Abstract
In this study, we have used immunoprecipitation and mass spectrometry to identify over 50 cellular and viral proteins that are associated with the herpes simplex virus 1 (HSV-1) ICP8 single-stranded DNA-binding protein. Many of the coprecipitating cellular proteins are known members of large cellular complexes involved in (i) DNA replication or damage repair, including RPA and MSH6; (ii) nonhomologous and homologous recombination, including the catalytic subunit of the DNA-dependent protein kinase, Ku86, and Rad50; and (iii) chromatin remodeling, including BRG1, BRM, hSNF2H, BAF155, mSin3a, and histone deacetylase 2. It appears that DNA mediates the association of certain proteins with ICP8, while more direct protein-protein interactions mediate the association with other proteins. A number of these proteins accumulate in viral replication compartments in the infected cell nucleus, indicating that these proteins may have a role in viral replication. WRN, which functions in cellular recombination pathways via its helicase and exonuclease activities, is not absolutely required for viral replication, as viral yields are only very slightly, if at all, decreased in WRN-deficient human primary fibroblasts compared to control cells. In Ku70-deficient murine embryonic fibroblasts, viral yields are increased by almost 50-fold, suggesting that the cellular nonhomologous end-joining pathway inhibits HSV replication. We hypothesize that some of the proteins coprecipitating with ICP8 are involved in HSV replication and may give new insight into viral replication mechanisms.
Herpes simplex virus 1 (HSV-1) is a large, double-strandedDNA virus that replicates in the host cell nucleus. HSV encodes over 80 gene products that contribute to viral replication in either cultured cells or animal hosts (76). Due to the limited size of the HSV-1 genome, the virus cannot code for every function required for its propagation; thus, HSV-1 must rely upon factors supplied by the host cell for replication. For example, HSV exclusively uses the host cell RNA polymerase II for the transcription of viral genes (4, 16). The exact number and identity of the cellular factors required for HSV replication is unknown, but the identification of such factors is an active area of research as it may shed light on mechanisms of viral replication, the cellular process, or the factor itself. It is this concept that induced us to identify cellular proteins that associate with HSV-1 ICP8.
The HSV-1 single-stranded DNA-binding protein, ICP8, is a 128-kDa multifunctional zinc metalloprotein (31, 37) encoded by the UL29 gene (61). ICP8, in concert with the other HSV DNA replication proteins, including the helicase-primase complex (UL5, UL8, UL52), the origin-binding protein (UL9), and the polymerase holoenzyme (UL30/UL42), is required for viral DNA synthesis (11, 12). While the seven HSV DNA replication proteins are known, it is currently unclear as to what host proteins are involved in viral DNA replication. In addition to its role in DNA synthesis, ICP8 has been shown to affect viral transcription in at least two ways: (i) by repressing transcription from input parental viral genomes (33-35) and (ii) by stimulating late gene transcription (32).
ICP8 and a number of other viral proteins, including the aforementioned viral replication proteins, the major viral transactivator ICP4, the immediate-early protein ICP27, and the major capsid protein VP5 accumulate within intranuclear structures referred to as replication compartments (9, 19, 47, 59, 71, 73). Many of the processes required for viral replication, including viral DNA synthesis (18, 71, 74), viral transcription (47, 57, 71, 74, 75), virion assembly, and DNA packaging (19, 51, 93, 96), occur within replication compartments. Because numerous viral processes take place in replication compartments, it is expected that cellular proteins that are required for viral replication may accumulate there as well. Indeed, the host cell RNA polymerase II is redistributed to replication compartments during HSV infection (57, 71, 75). Other proteins such as p53 and the cellular single-stranded DNA-binding protein replication protein A (RPA) have been observed in replication compartments (95), but their role in viral replication remains unknown.
We hypothesized that host proteins that coprecipitate with viral proteins in replication compartments might be cellular proteins that play a role in viral replication. We thought that ICP8 was a good candidate for this analysis because it is highly expressed in infected cells and it is believed to interact with multiple cellular or viral complexes to mediate its various functions during infection. Here, we report the identification of numerous cellular proteins that coprecipitate with ICP8, which suggests that they may have a functional role in HSV replication.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) and human epidermoid (HEp-2) cells obtained from the American Type Culture Collection (Manassas, Va.) were grown and maintained in Dulbecco's modified Eagle's medium (DMEM; Media Tech Inc., Herndon, Va.) supplemented with 5% fetal bovine serum (Gibco, Carlsbad, Calif.)-5% bovine calf serum (HyClone, Logan, Utah), streptomycin (100 μg/ml), and penicillin (100 U/ml) (DMEM-10% fetal calf serum [FCS]). V529 cells (17) were grown in DMEM-10% FCS supplemented with G418 (400 μg/ml). Normal and Ku70-deficient murine embryonic fibroblasts (MEFs) (36), kindly provided by David Sinclair, Harvard Medical School, Boston, Massachusetts, were grown and maintained in DMEM-10% FCS. Normal (number AG14591) and WRN-deficient (number AG00780H) primary human fibroblasts obtained from the Coriell Institute for Medical Research (Camden, N.J.) were grown and maintained in modified Eagle's medium (MEM) with Earle's balanced salt solution supplemented with 15% heat-inactivated fetal bovine serum, a 2× concentration of MEM essential and nonessential amino acids (Gibco), and vitamins (Gibco).
The HSV-1 wild-type (wt) KOS strain was propagated and assayed on Vero cells. The HSV-2-derived dl5 and dl29 mutant strains were propagated on V529 cells as previously described (17).
Antibodies.
The anti-HSV ICP8 39S mouse monoclonal (81) and 3-83 rabbit polyclonal (47) antibodies were described previously. The anti-RPA p70-9 mouse monoclonal antibody (23) was kindly provided by Bruce Stillman, Cold Spring Harbor Laboratory. The anti-HSV ICP4 4040II rabbit polyclonal antibody was a gift from Kent Wilcox, Medical College of Wisconsin. Paul Olivo, Washington University School of Medicine, provided the anti-HSV UL5 R252 rabbit polyclonal antibody (67). The anti-HSV ICP27 mouse monoclonal antibody H1119 was purchased from the Rumbaugh-Goodwin Institute for Cancer Research (Plantation, Fla.). The following antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.): rabbit polyclonal antibodies to BAF155 (H-76), hSNF2H (H-300), Rad50 (H-300), BRG1 (H-88), histone deacetylase 2 (HDAC2) (H-54), BLM (H-300), BRCA1 (C-20), Ku86 (H-300), MSH2 (N-20), mSin3a (K-20), and WRN (H-300) and mouse monoclonal antibodies to BRM (E-1) and the DNA-dependent protein kinase (DNA-PK) catalytic subunit (DNA-PKcs) (Ab-2 and G4). As expected, not all of the antibodies or antisera performed equally well for all assays; therefore, we have included results of certain assays for only some of the antibodies.
Viral infections and immunoprecipitations.
HEp-2 cells in 15-cm tissue culture dishes were mock infected or infected with wt HSV-1 at a multiplicity of infection (MOI) of 20 PFU per cell. At 6.5 h postinfection, approximately 5 × 107 cells were washed twice with cold phosphate-buffered saline (PBS) and then harvested by scraping into PBS. The cells were collected by centrifugation at 4°C and transferred to microcentrifuge tubes for further preparation. The cells were incubated on ice for 30 min in 0.5 ml of immunoprecipitation (IP)buffer (120 mM potassium-acetate, 20 mM Tris-acetate [pH 7.9], 5 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 0.1% Nonidet P-40, and 1 Complete protease inhibitor cocktail tablet [Roche Applied Science, Indianapolis, Ind.] per 25 ml). Ethidium bromide (EtBr) was added to some samples at this step and was maintained at a concentration of 20 or 50 μg/ml throughout the entire IP process, including wash steps. The cellular lysates were sonicated for 5 s and then clarified by centrifugation at 4°C in an Eppendorf microcentrifuge for 10 min. IP was performed by the addition of 5 μl of the 39S monoclonal antibody to 0.5 ml of the cellular lysate with gentle agitation at 4°C for 45 min. Protein G-coated paramagnetic beads (Dynal Biotech, Oslo, Norway) were added, and the lysates were incubated with gentle agitation for an additional 45 min at 4°C. After 4 washes with IP buffer, the precipitates were boiled in sample buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
SDS-PAGE and Western blotting.
Proteins in the IPs were separated on 8 to 10% polyacrylamide gels. The proteins were visualized by using a colloidal blue staining kit (Invitrogen, Carlsbad, Calif.) according to manufacturer's instructions. For immunoblotting, the proteins were transferred to a polyvinylidene difluoride membrane (Perkin-Elmer Life Sciences, Boston, Mass.) by electroblotting and the membranes were blocked in 5% milk in Tris-buffered saline with Tween 20 (TBST; 20 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.1% Tween 20), probed with the indicated primary antibody in TBST, and visualized by enhanced chemiluminescence (Perkin-Elmer Life Sciences). The anti-ICP8 3-83, anti-UL5 R252, anti-ICP4 4040II, and anti-ICP27 H1119 antibodies were used at a dilution of 1:1,000. All other antibodies were used at a dilution of 1:200. The goat anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used at a dilution of 1:10,000. The immunoblots were stripped (4% SDS, 62.5 mM Tris-HCl [pH 6.8], 100 mM 2-mercaptoethanol) at 55°C for 45 min before reprobing.
Mass spectrometry.
Bands excised from colloidal blue-stained gels were analyzed at the Taplin Biological Mass Spectrometry (MS) Facility, Harvard Medical School, by microcapillary liquid chromatography-tandem MS with a LCQ DECA ion-trap mass spectrometer (Thermo Finnigan, San Jose, Calif.).
Indirect immunofluorescence.
For indirect immunofluorescence, HEp-2 cells plated on 12-mm coverslips in a 24-well tissue culture plate were mock infected or infected with wt HSV-1 at an MOI of 20. At 6.5 h postinfection, the cells were rinsed in cold PBS and then fixed for 10 min in 3.7% formaldehyde in PBS. After washing with PBS for 5 min, the cells were subsequently permeabilized with −20°C acetone for 2 min. The cells were rinsed briefly in distilled water and then PBS for 5 min. Primary antibodies were then applied for 30 min in a humid chamber at 37°C. After a 5-min wash in PBS, the secondary antibodies were applied for 30 min in a humid chamber at 37°C. After washing in PBS and rinsing in distilled water, the coverslips were mounted for observation. The anti-ICP8 39S monoclonal and the 3-83 polyclonal antibodies were used at a dilution of 1:200. All other primary antibodies were used at a dilution of 1:30. Fluorescein isothiocyanate or rhodamine-conjugated secondary antibodies (Cappel, ICN, Irvine, Calif.) were used at a dilution of 1:200.
Measurements of viral growth kinetics.
Cells were infected at the indicated MOI with wt HSV-1. At 1 h postinfection, the virus was removed and the cells were washed twice (135 mM NaCl, 10 mM KCl, 40 mM sodium-citrate, pH 3.0) for 30 s before being overlaid with fresh medium. At 2 or 24 h postinfection, the cells were harvested by scraping into the medium and frozen at −80°C. After thawing, the lysate was sonicated for 15 s on ice. The viral yield was determined by titration on Vero cells.
Viral recombination assay.
Normal or WRN-deficient human fibroblasts were infected either singly or doubly with dl5 or dl29. At 24 h postinfection, the virus was harvested and titered on Vero and V529 cells. Recombination frequency was determined as the percentage of viral progeny that contained wt alleles of UL5 and UL29 and could form plaques on Vero cells.
RESULTS
Coprecipitation of cellular and viral proteins with ICP8.
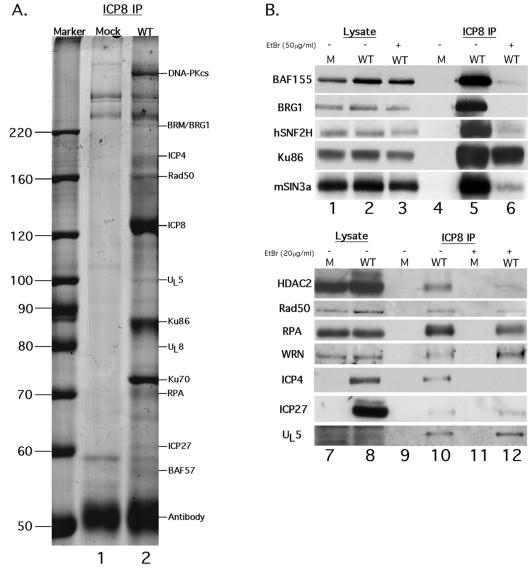
To identify potential ICP8-associated cellular and viral proteins, we immunoprecipitated ICP8 and associated molecules from wt HSV-infected or mock-infected whole-cell HEp-2 lysates prepared at 6.5 h postinfection using the conformation-specific 39S monoclonal antibody, which predominantly recognizes ICP8 at prereplicative sites or within HSV replication compartments (89). At this time of infection, the bulk of ICP8 is in replication compartments (18, 72), and the majority of ICP8 is associated with replication complexes or progeny viral DNA (48). The IPs were separated by SDS-PAGE, and the resolved proteins were visualized by Coomassie blue staining (Fig. 1A). Protein bands present only in IPs from virus-infected cells were excised, digested with trypsin, and analyzed by tandem MS.
FIG. 1.
Coprecipitation of cellular and viral proteins with ICP8 using the anti-ICP8 39S monoclonal antibody. (A) IPs of ICP8-associated proteins from mock-infected (lane 1) or wt HSV-1-infected (lane 2) HEp-2 cells were resolved in an SDS-8% polyacrylamide gel and then stained with Coomassie blue. The proteins indicated at the right were identified by MS. The molecular mass markers are shown at the left in kilodaltons. (B) Immunoprecipitations were performed in the presence or absence of EtBr and then an equal volume of each lysate or IP was separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The blot was probed with antibodies against the indicated cellular or viral proteins. Lane 1, mock (M) lysate; Lane 2, wt (WT) KOS lysate; Lane 3, wt KOS lysate with EtBr; Lane 4, mock IP; lane 5, wt KOS IP; lane 6, wt KOS IP with EtBr; lane 7, mock lysate; lane 8, wt KOS lysate; lane 9, mock IP; lane 10, wt KOS IP; lane 11, mock IP with EtBr; lane 12, wt KOS IP with EtBr.
We identified, in total, over 50 cellular and viral proteins that coprecipitated with ICP8 with 39S antibody (Table 1). Table 1 lists the majority of the coprecipitating proteins categorized by general function with the corresponding number of peptides detected by MS from a representative experiment. Some proteins, such as cytoplasmic constituents or cytoskeletal elements, were not included in this analysis, but the entire list of coprecipitating proteins may be found at http://knipelab.med.harvard.edu/. Certain proteins identified by one peptide were included in Table 1 because of their relationship or interaction with other coprecipitating proteins on the list.
TABLE 1.
Proteins that coimmunoprecipitate or colocalize with ICP8 categorized by function
| Protein | No. of peptides by MSa | Confirmed by IP-Western blotb | Replication compartment localizationc | DNA- independent ICP8 associationd |
|---|---|---|---|---|
| Replication/repair/recombination | ||||
| DNA-PKcs | 51 | Yes | This study | |
| Rad50 | 29 | Yes | This study | + |
| Ku86 | 26 | Yes | This study | + |
| Ku70 | 23 | |||
| Poly(ADP-ribose) polymerase-1 (PARP-1) | 16 | |||
| MutS homolog 6 (MSH6) | 5 | |||
| Meiotic recombination 11 (Mre11) | 4 | |||
| RPA | 4 | Yes | 95 | + |
| DNA methyltransferase-associated protein-1 (DNMTAP-1) | 3 | |||
| Dead-box p68 | 3 | |||
| Dead-box protein | 2 | |||
| Minichromosome maintenance protein-2 (MCM2) | 2 | |||
| X-ray repair cross complementing protein 4 (XRCC4) | 1 | |||
| MSH3 | 1 | |||
| Proliferating cell nuclear antigen (PCNA) | 1 | 96 | ||
| Werner syndrome gene product (WRN)e | Yes | This study | ++ | |
| MSH2e | This study | |||
| Breast cancer-associated gene 1 protein (BRCA1)e | This study | |||
| Bloom syndrome gene product (BLM)c | This study | |||
| Chromatin remodeling | ||||
| hSNF2H | 18 | Yes | This study | +/− |
| Facilitates chromatin transcription p140 (FACT p140) | 16 | |||
| Brahma protein (BRM) | 15 | This study | ||
| Brahma-related gene-1 protein (BRG1) | 13 | Yes | This study | − |
| BRG1 or BRM-associated factor 155 (BAF155) | 11 | Yes | This study | +/− |
| Structural maintenance of chromosomes-1 (SMC1) | 9 | |||
| BRG1- or BRM-associated factor 57 (BAF57) | 9 | |||
| BRG1- or BRM-associated factor 170 (BAF170) | 8 | |||
| Chromo-helicase-DNA-binding protein-3-interacting protein (CHD3-IP) | 8 | |||
| mSIN3a | 7 | Yes | This study | +/− |
| hSNF2L | 6 | |||
| DEK | 4 | |||
| BRG1- or BRM-associated factor 60a (BAF60a) | 3 | |||
| Histone deacetylase-2 (HDAC2) | 3 | Yes | This study | +/− |
| Nucleosome-associated protein-1-like (NAP-1-like) | 3 | |||
| Retinoblastoma-associated protein of 48 kDa (RbAp48) | 2 | |||
| RNA binding/splicing | ||||
| Spliceosome-associated protein 130 (SAP130) | 7 | |||
| Spliceosome-associated protein 155 (SAP155) | 5 | |||
| Glycine, arginine, tyrosine-rich RNA-binding protein (GRY-RBP) | 3 | |||
| Nuclear matrix protein 200 (NMP200) | 3 | |||
| Transcription factors | ||||
| General transcription factor II-I (GTF II-I) | 7 | |||
| TATA-binding protein-associated factor of 172 kDa (TAF172) | 3 | |||
| Activity-dependent neuroprotective protein (ADNP) | 3 | |||
| REST corepressor | 2 | |||
| Other | ||||
| Inosine-5′-monophosphate dehydrogenase-2 (IMPDH-2) | 12 | |||
| Herpesvirus-associated ubiquitin-specific protease (HAUSP) | 6 | |||
| Emerin | 3 | |||
| Nuclear matrix protein 238 (NMP238) | 2 | |||
| Protein phosphatase 1A subunit (PP1A subunit) | 2 | |||
| Lamin A/C | 2 | |||
| Viral proteins | ||||
| UL5 | 25 | Yes | 59 | ++ |
| ICP4 | 19 | Yes | 47 | − |
| UL12 | 8 | 87 | ||
| UL42 | 6 | 19 | ||
| ICP27 | 4 | Yes | 19 | ++ |
| UL8 | 3 | 59 |
Number of peptides identified by MS after trypsin digestion of an excised polyacrylamide gel band.
Coimmunoprecipitation was confirmed by IP-Western blot.
Immunofluorescence was used to determine if the indicated protein localized to viral replication compartments during infection. References are given for previously published observations.
DNA-independent ICP8 association was determined by IP in the presence of EtBr. ++, increased coimmunoprecipitation in the presence of EtBr; +, similar or slightly decreased coimmunoprecipitation in the presence of EtBr; +/−, more than 50% decreased coimmunoprecipitation in the presence of EtBr; −, no coimmunoprecipitation in the presence of EtBr.
Protein that was predicted to associate with ICP8 or localize to replication compartments based upon its interaction with another protein shown in Table 1.
To confirm the identity of these proteins and the authenticity of their association with ICP8, we performed several lines of experiments. First, we confirmed the presence of representative members of the larger protein complexes in ICP8 IPs by Western blotting with specific antibodies (Fig. 1B). Second, we determined if DNA plays a role in the coprecipitation (Fig. 1B). Third, we used immunofluorescence to determine if the coprecipitating proteins colocalize with ICP8 in viral replication compartments (Fig. 2). Finally, to determine if a cellular protein plays a role in viral replication, we have initiated studies to analyze HSV-1 replication in cells deficient for the cellular protein (Tables 2, 3, and 4).
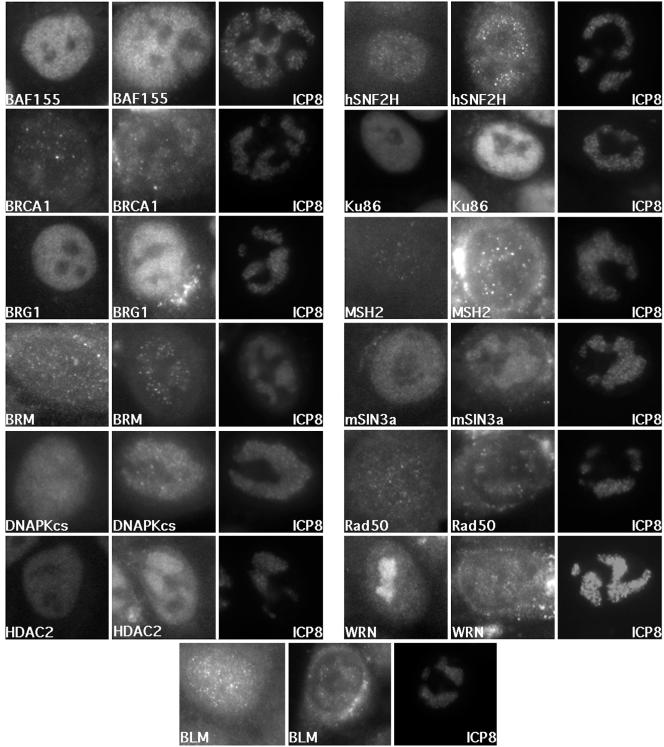
FIG. 2.
Distribution of cellular proteins in HSV-1-infected cells. HEp-2 cells infected with wt KOS or mock infected were processed for immunofluorescence at 6.5 h postinfection. The first panel (left) of each protein series shows the protein distribution in mock-infected cells. The next panel (middle) shows the distribution of the cellular protein in an HSV-1-infected cell. The last panel (right) shows the distribution of ICP8 in the same cell as that in the middle panel.
TABLE 2.
HSV-1 growth in human WRN-deficient fibroblast cells
| h postinfectiona | Yield (PFU/ml)b
|
|
|---|---|---|
| Normal fibroblasts | WRN-deficient fibroblasts | |
| Expt 1 | ||
| 2 | 4.1 × 103 | 7.2 × 102 |
| 24 | 6.9 × 107 | 1.8 × 107 |
| Expt 2 | ||
| 2 | 2.9 × 102 | 1.6 × 102 |
| 24 | 8.3 × 107 | 1.5 × 107 |
Duplicate samples of WRN-deficient or normal human fibroblasts were infected at an MOI of 20 with HSV-1 KOS.
Yield was determined by titration on Vero cells and is the average of duplicate samples.
TABLE 3.
HSV recombination in human WRN-deficient fibroblast cellsb
| Virus | Normal fibroblasts
|
WRN-deficient fibroblasts
|
||||
|---|---|---|---|---|---|---|
| Titer (106 PFU/ml)a
|
RF (%) | Titer (106 PFU/ml)
|
RF (%) | |||
| Vero | V529 | Vero | V529 | |||
| dl5 | <0.001 | 0.015 | <0.001 | 0.008 | ||
| dl29 | 0.0014 | 0.011 | <0.001 | 0.011 | ||
| dl5/dl29 | 2.5 | 9.0 | 28 | 0.58 | 2.8 | 21 |
| dl5/dl29 | 1.9 | 7.3 | 26 | 0.38 | 2.3 | 16 |
| dl5/dl29 | 1.4 | 6.5 | 22 | 0.34 | 1.9 | 18 |
| Average | 25 | 18 | ||||
Normal or WRN-deficient fibroblasts were either singly or doubly infected with dl5 or dl29 at an MOI of 20. At 24 h postinfection, the virus was harvested and titered on both Vero and V529 cells.
Recombination frequency (RF) was determined by dividing the titer on the noncomplementing cell line (Vero) by the titer on the complementing cell line (V529).
TABLE 4.
HSV-1 growth in Ku70-deficient MEF cells
| h postinfectiona | Yield (PFU/ml)b
|
|
|---|---|---|
| Normal MEFs | Ku70-deficient MEFs | |
| Expt 1c | ||
| 2 | 4.4 × 103 | 4.5 × 102 |
| 24 | 1.5 × 105 | 9.4 × 106 |
| Expt 2 | ||
| 2d | 1.1 × 102 | 8.0 × 101 |
| 24d | 2.8 × 104 | 7.9 × 105 |
| 24e | 4.7 × 102 | 2.6 × 104 |
Duplicate samples of Ku70-deficient or normal MEFs were infected with HSV-1 KOS.
Yield was determined by titration on Vero cells and is the average of duplicate samples.
MEFs were infected at an MOI of 50.
MEFs were infected at an MOI of 20.
MEFs were infected at an MOI of 1.
As expected, we identified several coprecipitating viral DNA replication proteins, including UL5, UL8, and UL42 (Table 1), which are known to associate with ICP8 in the viral DNA replication complex (6, 7, 9, 39, 59). In agreement with previous reports (84, 86, 99), we also identified UL12, ICP27, and ICP4 as ICP8-coprecipitating proteins.
We also identified numerous cellular proteins (Table 1) that are predominantly involved in cellular DNA replication and damage repair, including RPA and MSH6; homologous and nonhomologous recombination, including Rad50, DNA-PKcs, Mre11, and Ku86; chromatin remodeling, including BRG1, BRM, hSNF2H, BAF155, mSin3a, and HDAC2; and mRNA splicing, including SAP130, SAP155, and NMP200. Some of the other coprecipitating proteins have been identified as possibly playing a role during HSV replication, including HAUSP, a ubiquitin-specific protease that has been shown to associate with the HSV-1 immediate-early ICP0 protein (26); the mRNA splicing factors SAP130 and SAP155, which interact with SAP145 which in turn binds HSV-1 ICP27 (8); and Ku70, which has been demonstrated to bind the downstream activating sequence present in some HSV late gene promoter sequences (70).
We isolated multiple members of individual complexes, arguing that the coprecipitation of these complexes was specific. For example, we identified 4 of the 5 known protein partners in the cellular nonhomologous end-joining (NHEJ) complex (10), including DNA-PKcs, Ku86, Ku70, and XRCC4. Based on the coprecipitation of DNA repair and recombination complexes, we hypothesized that BRCA1, WRN, BLM, and MSH2, each known to interact with other proteins shown in Table 2 (5, 14, 28, 29, 45, 98, 100), may also coprecipitate or colocalize with ICP8 in infected cells and were included in this study as described in more detail below.
Role of DNA in association of the proteins with ICP8.
Many of the cellular complexes identified have DNA-binding components, and ICP8 is also bound to viral DNA in infected cells (48, 53), so we attempted to determine if the associations were mediated by DNA-protein or protein-protein interactions by performing the immunoprecipitations in the presence of EtBr at concentrations known to disrupt DNA-protein but not protein-protein interactions (50). The presence of EtBr did not significantly disrupt the coprecipitation of the host proteins Ku86, Rad50, RPA, and WRN, all of which are involved in DNA repair or recombination, or the viral proteins ICP27 and UL5 (Fig. 1B). As observed previously for ICP8 and ICP27 (99), disrupting DNA binding increased the coprecipitation of ICP8 with ICP27, UL5, and WRN (Fig. 1B). This may be due to the release of ICP8 or one of the proteins from DNA, allowing them to associate more readily. In contrast, coprecipitation of BAF155, hSNF2H, mSin3a, and HDAC2, which are all involved in chromatin remodeling, was reduced by greater than 50%, and ICP4 and BRG1 coprecipitation was abolished in the presence of EtBr (Fig. 1B).
These results suggested that a number of proteins, including Ku86, Rad50, RPA, WRN, ICP27, and UL5, associate with ICP8, either directly or indirectly, via protein-protein interactions. Other proteins, including BAF155, hSNF2H, mSin3a, HDAC2, BRG1, and ICP4, may require DNA to at least partially mediate or stabilize the association with ICP8.
Accumulation of cellular proteins in HSV replication compartments.
If the ICP8-coprecipitating proteins physically associated with ICP8, it was expected that they would colocalize with ICP8 within the nucleus of an infected cell. To test this hypothesis, we used immunofluorescence to examine the distribution of several representative proteins in HSV-infected HEp-2 cells. Proteins involved in NHEJ (DNA-PKcs and Ku86), homologous recombination and DNA repair (BLM, BRCA1, MSH2, Rad50, and WRN), and chromatin remodeling (BAF155, BRG1, BRM, HDAC2, hSNF2H, and mSin3a) were redistributed from their normal nuclear distribution (Fig. 2, left panels) to viral replication compartments after infection (Fig. 2, middle panels) as shown by their costaining with ICP8 or localization within replication compartments (Fig. 2, right panels). Similar staining patterns were observed in single-stained cells (data not shown). One of the most dramatic alterations involved the redistribution of WRN from its normal nucleolar pattern (Fig. 2, left WRN panel) (90) to viral replication compartments (Fig. 2, middle WRN panel). The redistribution of WRN from the nucleolus normally takes place in the presence of DNA-damaging agents (78), suggesting that viral infection may induce intranuclear signaling events that lead to altered nuclear structure. The accumulation of numerous ICP8-coprecipitating proteins in viral replication compartments suggested that they have a functional role in the HSV life cycle or that these proteins were recruited to damaged viral DNA. It is also possible that some of the proteins were sequestered within replication compartments to prevent possible antiviral functions.
Growth of HSV-1 in WRN-deficient human fibroblasts.
WRN is a mammalian RecQ helicase family member (41) and is thought to function in cellular DNA replication, repair, and recombination pathways via its helicase and exonuclease domains (68, 80). To determine if WRN was required for HSV replication, we examined viral growth and recombination in WRN-deficient primary human fibroblasts. The viral yield was decreased by three- to fivefold in WRN-deficient cells compared to control human fibroblast cells (Table 2). This result suggested that WRN might promote viral replication slightly but that it was not essential for HSV replication.
We next determined if HSV recombination was altered in the WRN-deficient cells by infecting normal or WRN-deficient fibroblasts singly or doubly with HSV-2 dl5 and dl29 strains (17), which contain mutations in the UL5 and UL29 genes, respectively, and measured the number of wt plaques that formed on Vero cells. We observed a recombination frequency of ∼25% (Table 3) in normal fibroblast cells. In contrast, we observed a lower average recombination frequency of ∼18% in WRN-deficient cells (Table 3). This decrease suggested that HSV recombination was not as efficient or did not occur as readily in WRN-deficient cells. In agreement with the above results with HSV-1 infection, we also observed an approximate threefold decrease in total HSV-2 yields in WRN-deficient cells compared to control human fibroblast cells.
Growth of HSV-1 in Ku70-deficient MEFs.
Ku70 and Ku86 form the heterodimeric DNA-binding portion of the DNA-PK complex. Once the Ku70/Ku86 heterodimer is bound to DNA, the other components of the DNA-PK complex are recruited to the double-strand break to orchestrate NHEJ. In the absence of the Ku70 subunit in mammalian cells, the NHEJ pathway is disrupted. To determine the role of Ku70 during viral replication, we examined HSV growth in Ku70-deficient MEFs.
Viral yields were increased by 30- to 50-fold in Ku70-deficient MEFs compared with control MEFs (Table 4). The increased yield was observed over a range of input virus from an MOI of 1 to 50 (Table 4). These results suggested that HSV growth did not depend upon Ku70 but rather was inhibited by Ku70. This result implied that the NHEJ pathway inhibited HSV replication in some manner.
DISCUSSION
We hypothesized that host proteins involved in HSV replication may be identified as proteins that coimmunoprecipitate with viral proteins in nuclear replication compartments, and we therefore used the HSV ICP8 DNA-binding protein as the prototype viral protein for IP studies. We first identified protein bands in ICP8 IPs by using MS. IP and Western blotting with specific antibodies confirmed the association of the host protein with ICP8. We expected that proteins associating with ICP8 would colocalize in viral replication compartments, so we next demonstrated that representative ICP8-coprecipitating proteins accumulated within viral replication compartments. We also initiated studies to analyze the functional role of these host proteins during HSV replication by examining viral growth and recombination in mutant cell lines. We hope to extend these studies by depleting specific host factors by RNA interference techniques.
In this study, we report the identification of numerous cellular proteins that coprecipitated with HSV ICP8. The majority of the proteins were components of cellular complexes that coordinate DNA recombination and repair or chromatin remodeling (for reviews, see references 2, 21, 27, 41, 46, 63, and 83). We examine the potential roles of the coprecipitating proteins in HSV replication in greater detail below.
Interactions with cellular DNA repair and recombination complexes.
Double-strand DNA breaks (DSB) and other DNA damage are experimentally induced by irradiation, UV exposure, and chemical treatment; however, this damage may also arise naturally as a consequence of DNA replication. Many of the repair and recombination proteins reported to redistribute to DSB after irradiation or chemical treatment colocalize with cellular DNA replication sites during S phase. For example, the Rad50/Mre11/NBS complex localizes to cellular replication structures during S phase as shown by costaining with RPA or BrdU (13, 60, 62). It is believed that the presence of stalled replication forks or stretches of single-stranded DNA are responsible for recruiting these factors. In the absence of such repair functions, genomic integrity degrades. As evidence for the critical role of these proteins in genomic integrity, many of the proteins involved in DNA repair and recombination are mutated or deleted in human genetic disorders that are associated with an increased risk of cancer (58, 87).
Two major pathways in mammalian cells repair DSB: (i) NHEJ and (ii) homologous recombination repair (HRR). The NHEJ and HRR pathways are believed to compete within the cell as they both act on DSB (3, 38, 44). HSV replication and recombination are coupled in infected cells (24, 25, 94), but the significance of this process remains unclear. It is known that the HSV genome undergoes an inversion of the L and S segments (20, 40) and that recombination frequencies are high in HSV-infected cells (24, 25, 79, 82), indicating that recombination may have a role in HSV replication. Members of both NHEJ and HRR complexes coprecipitated with ICP8, suggesting that these factors may also have a role in repairing DSB that occur during HSV DNA synthesis or have an as-yet-undefined function required for viral DNA replication. It has been recently proposed that HSV DNA replication occurs initially on a linear molecule (43); thus, recombination may play a role in HSV DNA replication as observed for bacteriophage T4 DNA replication (49).
We found that HSV replication and recombination levels were decreased modestly in WRN-deficient cells. WRN-deficient cells have a decreased ability to resolve homologous recombination products (77), which may account for the lower levels of homologous recombination that we observed in our assays. The presence of other RecQ helicases in the cell, such as BLM, raises the possibility that other cellular proteins may functionally substitute for WRN, providing a possible explanation for why we did not see a significant decrease in viral replication in WRN-deficient cells. We are currently investigating whether HSV replication is inhibited in other cell lines that have reduced homologous recombination activity.
In contrast to the WRN-deficient cells, we found that HSV replication is significantly increased in Ku70-deficient cells, which suggested that NHEJ may inhibit HSV replication. In support of this, it has been shown that HSV growth is increased in cells lacking another NHEJ component, DNA-PKcs (69). Although it has been suggested that HSV-1 infection causes a degradation of DNA-PKcs (54, 69), the extent of this effect is cell type and/or virus strain specific (69). We were surprised to see DNA-PKcs as one of the major proteins coprecipitating with ICP8, but further studies have shown that DNA-PKcs decreases by less than 50% in HEp-2 and other cells infected with HSV-1 KOS (T. J Taylor, G. Melroe, and D. M. Knipe, unpublished data). Therefore, DNA-PKcs could exert an effect during infection under our conditions. Indeed, we found that DNA-PKcs accumulated in viral replication compartments in HEp-2 cells infected with HSV-1 KOS. The accumulation of proteins involved in NHEJ within replication compartments may not reflect a functional role in HSV replication but rather the nonspecific binding of these proteins to DSBs within replication compartments.
The increased growth of HSV in cell lines deficient for NHEJ suggests that this pathway inhibits HSV replication. The disruption of the NHEJ pathway may increase HSV replication in at least two ways. First, it has been suggested that HSV may utilize a recombination-dependent replication mechanism similar to that used by bacteriophage T4 to replicate linear viral DNA later during infection (66). If so, then the Ku70/Ku86 DNA-binding heterodimer may block access of viral or cellular recombination proteins to DNA by competitively binding DSB. In the absence of Ku70, the competition for DSB would decrease, thus allowing greater access of other recombination factors. The loss of DNA-PKcs activity would also disrupt the NHEJ pathway because DNA-PKcs regulates the function of proteins involved in DNA repair by phosphorylation (3, 44); however, the effect on HSV replication might not be as pronounced in DNA-PKcs-deficient cells compared to Ku70-deficient cells in that Ku70 acts upstream of DNA-PKcs in the NHEJ pathway and could still bind DSB to some degree in DNA-PKcs-deficient cells. If HSV uses a recombination-dependent replication mechanism for replication, then it might be expected that viral replication occurs more readily in the absence of the competing NHEJ pathway. Second, it has recently been suggested that HSV genome circularization may actually repress the onset of HSV infection (43). If the NHEJ pathway is responsible for genome circularization, then the absence of this cellular pathway may promote the initiation of the viral life cycle. Further studies are needed to define the mechanisms of the inhibitory effect of NHEJ during HSV replication.
At least two proteins involved in DNA repair and recombination, NBS and BLM, accumulate in or near intranuclear ND10 sites (64, 91). As it is believed that HSV DNA targets to ND10 sites and replication compartments form adjacent to the ND10 sites (42, 88), it is possible that ICP8 or other viral proteins associate with one or more of these proteins for proper intranuclear localization early during infection. It will be of interest to determine if a recently identified region of ICP8 required for proper intranuclear localization (85) mediates the interaction with one or more of the ICP8-coprecipitating proteins.
Interactions with cellular chromatin remodeling complexes.
Two main groups of chromatin remodeling complexes exist in mammalian cells: (i) those that require ATP hydrolysis to alter histone-DNA contacts, such as the mating type switch/sucrose nonfermenting (SWI/SNF) and imitation switch (ISWI) complexes, and (ii) those that covalently modify histone proteins by a variety of posttranslational modifications (phosphorylation, acetylation, methylation), such as histone acetyltransferases and histone deacetylase complexes. We identified members of both types of complexes as ICP8-coprecipitating proteins.
The SWI/SNF and ISWI complexes have related core subunits but differ in the makeup of accessory proteins that are believed to regulate function or specificity (83). The SWI/SNF and ISWI complexes have similar but nonoverlapping functions that differ by substrate specificity and mechanism of action (1, 52, 83), again possibly regulated by various binding partners. Several ATPase subunits associated with SWI/SNF or ISWI complexes, such as BRM, BRG1, hSNF2H, and hSNF2L, coprecipitated with ICP8, suggesting a role for these proteins in viral replication. The role of chromatin remodeling proteins in HSV productive infection is not immediately obvious because it has been reported that HSV genomic DNA is kept in a relatively nucleosome-free form during lytic replication (55, 56, 72). It is possible that SWI/SNF complexes may be involved in maintaining this nucleosome-free state. In contrast to lytic infection, it is thought that latent HSV genomes are nucleosome associated (22), suggesting that SWI/SNF complexes may be involved in generating nucleosome-free genomes during HSV reactivation.
There is evidence that interactions with transcription factors may target SWI/SNF complexes to specific promoters to regulate transcription (1, 15, 52, 65, 83, 97). It is possible that the virus uses a similar form of targeted regulation by recruiting SWI/SNF complexes to weak viral promoters early in infection or during reactivation to enhance transcription.
Other ICP8-coprecipitating proteins.
Many other proteins that do not fall into the above categories also coprecipitated with ICP8. The most numerous of those were proteins involved in mRNA splicing or transcription factors. HSV has been shown to alter host cell mRNA maturation through the immediate-early protein ICP27. As we also identified ICP27 as a coprecipitating protein, it is possible that the mRNA splicing proteins were interacting directly with ICP27 and not ICP8. The transcription factors may have been associated with viral DNA or with RNA polymerase II, which has been shown to associate with ICP8 (99). We postulate that these interactions may play a role in virus early and/or, more likely, late gene transcription.
Role of DNA in mediating ICP8 coprecipitation.
The majority of the ICP8-coprecipitating proteins tested associated with ICP8 in a DNA-independent manner, suggesting that they physically associate with ICP8 directly or indirectly through other binding partners. The coprecipitation of proteins involved in chromatin remodeling required DNA for optimal association. In agreement with a recent study (84), we coprecipitated ICP4 with ICP8; however, this did not appear to be an authentic direct protein-protein interaction as it was dependent upon the presence of DNA.
As we precipitated ICP8 using the conformation-specific 39S monoclonal antibody that preferentially recognized ICP8 within replication compartments (89), we believe that viral DNA, not contaminating cellular DNA, mediated these interactions because ICP8 is bound to viral DNA in replication compartments (48). It is possible that ICP8 recruited these factors to replication compartments or viral DNA and was not required for their retention once assembled onto viral DNA.
Functional role of cellular proteins in HSV replication compartments.
The presence of representative members of the coprecipitating proteins in replication compartments argued for a potential role of these proteins in HSV replication. We observed that the distribution and number of foci within replication compartments differed from protein to protein. For example, there were relatively few MSH2, BRM, or hSNF2H foci within the boundaries of replication compartments compared to BRG1, mSin3a, or BAF155 (Fig. 2, middle panels). This finding suggested that these cellular complexes targeted to distinct subcompartments or structures within replication compartments. This sort of subcompartmentalization has been described for viral proteins in that ICP4, ICP8, and VP5 have distinct distribution patterns within replication compartment boundaries (19). The different distributions of cellular proteins may reflect functional domains within replication compartments in which similar or related cellular machinery are recruited to aid HSV replication or sequestered to prevent possible antiviral activities.
It remains to be established that the ICP8-coprecipitating proteins have a role in viral replication. We demonstrated that the WRN and Ku70 proteins were not absolutely required for HSV growth. Further investigation is needed to examine the necessity of the other coprecipitating proteins during HSV replication. To do so, cells deficient in one or more proteins listed in Table 1 would be invaluable. This may prove to be difficult, as the proteins may be absolutely required for viability, making isolation of such cell lines difficult, or as is the case for WRN, redundant protein functions may complement the deficiency, thus masking any phenotype. However, with the increasing number of knockout mice, small-interfering RNA technology, and repositories that supply cells from humans with certain genetic disorders, it will rapidly become more feasible to investigate the role of the ICP8-coprecipitating proteins in HSV replication.
We believe that the large number of coprecipitating proteins reflects the multiple roles of ICP8 and replication compartments in viral DNA replication, viral gene expression, and capsid assembly. Not all of the coprecipitating proteins can interact with ICP8 directly, so we believe that some of the interactions are dependent upon intermediate binding partners. It remains to be determined which ICP8-coprecipitating proteins physically interact with ICP8 directly. Many of the proteins identified associate with numerous other proteins including other ICP8-coprecipitating proteins. It is possible that the association of ICP8 with one or two proteins may lead to the recruitment of numerous other proteins or complexes with a variety of functions. For example, the BRCA1-associated genome surveillance complex (BASC) is a 2-MDa “super complex” that is believed to contain over 40 proteins that are involved in genome integrity surveillance (92). The many components of BASC can detect and repair DNA damage as well as transduce signals to cell cycle control proteins to halt cell growth (30, 92). The association of ICP8 with BRCA1 or another member of this complex could recruit the numerous other BASC-associated proteins to viral replication compartments.
We hypothesize that HSV recruits some of these cellular proteins to replication compartments to participate directly in HSV replication. Alternatively, they may be targeted to damaged viral DNA that arises during replication. This demonstrates the complexity of determining the function of these proteins in the context of HSV infection. As many of the ICP8-coprecipitating proteins function in the major recombination repair pathways, it will be of interest to determine if these proteins have a necessary role in HSV replication or are recruited to sites of DNA damage or stalled replication forks and are not required for HSV replication. Further studies are needed to determine the specific role of the proteins identified in viral infection.
Acknowledgments
We thank Kent Wilcox, Bruce Stillman, Haim Cohen, and David Sinclair for generously providing reagents used in this study. We also thank Ross Tomaino and Steven Gygi for their aid in the MS analysis.
This study was supported by NIH grant AI 63106.
REFERENCES
- 1.Aalfs, J. D., G. J. Narlikar, and R. E. Kingston. 2001. Functional differences between the human ATP-dependent nucleosome remodeling proteins BRG1 and SNF2H. J. Biol. Chem. 276:34270-34278. [DOI] [PubMed] [Google Scholar]
- 2.Ahringer, J. 2000. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 16:351-356. [DOI] [PubMed] [Google Scholar]
- 3.Allen, C., A. Kurimasa, M. A. Brenneman, D. J. Chen, and J. A. Nickoloff. 2002. DNA-dependent protein kinase suppresses double-strand break-induced and spontaneous homologous recombination. Proc. Natl. Acad. Sci. USA 99:3758-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwine, J. C., W. L. Steinhart, and C. W. Hill. 1974. Transcription of herpes simplex type 1 DNA in nuclei isolated from infected HEp-2 and KB cells. Virology 60:302-307. [DOI] [PubMed] [Google Scholar]
- 5.Bochar, D. A., L. Wang, H. Beniya, A. Kinev, Y. Xue, W. S. Lane, W. Wang, F. Kashanchi, and R. Shiekhattar. 2000. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell 102:257-265. [DOI] [PubMed] [Google Scholar]
- 6.Boehmer, P. E., M. C. Craigie, N. D. Stow, and I. R. Lehman. 1994. Association of origin binding protein and single strand DNA-binding protein, ICP8, during herpes simplex virus type 1 DNA replication in vivo. J. Biol. Chem. 269:29329-29334. [PubMed] [Google Scholar]
- 7.Boehmer, P. E., and I. R. Lehman. 1993. Physical interaction between the herpes simplex virus 1 origin-binding protein and single-stranded DNA-binding protein ICP8. Proc. Natl. Acad. Sci. USA 90:8444-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant, H. E., S. E. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 75:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush, M., D. R. Yager, M. Gao, K. Weisshart, A. I. Marcy, D. M. Coen, and D. M. Knipe. 1991. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J. Virol. 65:1082-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calsou, P., C. Delteil, P. Frit, J. Drouet, and B. Salles. 2003. Coordinated assembly of Ku and p460 subunits of the DNA-dependent protein kinase on DNA ends is necessary for XRCC4-ligase IV recruitment. J. Mol. Biol. 326:93-103. [DOI] [PubMed] [Google Scholar]
- 11.Challberg, M. D. 1986. A method for identifying the viral genes required for herpesvirus DNA replication. Proc. Natl. Acad. Sci. USA 83:9094-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conley, A. J., D. M. Knipe, P. C. Jones, and B. Roizman. 1981. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J. Virol. 37:191-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantinou, A., M. Tarsounas, J. K. Karow, R. M. Brosh, V. A. Bohr, I. D. Hickson, and S. C. West. 2000. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 1:80-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper, M. P., A. Machwe, D. K. Orren, R. M. Brosh, D. A. Ramsden, and V. A. Bohr. 2000. Ku complex interacts with and stimulates the Werner protein. Genes Dev. 14:907-912. [PMC free article] [PubMed] [Google Scholar]
- 15.Cosma, M. P. 2002. Ordered recruitment: gene-specific mechanism of transcription activation. Mol. Cell 10:227-236. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo, F., G. Campadelli-Fiume, L. Foa-Tomasi, and E. Cassai. 1977. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J. Virol. 21:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Da Costa, X., M. F. Kramer, J. Zhu, M. A. Brockman, and D. M. Knipe. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 74:7963-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bruyn Kops, A., and D. M. Knipe. 1988. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell 55:857-868. [DOI] [PubMed] [Google Scholar]
- 19.de Bruyn Kops, A., S. L. Uprichard, M. Chen, and D. M. Knipe. 1998. Comparison of the intranuclear distributions of herpes simplex virus proteins involved in various viral functions. Virology 252:162-178. [DOI] [PubMed] [Google Scholar]
- 20.Delius, H., and J. B. Clements. 1976. A partial denaturation map of herpes simplex virus type 1 DNA: evidence for inversions of the unique DNA regions. J. Gen. Virol. 33:125-133. [DOI] [PubMed] [Google Scholar]
- 21.de Ruijter, A. J. M., A. H. van Gennip, H. N. Caron, S. Kemp, and A. B. P. van Kuilenburg. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmane, S. L., and N. W. Fraser. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 63:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Din, S., S. J. Brill, M. P. Fairman, and B. Stillman. 1990. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 4:968-977. [DOI] [PubMed] [Google Scholar]
- 24.Dutch, R. E., V. Bianchi, and I. R. Lehman. 1995. Herpes simplex virus type 1 DNA replication is specifically required for high-frequency homologous recombination between repeated sequences. J. Virol. 69:3084-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutch, R. E., R. C. Bruckner, E. S. Mocarski, and I. R. Lehman. 1992. Herpes simplex virus type 1 recombination: role of DNA replication and viral a sequences. J. Virol. 66:277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan, H.-Y., X. He, R. E. Kingston, and G. J. Narlikar. 2003. Distinct strategies to make nucleosomal DNA accessible. Mol. Cell 11:1311-1322. [DOI] [PubMed] [Google Scholar]
- 28.Franchitto, A., and P. Pichierri. 2002. Bloom's syndrome protein is required for correct relocalization of RAD50/MRE11/NBS1 complex after replication fork arrest. J. Cell Biol. 157:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchitto, A., P. Pichierri, R. Piergentili, M. Crescenzi, M. Bignami, and F. Palitti. 2003. The mammalian mismatch repair protein MSH2 is required for correct MRE11 and RAD51 relocalization and for efficient cell cycle arrest induced by ionizing radiation in G2 phase. Oncogene 22:2110-2120. [DOI] [PubMed] [Google Scholar]
- 30.Futaki, M., and J. M. Liu. 2001. Chromosomal breakage syndromes and the BRCA1 genome surveillance complex. Trends Mol. Med. 7:560-565. [DOI] [PubMed] [Google Scholar]
- 31.Gao, M., J. Bouchey, K. Curtin, and D. M. Knipe. 1988. Genetic identification of a portion of the herpes simplex virus ICP8 protein required for DNA-binding. Virology 163:319-329. [DOI] [PubMed] [Google Scholar]
- 32.Gao, M., and D. M. Knipe. 1991. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J. Virol. 65:2666-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godowski, P. J., and D. M. Knipe. 1985. Identification of a herpes simplex virus function that represses late gene expression from parental viral genomes. J. Virol. 55:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godowski, P. J., and D. M. Knipe. 1983. Mutations in the major DNA-binding protein gene of herpes simplex virus type 1 result in increased levels of viral gene expression. J. Virol. 47:478-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godowski, P. J., and D. M. Knipe. 1986. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc. Natl. Acad. Sci. USA 83:256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu, Y., K. J. Seidl, G. A. Rathbun, C. Zhu, J. P. Manis, N. van der Stoep, L. Davidson, H. L. Cheng, J. M. Sekiguchi, K. Frank, P. Stanhope-Baker, M. S. Schlissel, D. B. Roth, and F. W. Alt. 1997. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity 7:653-665. [DOI] [PubMed] [Google Scholar]
- 37.Gupte, S. S., J. W. Olson, and W. T. Ruyechan. 1991. The major herpes simplex virus type-1 DNA-binding protein is a zinc metalloprotein. J. Biol. Chem. 266:11413-11416. [PubMed] [Google Scholar]
- 38.Haber, J. E. 1998. The many interfaces of Mre11. Cell 95:583-586. [DOI] [PubMed] [Google Scholar]
- 39.Hamatake, R. K., M. Bifano, W. W. Hurlburt, and D. J. Tenney. 1997. A functional interaction of ICP8, the herpes simplex virus single-stranded DNA-binding protein, and the helicase-primase complex that is dependent on the presence of the UL8 subunit. J. Gen. Virol. 78:857-865. [DOI] [PubMed] [Google Scholar]
- 40.Hayward, G. S., R. J. Jacob, S. C. Wadsworth, and B. Roizman. 1975. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short segments. Proc. Natl. Acad. Sci. USA 72:4243-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickson, I. D. 2003. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer 3:169-178. [DOI] [PubMed] [Google Scholar]
- 42.Ishov, A. M., and G. G. Maul. 1996. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 134:815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson, S. A., and N. A. DeLuca. 2003. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Natl. Acad. Sci. USA 100:7871-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karmakar, P., J. Piotrowski, R. M. Brosh, Jr., J. A. Sommers, S. P. L. Miller, W.-H. Cheng, C. M. Snowden, D. A. Ramsden, and V. A. Bohr. 2002. Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro, and its catalytic activities are regulated by phosphorylation. J. Biol. Chem. 277:18291-18302. [DOI] [PubMed] [Google Scholar]
- 45.Karmakar, P., C. M. Snowden, D. A. Ramsden, and V. A. Bohr. 2002. Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus. Nucleic Acids Res. 30:3583-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klochendler-Yeivin, A., C. Muchardt, and M. Yaniv. 2002. SWI/SNF chromatin remodeling and cancer. Curr. Opin. Gen. Dev. 12:73-79. [DOI] [PubMed] [Google Scholar]
- 47.Knipe, D. M., D. Senechek, S. A. Rice, and J. L. Smith. 1987. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J. Virol. 61:276-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreuzer, K. N. 2000. Recombination-dependent DNA replication in phage T4. Trends Biochem. Sci. 25:165-173. [DOI] [PubMed] [Google Scholar]
- 50.Lai, J., and W. Herr. 1992. Ethidium bromide provides a simple tool for identifying genuine DNA-independent associations. Proc. Natl. Acad. Sci. USA 89:6958-6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamberti, C., and S. K. Weller. 1998. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J. Virol. 72:2463-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazzaro, M. A., and D. J. Picketts. 2001. Cloning and characterization of the murine Imitation Switch (ISWI) genes: differential expression patterns suggest distinct developmental roles for Snf2h and Snf2L. J. Neurochem. 77:1145-1156. [DOI] [PubMed] [Google Scholar]
- 53.Lee, C. K., and D. M. Knipe. 1985. An immunoassay for the study of DNA-binding activities of herpes simplex virus protein ICP8. J. Virol. 54:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lees-Miller, S., M. Long, M. Kilvert, V. Lam, S. Rice, and C. Spencer. 1996. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 70:7471-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leinbach, S. S., and W. C. Summers. 1980. The structure of herpes simplex virus type 1 DNA as probed by micrococcal nuclease digestion. J. Gen. Virol. 51:45-59. [DOI] [PubMed] [Google Scholar]
- 56.Lentine, A. F., and S. L. Bachenheimer. 1990. Intracellular organization of herpes simplex virus type 1 DNA assayed by staphylococcal nuclease sensitivity. Virus Res. 16:275-292. [DOI] [PubMed] [Google Scholar]
- 57.Leopardi, R., P. Ward, W. Ogle, and B. Roizman. 1997. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J. Virol. 71:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levitt, N. C., and I. D. Hickson. 2002. Caretaker tumour suppressor genes that defend genome integrity. Trends Mol. Med. 8:179-186. [DOI] [PubMed] [Google Scholar]
- 59.Liptak, L., S. Uprichard, and D. Knipe. 1996. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J. Virol. 70:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maser, R. S., O. K. Mirzoeva, J. Wells, H. Olivares, B. R. Williams, R. A. Zinkel, P. J. Farnham, and J. H. J. Petrini. 2001. Mre11 complex and DNA replication: linkage of E2F and sites of DNA synthesis. Mol. Cell. Biol. 21:6006-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 62.Mirzoeva, O. K., and J. H. J. Petrini. 2003. DNA replication-dependent nuclear dynamics of the Mre11 complex. Mol. Cancer Res. 1:207-218. [PubMed] [Google Scholar]
- 63.Muchardt, C., and M. Yaniv. 2001. When the SWI/SNF complex remodels the cell cycle. Oncogene 20:3067-3075. [DOI] [PubMed] [Google Scholar]
- 64.Naka, K., K. Ikeda, and N. Motoyama. 2002. Recruitment of NBS1 into PML oncogenic domains via interaction with SP100 protein. Biochem. Biophys. Res. Commun. 299:863-871. [DOI] [PubMed] [Google Scholar]
- 65.Narlikar, G. J., H.-Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 66.Nimonkar, A. V., and P. E. Boehmer. 2003. Reconstitution of recombination-dependent DNA synthesis in herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 100:10201-10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olivo, P. D., N. J. Nelson, and M. D. Challberg. 1989. Herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J. Virol. 63:196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opresko, P. L., J.-P. Laine, R. M. Brosh, Jr., M. M. Seidman, and V. A. Bohr. 2001. Coordinate action of the helicase and 3′ to 5′ exonuclease of Werner syndrome protein. J. Biol. Chem. 276:44677-44687. [DOI] [PubMed] [Google Scholar]
- 69.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petroski, M. D., and E. K. Wagner. 1998. Purification and characterization of a cellular protein that binds to the downstream activation sequence of the strict late UL38 promoter of herpes simplex virus type 1. J. Virol. 72:8181-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phelan, A., J. Dunlop, A. Patel, N. Stow, and J. Clements. 1997. Nuclear sites of herpes simplex virus type 1 DNA replication and transcription colocalize at early times postinfection and are largely distinct from RNA processing factors. J. Virol. 71:1124-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pignatti, P. F., and E. Cassai. 1980. Analysis of herpes simplex virus nucleoprotein complexes extracted from infected cells. J. Virol. 36:816-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 74.Randall, R. E., and N. Dinwoodie. 1986. Intranuclear localization of herpes simplex virus immediate-early and delayed-early proteins: evidence that ICP4 is associated with progeny virus DNA. J. Gen. Virol. 67:2163-2177. [DOI] [PubMed] [Google Scholar]
- 75.Rice, S. A., M. C. Long, V. Lam, and C. A. Spencer. 1994. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J. Virol. 68:988-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincot Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 77.Saintigny, Y., K. Makienko, C. Swanson, M. J. Emond, and R. J. Monnat, Jr. 2002. Homologous recombination resolution defect in Werner syndrome. Mol. Cell. Biol. 22:6971-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakamoto, S., K. Nishikawa, S.-J. Heo, M. Goto, Y. Furuichi, and A. Shimamoto. 2001. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells 6:421-430. [DOI] [PubMed] [Google Scholar]
- 79.Schaffer, P. A., M. J. Tevethia, and M. Benyesh-Melnick. 1974. Recombination between temperature-sensitive mutants of herpes simplex virus type 1. Virology 58:219-228. [DOI] [PubMed] [Google Scholar]
- 80.Shen, J.-C., M. D. Gray, J. Oshima, A. S. Kamath-Loeb, M. Fry, and L. A. Loeb. 1998. Werner syndrome protein. I. DNA helicase and DNA exonuclease reside on the same polypeptide. J. Biol. Chem. 273:34139-34144. [DOI] [PubMed] [Google Scholar]
- 81.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smiley, J. R., M. J. Wagner, W. P. Summers, and W. C. Summers. 1980. Genetic and physical evidence for the polarity of transcription of the thymidine kinase gene of herpes simplex virus. Virology 102:83-93. [DOI] [PubMed] [Google Scholar]
- 83.Sudarsanam, P., and F. Winston. 2000. The SWI/SNF family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 16:345-351. [DOI] [PubMed] [Google Scholar]
- 84.Tang, Q., L. Li, A. M. Ishov, V. Revol, A. L. Epstein, and G. G. Maul. 2003. Determination of minimum herpes simplex virus type 1 components necessary to localize transcriptionally active DNA to ND10. J. Virol. 77:5821-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor, T. J, and D. M. Knipe. 2003. C-terminal region of herpes simplex virus ICP8 protein needed for intranuclear localization. Virology 309:219-231. [DOI] [PubMed] [Google Scholar]
- 86.Thomas, M. S., M. Gao, D. M. Knipe, and K. L. Powell. 1992. Association between the herpes simplex virus major DNA-binding protein and alkaline nuclease. J. Virol. 66:1152-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thompson, L. H., and D. Schild. 2002. Recombinational DNA repair and human disease. Mutation Res. 509:49-78. [DOI] [PubMed] [Google Scholar]
- 88.Uprichard, S. L., and D. M. Knipe. 1997. Assembly of herpes simplex virus replication proteins at two distinct intranuclear sites. Virology 229:113-125. [DOI] [PubMed] [Google Scholar]
- 89.Uprichard, S. L., and D. M. Knipe. 2003. Conformational changes in the herpes simplex virus ICP8 DNA-binding protein coincident with assembly in viral replication structures. J. Virol. 77:7467-7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Kobbe, C., and V. A. Bohr. 2002. A nucleolar targeting sequence in the Werner syndrome protein resides within residues 949-1092. J. Cell Sci. 115:3901-3907. [DOI] [PubMed] [Google Scholar]
- 91.Wang, X. W., A. Tseng, N. A. Ellis, E. A. Spillare, S. P. Linke, A. I. Robles, H. Seker, Q. Yang, P. Hu, S. Beresten, N. A. Bemmels, S. Garfield, and C. C. Harris. 2001. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 276:32948-32955. [DOI] [PubMed] [Google Scholar]
- 92.Wang, Y., D. Cortez, P. Yazdi, N. Neff, S. J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927-939. [PMC free article] [PubMed] [Google Scholar]
- 93.Ward, P., W. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weber, P. C., M. D. Challberg, N. J. Nelson, J. Levine, and J. C. Glorioso. 1988. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell 54:369-381. [DOI] [PubMed] [Google Scholar]
- 95.Wilcock, D., and D. P. Lane. 1991. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature 349:429-431. [DOI] [PubMed] [Google Scholar]
- 96.Yu, D., and S. K. Weller. 1988. Genetic analysis of the UL15 gene locus for the putative terminase of herpes simplex virus type 1. Virology 243:32-44. [DOI] [PubMed] [Google Scholar]
- 97.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong, Q., C.-F. Chen, S. Li, Y. Chen, C.-C. Wang, J. Xiao, P.-L. Chen, Z. D. Sharp, and W.-H. Lee. 1999. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 285:747-750. [DOI] [PubMed] [Google Scholar]
- 99.Zhou, C., and D. M. Knipe. 2002. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76:5893-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zink, D., C. Mayr, C. Janz, and L. Wiesmuller. 2002. Association of p53 and MSH2 with recombinative repair complex during S phase. Oncogene 21:4788-4800. [DOI] [PubMed] [Google Scholar]