Abstract
Helper-dependent adenovirus (HD-Ad) vectors with all adenoviral genes deleted mediate very long-term expression of therapeutic transgenes in a variety of animal models of disease. These vectors are associated with reduced toxicity and improved safety relative to traditional early region 1 deletion first-generation Ad (FG-Ad) vectors. Many studies have clearly demonstrated that FG-Ad vectors induce innate and adaptive immune responses in vivo; however, a comprehensive analysis of host immune responses to HD-Ad vectors has not yet been performed. In DBA/2 mice, intravenous injection of HD-Ad vectors encoding LacZ (HD-AdLacZ) or a murine secreted alkaline phosphatase (HD-AdSEAP) induced an early expression of inflammatory cytokine and chemokine genes in the liver, including interferon-inducible protein 10, macrophage inflammatory protein 2, and tumor necrosis factor alpha, and were expressed in a pattern similar to that induced by FG-Ad vectors encoding AdSEAP. Like AdSEAP, and consistent with the pattern of cellular gene expression, HD-AdLacZ and HD-AdSEAP induced the recruitment of CD11b-positive leukocytes to the transduced liver within hours of administration. AdSEAP also induced a second phase of liver inflammation, consisting of inflammatory gene expression and CD3-positive lymphocytic infiltrates 7 days posttransduction. In contrast, beyond 24 h no infiltrates or expression of inflammatory genes was detected in the livers of mice receiving HD-AdSEAP. Despite the lack of liver inflammation at 7 days, Ad-specific cytotoxic T lymphocytes could be detected in mice receiving HD-AdSEAP. This lack of liver inflammation was not due to reduced transduction since levels of transgene expression and the amounts of vector DNA in the liver were equivalent in mice receiving HD-AdSEAP and AdSEAP. These results demonstrate that HD-Ad vectors induce intact innate but attenuated adaptive immune responses in vivo.
Adenovirus (Ad) vectors are used clinically and experimentally for gene therapy (reviewed by Amalfitano and Parks in reference 1). The majority of gene therapy studies utilize first-generation Ad (FG-Ad) vectors that are rendered replication defective due to the deletion of early region 1 (E1). Although these vectors cannot replicate, FG-Ad vectors induce strong host innate and adaptive immune responses (10). The induction of adaptive immunity by FG-Ad is characterized by the generation of Ad-specific major histocompatibility complex class I-restricted CD8+ cytotoxic T lymphocytes (CTL). In immunocompetent hosts, this response limits the duration of transgene expression and ultimately results in Ad vector clearance within several weeks of administration. The ongoing expression of Ad genes borne by these vectors contributes significantly to the induction of adaptive immunity (31-33). In recent years, a variety of strategies have been developed to minimize or eliminate Ad gene expression in this vector system. Currently, among the most effective vehicles for Ad-mediated gene therapy are helper-dependent Ad (HD-Ad) vectors. HD-Ad vectors lack all viral protein coding sequences and contain only the cis-acting elements required to replicate and package the vector DNA (approximately 500 bp of Ad sequence). Therefore, HD-Ad vectors can accommodate up to 36 kb of foreign DNA. Helper Ads provide the necessary viral genes in trans to package the vector DNA but cannot themselves be packaged due to Cre- or Flp-mediated excision of the helper virus packaging signal (16, 19, 27). In animal models, HD-Ad vectors exhibit stable, long-term transgene expression (8, 23) and are associated with reduced toxicity (13, 14, 29, 35). The development of these agents is thus a major advance in the field of Ad-mediated gene delivery.
In addition to their impact on adaptive immunity, FG-Ad vectors also induce innate immune responses (15, 24, 34). Unlike adaptive responses, the induction of innate immunity is triggered by the Ad particle and does not require viral gene expression (11, 24). Within minutes of delivery in vivo, UV-psoralen-inactivated (i.e., transcription-defective) FG-Ad vectors induce the expression of host cytokines and chemokines, similar to transcription-competent FG-Ad vectors (9, 11, 12, 15, 24), resulting in the rapid loss of vector independent of the adaptive immune system (30). Furthermore, transcription-defective FG-Ad vectors can induce Ad-specific CTL, illustrating that adaptive immune mechanisms remain in play despite the apparent lack of viral gene expression (7, 11, 22). Although HD-Ad vectors might be expected to exhibit similar responses, FG-Ad vectors inactivated by UV-psoralen-treatment do not truly mimic HD-Ad vectors, and a detailed analysis of the host immune response to HD-Ad has yet to be performed.
We have developed a model of adenoviral gene transfer to study both innate and adaptive immune responses in vivo (11). In this model, intravenous administration of FG-Ad vectors produces a biphasic immune response in the liver of naïve mice. First, FG-Ad vectors trigger rapid (<24-h) induction of inflammatory gene expression within the liver, accompanied by leukocyte recruitment and acute hepatic inflammation. This first phase corresponds to the innate immune response and, in the absence of Ad gene transcription, does not persist beyond 24 h. Following the administration of transcription-competent FG-Ad vectors, a second peak of cytokine and/or chemokine gene expression and inflammation occurs within the liver at 5 to 7 days postinjection. This phase, characterized by predominantly lymphocytic infiltrates and the induction of Ad-specific CTL, represents the adaptive immune response (11). In this study, we employ this model to examine the host immune response to HD-Ad vectors in comparison to the response to FG-Ad vectors. We show that HD-Ad vectors induce innate immune responses similar to those induced by FG-Ad vectors and that they elicit attenuated adaptive immunity in vivo. Our results show that HD-Ad vectors provide an immunological advantage over their FG-Ad counterparts.
MATERIALS AND METHODS
Ad vectors.
Ad constructs were generated by using a combination of conventional cloning and RecA-mediated bacterial recombination (3, 5). Ad-SEAP is an E1/E3 deletion Ad vector that encodes a murine secreted alkaline phosphatase (mSEAP) cDNA under the regulation of the murine phosphoglycerate kinase (PGK) promoter and simian virus 40 polyadenylation sequence, replacing the E1 deletion. This virus was grown in 293 cells by using standard techniques (17). HD-AdSEAP encodes an expression cassette identical to that encoded by Ad-SEAP but lacking all Ad sequences, with the exception of the left and right inverted terminal repeat and the viral packaging signal. To maintain the DNA size of the vector within appropriate packaging limits (20), HD-AdSEAP also contains a 22-kb fragment from the human hypoxanthine-guanine phosphorybosyltransferase gene, as has been previously described (18). HD-AdLacZ is similar to HD-AdSEAP but encodes the Escherichia coli lacZ gene under the regulation of the murine cytomegalovirus immediate-early promoter or enhancer replacing the PGK-mSEAP expression cassette. HD-Ad vectors were grown as previously described (19). Ad vector particle titers were determined by measuring the optical density at 260 nm and were expressed as particles per animal. Helper virus contamination for the HD-Ad vectors was determined by plaque assay on 293 cells and was approximately 0.2% for both HD-AdLacZ and HD-AdSEAP. Endotoxin levels for all vector preparations measured <0.1 EU/ml.
Animal studies.
DBA/2 (H-2d) mice (Charles River Laboratories, Wilmington, Mass.) were housed under single barrier conditions. The animals at 8 to 10 weeks of age (25 to 30 g) were injected with Ad vectors in 100 μl of vehicle via the femoral vein. Control animals received 100 μl of vehicle alone. Animal studies were performed in accordance with the Animal Care Committee guidelines at The University of Calgary.
RNase protection assay.
Total RNA was isolated from mouse liver tissue with an RNeasy kit (QIAGEN) according to the manufacturer's protocol. An RNase protection assay was carried out by using the RiboQuant multiprobe system (BD Pharmingen). The mCK-3 and mCK-5 template sets or a customized template were used to transcribe radiolabeled antisense riboprobes for a set of murine cytokines and chemokines. The murine ribosomal protein L32 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were used as internal control genes. Five micrograms of RNA per sample was hybridized to 6 × 105 cpm of total probe overnight at 56°C. After RNase A plus T1 and proteinase K digestions, the protected RNA fragments were electrophoresed on a 5% acrylamide-8 M urea gel and visualized by autoradiography. For quantification, the gels were subjected to phosphorimager analysis by a Personal F/X Molecular Imager (Bio-Rad), and the signal intensities for the individual genes were analyzed with Quantity One software (Bio-Rad). To correct for RNA loading, each intensity score was normalized to the intensity of hybridization for the GAPDH gene and expressed as phosphorimager units.
Southern blot analysis.
To obtain total liver DNA, 30 mg of liver tissue was lysed in 500 μl of lysis buffer (100 mM NaCl, 10 mM Tris [pH 8.0], 25 mM EDTA [pH 8.0], 0.5% sodium dodecyl sulfate [SDS], 0.1 mg of proteinase K per ml) and incubated at 50°C overnight. Nucleic acids were thoroughly extracted with phenol-chloroform-isoamyl alcohol (25:24:1), and the DNA was isolated from the aqueous layer by ethanol precipitation and resuspended in double-distilled H2O. Two micrograms of liver DNA was digested overnight with HindIII or HindIII/NheI/NotI (for HD-AdSEAP), and the DNA was subjected to electrophoresis on a 0.8% agarose gel. DNA was transferred onto a positively charged Hybond XL nylon membrane (Amersham Pharmacia) by using 0.4 M NaOH and hybridized with [32P]dCTP-labeled full-length mSEAP cDNA. Hybridizations were performed by using 2 ng of labeled probe per ml in 10 ml of ExpressHyb solution (Clontech) at 60°C for 90 min. The membrane was then washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS for 30 min and twice in 0.2× SSC-0.1% SDS for 30 min at 50°C. For quantification, the blots were subjected to phosphorimager analysis by using a Personal F/X Molecular Imager (Bio-Rad), and the signal intensities for the individual genes were analyzed with Quantity One software (Bio-Rad). To correct for DNA loading, each gene intensity score was normalized to the density of total DNA in the agarose gel (prior to membrane transfer) and expressed as phosphorimager units.
Enzymatic assays.
The levels of alkaline phosphatase in serum were measured by colorimetric assay. Twenty microliters of serum was diluted in 80 μl of phosphate-buffered saline (PBS). A total of 100 μl of p-nitrophenyl-phosphate solution (Sigma) was added to diluted serum and incubated at 37°C for 20 min. The reaction was stopped with 20 μl of 3 M NaOH, and the intensity of the color reaction was determined spectrophotometrically (at 405 nm).
CTL Assay.
CTL assays were performed by measuring cytoplasmic lactate dehydrogenase released upon cell lysis, as described previously (4). Briefly, 7 days after the injection of Ad vectors, DBA/2 mice were sacrificed. Spleens were aseptically harvested, red blood cells were lysed, and the remaining cells were resuspended in RMPI media containing 0.02 mM β-mercaptoethanol. To exclude transgene effects, target and effector cells were treated with different FG serotype 5 Ad vectors. Splenocytes were stimulated with Ad green fluorescent protein at 100 particles per cell for 5 days. The human coxsackievirus and Ad receptor (hCAR)-expressing syngeneic P815 mastocytoma cell line (P815-hCAR) was used as a target (2). P815-hCAR cells were pulsed with AdLacZ at 1,000 particles per cell for 24 h prior to CTL assays. Effector cells were harvested by centrifugation at 200 × g for 20 min through Lymphocyte-M (Cedarlane Laboratories) and incubated at standard effector/target ratios in 96-well round-bottom plates with 2 × 104 target cells/well for 5 h at 37°C. Maximum lysis was determined by adding 20 μl of 0.9% Triton X-100 in PBS per well. For spontaneous lysis, targets or effectors were incubated with media alone. After a 6-h incubation, the plates were centrifuged at 1,000 rpm for 2 min, and 50 μl of the supernatant was transferred to 96-well flat-bottom plates and mixed with 60 μl of solution A [36 mg of l-(+)-lactic acid per ml in 10 mM Tris (pH 8.5), 2 mg of ρ-iodonitrotetrazolium violet per ml in PBS, 3 mg of NAD+ per ml, 13.5 U of diaphorase in 0.03% bovine serum albumin, 1.2% sucrose in PBS] and incubated for 20 min at 37°C before the reaction was stopped by adding 20 μl of solution B (16.6 mg of oxamic acid/ml in PBS). The intensity of the color reaction was determined spectrophotometrically (at 490 nm), and specific lysis was determined by the following calculation: [experimental lysis − (spontaneous effector and target lysis)]/(maximum lysis − spontaneous target lysis) × 100.
Histology.
At predetermined time points, mice were sacrificed and livers were fixed in 10% formalin. A portion of the liver was also quick-frozen in OCT compound (Tissue Tek; Miles Diagnostics). Formalin-fixed tissues were embedded in paraffin. Sections (5 μm) were stained with eosin and hematoxylin and analyzed under a light microscope in a blind fashion. Immunohistochemistry was performed on frozen sections. To stain leukocytes, frozen sections were incubated with rat anti-mouse CD3 (for T lymphocytes) or CD11b (for monocytes, natural killer cells, and neutrophils) antibodies (BD Pharmingen). The slides were then processed by using a Vectastain ABC kit according to the manufacturer's instructions (Vector Laboratories). The reaction was developed with 3,3′-diaminobenzidine tetrahydrochloride and analyzed by microscopy.
Statistical analysis.
All experiments were performed at least in triplicate. Values are expressed as the mean ± standard deviation of individual samples.
RESULTS
HD-Ad vector induction of innate immune responses.
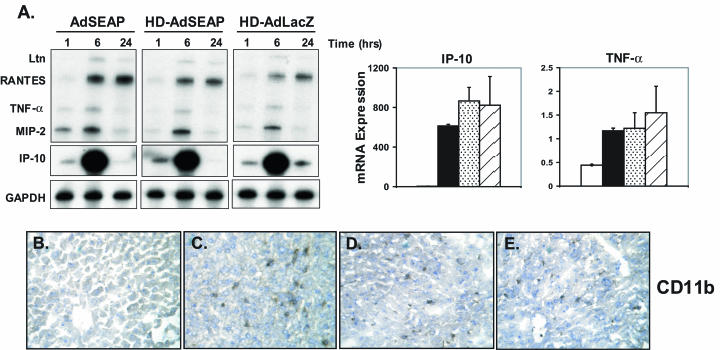
FG and transcription-defective Ad vectors activate the innate immune system (11). To determine whether HD-Ad vectors induced similar responses, DBA/2 mice were injected intravenously with Ad vectors. The innate response to Ad vectors is dose dependent (15). To remove potential confounding variables that may be associated with very high dose Ad vector administration, animals were administered an Ad vector particle titer sufficient to activate markers of the innate immune system but insufficient to cause liver toxicity. Mice (n = 3 per time point) therefore received 5 × 1010 particles of HD-AdSEAP or HD-AdLacZ. Control mice received 5 × 1010 particles of an FG-AdSEAP vector or vehicle alone. Quantification of cytokine and chemokine mRNA expression in the liver was determined at 1, 6, and 24 h following administration by using RNase protection assays. All Ad vectors induced cytokine and chemokine mRNA expression in the liver at 1 h (Fig. 1A). Tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein 2 (MIP-2), interferon-inducible protein 10 (IP-10), lymphotactin, and RANTES mRNA levels were all increased by Ad vectors over baseline at all time points, with the highest expression occurring at 6 h. There was no significant difference in the levels of induction of these inflammatory genes between Ad-SEAP and the HD vectors HD-AdSEAP and HD-AdLacZ. There was no cytokine or chemokine mRNA expression in mice receiving vehicle alone (data not shown). To confirm that the inflammatory gene expression in the liver was associated with leukocyte recruitment, liver sections were analyzed by immunohistochemistry (Fig. 1B to E). CD11b is a β2-integrin that is expressed on monocytes, natural killer cells, and activated neutrophils. Vehicle-treated animals had negligible CD11b-positive cells in the liver. Following Ad administration, the number of CD11b-expressing cells increased significantly in the liver at 1 h and persisted up to 24 h. Similar to the pattern of cytokine and chemokine gene expression, there was no difference in liver CD11b staining between AdSEAP, HD-AdSEAP, and HD-AdLacZ at all time points. Liver transaminase levels were unchanged and remained within normal limits in all animals receiving Ad vectors (data not shown). Consistent with our hypothesis that the early immune responses to Ad are mediated primarily through activation by Ad capsid (11), these results show that HD-Ad and FG-Ad vectors elicit similar innate immune responses in vivo.
FIG. 1.
Innate immune responses to HD-Ad vectors. (A) Cytokine and chemokine mRNA expression in the livers of mice receiving intravenous AdSEAP, HD-AdSEAP, or HD-AdLacZ vector (RNase protection assay). The graphs on the right show the relative expression of TNF-α and IP-10 mRNA at 6 h. Open bar, vehicle; filled bar, AdSEAP; speckled bar, HD-AdSEAP; hatched bar, HD-AdLacZ. (B to E) CD11b immunohistochemistry of mouse liver at 6 h following the intravenous administration of (in order from left to right) vehicle, AdSEAP, HD-AdSEAP, and HD-AdLacZ. Representative samples of at least three mice per group are shown.
HD-Ad vector induction of adaptive immune responses.
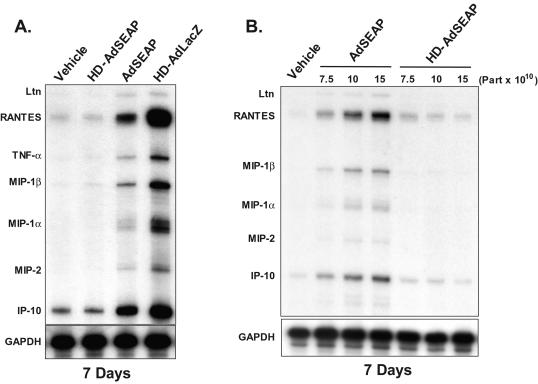
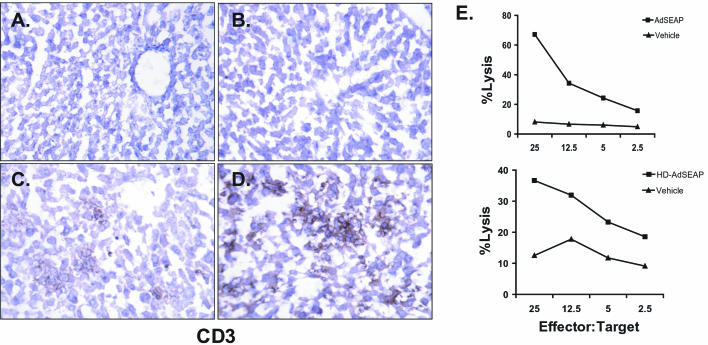
Studies utilizing UV-psoralen-inactivated Ad particles have suggested that Ad vectors can stimulate adaptive immune responses in the absence of viral gene transcription (7, 11). To test the impact of HD-Ad vectors on the adaptive immune response, DBA/2 mice were first injected with 5 × 1010 particles of HD-AdLacZ (n = 3), and 7 days later, livers were harvested and analyzed for cytokine and chemokine gene expression by an RNase protection assay. Similar to FG-Ad vectors, HD-AdLacZ vectors triggered a significant inflammatory response characterized by high levels of TNF-α, RANTES, MIP-1α, MIP-1β, MIP-2, and IP-10 mRNA expression (Fig. 2A). Consistent with these findings, at 7 days the livers of mice receiving vector HD-AdLacZ contained intense lymphocytic infiltrates that were CD3 positive, indicating a substantial number of T lymphocytes (Fig. 3D). Ad-specific CTL could also be detected at 7 days in mice receiving the vector HD-AdLacZ (data not shown). These results show that HD-AdLacZ induces a significant adaptive immune response similar to that induced by FG-Ad vectors.
FIG. 2.
Cytokine and chemokine gene expression 7 days following Ad vector administration. (A) RNase protection assay of total liver RNA 7 days following the intravenous administration of vehicle, HD-AdSEAP, AdSEAP, or HD-AdLacZ. (B) Chemokine mRNA expression in mouse liver following the administration of increasing titers of AdSEAP and HD-AdSEAP (RNase protection assay). Representative samples of at least three mice per group are shown. Part, particles.
FIG. 3.
Adaptive immune response to HD-Ad vectors. CD3 immunohistochemistry of mouse liver at 7 days following intravenous administration of vehicle (A), HD-AdSEAP (B), AdSEAP (C), and HD-AdLacZ (D). Panel E shows the results of a cytotoxic T-lymphocyte assay of splenocytes from mice receiving HD-AdSEAP, AdSEAP, or vehicle. Data shown are a representative sample of an experiment performed four times.
In addition to the Ad capsid, other factors may be contributing to the adaptive immune response induced by vector HD-AdLacZ. First, all HD-Ad vectors contain a small amount of helper virus contamination, and, second, the transgene may also be immunogenic, as has been demonstrated for β-galactosidase (25, 32). Finally, previous studies have suggested that vector promoters may influence host immune responses (21, 28). To address these potential confounding issues, we tested our HD-Ad vector expressing the nonimmunogenic transgene for HD-AdSEAP, driven by the ubiquitous mouse PGK promoter. An FG-Ad vector encoding the same expression cassette was used as a control (AdSEAP). DBA/2 mice were injected with 5 × 1010 particles of HD-AdSEAP (n = 5) or AdSEAP (n = 5), and livers were analyzed at 7 days. An RNase protection assay of liver RNA revealed a significant induction of chemokine and cytokine gene expression by AdSEAP, including TNF-α, RANTES, MIP-1α, MIP-1β, MIP-2, and IP-10 mRNA (Fig. 2A). Interestingly, the level of expression was less than that observed following a similar dose of HD-AdLacZ. The induction of chemokine and cytokine gene expression following AdSEAP administration was associated with CD3+ lymphocytic infiltrates in the liver at 7 days (Fig. 3C); however, compared to results with HD-AdLacZ, the extent of CD3+ hepatic inflammation was less. In contrast, animals that received HDAdSEAP completely lacked liver inflammation at 7 days (Fig. 2 and 3B). HD-AdSEAP-treated mice expressed no liver chemokine and cytokine genes, and, histologically, the livers were normal, with no CD3 detectable. To confirm that the second peak of inflammation was not simply delayed, mice receiving vector HD-AdSEAP were also analyzed at 14 days (n = 4). Again, livers were histologically normal, and no inflammatory gene expression could be detected (data not shown). The lack of a second peak of inflammation induced by HD-AdSEAP in this animal model suggests an attenuated adaptive immune response to this vector. To determine the extent of adaptive immune activation, the presence of Ad-specific CTL was examined in mice receiving vector HD-AdSEAP. At 7 days, Ad-specific CTL were easily detectable in mice receiving HD-AdSEAP, similar to the results with AdSEAP vector (Fig. 3E). Taken together, these results confirm that in the absence of an immunogenic transgene, HD-Ad vectors elicit adaptive immune responses that are attenuated compared to FG-Ad vector responses. Although these agents induce Ad-specific T-cell clones, viral gene expression is likely required to fully realize their effector functions in vivo.
HD-Ad vector transduction and transgene expression.
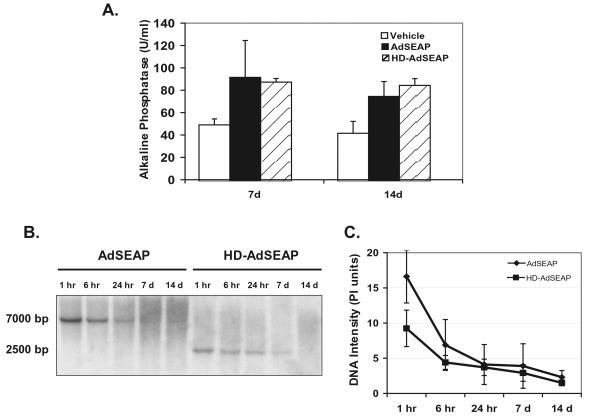
The induction of host innate and adaptive immune responses by Ad vectors is dose dependent (15, 22, 26). To confirm that the differences in host responses to vectors AdSEAP and HD-AdSEAP were not due to differences in transduction (i.e., the amount of vector that the liver received), transgene expression and vector genomes were measured. Serum alkaline phosphatase levels at 7 and 14 days were similar in animals receiving AdSEAP and HD-AdSEAP (AdSEAP versus HD-AdSEAP at 7 days: 92 ± 33 versus 88 ± 3 U/ml; P was not significant) (Fig. 4A). To further assess transduction, Ad vector genomes were measured in the liver by using Southern blot analysis (Fig. 4B). In the first 24 h following vector administration, AdSEAP and HD-AdSEAP genomes in the liver decreased rapidly. Compared to levels at 1 h, vector genomes at 24 h diminished more than 50% in animals receiving either vector AdSEAP or HD-AdSEAP. These results are consistent with the similar innate responses induced by these vectors. At 7 and 14 days, liver vector genomes were equivalent in animals receiving HD-AdSEAP and AdSEAP. Analysis of the vector genomes at 1 h consistently revealed higher genome levels in the livers of mice receiving AdSEAP than levels in mice receiving HD-AdSEAP, suggesting that the differences in host immunity triggered by these vectors may be due to differences in the administered titer. To further address this issue, mice were administered increasing titers of AdSEAP and HD-AdSEAP (7.5 × 1010, 10 × 1010, and 15 × 1010 particles per mouse) and the livers were analyzed at 7 days (n = 3). Increasing titers of AdSEAP induced chemokine gene expression in the liver in a dose-dependent manner. In contrast, increasing titers of HD-AdSEAP resulted in no inflammatory gene expression at 7 days, similar to the results for vehicle-treated mice (Fig. 2B). Histologically, the livers of mice receiving AdSEAP contained significant lymphocytic infiltrates. The higher dose of HD-AdSEAP did not induce any histological liver changes, consistent with the lack of inflammatory gene expression seen (data not shown). These results confirm that the reduced adaptive response seen in mice receiving HD-AdSEAP is not due to reduced liver transduction.
FIG. 4.
Transgene expression and persistence of HD-Ad vectors. (A) Serum alkaline phosphatase levels from mice receiving vehicle, AdSEAP, or HD-AdSEAP at 7 and 14 days (U/ml). (B) Southern analysis of total DNA isolated from mouse liver transduced with AdSEAP or HD-AdSEAP. (C) Quantitative analysis of liver vector genomes (mean ± standard deviation).
DISCUSSION
HD-Ad vectors represent a tremendous potential in gene therapy and provide many advantages over FG-Ad vectors. In this study, we characterized the host immune response to these agents. We show that, similar to FG-Ad vectors, HD-Ad vectors trigger innate immunity in vivo. This finding is consistent with studies that demonstrate a dependence on the adenoviral capsid or particle for this response (11, 24). Furthermore, our results show that in comparison to FG-Ad vectors, HD-Ad vectors induce attenuated adaptive immune responses in vivo. Therefore, it appears that HD-Ad vectors, while not completely devoid of immune-stimulating properties, provide an immunologic advantage over their predecessors.
The adaptive response to FG-Ad vectors has been well characterized (31, 33). The expression of viral genes still encoded within Ad vectors plays a significant role in mediating this response. A number of studies have examined adaptive immune responses to the adenoviral capsid or particle (7, 11, 22). Using UV-psoralen-inactivated Ad vectors, Ad-specific T-lymphocyte clones can be induced and detected in standard CTL assays (7, 11). Furthermore, Kafri et al. have suggested that, in muscle-directed gene therapy, the Ad particle can elicit persistent and late inflammation similar to a replication-competent FG vector (7). In studies employing liver-directed gene therapy, we have also detected anti-Ad CTL following systemic administration of UV-psoralen-inactivated FG-Ad vectors. However, late or persistent inflammation beyond the early innate response was not seen (11). In vitro, Roth et al. have shown that HD-Ad vectors transduce dendritic cells and stimulate Ad-specific T-cell responses. The dendritic cell-induced T-cell responses were mediated by the Ad capsid, independent of viral gene transcription (22). These studies suggest that HD-Ad vectors may not be dissimilar to FG-Ad vectors with respect to host adaptive immunity. The results in our study illustrate that the ongoing expression of viral genes is necessary to elicit an optimal adaptive immune response following liver transduction in vivo. While Ad-specific CTL are generated by HD-Ad vectors, the expression of viral genes is required for T cells to exert their effector functions in the liver. In immunologically naïve hosts, this finding possibly explains the vector persistence and improved transgene expression following transduction with HD-Ad vectors compared to results with Ad lacking E1, as has been observed in a number of studies (8, 23).
Immune responses to the transgene are well documented (25, 32). Numerous transgene products, including β-galactosidase, can elicit specific CTL responses in vitro and in vivo (6, 25, 32). Our results confirm that the transgene can clearly affect the immune properties of HD-Ad vectors. The expression of the immunogenic transgene β-galactosidase, in the context of an HD-Ad vector, enhanced the host response to the vector. Therefore, careful selection of the transgene and promoter within the expression cassette is essential to realize the immunologic benefit of HD-Ad vectors. Helper virus contamination did not play a significant role in enhancing host immunity to HD-AdLacZ. All HD-Ad vector systems are contaminated with helper virus (16, 19), but our results suggest that this contamination is not relevant at low vector titers. Since both vectors HD-AdSEAP and HD-AdLacZ contained similar amounts of helper contamination, the complete lack of inflammation induced by HD-AdSEAP at 7 and 14 days rules out helper virus as the cause of the inflammatory response to HD-AdLacZ. This finding is consistent with studies showing the dose-dependent nature of the immune response to Ad vectors (15, 22, 26). This study, however, cannot estimate the impact of helper contamination following very high dose HD-Ad vector administration.
HD-Ad vectors stimulated innate responses in this study that were similar to those stimulated by FG-Ad vectors. This is not unexpected since the innate response to Ad vectors has clearly been shown to be dependent on the viral capsid or particle (11, 24). HD-Ad vectors are packaged in an intact viral particle; thus, innate responses remained unaltered. This finding has several implications. First, since the innate response can induce acute inflammation, it is possible that at high titers, HD-Ad vectors may induce significant toxicity, as has been observed for Ad lacking E1 (15, 24). Although liver toxicity was not observed in this study due to the low Ad vector titers used, recently Brunetti-Pierri and colleagues reported significant toxicity in nonhuman primates following the administration of high-dose HD-Ad vectors (2a). Second, as seen by others and us, the innate response clears Ad vectors very efficiently from transduced tissues (30). The loss of HD-Ad vectors in the first 24 h mirrored that seen with FG-Ad vectors. Therefore, reduced gene transfer efficiency can be expected in various applications employing these agents.
This study adds to our understanding of the host immune response to HD-Ad vectors in vivo. In immunologically naïve hosts, HD-Ad vectors provide an immunological advantage over FG vectors, eliciting an attenuated adaptive immune response. This finding is consistent with prior studies demonstrating improved transgene expression and reduced toxicity following HD-Ad vector-mediated gene transfer. However, problems involving the innate immune system still exist and remain the final hurdle to designing nonimmunogenic Ad vectors. To improve the safety and overall effectiveness of Ad vectors for human gene therapy, future studies should focus on the biology of the adenoviral capsid and its interaction with the innate immune system.
Acknowledgments
We thank Frank L. Graham for critical evaluation of the manuscript.
This research was supported by funds provided by the Alberta Heritage Foundation for Medical Research (D.A.M.), Canadian Institutes of Health Research (CIHR) (D.A.M. and R.J.P.), CIHR/Muscular Dystrophy Association of Canada/Amyotrophic Lateral Sclerosis Society of Canada Partnership Grant (R.J.P.), Premier's Research Excellence Award (R.J.P.), CIHR Institute Strategic Initiative (R.J.P). D.A.M. and R.J.P. are recipients of CIHR New Investigator Awards. P.J.R. is supported by a Natural Sciences and Engineering Research Council (NSERC) scholarship.
REFERENCES
- 1.Amalfitano, A., and R. J. Parks. 2002. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr. Gene Ther. 2:111-133. [DOI] [PubMed] [Google Scholar]
- 2.Bowen, G. P., S. L. Borgland, M. Lam, T. A. Libermann, N. C. Wong, and D. A. Muruve. 2002. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum. Gene Ther. 13:367-379. [DOI] [PubMed] [Google Scholar]
- 2a.Brunetti-Pierri, N., D. J. Palmer, A. L. Beaudet, K. D. Carey, M. Finegold, and P. Ng. 2004. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 15:35-46. [DOI] [PubMed] [Google Scholar]
- 3.Chartier, C., E. Degryse, M. Gantzer, A. Dieterle, A. Pavirani, and M. Mehtali. 1996. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decker, T., and M. L. Lohmann-Matthes. 1988. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 115:61-69. [DOI] [PubMed] [Google Scholar]
- 5.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jooss, K., H. C. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72:2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kafri, T., D. Morgan, T. Krahl, N. Sarvetnick, L. Sherman, and I. Verma. 1998. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc. Natl. Acad. Sci. USA 95:11377-11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, I. H., A. Jozkowicz, P. A. Piedra, K. Oka, and L. Chan. 2001. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA 98:13282-13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, Y., D. A. Muruve, R. G. Collins, S. S. Lee, and P. Kubes. 2002. The role of selectins and integrins in adenovirus vector-induced neutrophil recruitment to the liver. Eur. J. Immunol. 32:3443-3452. [DOI] [PubMed] [Google Scholar]
- 10.Liu, Q., and D. A. Muruve. 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10:935-940. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Q., A. K. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. J. Wickham, and D. A. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14:627-643. [DOI] [PubMed] [Google Scholar]
- 12.McCoy, R. D., B. L. Davidson, B. J. Roessler, G. B. Huffnagle, S. L. Janich, T. J. Laing, and R. H. Simon. 1995. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum. Gene Ther. 6:1553-1560. [DOI] [PubMed] [Google Scholar]
- 13.Morral, N., R. J. Parks, H. Zhou, C. Langston, G. Schiedner, J. Quinones, F. L. Graham, S. Kochanek, and A. L. Beaudet. 1998. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum. Gene Ther. 9:2709-2716. [DOI] [PubMed] [Google Scholar]
- 14.Morsy, M. A., M. Gu, S. Motzel, J. Zhao, J. Lin, Q. Su, H. Allen, L. Franlin, R. J. Parks, F. L. Graham, S. Kochanek, A. J. Bett, and C. T. Caskey. 1998. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 95:7866-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 16.Ng, P., C. Beauchamp, C. Evelegh, R. Parks, and F. L. Graham. 2001. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 3:809-815. [DOI] [PubMed] [Google Scholar]
- 17.Ng, P., C. Evelegh, D. Cummings, and F. L. Graham. 2002. Cre levels limit packaging signal excision efficiency in the Cre/loxP helper-dependent adenoviral vector system. J. Virol. 76:4181-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks, R. J., J. L. Bramson, Y. Wan, C. L. Addison, and F. L. Graham. 1999. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J. Virol. 73:8027-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks, R. J., and F. L. Graham. 1997. A helper-dependent system for adenovirus vector production helps define a lower limit for efficient DNA packaging. J. Virol. 71:3293-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastore, L., N. Morral, H. Zhou, R. Garcia, R. J. Parks, S. Kochanek, F. L. Graham, B. Lee, and A. L. Beaudet. 1999. Use of a liver-specific promoter reduces immune response to the transgene in adenoviral vectors. Hum. Gene Ther. 10:1773-1781. [DOI] [PubMed] [Google Scholar]
- 22.Roth, M. D., Q. Cheng, A. Harui, S. K. Basak, K. Mitani, T. A. Low, and S. M. Kiertscher. 2002. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J. Immunol. 169:4651-4656. [DOI] [PubMed] [Google Scholar]
- 23.Schiedner, G., N. Morral, R. J. Parks, Y. Wu, S. C. Koopmans, C. Langston, F. L. Graham, A. L. Beaudet, and S. Kochanek. 1998. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18:180-183. [DOI] [PubMed] [Google Scholar]
- 24.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 25.Song, W., H. L. Kong, P. Traktman, and R. G. Crystal. 1997. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenoviral vectors. Hum. Gene Ther. 8:1207-1217. [DOI] [PubMed] [Google Scholar]
- 26.Thomas, C. E., D. Birkett, I. Anozie, M. G. Castro, and P. R. Lowenstein. 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3:36-46. [DOI] [PubMed] [Google Scholar]
- 27.Umana, P., C. A. Gerdes, D. Stone, J. R. Davis, D. Ward, M. G. Castro, and P. R. Lowenstein. 2001. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 19:582-585. [DOI] [PubMed] [Google Scholar]
- 28.Van Linthout, S., D. Collen, and B. De Geest. 2002. Effect of promoters and enhancers on expression, transgene DNA persistence, and hepatotoxicity after adenoviral gene transfer of human apolipoprotein A-I. Hum. Gene Ther. 13:829-840. [DOI] [PubMed] [Google Scholar]
- 29.Van Linthout, S., M. Lusky, D. Collen, and B. De Geest. 2002. Persistent hepatic expression of human apo A-I after transfer with a helper-virus independent adenoviral vector. Gene Ther. 9:1520-1528. [DOI] [PubMed] [Google Scholar]
- 30.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 31.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 32.Yang, Y., K. U. Jooss, Q. Su, H. C. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3:137-144. [PubMed] [Google Scholar]
- 33.Yang, Y., F. A. Nunes, K. Berencsi, E. E. Furth, E. Gonczol, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91:4407-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 35.Zou, L., H. Zhou, L. Pastore, and K. Yang. 2000. Prolonged transgene expression mediated by a helper-dependent adenoviral vector (hdAd) in the central nervous system. Mol. Ther. 2:105-113. [DOI] [PubMed] [Google Scholar]