Abstract
The human immunodeficiency virus (HIV) protein Nef has been shown to increase the infectivity of HIV at an early point during infection. Since Nef is known to interact with proteins involved in actin cytoskeleton rearrangements, we tested the possibility that Nef may enhance HIV infectivity via a mechanism that involves the actin cytoskeleton. We find that disruption of the actin cytoskeleton complements the Nef infectivity defect. The ability of disruption of the actin cytoskeleton to complement the Nef defect was specific to envelopes that fuse at the cell surface, including a variety of HIV envelopes and the murine leukemia virus amphotropic envelope. In contrast, the infectivity of HIV virions pseudotyped to enter cells via endocytosis, which is known to complement the HIV Nef infectivity defect and can naturally penetrate the cortical actin barrier, was not altered by actin cytoskeleton disruption. The results presented here suggest that Nef functions to allow the HIV genome to penetrate the cortical actin network, a known barrier for intracellular parasitic organisms.
The human immunodeficiency virus (HIV) accessory protein Nef is a small, myristoylated protein of ∼27 kDa with at least three separate activities (reviewed in reference 5). First, Nef is known to deregulate cellular signaling pathways (42). Second, Nef down regulates the cell surface expression of cellular proteins, including CD4 and major histocompatibility complex class I proteins (3, 40). Last, Nef has been shown to increase the infectivity of HIV type 1 (HIV-1) virions (4, 31, 39). While the mechanisms through which Nef stimulates cellular signaling pathways and alters the cell surface expression of cellular proteins have been established, the mechanism by which Nef enhances the infectivity of virions remains enigmatic. It is known that the Nef-mediated increase in infectivity is manifested at an early point in infection prior to the completion of reverse transcription (4, 39). Nef has been shown to be incorporated into virions (8, 12, 48, 49) via an N-terminal bipartite membrane-targeting signal (8, 12, 48). While the number of Nef molecules incorporated into virions is small (∼10 molecules/virion [49]), disruption of this membrane-targeting signal eliminates the incorporation of Nef into virions and the ability of Nef to increase the infectivity of virions (4, 48), suggesting that incorporation into virions is required for Nef to increase the infectivity of virions. Once in the virion, numerous reports have indicated that Nef is cleaved by the viral protease (8, 12, 38, 49), although it does not appear that cleavage by the viral protease is required for Nef to increase the infectivity of virions (12, 30).
A number of reports have found that pseudotyping virions with pH-dependent envelope proteins, such as that of vesicular stomatitis virus (VSV) or Ebola virus reduces or eliminates the infectivity defect of virions lacking a functional Nef protein (2, 11, 26). However, the infectivity enhancement of virions with a functional Nef protein is conserved when HIV-1 virions are pseudotyped with the pH-independent envelope protein of amphotropic murine leukemia virus (MLV), which fuses at the plasma membrane (4). Therefore, the mechanism by which Nef enhances infectivity does not appear to involve or specifically require the HIV envelope protein, while the stage of infection at which Nef enhances infectivity can be bypassed when virions infect cells following endocytosis via a pH-dependent envelope protein.
Numerous reports indicate that the host cell actin cytoskeleton plays a role in the entry steps of the infectious pathway utilized by HIV-1. Actin microfilaments have been suggested to facilitate receptor clustering during virion fusion to the host cell (20, 46). Other studies suggest that the establishment of a functional reverse transcription complex involves the actin cytoskeleton (9). Live-cell microscopy has observed HIV-1 virions trafficking in a microtubule-independent manner in the periphery of the cytoplasm after entry into the cytoplasm. This movement is likely due to an interaction with the actin cytoskeleton in the host cell cytoplasm (29).
Nef is one viral protein that could participate in viral interaction with the actin cytoskeleton early in infection. Nef has been shown to colocalize with actin in cells and form noncovalent high-molecular-weight complexes with actin in B cells and T cells (14). Nef has also been reported to interact with a number of proteins and molecules associated with actin microfilament reorganization. Specifically, Nef associates with and activates the Rho GTPase exchange factor Vav, inducing cytoskeletal rearrangements in cells (15). Additionally, Nef also associates with and activates a member of the p21-activated kinase (PAK) family (25, 27, 35). PAK proteins are serine/threonine kinases known to mediate actin cytoskeletal organization and rearrangement in mammalian cells (13, 41). Moreover, the regions of Nef required for its association with Vav (15) and PAK (50) have been shown to be within the SH3 binding domain and other elements of the folded core domain of the protein. This region has also been identified as the region required for Nef's ability to increase infectivity (18). Nef has also been shown to associate with the viral core (16, 22), consistent with the notion that Nef may facilitate a postfusion trafficking of the core via a mechanism that involves the actin cytoskeleton.
Given the potential of Nef to influence the actin cytoskeleton during infection, we examined the effect of actin filament depolymerization on the infectivity of wild-type (WT) virions and virions lacking a functional Nef protein (ΔNef). We reasoned that if Nef were enhancing the infectivity of HIV-1 virions through an interaction with the actin cytoskeleton, agents that disrupt the actin cytoskeleton should prevent Nef from improving the infectivity of virions. Instead, we found that disruption of the actin cytoskeleton had the opposite effect, complementing the infectivity of ΔNef virions to levels comparable to WT HIV controls receiving the same drug treatment. This was true for two HIV strains and both CXCR4- and CCR5-tropic envelopes. The ability of actin cytoskeletal disruption to complement ΔNef virions was specific to pH-independent envelopes that are believed to fuse at the cell surface. In contrast, the infectivity of HIV virions pseudotyped with the VSV-g envelope protein was not altered by actin cytoskeleton disruption. Complementation was also specific to the ΔNef virions because HIV virions impaired in infectivity by treatment with cyclosporine A (CsA) were not complemented by actin cytoskeleton disruption. Further, the WT and ΔNef virions were found to fuse with target cells at similar levels, and cytoskeletal disruption did not significantly alter the levels of virion fusion. The results presented here, along with the known ability of pH-dependent envelopes to complement the Nef infectivity defect suggest that Nef functions to allow the HIV genome to penetrate the cortical actin network which lies directly below the plasma membrane and is a known barrier for a number of intracellular parasitic organisms.
MATERIALS AND METHODS
Cell culture and virus production.
Magi+/+ (21), GHOST (32), and human osteosarcoma cell (HOS) CD4 (23) cells were cultured in complete Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum, penicillin, and streptomycin. ΔNef versions of HIV strains LAI and NL43 were generated by removing the unique XhoI site by filling in 5′ overhangs with Klenow fragment and religation. Virus was produced by CaPO4 transfection of 293T cells with 12 μg of WT or ΔNef proviral constructs. Two days following transfection, virus was collected, passed through a 0.45-μm-pore-size filter, and normalized for viral content using a p24 enzyme-linked immunosorbent assay kit (Perkin-Elmer). Amphotropic MLV, VSV-g, and JRFL envelope pseudotyped virions were produced in similar fashion following cotransfection of 4 μg of WT LAI and LAIΔNef proviral constructs in which the env gene has been deleted with 20 μg of the indicated envelope expression plasmids. Vpr-β-lactamase virions were produced by cotransfection of 293T cells with 10 μg of Vpr-β-lactamase expression plasmid with 10 μg of LAI or LAIΔNef proviral construct.
Infection assays.
Magi+/+ cells were plated the day prior to infection at 10,000 cells per well in 96-well plates. One hour prior to infection, cells were treated with 3 μM cytochalasin D (CytD) (Sigma), 5 μM latrunculin B (LatB) (Sigma), or 125 nM jasplakinolide (Jas) (Fisher). These concentrations were determined by titrating each drug to determine the minimal concentration that had the maximal ability to stimulate the infectivity of ΔNef virions. Drugs were added to virus stocks at the same concentration as in the pretreatment and infected 12 to 14 h overnight. The medium was replaced with DMEM plus 200 μM zidovudine (Sigma) immediately following virus removal. At 36 h postinfection (p.i.), cells were assayed for β-galactosidase (β-Gal) expression using either a liquid or fixed cell β-Gal assay. For the liquid assay, cells were lysed in sodium phosphate buffer with 0.2% Triton X-100, and β-Gal expression was assayed by monitoring cleavage of the colorimetric β-Gal substrate o-nitrophenyl-β-d-galactopyranoside (ONPG) using a 96-well microplate reader measuring absorbance at 405 nm. The average background absorbance of more than three uninfected wells was then subtracted from the values of infected wells prior to analysis. For the foci assay, cells were fixed 36 h p.i. in 1% formaldehyde with 0.2% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) for 90 min in 4 mM potassium ferrocyanide-4 mM potassium ferricyanide at 37°C (21). Results are the averages ± standard deviations of three infections. Data are representative of at least three experiments.
GHOST cell assays were performed in 12-well dishes containing 80,000 cells/well as described above. At 12 to 14 h after infection, virus was replaced with 1 μM AMD3100 (AIDS Reagent Program) to prevent formation of syncytium. At 36 h p.i., cells were harvested by treating the cells with trypsin (Invitrogen), fixed in 1% paraformaldehyde in phosphate-buffered saline, and analyzed for green fluorescent protein (GFP) expression using a FACSCalibur flow cytometer (Becton Dickinson).
Microscopy.
Magi+/+ cells were transfected in six-well plates with 0.5 μg of yellow fluorescent protein (YFP)-actin expression plasmid (Clontech) using Effectene transfection reagent (Qiagen) according to the manufacturer's protocol. At 24 h posttransfection, cells were plated on ΔT dishes (Bioptechs, Butler, Pa.) and allowed to adhere overnight. During imaging, chamber and objective heaters were used to maintain the temperature of DMEM, which was supplemented with 50 mM HEPES (pH 7.5) at 37°C. Images were captured in a z-series on a charge-coupled device digital camera mounted on a DeltaVision system. Out-of-focus light was digitally removed using Softworks deconvolution software. Volume projections were generated using Softworks software. The effects of photobleaching were minimized using the Equalize Time Points Softworks function (Applied Precision, Inc).
Real-time PCR.
The day prior to infection, target cells were plated in 12-well plates at 100,000 cells/well. Target cells were pretreated and infected as described above. At 12 h p.i., cells were harvested by treating the cells with trypsin. Following a 5-min incubation with RNase A (2 mg/ml) to remove residual viral RNA, genomic DNA was purified using a DNeasy tissue kit (Qiagen) according to the manufacturer's instructions. Genomic samples were normalized for DNA content and then digested with DpnI for 2 h to remove residual plasmid DNA. Proviral DNA present in each infection was quantitated in triplicate using the primers 5′-GAGTCCTGCGTCGAGAGAGC-3′ and TGTGTGCCCGTCTGTTGTGT, which are specific for HIV late reverse transcription products using an Icycler (Bio-Rad) and Syber green PCR core reagents (Perkin-Elmer Biosystems) according to the manufacturer's instructions. The proviral DNA calculated in each sample was then normalized to the amount of β-actin genomic DNA observed in the same digest to calculate the number of proviral genomes present per cell. Standard curves were generated using dilutions of LAI and human β-actin plasmid DNA to allow quantitation of proviral DNA per cell. The error bars represent the standard errors of the means of the number of proviral genomes calculated following actin normalization. Data are representative of at least three experiments.
Virion fusion assays.
HOS CD4 cells were plated at 40,000 cells/well in a 24-well plate 6 to 12 h prior to infection. Cells were pretreated as described above for the infection assays; cells were pretreated for 1 h prior to infection and infected for 3 h with equivalent amounts of LAI or LAIΔNef virions in the presence of drug. The medium was then replaced with DMEM lacking phenol red and fetal bovine serum but containing penicillin, streptomycin, glutamine, 50 mM HEPES (pH 7.5), and CCF2 Gene Blazer loading solution (Aurora Biosciences, San Diego, Calif.) prepared according to the manufacturer's protocol for 14 h at room temperature. Cells were then harvested by treatment with trypsin and resuspended in 1% paraformaldehyde. Cleavage of the CCF2 substrate was measured using a three-laser FACSVantage flow cytometer (Becton Dickinson). Data are representative of at least three experiments.
RESULTS
Actin depolymerization complements the infectivity defect of HIV-1 virions lacking Nef.
To test a possible relationship between HIV Nef and the cytoskeleton, we determined the infectivity of WT and ΔNef HIV in a reporter cell line after treatment with two drugs known to disrupt the actin cytoskeleton, CytD and LatB. Treatment with CytD causes a collapse of the actin cytoskeleton, potentially by disrupting interaction of the barbed end of actin filaments with the plasma membrane (47). In contrast, LatB binds to monomeric actin and prevents repolymerization of filamentous actin during normal actin turnover. The reporter cell line, Magi+/+, was utilized to quantitate single-cycle infectivity. Magi+/+ cells are HeLa cells that stably express CD4 and CCR5 on the cell surface and contain a stably integrated copy of the β-Gal gene under the control of the HIV-1 long terminal repeat (LTR) (21). After integration, the HIV transactivator protein Tat activates β-Gal expression, allowing quantification of infection.
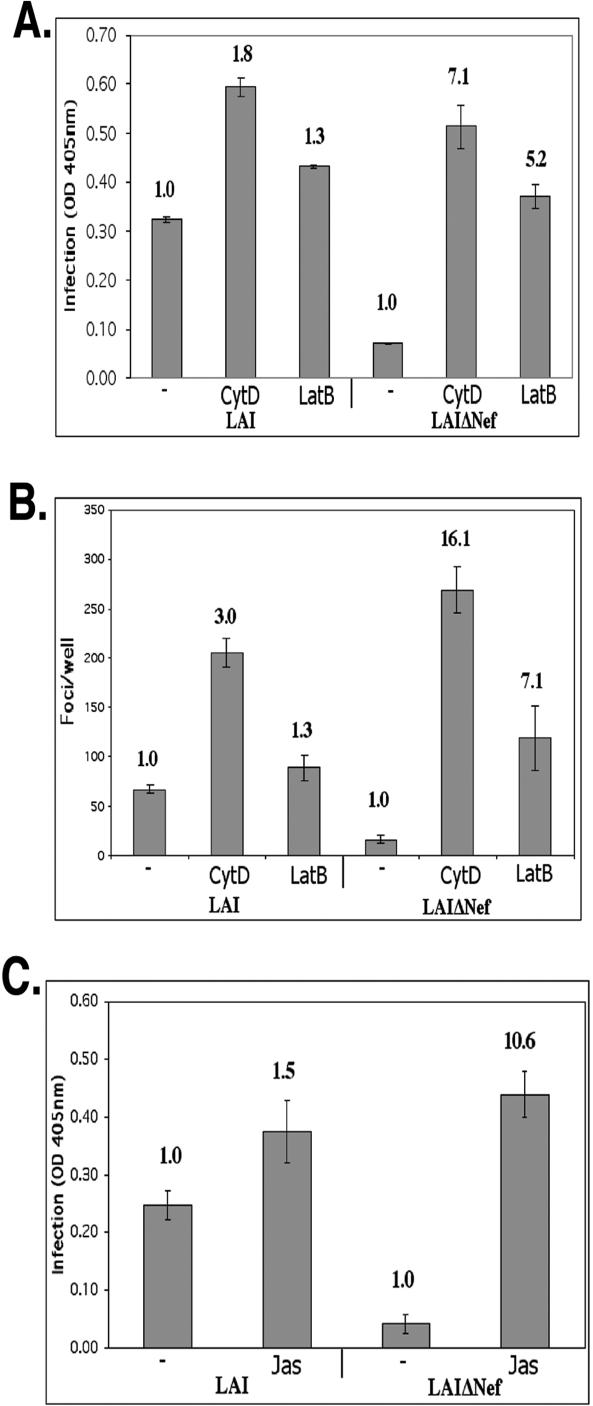
Comparison of WT and ΔNef derivatives of the HIV strain LAI revealed that the ΔNef HIV was 7.8-fold (±0.8-fold) (n = 20) less infectious than WT LAI in repeated experiments using this reporter cell line after a single cycle of infection with viral supernatants normalized to p24, consistent with previous reports (4) (Fig. 1A). When the infection was performed in the presence of CytD, the infectivity of the ΔNef virus became comparable to that of the WT virus (Fig. 1A). Likewise, treatment with LatB also appeared to relieve the ΔNef phenotype relative to WT HIV (Fig. 1A). It is important to note that treatment with both drugs caused a modest increase in infection of the WT virus. However, comparing the fold difference relative to untreated virus revealed that the disruption of the actin cytoskeleton had a much greater stimulatory effect on the infectivity of ΔNef HIV compared to the infectivity of WT HIV (Fig. 1A and Table 1). In these studies, treatment with CytD consistently had a greater stimulatory effect on infectivity relative to treatment with LatB for both WT and ΔNef virions (Fig. 1A and Table 1). Interestingly, although treatment with CytD had a greater stimulatory effect, treatment with either CytD or LatB complemented ΔNef virion infectivity to a similar degree. When compared to fold stimulation of ΔNef infectivity relative to the infectivity of WT virus, the fold stimulation of the ΔNef virus was 4.2-fold (±0.6-fold) and 4.2-fold (±0.3-fold) greater than the observed WT stimulation, for CytD and LatB, respectively. The ability of CytD and LatB to complement Nef function was detected over a range of viral dilutions, ensuring that the differential enhancement of WT and ΔNef virions observed was not a result of complications involving a multiplicity of infection (data not shown).
FIG. 1.
Actin depolymerization complements the infectivity defect of ΔNef virions. Magi+/+ cells were infected in the presence of 3 μM CytD, 5 μM LatB, or dimethyl sulfoxide without drug (−) as a negative control. Infection was quantified using a liquid β-Gal assay (A) or by fixing infected cells and counting the number of β-Gal-positive infected foci (B). (C) Cells were infected in the presence of 125 nM Jas or in the absence of Jas (−) as a negative control, and β-Gal expression was quantified using a liquid β-Gal assay. Bold numbers above the bars represent the fold increase in infection relative to the no-drug infection (−) (negative control). OD 405 nm, optical density at 405 nm.
TABLE 1.
Average enhancement in infectivity and average increase in reverse transcription of virions caused by three drugs
| Virus | Fold infectivity enhancementa
|
Fold reverse transcription enhancementb
|
||||
|---|---|---|---|---|---|---|
| CytD | LatB | Jas | CytD | LatB | Jas | |
| LAI | 1.97 ± 0.21 (16) | 1.24 ± 0.16 (14) | 1.29 ± 0.32 (4) | 4.18 ± 0.81 (5) | 2.33 ± 0.45 (5) | 1.25 ± 0.30 (3) |
| LAIΔNef | 7.48 ± 0.93 (16) | 5.18 ± 0.72 (14) | 9.47 ± 2.36 (4) | 11.72 ± 1.22 (5) | 5.60 ± 0.98 (5) | 7.34 ± 1.46 (3) |
| LAI VSV | 0.93 ± 0.30 (3) | 0.71 ± 0.13 (3) | 0.585 ± 0.14 (4) | ND | ND | ND |
| LAIΔNef VSV | 1.42 ± 0.34 (3) | 1.59 ± 0.37 (3) | 0.878 ± 0.21 (4) | ND | ND | ND |
Average fold enhancement observed in the presence of the indicated drug relative to control infection. The number of infections (n) is shown in parentheses and is similar to the infections shown in Fig. 1A and C. Data are means ± standard errors of the means obtained from averaging n experiments.
Average increase in late reverse transcription products per cell calculated in the presence of the indicated drug relative to control infection. The number of infections (n) is shown in parentheses and is similar to the infections shown in Fig. 3A and C. Data are means ± standard errors of the means obtained from averaging n experiments. ND, not determined.
To ensure that the increase in infectivity we observed in response to actin depolymerization was not a result of an increase in LTR activity or differences in syncytium formation in response to actin-depolymerizing agents, individual infected cells were stained for β-Gal expression and quantified by direct counting. No difference in syncytium size or β-Gal expression was observed in response to drug treatment (data not shown). Quantification of a representative experiment is shown in Fig. 1B. The ability of the actin cytoskeleton-disrupting drugs to complement the Nef infectivity defect was observed in both a lysed-cell assay, which gives the cumulative stimulatory effect on the entire culture, and a direct quantification of individual β-Gal-positive cells. To extend these observations, we assayed the effect of another drug known to alter the normal actin cytoskeleton, Jas. Jas has been reported to stabilize actin microfilaments by binding filamentous actin (7). Treatment with Jas, like CytD and LatB, caused a specific increase in the infectivity of ΔNef HIV relative to WT HIV as shown in Fig. 1C. The stimulatory effect of treatment with CytD, LatB, and Jas was specific to cytoskeleton structure and dynamics, rather than the function of an actin-based motor, because treatment with ML-7, an inhibitor of actin-based motor function (36), had no effect on virus infectivity in the studies presented here (data not shown) The phosphatidylinositol 3-kinase inhibitor LY294002 also had no differential effect on the infectivity of WT versus ΔNef virions, reducing the infectivity of both viruses to a similar degree (data not shown).
One of the known aspects of the ability of HIV Nef to enhance HIV infectivity is that the stimulatory effect is variable, depending on virus preparation and target cells (4, 31). To address potential variability, we determined the average stimulatory effect that the three drugs that modify the actin cytoskeleton had on multiple preparations of virions in numerous liquid β-Gal infection assays relative to untreated virions. This analysis reveals the reproducibility of the ability of the three compounds to complement the lack of Nef protein in HIV infectivity (Table 1).
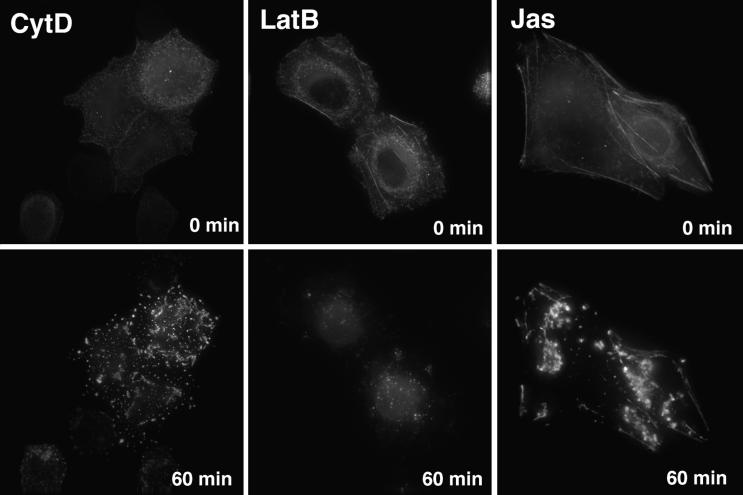
The ability of all three drugs to complement the Nef effect was confounding because while CytD and LatB disrupt the actin cytoskeleton, Jas has been reported to stabilize actin filaments (7). Therefore, to gain insights into how drug treatment was altering the actin cytoskeleton of the target cells, we directly observed the consequences of drug treatment on the reporter cells by deconvolution microscopy. To observe the dynamics of the actin cytoskeleton over time, target cells were transfected with a YFP-actin fusion protein. Prior to the addition of drug, filamentous actin was present in the form of stress fibers located on the ventral side of the cells as well as in a layer of cortical actin, including microvilli and other cell surface projections, located below the plasma membrane on the dorsal, medium-exposed side of the adherent, transfected cells (Fig. 2). As seen in Fig. 2, CytD induced the rapid collapse of actin filament structures into punctate actin foci (movie 1 at website http://www.uic.edu/depts/mcmi/hope/movies). LatB also induced the rapid disappearance of cellular microfilaments (Fig. 2) (movie 2 at website given above). Compared to the collapse of the actin cytoskeleton induced by CytD, LatB treatment caused the actin cytoskeleton to essentially dissolve. In contrast, Jas caused a localized aggregation of the cortical actin cytoskeleton while having less effect on stress fibers (Fig. 2F) (movie 3 at website given above), consistent with recent reports (24, 34, 43). Therefore, although all three drugs affect actin microfilament dynamics by separate mechanisms, all three induce the disruption of cortical actin structures and similarly induce an increase in the infectivity of LAIΔNef virions.
FIG. 2.
CytD, LatB, and Jas disrupt cortical actin structures. Magi+/+ cells expressing a YFP-actin fusion protein were imaged before (0 min) and after a 60-min treatment with 3 μM CytD, 5 μM LatB, or 125 nM Jas.
Disruption of the actin cytoskeleton complements the reverse transcription defect in HIV-1 virions lacking Nef.
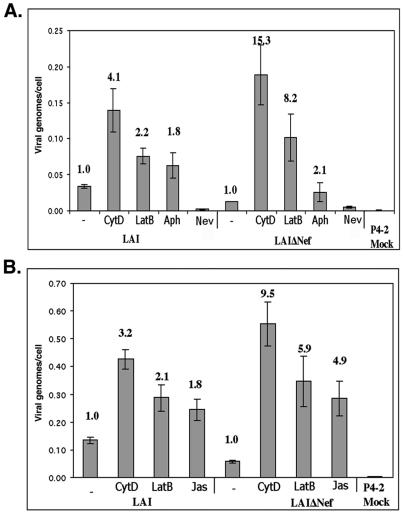
Virions lacking HIV Nef are known to have a defect in reverse transcription (4, 39). The studies described above revealed that disruption of the actin cytoskeleton can largely complement the infectivity defect of virions lacking HIV Nef. We surmised that if treatment of target cells with either CytD, LatB, or Jas was complementing Nef function, treatment with these compounds should rescue the reverse transcription defect of ΔNef virions. Therefore, quantitative PCR for HIV late reverse transcription products was performed on target cell DNA samples following infection with equivalent amounts of WT and ΔNef HIV-1. A control sample treated with the agent aphidicholine (Aph), which prevents cell division by blocking DNA replication, was included to account for the reduced proliferation of cells treated with CytD or LatB. Genomic DNA was harvested, and the number of viral DNA genomes per cell was measured following infections in the presence or absence of CytD or LatB. As previously reported, ΔNef virions were deficient in the formation of late reverse transcription products in the absence of drug (Fig. 3). Treatment with Aph showed an approximately twofold increase in late reverse transcription products of both WT and ΔNef virions after normalization to cellular actin, indicating that the cells in the untreated culture divided on average once during the 12-h experiment (Fig. 3A). However, infection in the presence of CytD or LatB led to similar levels of late reverse transcription products for both WT and ΔNef virions (Fig. 3). Jas also complemented the ΔNef defect in production of late reverse transcription products (Fig. 3B). A mock infection (no virus) and nevirapine, a reverse transcription inhibitor, were included as controls to ensure that the PCR products being measured were a result of proviral DNA generated following infection (Fig. 3A). Therefore, disrupting actin structures in target cells complements the known defect in the production of reverse transcription products by ΔNef virions.
FIG. 3.
Actin depolymerization complements the reverse transcription defect of ΔNef virions. (A) Magi+/+ cells were infected in the presence of 3 μM CytD, 5 μM LatB, 5 μg of Aph per ml, or 10 μM nevirapine (Nev), or in the absence of drug (−) as a negative control. (B) Magi+/+ cells were infected in the presence of 3 μM CytD, 5 μM LatB, or 125 nM Jas or in the absence of drug (−) as a negative control. Genomic DNA was isolated from the target cells, and the number of viral genomes present in each samples was calculated. This value was normalized to the number of genomic actin copies present in an identical sample to calculate the number of late reverse transcription products per cell. Bold numbers above the bars represent the fold increase in infection relative to infection in the absence of drug (−).
The infectivity enhancement of HIVΔNef virions in response to actin depolymerization occurs independently of coreceptor tropism and in multiple viral strains.
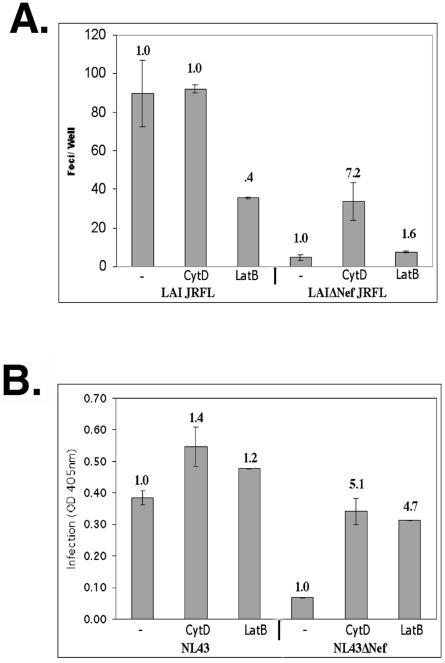
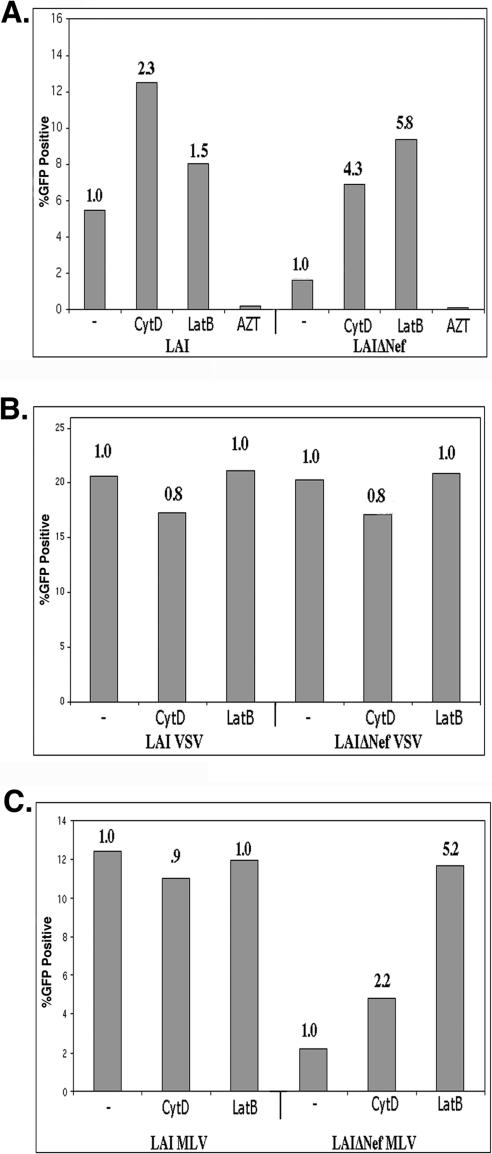
To determine whether the improved infectivity of LAIΔNef virions in response to actin depolymerization was specific to entry mediated by the CXCR4 coreceptor, we pseudotyped the envelope-deficient (ΔEnv) versions of WT and ΔNef LAI virions with the CCR5-tropic JRFL envelope protein. When reporter cells were infected with these virions in the presence or absence of CytD or LatB, the WT virions were again differentially affected by the drugs (Fig. 4A). Unlike CXCR4-tropic HIV, LatB treatment inhibited the infectivity of the WT CCR5-tropic virus by more than 50% (Fig. 4A). In contrast, LatB treatment did not markedly alter the infectivity of the ΔNef CCR5-tropic HIV (Fig. 4A). In contrast, CytD treatment had no effect on the infectivity of the WT CCR5-tropic HIV, while stimulating the infectivity of the CCR5-tropic ΔNef HIV sevenfold (Fig. 4A). Using pseudotyped virions of this type, actin depolymerization with CytD never fully complemented the infectivity defect of ΔNef virions, although the fold enhancement observed was similar to stimulation seen in similar experiments using CXCR4 virus (Fig. 1B). It should be noted that in the absence of actin-depolymerizing agents, the infectivity defect of JRFL pseudotyped virions lacking Nef was more substantial (15- to 20-fold) than WT virions expressing endogenous CXCR4-specific envelope.
FIG. 4.
Actin depolymerization complements the infectivity defect of ΔNef virions independently of coreceptor tropism and in other viral strains. (A) Cells were infected with equivalent amounts of WT and ΔNef viruses in which the envelope has been deleted that were pseudotyped with the JRFL envelope protein in the presence of 3 μM CytD or in the absence of drug (−) as a negative control. Infection was measured 36 h p.i. using a fixed cell β-Gal assay. (B) Cells were also infected with equivalent amounts of NL43 and NL43ΔNef in the presence of 3 μM CytD, 5 μM LatB, or dimethyl sulfoxide (negative control) (−). Infection was assayed using a liquid β-Gal assay. OD 405nm, optical density at 405 nm.
We also examined the ability of actin depolymerization to specifically enhance the infectivity of other HIV strains lacking a functional Nef protein. For these studies, the strain NL43 was utilized. As shown in Fig. 4B, WT NL43 was approximately sixfold more infectious than its ΔNef counterpart. The infectivity of NL43ΔNef virions was enhanced in response to actin depolymerization (Fig. 4B), suggesting that this increased infectivity in response to actin depolymerization is not strain specific.
The infectivity of virions produced in CsA is not specifically enhanced in response to actin depolymerization.
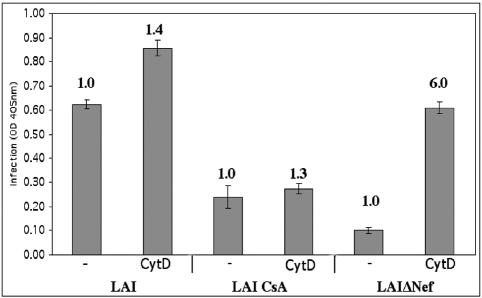
HIV-1 virions produced in the presence of CsA are less infectious than WT virions. This reduced infectivity of CsA-treated virions is due to CsA binding the cellular protein cyclophilin A (CpA), preventing CpA from associating with the viral core and enhancing infection (17). This reduced infectivity is also complemented to near WT levels by pseudotyping virions with the VSV-g envelope protein (2). This phenotype is very similar to that seen for HIV lacking Nef. We therefore asked whether actin cytoskeleton disruption can also increase the infectivity of virions produced in 1 μg of CsA per ml. As expected, equivalent amounts of virions produced in the presence of CsA were less infectious than WT virus (Fig. 5). CytD treatment minimally increased the infectivity of HIV produced in the presence of CsA to a degree similar to the enhancement observed for WT virions, while the infectivity of the LAIΔNef virus was enhanced to a greater degree (Fig. 5), as seen in previous experiments. Therefore, actin depolymerization specifically complements the infectivity defect of ΔNef virions but does not complement the infectivity defect of virions produced in the presence of CsA.
FIG. 5.
Actin depolymerization does not complement the infectivity defect of virions produced in the presence of CsA. Cells were infected with equivalent amounts of WT virus, ΔNef virus, or WT virus produced in 1 μM CsA. Cells were infected in the presence of 3 μM CytD or in the absence of drug (−) as a negative control. Infection was quantified using a liquid β-Gal assay. OD 405nm, optical density at 405 nm.
The ability of the disruption of the actin cytoskeleton to stimulate the infectivity of ΔNef HIV is dependent on the mode of viral entry.
To determine whether actin depolymerization complemented the infectivity defect of ΔNef virions in other cell lines, we utilized another reporter cell line, GHOST, developed from human osteosarcoma cells (HOS cells). GHOST cells express CD4 and CXCR4 and have a stably integrated LTR-GFP construct that causes the Tat-dependent expression of GFP following infection. Infected cells can be identified by flow cytometry. As shown in Fig. 6A, treatment with CytD and LatB complemented the Nef defect in GHOST cells. This result demonstrates that the ability of the disruption of the actin cytoskeleton to complement the infectivity defect of ΔNef HIV is not specific to HeLa cells.
FIG. 6.
Actin depolymerization complements the infectivity defect of ΔNef HIV-1 virions in GHOST cells. GHOST cells were infected in duplicate in the presence of 3 μM CytD or 5 μM LatB or in the absence of drug (−) as a negative control. Cells were fixed in 1% paraformaldehyde and analyzed for GFP expression following infection with WT LAI and LAIΔNef virions (A) or Env-deficient versions of WT and ΔNef virions pseudotyped with VSV-g envelope protein (B) or amphotropic MLV envelope protein (C). The bars represent the mean percentages of infected cells of duplicate infections determined by analyzing more than 5,000 cells/infection.
It has been previously reported that the ability of Nef to enhance the infectivity of HIV is dependent on the pathway of entry of HIV cores. The infectivity of HIV virions pseudotyped with pH-dependent envelopes that enter the cell via receptor-mediated endocytosis is not enhanced by the presence of Nef (2, 11). In contrast, the infectivity of HIV virions pseudotyped with amphotropic MLV envelope, which is pH independent and fuses at the cell surface, can be enhanced by the presence of Nef (4). As previously reported (4), the presence of Nef had no stimulatory effect on HIV pseudotyped with VSV-g (Fig. 6B). Furthermore, treatment with CytD or LatB had no effect on the infectivity of VSV-g pseudotyped HIV (Fig. 6B). Similar results were obtained using Magi+/+ reporter cells as targets (Table 1 and data not shown). In contrast, actin depolymerization specifically enhanced the infectivity of ΔNef virions pseudotyped with the amphotropic MLV envelope protein (Fig. 6C). We find that in the absence of drug, the WT clone pseudotyped with MLV amphotropic envelope is 3.5-fold (±0.71-fold) (n = 4) more infectious than its ΔNef counterpart, consistent with previous reports (4). However, actin depolymerization specifically increased the infectivity of the ΔNef HIV. For amphotropic MLV envelope pseudotyped HIV, treatment with LatB complemented Nef, while treatment with CytD, had a partial stimulatory effect (Fig. 6C).
The infectivity defect of LAIΔNef virions is manifested at a postfusion step in infection.
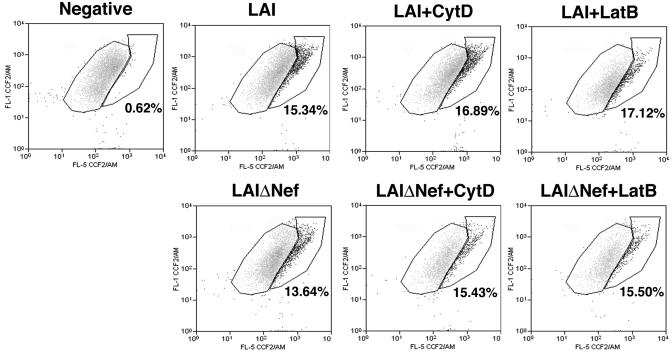
Actin has been implicated at two events occurring early in HIV infection, namely, fusion (20, 46) and postfusion trafficking events (9, 29). We therefore measured the ability of WT and ΔNef HIV to fuse with target cells using the recently developed Vpr-β-lactamase entry assay (10, 33). This assay measures fusion of target cells with virions which have incorporated the Vpr-β-lactamase fusion protein produced in 293T cells. Target cells are loaded with a fluorescent substrate of β-lactamase that changes its fluorescent emission following cleavage by the enzyme, which occurs only after productive entry of viral cores into the cytoplasm (10). This shift in fluorescence is observed using flow cytometry. Using this assay, a similar amount of viral fusion was measured when HOS CD4 target cells were incubated for 4 h with equal amounts of either WT or ΔNef virions (Fig. 7), as recently reported (44). Moreover, viral fusion of either clone did not change significantly when target cells were infected in the presence of CytD or LatB. These results reveal that the increased infectivity of ΔNef virions in response to actin depolymerization is manifested at a postfusion point early in the HIV life cycle.
FIG. 7.
LAI and LAIΔNef HIV-1 virions productively enter cells at similar levels and in the presence or absence of actin-depolymerizing agents. HOS CD4 cells were infected for 3 h with equivalent amounts of LAI or LAIΔNef virus containing the Vpr-β lactamase fusion protein in the presence of 3 μM CytD or 5 μM LatB or in the absence of drug as a negative control. Fusion was measured as cleavage of CCF2 substrate as previously described (10). The percent fusion is indicated by the bold number below the positive fusion population. Data are representative of at least three independent experiments.
DISCUSSION
The mechanism of how the HIV protein Nef facilitates the infectivity of HIV has remained elusive. Two facts are clear. Nef acts at a point prior to the completion of reverse transcription (4, 39), and it is possible to complement HIV lacking the Nef protein by pseudotyping with viral envelopes that direct entry via a pH-dependent endocytosis pathway (2, 11, 26). We report here an additional way to complement Nef function by disrupting the normal composition of the actin cytoskeleton. This complementation can be accomplished in most instances using three different drugs: CytD, LatB, and Jas. While CytD and LatB act to disrupt both the cortical actin network and stress fibers, Jas acts primarily on the cortical actin network while leaving some stress fibers intact, as has been previously reported (24, 34, 43) and confirmed by the time-lapse studies presented in Fig. 2. The ability of all three drugs to replace the need for Nef suggests that it is the cortical actin barrier, rather than general actin depolymerization or prevention of actin polymerization, that is important for complementation. Although there is always a concern of pleotropic effects when using drugs that have such a large effect on cellular function, since drug treatment is not inhibitory, but rather stimulatory in this case, such concerns are minimal. Therefore, it is likely that interaction between Nef and the cortical actin network is key in the ability of Nef to enhance HIV infectivity.
In the studies presented here, treatment with either CytD, LatB, or Jas stimulated HIV infection of both WT virions and Nef-deficient virions. However, the stimulatory effect on the ΔNef HIV was much greater than that seen for WT HIV. The difference in the stimulation of infectivity relating to actin disruption was clear when either the absolute value of infection or the fold increase in infectivity after drug treatment was considered. As seen in Fig. 1, disruption of the actin cytoskeleton with CytD or LatB stimulated infectivity of the ΔNef HIV to infectivity levels similar to that seen for the WT HIV after treatment. This observation is striking, considering the fact that the WT virus was eight times more infectious than ΔNef HIV in the absence of drug treatment. This was true if infectivity was assayed using a liquid β-Gal assay (Fig. 1A) or by counting infected-cell foci (Fig. 1B). The same was seen after treatment with Jas (Fig. 1C). Given the known variability of the importance of Nef for HIV infectivity, we compared the average fold stimulation in infectivity of ΔNef HIV and WT HIV, as quantified with the liquid β-Gal assay, after treatment with each of the three drugs (Table 1). In multiple experiments, both CytD and LatB increased the infectivity of ΔNef HIV fourfold more than the stimulatory effect of Nef-containing HIV. Jas treatment consistently had the greatest stimulatory effect on ΔNef HIV, increasing infectivity sevenfold relative to stimulation of WT HIV.
The increased stimulatory effect of cortical actin disruption on ΔNef HIV was observed in two different reporter cell lines, which demonstrate differential sensitivity to the presence of Nef. For example, the presence of Nef increased HIV infectivity two- to fourfold in the human osteosarcoma-based reporter compared to a six- to eightfold stimulatory effect in the Magi+/+ HeLa cell-based reporter cell line. Likewise, pseudotyping with VSV-g was able to complement ΔNef HIV to WT levels in the human osteosarcoma-based reporter cell line, while VSV-g pseudotyping only partially complemented ΔNef HIV in the HeLa cell-based reporter cell line, as previously reported (26). It is possible that subtle differences in the cortical actin cytoskeleton between these two cell lines reflect this differential sensitivity to the action of Nef.
The ability of cortical actin cytoskeletal disruption to complement ΔNef HIV infectivity appears to be specific to the action of Nef. Specificity is demonstrated by the inability of cortical actin disruption to complement CsA-treated virions and HIV pseudotyped with VSV-g. A previous report found that, similar to Nef-deficient virions, it is possible to complement the infectivity defect of HIV treated with CsA by pseudotyping with VSV-g (2), suggesting a possible relationship between the mechanism of the enhancement of HIV infectivity by Nef and the presence of CpA in virions. However, as shown in Fig. 5, it is not possible to complement the defect in HIV infectivity caused by treatment with CsA by disruption of the actin network with CytD. This result suggests that the mechanism used by Nef to enhance HIV infectivity is distinct from the enhancement derived from the presence of CpA in the virion, as has been previously reported (1). Consistent with this interpretation, it has recently been reported that CpA incorporation is involved with masking a determinant in HIV Gag that is the target of innate antiviral factors present in certain target cells (45).
Additional support for the interpretation that actin cytoskeleton disruption is specifically complementing Nef comes from analysis of HIV pseudotyped with VSV-g or the Ebola virus envelope protein. In this context, Nef is no longer needed for maximal infectivity (2, 11) (Fig. 6B), and actin cytoskeleton disruption does not have a stimulatory effect on HIV infectivity pseudotyped with VSV-g (Fig. 6B). However, Nef does increase virion infectivity when virion entry is mediated by the pH-independent amphotropic MLV envelope protein (4) (Fig. 6C), which fuses at the cell surface. Moreover, infection by ΔNef virions pseudotyped with this envelope protein is stimulated by actin cytoskeletal disruption, suggesting that it is the mode of entry, not the envelope protein itself, that allows Nef to increase infectivity and actin depolymerization to complement this Nef-mediated increase.
Consistent with this notion, the ability of cortical actin cytoskeleton disruption to complement Nef-deficient HIV is independent of envelope tropism, complementing LAIΔNef possessing both CCR5- and CXCR4-dependent envelopes, and is seen for the several different virus isolates tested (Fig. 4). However, the effect of drug treatment on CCR5-tropic HIV was more complex than seen for the three CXCR4-tropic envelopes tested. While drug treatment increased the infectivity of CXCR4-tropic HIV and complemented the Nef-deficient CXCR4-tropic HIV (Fig. 1), it either had no effect or inhibited the WT CCR5-tropic WT HIV (Fig. 4A). However, drug treatment differentially affected the Nef-deficient CCR5-tropic HIV relative to WT HIV. The result was a decrease in the infectivity defect of ΔNef CCR5-tropic HIV relative to WT HIV (Fig. 4A). This differential effect is likely similar to previous reports that found that disruption of the actin cytoskeleton inhibits HIV infectivity (9, 20). However, recently it has been reported that that the inhibitory effect of CytD on HIV fusion depends on the density of receptors and coreceptors on the target cell (46). Inhibition was observed only when receptors and coreceptors were expressed at low levels. Therefore, cell lines which overexpress receptor are useful tools to dissect the role of the actin cytoskeleton in postfusion events during infection.
It has previously been suggested that Nef-deficient virions are less infectious because of decreased efficiency of fusion with target cells (51). However, using a new assay which directly monitors the fusion step of HIV entry, as shown in Fig. 7, we demonstrate that fusion of HIV virions is the same in the presence or absence of Nef. This result is consistent with a recent report (44). Further, CytD and LatB had little effect on efficiency of fusion using the Vpr-β-lactamase assay as shown in Fig. 7, consistent with other findings (46). Therefore, drug treatment is not influencing fusion, but some postfusion step in the HIV life cycle. After the membrane barrier, the potentially dense cortical actin barrier likely forms an obstacle. The results presented here also suggest new insights into the cascade of events leading to the formation of the HIV provirus. Since fusion is normal, yet reverse transcription does not efficiently generate late reverse transcription products, perhaps the cytoplasmic complex must pass through the cortical actin barrier before reverse transcription can proceed normally. One obvious downstream event is engagement of microtubules. We have previously reported that evidence of reverse transcription can be detected in viral complexes on the microtubules (29). If microtubule engagement facilitates reverse transcription, it would be consistent with the Nef phenotype of a defect in reverse transcription because a HIV core trapped in the cortical actin meshwork would not interact with microtubules.
The possibility of cortical actin structures acting as a barrier to viral infection has also been suggested by the work of Marsh and Bron, who noted a cell type-specific, postfusion block to infection by Semliki forest virus (SFV) in CHO cells following forced fusion of the pH-dependent SFV envelope at the plasma membrane (28). They speculated that this barrier to infection is due to cortical actin structures in CHO cells (28). This would be similar to larger intracellular pathogens that have a variety of mechanisms they utilize to breach the cortical actin barrier. For instance, Salmonella injects bacterial proteins into host cells at the site of invasion, such as SopE, leading to cortical actin cytoskeleton reorganization facilitating productive crossing of the cortical actin barrier (reviewed in references 6 and 19). SopE acts as a highly active Rho GTPase exchange factor, and this exchange factor activity is thought to facilitate entry of the bacterium into the host cell by inducing local remodeling of the host cell actin cytoskeleton. In light of this fact, the finding that Nef binds and activates Vav, a cellular Rho GTPase exchange factor, and induces cytoskeletal rearrangements in cells (15) represents a very plausible mechanism as to how Nef might allow the virus to circumvent the barrier to infection posed by the cortical actin cytoskeleton.
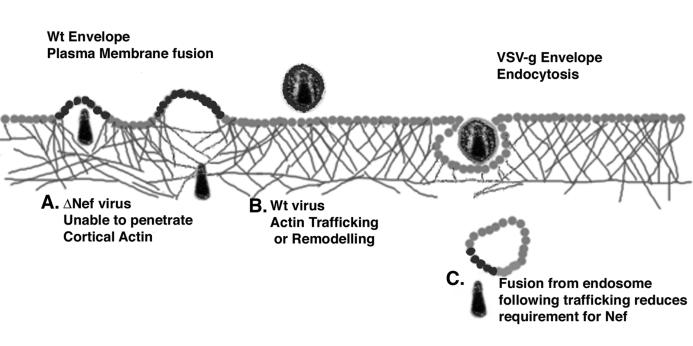
The results presented here suggest the following model for the role of Nef in facilitating HIV reverse transcription and infectivity (Fig. 8). Following fusion at the plasma membrane, Nef acts to induce local actin remodeling to facilitate the movement of the viral core past a potentially obstructive cortical actin barrier. This is likely accomplished through known interactions of Nef and proteins involved in actin cytoskeleton dynamics, such as Vav and PAK. In the absence of actin reorganization, the HIV core released into the cytoplasm could be caught in the cortical actin meshwork and not be allowed to proceed deeper into the cytoplasm to complete reverse transcription. This model also explains the previously enigmatic observation that pseudotyping HIV virions with pH-dependent envelopes reduces or eliminates the infectivity-enhancing properties of Nef. Infection with a pH-dependent envelope occurs only after endocytosis of the virion through normal cellular pathways (2, 4, 11). Using this path, the endosome that normally must traverse the cortical actin barrier would relieve the need for Nef by depositing the HIV core into the cytoplasm after crossing the barrier (Fig. 8). Likewise, HIV infection via fusion at the plasma membrane with amphotropic MLV envelope is enhanced by the presence of Nef (Fig. 6C) (4). This model is also consistent with the partial defect associated with the infectivity of Nef-deficient virions. A minority of fusion events would take place in areas where the cortical actin barrier is minimal. HIV cores entering the cells in these areas can proceed into the cytoplasm and through the HIV life cycle without blockage. This model may also help explain the results of Schaeffer et al. (37). They found that WT virions entered the cytoplasm of target cells at a higher frequency than their ΔNef counterparts. Although this result appears inconsistent with our results and those of others (44) indicating that ΔNef virions fuse with target cells at similar levels, this apparent contradiction could easily be explained if ΔNef virions were trapped in cortical actin structures. The cell fractionation protocol used by Schaeffer et al. to purify cytoplasmic fractions would have removed the cytoskeletal fraction during purification, thus leading to a decreased amount of ΔNef virions in the cytoplasmic fraction. Furthermore, since the extent of the cortical actin meshwork varies as a result of different culture conditions of a single cell line and varies for different cell lines, this model is also consistent with the known variability of the Nef infectivity defect (4, 31) Therefore, this model for the function of HIV Nef in infectivity is able to account for the known characteristics associated with Nef-deficient HIV.
FIG. 8.
Model for Nef's infectivity enhancement of HIV-1 virions. Following fusion at the plasma membrane, viral cores encounter and must traverse a dense layer of cortical actin capable of hindering virion mobility. (A) Depending on the local actin environment encountered after fusion, a great number of ΔNef virions may be unable to traverse this barrier. (B) WT virions rely on the ability of Nef to alter the host cell actin cytoskeleton following fusion and are therefore more likely to cause infection. Following depolymerization of the actin cytoskeleton of the target cell, both WT and ΔNef virions have similar infectivity. (C) This model also explains why HIV-1 virions pseudotyped with pH-dependent envelope protein no longer require Nef for maximal infectivity. Trafficking of the endosome induced by the pH-dependent envelope would complement Nef-deficient virions, since the endosome would be trafficked past cortical actin structures by cellular endocytic machinery.
Acknowledgments
HOS CD4 and GHOST cells were provided by Ned Landau. We thank the contributors to the NIH AIDS Reagent Program for materials, specifically Michael Emerman for Magi+/+ cells and NIAID for AMD3100. We thank Jenny Anderson and Christopher O'Connor for thoughtful suggestions regarding the manuscript.
This work was supported in part by NIH grant R01-AI47770 to T.J.H.
REFERENCES
- 1.Aiken, C. 1998. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology 248:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 4.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, J. L., and T. J. Hope. 2003. Recent insights into HIV accessory proteins. Curr. Infect. Dis. Rep. 5:439-450. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri, J. T., M. J. Riese, and K. Aktories. 2002. Bacterial toxins that modify the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 18:315-344. [DOI] [PubMed] [Google Scholar]
- 7.Bubb, M. R., A. M. Senderowicz, E. A. Sausville, K. L. Duncan, and E. D. Korn. 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269:14869-14871. [PubMed] [Google Scholar]
- 8.Bukovsky, A. A., T. Dorfman, A. Weimann, and H. G. Gottlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 71:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinskaya, A., B. Brichacek, A. Mann, and M. Stevenson. 1998. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 188:2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 11.Chazal, N., G. Singer, C. Aiken, M. L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Y. L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 72:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 14.Fackler, O. T., N. Kienzle, E. Kremmer, A. Boese, B. Schramm, T. Klimkait, C. Kucherer, and N. Mueller-Lantzsch. 1997. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur. J. Biochem. 247:843-851. [DOI] [PubMed] [Google Scholar]
- 15.Fackler, O. T., W. Luo, M. Geyer, A. S. Alberts, and B. M. Peterlin. 1999. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3:729-739. [DOI] [PubMed] [Google Scholar]
- 16.Forshey, B. M., and C. Aiken. 2003. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J. Virol. 77:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruenheid, S., and B. B. Finlay. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 20.Iyengar, S., J. E. Hildreth, and D. H. Schwartz. 1998. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J. Virol. 72:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotov, A., J. Zhou, P. Flicker, and C. Aiken. 1999. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 73:8824-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landau, N. R., and D. R. Littman. 1992. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J. Virol. 66:5110-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, E., E. A. Shelden, and D. A. Knecht. 1998. Formation of F-actin aggregates in cells treated with actin stabilizing drugs. Cell Motil. Cytoskeleton 39:122-133. [DOI] [PubMed] [Google Scholar]
- 25.Linnemann, T., Y. H. Zheng, R. Mandic, and B. M. Peterlin. 2002. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294:246-255. [DOI] [PubMed] [Google Scholar]
- 26.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241:224-233. [DOI] [PubMed] [Google Scholar]
- 27.Manninen, A., M. Hiipakka, M. Vihinen, W. Lu, B. J. Mayer, and K. Saksela. 1998. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology 250:273-282. [DOI] [PubMed] [Google Scholar]
- 28.Marsh, M., and R. Bron. 1997. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110:95-103. [DOI] [PubMed] [Google Scholar]
- 29.McDonald, D., M. A. Vodicka, G. Lucero, T. M. Svitkina, G. G. Borisy, M. Emerman, and T. J. Hope. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, M. D., M. T. Warmerdam, S. S. Ferrell, R. Benitez, and W. C. Greene. 1997. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology 234:215-225. [DOI] [PubMed] [Google Scholar]
- 31.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morner, A., A. Bjorndal, J. Albert, V. N. Kewalramani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou, G. S., Z. L. Chen, and M. Yuan. 2002. Jasplakinolide reversibly disrupts actin filaments in suspension-cultured tobacco BY-2 cells. Protoplasma 219:168-175. [DOI] [PubMed] [Google Scholar]
- 35.Renkema, G. H., A. Manninen, and K. Saksela. 2001. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nck or β-PIX. J. Virol. 75:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders, L. C., F. Matsumura, G. M. Bokoch, and P. de Lanerolle. 1999. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283:2083-2085. [DOI] [PubMed] [Google Scholar]
- 37.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schorr, J., R. Kellner, O. Fackler, J. Freund, J. Konvalinka, N. Kienzle, H. G. Krausslich, N. Mueller-Lantzsch, and H. R. Kalbitzer. 1996. Specific cleavage sites of Nef proteins from human immunodeficiency virus types 1 and 2 for the viral proteases. J. Virol. 70:9051-9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz, O., V. Marechal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 41.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 42.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 43.Simpson-Holley, M., D. Ellis, D. Fisher, D. Elton, J. McCauley, and P. Digard. 2002. A functional link between the actin cytoskeleton and lipid rafts during budding of filamentous influenza virions. Virology 301:212-225. [DOI] [PubMed] [Google Scholar]
- 44.Tobiume, M., J. E. Lineberger, C. A. Lundquist, M. D. Miller, and C. Aiken. 2003. Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J. Virol. 77:10645-10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 46.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. W. Ruscetti, J. M. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakatsuki, T., B. Schwab, N. C. Thompson, and E. L. Elson. 2001. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 114:1025-1036. [DOI] [PubMed] [Google Scholar]
- 48.Welker, R., M. Harris, B. Cardel, and H. G. Krausslich. 1998. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J. Virol. 72:8833-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welker, R., H. Kottler, H. R. Kalbitzer, and H. G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219:228-236. [DOI] [PubMed] [Google Scholar]
- 50.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224:292-301. [DOI] [PubMed] [Google Scholar]
- 51.Zheng, Y. H., A. Plemenitas, C. J. Fielding, and B. M. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA 100:8460-8465. [DOI] [PMC free article] [PubMed] [Google Scholar]