions to improve the manuscript. Mike Roof (Boehringer Ingelheim Vetmedica, Ames, Iowa) provided research materials.
The research was supported by Minnesota Rapid Response funds and a grant from the Minnesota Agricultural Experiment Station.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allende, R., W. W. Laegreid, G. F. Kutish, J. A. Galeota, R. W. Wills, and F. A. Osorio. 2000. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J. Virol. 74:10834-10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, G. W., R. R. Rowland, G. A. Palmer, C. Even, and P. G. Plagemann. 1995. Lactate dehydrogenase-elevating virus replication persists in liver, spleen, lymph node, and testis tissues and results in accumulation of viral RNA in germinal centers, concomitant with polyclonal activation of B cells. J. Virol. 69:5177-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnheiter, H., and O. Haller. 1983. Mx gene control of interferon action: different kinetics of the antiviral state against influenza virus and vesicular stomatitis virus. J. Virol. 47:626-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista, E. M., and T. W. Molitor. 1997. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 10:83-94. [DOI] [PubMed] [Google Scholar]
- 6.Bautista, E. M., and T. W. Molitor. 1999. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch. Virol. 144:1191-1200. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Bierk, M. D., S. A. Dee, K. D. Rosssow, S. Otake, J. E. Collins, and T. W. Molitor. 2001. Transmission of porcine reproductive and respiratory syndrome virus from persistently infected sows to contact controls. Can. J. Vet. Res. 65:261-266. [PMC free article] [PubMed] [Google Scholar]
- 8.Carding, S. R., W. Allan, S. Kyes, A. Hayday, K. Bottomly, and P. C. Doherty. 1990. Late dominance of the inflammatory process in murine influenza by γ/δ + T cells. J. Exp. Med. 172:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carding, S. R., and P. J. Egan. 2002. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336-345. [DOI] [PubMed] [Google Scholar]
- 10.Castelli, J. C., B. A. Hassel, K. A. Wood, X. L. Li, K. Amemiya, M. C. Dalakas, P. F. Torrence, and R. J. Youle. 1997. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J. Exp. Med. 186:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanagh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. [PubMed] [Google Scholar]
- 12.Chan, S. P., C. O. Onyekaba, J. T. Harty, and P. G. Plagemann. 1989. Persistent infection of mice by lactate dehydrogenase-elevating virus: transient virus replication in macrophages of the spleen. Virus Res. 14:317-326. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., K. S. Faaberg, and P. G. Plagemann. 1994. Detection of negative-stranded subgenomic RNAs but not of free leader in LDV-infected macrophages. Virus Res. 34:167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesler, D. A., and C. S. Reiss. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13:441-454. [DOI] [PubMed] [Google Scholar]
- 15.Choi, I. S., R. Hokanson, and E. W. Collisson. 2000. Anti-feline immunodeficiency virus (FIV) soluble factor(s) produced from antigen-stimulated feline CD8+ T lymphocytes suppresses FIV replication. J. Virol. 74:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens, M. J., and A. Elia. 1997. The double-stranded RNA-dependent protein kinase PKR: structure and function. J. Interferon Cytokine Res. 17:503-524. [DOI] [PubMed] [Google Scholar]
- 17.Collins, J. E., D. A. Benfield, W. T. Christianson, L. Harris, J. C. Hennings, D. P. Shaw, S. M. Goyal, S. McCullough, R. B. Morrison, H. S. Joo, D. E. Gorcyca, and D. W. Chladek. 1992. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Investig. 4:117-126. [DOI] [PubMed] [Google Scholar]
- 18.Dalod, M., M. Dupuis, J.-C. Deschemin, D. Sicard, D. Salmon, J.-F. Delfraissy, A. Venet, M. Sinet, and J.-G. Guillet. 1999. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J. Virol. 73:7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan, X., H. J. Nauwynck, and M. B. Pensaert. 1997. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Microbiol. 56:9-19. [DOI] [PubMed] [Google Scholar]
- 20.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Even, C., R. R. Rowland, and P. G. Plagemann. 1995. Cytotoxic T cells are elicited during acute infection of mice with lactate dehydrogenase-elevating virus but disappear during the chronic phase of infection. J. Virol. 69:5666-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fawcett, D. W. 1994. A textbook of histology, 12th ed., p. 575-576. Chapman & Hall, New York, N.Y.
- 23.Flynn, J. N., S. Dunham, A. Mueller, C. Cannon, and O. Jarrett. 2002. Involvement of cytolytic and non-cytolytic T cells in the control of feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 85:159-170. [DOI] [PubMed] [Google Scholar]
- 24.Foss, D. L., M. J. Zilliox, W. Meier, F. Zuckermann, and M. P. Murtaugh. 2002. Adjuvant danger signals increase the immune response to porcine reproductive and respiratory syndrome virus. Viral Immunol. 15:557-566. [DOI] [PubMed] [Google Scholar]
- 25.Fruh, K., E. Bartee, K. Gouveia, and M. Mansouri. 2002. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 88:55-69. [DOI] [PubMed] [Google Scholar]
- 26.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. deq Uiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 27.Green, W. R. 1999. Cytotoxic T lymphocytes to endogenous mouse retroviruses and mechanisms of retroviral escape. Immunol. Rev. 168:271-286. [DOI] [PubMed] [Google Scholar]
- 28.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 29.Horisberger, M. A., P. Staeheli, and O. Haller. 1983. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl. Acad. Sci. USA 80:1910-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins, M. K., A. Khoruts, E. Ingulli, D. L. Mueller, S. J. McSorley, R. L. Reinhardt, A. Itano, and K. A. Pape. 2001. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19:23-45. [DOI] [PubMed] [Google Scholar]
- 31.Kapur, V., M. R. Elam, T. M. Pawlovich, and M. P. Murtaugh. 1996. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 77:1271-1276. [DOI] [PubMed] [Google Scholar]
- 32.Krause, W. J., and J. H. Cutts. 1994. Essentials of histology, p. 365-367. Little, Brown and Co., Boston, Mass.
- 33.Kuster, H., M. Opravil, P. Ott, E. Schlaepfer, M. Fischer, H. F. Gunthard, R. Luthy, R. Weber, and R. W. Cone. 2000. Treatment-induced decline of human immunodeficiency virus-1 p24 and HIV-1 RNA in lymphoid tissue of patients with early human immunodeficiency virus-1 infection. Am. J. Pathol. 156:1973-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwang, J., F. Zuckermann, G. Ross, S. Yang, F. Osorio, W. Liu, and S. Low. 1999. Antibody and cellular immune responses of swine following immunisation with plasmid DNA encoding the PRRS virus ORF's 4, 5, 6 and 7. Res. Vet. Sci. 67:199-201. [DOI] [PubMed] [Google Scholar]
- 35.Larsen, D. L., A. Karasin, F. Zuckermann, and C. W. Olsen. 2000. Systemic and mucosal immune responses to H1N1 influenza virus infection in pigs. Vet. Microbiol. 74:117-131. [DOI] [PubMed] [Google Scholar]
- 36.Lawson, S. R., K. D. Rossow, J. E. Collins, D. A. Benfield, and R. R. Rowland. 1997. Porcine reproductive and respiratory syndrome virus infection of gnotobiotic pigs: sites of virus replication and co-localization with MAC-387 staining at 21 days post-infection. Virus Res. 51:105-113. [DOI] [PubMed] [Google Scholar]
- 37.Lopez Fuertes, L., N. Domenech, B. Alvarez, A. Ezquerra, J. Dominguez, J. M. Castro, and F. Alonso. 1999. Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 64:33-42. [DOI] [PubMed] [Google Scholar]
- 38.Lubaki, N. M., M. E. Shepherd, R. S. Brookmeyer, H. Hon, T. C. Quinn, M. Kashamuka, M. Johnson, R. Gottle, J. Devers, H. M. Lederman, and R. C. Bollinger. 1999. HIV-1-specific cytolytic T-lymphocyte activity correlates with lower viral load, higher CD4 count, and CD8+CD38−DR− phenotype: comparison of statistical methods for measurement. J. Acquir. Immune Defic. Syndr. 22:19-30. [DOI] [PubMed] [Google Scholar]
- 39.Mahlum, C. E., S. Haugerud, J. L. Shivers, K. D. Rossow, S. M. Goyal, J. E. Collins, and K. S. Faaberg. 2002. Detection of bovine viral diarrhea virus by TaqMan reverse transcription polymerase chain reaction. J. Vet. Diagn. Investig. 14:120-125. [DOI] [PubMed] [Google Scholar]
- 40.Meier, W. A., J. Galeota, F. A. Osorio, R. J. Husmann, W. M. Schnitzlein, and F. A. Zuckermann. 2003. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 309:18-31. [DOI] [PubMed] [Google Scholar]
- 41.Meulenberg, J. J., M. M. Hulst, E. J. de Meijer, P. L. Moonen, A. den Besten, E. P. de Kluyver, G. Wensvoort, and R. J. Moormann. 1993. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 192:62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Migueles, S. A., and M. Connors. 2001. Frequency and function of HIV-specific CD8+ T cells. Immunol. Lett. 79:141-150. [DOI] [PubMed] [Google Scholar]
- 43.Molitor, T. W., E. M. Bautista, and C. S. Choi. 1997. Immunity to PRRSV: double-edged sword. Vet. Microbiol. 55:265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murtaugh, M. P., Z. Xiao, and F. Zuckermann. 2002. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 15:533-547. [DOI] [PubMed] [Google Scholar]
- 45.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen, J., and A. Botner. 1997. Hematological and immunological parameters of 4 1/2-month old pigs infected with PRRS virus. Vet. Microbiol. 55:289-294. [DOI] [PubMed] [Google Scholar]
- 47.Novitsky, V., P. Gilbert, T. Peter, M. F. McLane, S. Gaolekwe, N. Rybak, I. Thior, T. Ndung'u, R. Marlink, T. H. Lee, and M. Essex. 2003. Association between virus-specific T-cell responses and plasma viral load in human immunodeficiency virus type 1 subtype C infection. J. Virol. 77:882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 49.Onyekaba, C. O., J. T. Harty, C. Even, B. G. Hu, and P. G. Plagemann. 1989. Persistent infection of mice by lactate dehydrogenase-elevating virus: effects of immunosuppression on virus replication and antiviral immune responses. Virus Res. 14:297-315. [DOI] [PubMed] [Google Scholar]
- 50.Pescovitz, M. D., J. K. Lunney, and D. H. Sachs. 1985. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J. Immunol. 134:37-44. [PubMed] [Google Scholar]
- 51.Rodriguez-Carreno, M. P., L. Lopez-Fuertes, C. Revilla, A. Ezquerra, F. Alonso, and J. Dominguez. 2002. Phenotypic characterization of porcine IFN-gamma-producing lymphocytes by flow cytometry. J. Immunol. Methods 259:171-179. [DOI] [PubMed] [Google Scholar]
- 52.Rossow, K. D. 1998. Porcine reproductive and respiratory syndrome. Vet. Pathol. 35:1-20. [DOI] [PubMed] [Google Scholar]
- 53.Rowland, R. R., B. Robinson, J. Stefanick, T. S. Kim, L. Guanghua, S. R. Lawson, and D. A. Benfield. 2001. Inhibition of porcine reproductive and respiratory syndrome virus by interferon-gamma and recovery of virus replication with 2-aminopurine. Arch. Virol. 146:539-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saalmuller, A., W. Hirt, and M. J. Reddehase. 1990. Porcine γ/δ T lymphocyte subsets differing in their propensity to home to lymphoid tissue. Eur. J. Immunol. 20:2343-2346. [DOI] [PubMed] [Google Scholar]
- 55.Saalmuller, A., M. J. Reddehase, H. J. Buhring, S. Jonjic, and U. H. Koszinowski. 1987. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur. J. Immunol. 17:1297-1301. [DOI] [PubMed] [Google Scholar]
- 56.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 57.Shin, J., E. M. Bautista, Y. B. Kang, and T. W. Molitor. 1998. Quantitation of porcine reproductive and respiratory syndrome virus RNA in semen by single-tube reverse transcription-nested polymerase chain reaction. J. Virol. Methods 72:67-79. [DOI] [PubMed] [Google Scholar]
- 58.Shin, J. H., and T. W. Molitor. 2002. Assessment of porcine reproductive and respiratory syndrome virus RNA load in sera and tissues during acute infection. J. Vet. Sci. 3:75-86. [PubMed] [Google Scholar]
- 59.Sopper, S., D. Nierwetberg, A. Halbach, U. Sauer, C. Scheller, C. Stahl-Hennig, K. Matz-Rensing, F. Schafer, T. Schneider, V. ter Meulen, and J. G. Muller. 2003. Impact of simian immunodeficiency virus (SIV) infection on lymphocyte numbers and T-cell turnover in different organs of rhesus monkeys. Blood 101:1213-1219. [DOI] [PubMed] [Google Scholar]
- 60.Tough, D. F., S. Sun, X. Zhang, and J. Sprent. 1999. Stimulation of naive and memory T cells by cytokines. Immunol. Rev. 170:39-47. [DOI] [PubMed] [Google Scholar]
- 61.van den Broek, M. F., R. Sporri, C. Even, P. G. Plagemann, E. Hanseler, H. Hengartner, and R. M. Zinkernagel. 1997. Lactate dehydrogenase-elevating virus (LDV): lifelong coexistence of virus and LDV-specific immunity. J. Immunol. 159:1585-1588. [PubMed] [Google Scholar]
- 62.Van Reeth, K., G. Labarque, H. Nauwynck, and M. Pensaert. 1999. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 67:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waters, W. R., R. E. Sacco, A. D. Dorn, R. Hontecillas, F. A. Zuckermann, and M. J. Wannemuehler. 1999. Systemic and mucosal immune responses of pigs to parenteral immunization with a pepsin-digested Serpulina hyodysenteriae bacterin. Vet. Immunol. Immunopathol. 69:75-87. [DOI] [PubMed] [Google Scholar]
- 64.Wensvoort, G., C. Terpstra, J. M. Pol, E. A. ter Laak, M. Bloemraad, E. P. de Kluyver, C. Kragten, L. van Buiten, A. den Besten, F. Wagenaar, J. M. Broekhuijsen, P. L. J. M. Moonen, T. Zetstra, E. A. de Boer, H. J. Tibben, M. F. de Jong, P. van't Veld, G. J. R. Groenland, J. A. van Gennep, M. T. Voets, J. H. M. Verheijden, and J. Braamskamp. 1991. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 13:121-130. [DOI] [PubMed] [Google Scholar]
- 65.Westermann, J., E. M. Ehlers, M. S. Exton, M. Kaiser, and U. Bode. 2001. Migration of naive, effector and memory T cells: implications for the regulation of immune responses. Immunol. Rev. 184:20-37. [DOI] [PubMed] [Google Scholar]
- 66.Westermann, J., and R. Pabst. 1992. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin. Investig. 70:539-544. [DOI] [PubMed] [Google Scholar]
- 67.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wills, R. W., A. R. Doster, J. A. Galeota, J. H. Sur, and F. A. Osorio. 2003. Duration of infection and proportion of pigs persistently infected with porcine reproductive and respiratory syndrome virus. J. Clin. Microbiol. 41:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wills, R. W., J. J. Zimmerman, K. J. Yoon, S. L. Swenson, M. J. McGinley, H. T. Hill, K. B. Platt, J. Christopher-Hennings, and E. A. Nelson. 1997. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet. Microbiol. 55:231-240. [DOI] [PubMed] [Google Scholar]
- 70.Ziebuhr, J., E. J. Snijder, and A. E. Gorbalenya. 2000. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 81:853-879. [DOI] [PubMed] [Google Scholar]
- 71.Zuckermann, F. A., R. J. Husmann, R. Schwartz, J. Brandt, E. Mateu de Antonio, and S. Martin. 1998. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Vet. Immunol. Immunopathol. 63:57-67. [DOI] [PubMed] [Google Scholar]
- 72.Zuckermann, F. A., S. Martin, R. J. Husmann, and J. Brandt. 1999. Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv. Vet. Med. 41:447-461. [DOI] [PubMed] [Google Scholar]