Abstract
Epstein-Barr virus (EBV) is a human gammaherpesvirus associated with malignancies of both epithelial and lymphoid origin. Efficient infection of the latent host reservoir B lymphocytes involves the binding of glycoproteins gp350/220 for initial attachment, followed by the concerted action of gH, gL, gB, and gp42 for membrane fusion. The type II membrane protein gp42 is required for infection of B cells and assembles into a complex with gH and gL. The cellular host receptor for gp42, class II human leukocyte antigen (HLA), has been structurally verified by crystallization analyses of gp42 bound to HLA-DR1. Interestingly, the crystal structure revealed a hydrophobic pocket consisting of many aromatic and aliphatic residues from the predicted C-type lectin domain of gp42 that in other members of the C-type lectin family binds major histocompatibility complex class I or other diverse ligands. Although the hydrophobic pocket does not bind HLA class II, mutational analyses presented here indicate that this domain is essential for EBV-induced membrane fusion. In addition, mutational analysis of the region of gp42 contacting HLA class II in the gp42-HLA-DR1 cocrystal confirms that this region interacts with HLA class II and that this interaction is also important for EBV-induced membrane fusion.
Epstein-Barr virus (EBV) is a ubiquitous member of the human gammaherpesvirus subfamily that is able to establish lifelong latency in host B cells. The double-stranded enveloped virus has been associated with diseases of both lymphoid and epithelial origin (3, 9). Infection in infancy is generally asymptomatic but during adolescence can cause infectious mononucleosis. EBV infection is associated with Burkitt's lymphoma, Hodgkin's disease, and lymphoproliferative diseases in AIDS patients (for reviews, see references 25 and 32). Epithelial diseases associated with EBV infection include oral hairy leukoplakia and nasopharyngeal carcinoma (for a review, see reference 32). Although EBV encodes as many as 11 glycoproteins, efficient entry of EBV into B cells requires only 5 glycoproteins: gp350/220, gH, gL, gB, and gp42 (for reviews, see references 16, 33, and 34). The type II membrane protein gp42 of the BZLF2 open reading frame is required for entry into B lymphocytes but not epithelial cells and has two alternately processed forms: a 42- and a 38-kDa protein (1, 24, 37). The receptor of gp350 has been identified as complement receptor 2 (CR2) or CD21 (10, 29, 36). Expression of gp350 is not required for membrane fusion, but as a binding factor, it greatly enhances infection efficiency, similar to herpes simplex virus (HSV) gC (17, 20). Transmembrane gH dimerizes with gL, which exists as membrane-bound and soluble forms and is required for correct folding and transport of gH (14, 24, 40). These dimers have been demonstrated to bind gp42 (38). The minimal glycoproteins required for membrane fusion of B cells using experimentally transfected cells are gB, gH, gL, and gp42 (12). It was previously demonstrated that gB is required for lytic replication as well as the production of transforming virus (15, 22). EBV gB has been shown to be functionally distinct from other human herpesvirus subfamily gBs, and this could be due to the different regulatory domains of the cytoplasmic tail that are involved in membrane fusion and virion transport (12, 21, 22). Recently, Neuhierl et al. demonstrated that EBV gB dramatically enhances infection of human cells and constitutes an important virulence factor that determines infection of non-B cells (30).
It has been theorized that expression levels of gp42 on the surface of the virion decrease or increase as the virus alternates between infection of epithelial cells and lymphocytes in the human host, the so-called “cell-switching and kissing” model (2). It was demonstrated in vitro that when the virus is produced in epithelial cells, the virion contains abundant gp42, which allows very efficient infection of B cells. However, as gp42 is not required for entry into epithelial cells, the high surface expression might sterically interfere with receptor binding, which would lead to the reduced efficiency of epithelial cell infection that was observed. Conversely, when the virus is produced in B cells, the amount of gp42 in the virion is reduced, possibly due to colocalization with the EBV B-cell receptor, which allows efficient infection of epithelial cells but decreased infection of other B cells, as was demonstrated. This provides a plausible route of oral EBV infection in humans via epithelial cells and then B cells, where the virus can establish latency. The B-cell receptor of gp42 has been identified as the human leukocyte antigen (HLA) class II, and the DR, DP, and DQ alleles are all functional (11, 23, 35). Interestingly, HLA-DQ β*02 is the only functional DQ allele (13).
Recently, the crystal structure of a baculovirus-produced soluble form of gp42 (sgp42) bound to the HLA-DR1 allele was solved (28). The predicted C-type lectin domain (CTLD) lies between residues 94 and 223 of the protein, and though it shows some structural homology to other CTLD-containing proteins, such as Ly49A, it was revealed that binding of gp42/HLA-DR1 was not homologous to Ly49A binding to its receptor, the major histocompatibility complex (MHC) class I molecule H-2Dd, nor any other CTLD-containing protein and its receptor. Interestingly, gp42 dimerized in the crystal form between residues 87 and 94, a region of the ectodomain. Dimerization of gp42, either before or after binding to HLA class II, may be important for fusion since it would result in more receptors being available to trigger fusion. The initiation of fusion by gp42 mediated by the coordinated action of gB, gH, and gL may result from conformational changes in gp42 after its binding to HLA class II. This conformational change may allow other EBV-encoded glycoproteins or additional cellular receptors to bind to the fusion complex, or it may rearrange receptors on the virion or cell surface. Crystallization studies of gp42 alone should reveal if such a conformational change occurs upon binding with HLA class II. Adding to its inimitable character as a novel herpesvirus glycoprotein is a region of several aliphatic and aromatic residues that create a hydrophobic pocket with a yet unknown binding partner. The specific tropism that gp42 lends EBV, along with its unique binding structure and hydrophobic pocket, makes it an interesting target for functional studies of membrane fusion to not only characterize EBV fusion mechanisms but also increase our overall understanding of herpesvirus infection. We have undertaken mutational studies of gp42 to confirm the predicted interaction of gp42 with HLA class II from structural studies and to investigate the function of other gp42 domains such as the hydrophobic pocket in EBV-induced membrane fusion.
MATERIALS AND METHODS
Cells and plasmids.
Chinese hamster ovary cells (CHO-K1), kindly provided by Nanette Susmarski, were grown in Ham's F-12 media with 10% fetal bovine serum and 1% penicillin-streptomycin, referred to as complete media (all from BioWhittaker). EBV-positive HLA class II- and CD21-expressing Daudi B lymphocytes were obtained from American Type Culture Collection (Manassas, Va.) and were grown in RPMI complete media (BioWhittaker). To more easily monitor membrane fusion, a Daudi cell line stably transfected and expressing T7 RNA polymerase was constructed. Briefly, Daudi cells were electroporated with 40 μg of pOS2 plasmid DNA, containing a G418 selectable marker and bacteriophage T7 RNA polymerase under transcriptional control of the simian virus 40 promoter. This plasmid was kindly provided by Stanley Lemon (39). Following transfection, the cells were plated at 10,000 and 50,000 cells per well in 96-well plates and selected with G418 (0.9, 1.1, or 1.3 mg/ml). Clones emerged approximately 3 to 4 weeks postplating. Approximately 50 clones were expanded from the 96-well plates and were tested in the fusion assay as described below. Of the tested clones, cell lines 22, 29, 31, and 36 provided the highest levels of luciferase expression in the fusion assay. Of these lines, lines 29 and 36 were used for all subsequent experiments and were maintained in RPMI complete media with 1.1 and 1.3 mg of G418/ml, respectively. Cells were grown in 75-cm2 cell culture flasks (Corning), and adherent cells were detached by using either trypsin-Versene (BioWhittaker) or Versene (phosphate-buffered saline [PBS]-1 mM EDTA). The various plasmids used in the present studies are shown in Table 1.
TABLE 1.
Plasmids utilized for present studies
| Plasmid or mutant | Expressed protein or substitutiona | Restriction site | Reference or source |
|---|---|---|---|
| pCAGGS.MCS | Ampicillin selec. marker | Multiple | 19 |
| pF42 | EBV gp42 | EcoRI/BgIII | 12 |
| pF25 | EBV gL | EcoRI/BgIII | 12 |
| pF85 | EBV gH | ClaI/BgIII | 12 |
| pF110 | EBV gB | EcoRI/ClaI | 12 |
| pEGFP-N1 | EGFP | Multiple | Clontech |
| pOS2 | T7 RNA polymerase, G418 selec. marker | 39 | |
| pT7EMCLuc | T7 luciferase | 31 | |
| LI12 | EBV gp42 LFKQL-F | PmeI | This study |
| LI27 | CLNNF-L | PmeI | This study |
| LI82 | CLNNW-T | PmeI | This study |
| LI93 | MFKHQ-N | PmeI | This study |
| LI104 | CLNSN-T | PmeI | This study |
| LI112 | CLNTY-K | PmeI | This study |
| LI118 | CLNIY-F | PmeI | This study |
| LI122 | MFKQK-K | PmeI | This study |
| LI134 | CLNSA-E | PmeI | This study |
| LI148 | MFKHD-I | PmeI | This study |
| LI149 | CLNNI-L | PmeI | This study |
| LI179 | CLNID-G | PmeI | This study |
| LI193 | CLNNC-T | PmeI | This study |
| LI195 | CLNTY-V | PmeI | This study |
| LI206 | CLNTH-H | PmeI | This study |
| LI210 | LFKHS-F | PmeI | This study |
| LI216 | CLNSF-C | PmeI | This study |
| W44A | Trp to Ala | Acc65I | This study |
| Q89A | Gln to Ala | Acc65I | This study |
| T104A | Thr to Ala | XhoI | This study |
| Y107A | Tyr to Ala | BsaJI | This study |
| W125G | Trp to Gly | BsrDI | This study |
| R154A | Arg to Ala | MunI | This study |
| E160A | Glu to Ala | Esp3I | This study |
| Y185F | Tyr to Phe | BglII | This study |
| F210A | Phe to Ala | AflII | This study |
| R220A | Arg to Ala | HindIII | This study |
The mutant EBV gp42-expressed protein reveals either the 5-amino-acid insert followed by the residue (after the hyphen) indicating the location (LI12 contains LFKQL inserted before residue 12F) or the change in point mutation (W44A is tryptophan to alanine).
Generation of mutants.
Mutations were generated using either a GPS-LS linker scanning system (New England Biolabs [NEB]) or a QuikChange kit (Stratagene) on an EBV gp42-containing pCAGGS/MCS vector (19) and isolated by cesium chloride density gradients. The NEB kit uses a transposon-based random mutagenesis to introduce a selection marker. After selection, clones are diagnostically digested and then sequenced to determine the exact location of the mutation. Collapse of the marker leaves a 5-amino-acid insertion introducing a rare PmeI site and a repeat of five host base pairs. The QuikChange kit uses PCR to introduce a specific mutation via primers designed with a silent mutation for diagnostic purposes. The suggested PCR protocol was followed to generate mutant clones, which were then diagnostically digested and sequenced.
Transfection. (i) Fusion assay.
CHO cells were seeded in plastic 24-well plates (Corning), grown 24 h to approximately 90% confluency, and transiently transfected with 0.125 μg each of EBV gH, gL, and gB, 0.5 μg of gp42 or gp42 mutant, and 0.2 μg of a luciferase-containing reporter plasmid with a T7 promoter (31). Transfections utilized 700 μl of Opti-Mem (Gibco) and 1 μl of Lipofectamine 2000 (Invitrogen) per well.
(ii) Western blotting and cell-based enzyme-linked immunosorbent assay (CELISA).
CHO cells were seeded into six-well plastic plates (Corning) and transfected with 4 μg of plasmid DNA with 2.5 ml of Opti-Mem and 5 μl of Lipofectamine 2000. The transfection efficiency was always simultaneously assessed by transfection of pEGFP-N1 and expression of enhanced green fluorescent protein.
Expression of mutants. (i) Western blotting.
CHO cells were transfected as previously stated. Media was changed 12 h later and cells were collected 24 h later. Cells were scraped, washed 2 to 3 times in PBS, and lysed using a 1% Triton X buffer with 1 mM sodium vanadate, 10 mM sodium fluoride, leupeptin (0.5 mg/ml), pepstatin (0.7 mg/ml), and 0.2 mM phenylmethylsulfonyl fluoride. Lysates were run on Bio-Rad 12.5% criterion gels in sodium dodecyl sulfate running buffer at 120 V for 90 min. Proteins were transferred to Immobilon-P membranes in transfer buffer at 90 V for 90 min with cooling or at 15 V overnight. Blots were blocked in Tris-buffered saline with Tween with 3% milk for an hour at room temperature (RT) or overnight at 4°C and then incubated for an hour at RT with a rabbit polyclonal anti-gp42 antibody (PB1114) diluted 1:1,000 in blocking solution. Blots were washed, and a secondary protein A-horseradish peroxidase (HRP)-conjugated antibody (Amersham) was applied for half an hour at RT. Blots were then mixed in equal volumes of ECL solutions and exposed to hyperfilm (Amersham Biosciences).
(ii) CELISA.
CHO cells were transfected as previously stated. After 12 h media was changed, and then 12 h later cells were detached with Versene and transferred to Corning 96-well plates, 3 wells per sample. After 16 h of incubation at 37oC, cells were washed with PBS-ABC, incubated for 30 min at RT with a rabbit polyclonal anti-gp42 antibody (PB1112) diluted 1:1,000 in PBS-ABC with 3% bovine serum albumin (PBS-BSA), and then fixed for 10 min in PBS with 2% formaldehyde and 0.2% glutaraldehyde. Cells were washed three times with PBS-BSA, incubated with a biotinylated goat anti-rabbit immunoglobulin G (IgG) (Sigma) at 1:500 for 30 min, washed five times, and then incubated with a streptavidin-HRP antibody (1:20,000) (Amersham) for 30 min, all at RT. Cells were then mixed with a peroxide substrate (BioFX Laboratories) and read with a Victor plate reader at 370 nm for 0.1 s.
Cell-cell membrane fusion assay.
The cell-cell membrane fusion assay was slightly modified from a previously published protocol (12). Briefly, CHO cells were transfected as stated previously. After 12 h, these cells were washed and overlaid with 5.0 × 105 target HLA class II-expressing Daudi B cells that had been stably transfected to express T7 RNA polymerase. After 24 h of incubation, the cells were washed twice with PBS and lysed, and 100 μl of firefly luciferin substrate was added to 20 μl of lysate (Promega luciferase assay system). Relative luciferase activity was measured in Visibottom 96-well plates by a Victor plate reader at 370 nm for 0.1 s.
Quantification of sgp42 levels by enzyme-linked immunosorbent assay (ELISA).
A baculovirus-generated soluble gp42 (sgp42) was used as a positive control in addition to transfected soluble gp42. Twenty-five microliters of transfected CHO cell supernatants diluted in PBSN (PBS with 0.02% sodium azide) was added to Nunc round-bottom plates (3 wells per samples), covered with plastic wrap, and allowed to incubate overnight at RT. Samples were blocked with PBS-1% BSA (blocking buffer) for 1 h at 37°C, followed by PB1112 diluted 1:1,000 in blocking buffer for 2 h at 37oC, goat anti-rabbit IgG diluted 1:500 in blocking buffer for 2 h at 37°C, and streptavidin-HRP diluted 1:20,000 in blocking buffer for 2 h at 37°C. Peroxide substrate was added, and plates were read on a Victor plate reader at 405 nm for 0.1 s.
Binding of sgp42 to HLA class II-expressing cells by flow cytometry.
This assay was slightly modified from the previously published protocol (26). After measuring relative levels of soluble gp42 in CHO cell supernatants by ELISA, supernatants were diluted in Ham's F-12 complete media and incubated with 5.0 × 105 Daudi cells in approximately 250 μl of RPMI complete media while rotating at 4°C for 20 min. Cells were then washed with fluorescence-activated cell sorting (FACS) buffer (PBS with 0.01% sodium azide, 1% HEPES buffer [BioWhittaker], and 1% fetal bovine serum), followed by incubation with PB1114 (diluted in FACS buffer to 1:250) and a biotinylated anti-DQ Ia3 antibody (1:100) on ice for 15 min. After washing with FACS buffer, secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit whole IgG and allophycocyanin-conjugated streptavidin (both at 1:250) (Pharmingen), also on ice for 15 min. Cells were washed and resuspended in 350 μl of FACS buffer. Immunofluorescence was measured using a Becton-Dickinson FACS-Sort cytometer and analyzed by using Cell Quest Pro. As a positive control, a baculovirus-generated sgp42 of residues 33 to 223 was utilized.
RESULTS
Construction of gp42 mutants.
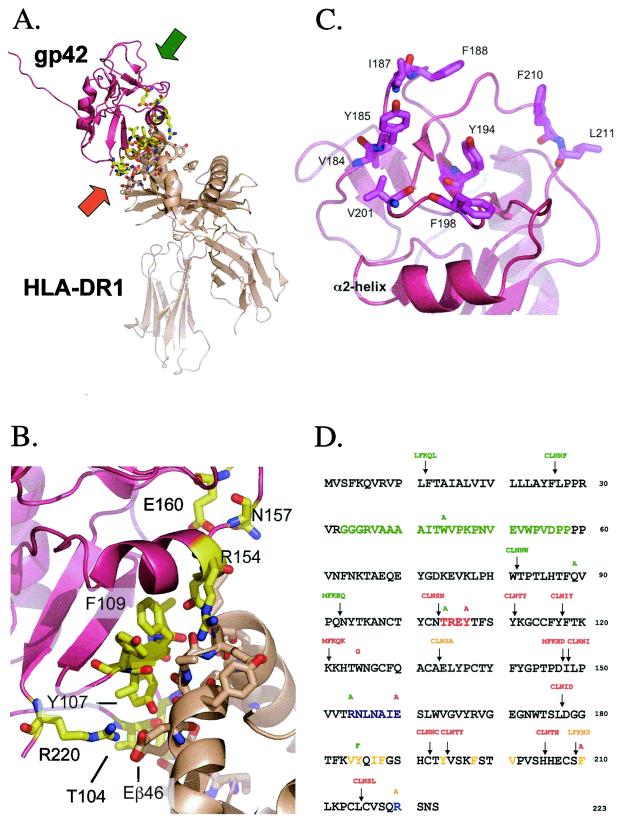
In order to better understand the mechanism of EBV-induced membrane fusion, we wanted to define functional domains of gp42. Initially, we utilized a transposon-based linker insertion mutagenesis to generate random 5-amino-acid inserts spaced throughout gp42. The NEB GPS-LS linker scanning system creates mutants with an insertion of five amino acids containing a 5-bp repeat from the host sequence along with the addition of a unique PmeI site. During this time, the crystal structure of a baculovirus-expressed soluble form of gp42 (sgp42) from residues 33 through 223 bound to HLA class II was solved, which revealed a short stretch of residues 104 through 107, an α-helix of residues 154 through 160, and arginine 220 all as areas of interaction with the HLA-DR1 β-chain (Fig. 1A). The HLA class II Εβ46 forms a salt bridge with gp42 R220 and hydrogen bonds with T104 and Y107, Rβ72 forms hydrogen bonds with T104 and Y107, and Nβ62, Sβ63, and Kβ65 all form hydrogen bonds with the α-helix (26, 28) (Fig. 1B). Although the ectodomain up to residue 85 was disordered, other structural features were also discovered. Ten cysteine residues of the native protein form five disulfide bonds (99 with 138, 102 and 115, 128 and 214, 132 and 216, and 192 with 208), and there are four potential N-linked glycosylation sites at residues 64, 93, 98, and 173. A potential dimerization site was delineated between residues 87 and 94, and a hydrophobic pocket was observed consisting of several aliphatic and aromatic residues between residues 161 and 201 (Fig. 1C). The proposed binding site with the gH/gL dimer located in the amino terminus was in the disordered region. Although the linker insertion mutants spanned all of these domains, we constructed additional site-directed mutations of individual residues to introduce specific mutations within certain domains. For these mutations, a Stratagene QuikChange kit was utilized. For each site-specific mutation, a unique restriction site was silently incorporated into the reading frame to allow easy identification of each mutant. More detailed procedures and the various plasmid clones used in these studies are described in Materials and Methods and in Table 1. Plasmid DNA was isolated by cesium chloride density gradients and sequenced to locate and confirm the nature of each mutation shown in Fig. 1D. Linker insertion mutants are named based on the residue number that follows the 5-amino-acid insert and are shown along with the locations of the point mutations.
FIG. 1.
Important structural features of EBV gp42 and residue sequence mapping location of mutations. (A) Three-dimensional ribbon model of soluble EBV gp42 (rose) bound to class II HLA-DR1 (beige). The amino terminus of residues 33 to 85 was disordered in the crystal structure. Backbones of residues interacting with HLA class II are yellow, oxygen atoms are red, and nitrogen atoms are blue. The orange and green arrows indicate the regions enlarged in panels B and C, respectively. (B) Close-up depicting key gp42 contact residues at the HLA class II interface. The color scheme is the same as in panel A. (C) Close-up of gp42 hydrophobic pocket highlighting aliphatic and aromatic residues. (D) The residue sequence of gp42 reveals the locations of point mutations above residues and linker insertion mutations containing 5-amino-acid inserts above arrows. Linker insertion mutants are named based on the residue that their mutation precedes, e.g., LI12, and point mutants reveal original and mutation residue, e.g., W44A. The baculovirus-produced soluble gp42 (sgp42) used as a positive control in many experiments spans residues 33 through 223 and was kindly provided by Maureen Mullen. Residues of the potential gH/gL binding site are in green. HLA class II contact sites are indicated as follows: aromatic ring residues in red, α-2-helix in purple, and arginine 220 in blue. Hydrophobic residues in the pocket are in yellow. Mutants are color-coded by their function in the fusion assay: those in green have levels similar to the wild-type levels, those in orange have reduced levels of fusion, and those in red do not mediate fusion.
Analysis of cell surface expression, whole-cell expression, and secretion of the gp42 mutants.
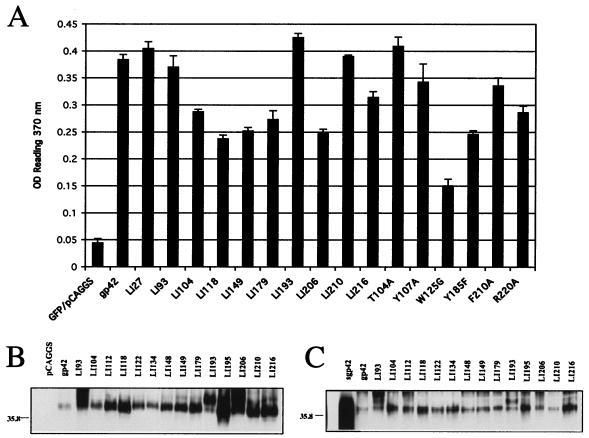
Prior to using the gp42 mutants in a cell-based fusion assay, we verified the expression of each of the mutants following transfection in CHO cells. Cell surface expression for each of the mutants was detected by CELISA at 40 h posttransfection. Only a subset of the mutants are shown in Fig. 2A, but all were tested. All mutants were expressed on the surface at detectable levels (threefold above background at the left of Fig. 2A), and many were expressed at levels similar to that of wild-type gp42. The W125G mutant was expressed the least, with an approximate 50% reduction compared to wild-type gp42. Total cellular expression of the gp42 mutants was measured by Western immunoblotting, and although the expression level for each of the mutants was somewhat variable, each was expressed at levels greater than that observed for wild-type gp42. Fourteen of the seventeen linker insertion mutants are shown in Fig. 2B. The linker insertion mutant LI193 consistently migrated higher than wild-type or other mutant gp42 samples on a gel (Fig. 2B, lane LI193). This mutant contains two asparagine residues in the insert, likely introducing a new glycosylation site at the first asparagine, resulting in the altered mobility. Finally, we investigated the levels of a secreted form of gp42 found in the media of the CHO-transfected cells 24 h posttransfection. As shown in Fig. 2C, the relative levels of gp42 in the medium supernatant for each of the gp42 linker insertion mutants were equal to or greater than that observed for wild-type gp42. Analyzed but not shown, the gp42 site-specific mutants also had expression levels similar to those of linker insertion mutants shown in Fig. 2C, except for W125G, which showed reduced levels similar to those obtained with the CELISA.
FIG. 2.
Verification of mutant gp42 surface expression by CELISA and of whole-cell expression by Western blotting. (A) CHO cells were transiently transfected to express wild-type or mutant gp42 and transferred to 96 wells, 3 wells per sample. Shown is a representative CELISA experiment with a random sampling of linker insertion mutants followed by point mutants in sequential order from amino terminus to carboxyl terminus. Positive expression is at least a threefold higher average reading of triplicate samples than the vector control shown at left. OD, optical density. (B and C) Mutant gp42 expression was verified as both a transmembrane form in whole lysates (B) and a secreted form in supernatants (C) of transiently transfected CHO cells by Western blotting. Molecular mass (kDa) is noted to the left of blots.
Function of gp42 mutants in membrane fusion.
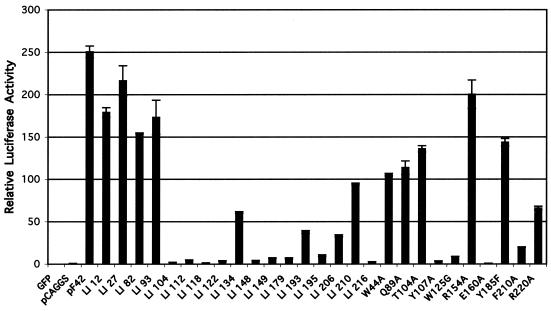
Since most gp42 mutants were expressed with levels similar to that of the wild type, we screened them for their ability to mediate membrane fusion in a virus-free assay (Fig. 3) as previously described (12). Briefly, effector CHO cells were transfected with expression constructs for gH, gL, gB, and either wild-type or one of the mutant forms of gp42. In addition, a plasmid with the luciferase gene under control of the T7 RNA polymerase was included. The effector cells were then overlaid with Daudi cells constitutively expressing T7 RNA polymerase. The T7 RNA polymerase-expressing cells were constructed as detailed in Materials and Methods. Upon fusion, luciferase expression is upregulated, which provides a quantitative measure of fusion between the two cell types. The fusion assay was repeated at least five times for each mutant, with clear patterns of the fusion competence of each mutant emerging. Results from a representative experiment with all of the gp42 mutants are shown in Fig. 3. Although surface expression levels vary as demonstrated in Fig. 2A, it is not a completely accurate gauge of fusion capability. Fusion levels consistently appear similar even if one mutant surface expression level is measurably higher than another, indicating a quantitative threshold of fusion and that detectable levels of surface expression are sufficient to assess the ability to mediate membrane fusion (compare LI93 and Y185F in Fig. 2A and 3). The mutants can be categorized by their fusion activity and, as might be expected from the crystallization studies, the various groups of mutations localized to specific domains of gp42, some in domains known to be functionally important and, interestingly, in others with no function currently ascribed. The first group of mutants, all located within the amino-terminal domain of gp42 or near the membrane-spanning domain of gp42, is competent in mediating fusion (LI12, LI27, LI82, LI93, W44A, and Q89A [Fig. 3]). Of the nine gp42 mutants with mutations localized in areas predicted to be important for binding to HLA class II, six had clear defects in fusion (LI104, LI112, LI148, LI149, Y107A, and E160A [Fig. 3]). Two of the gp42 mutations localized in these regions had no effect on membrane fusion (T104A and R154A [Fig. 3]), with the mutants having levels of fusion similar to that of wild-type gp42, whereas the R220A mutant was reduced in membrane fusion when compared to wild-type gp42. With the exception of the Y185F and LI210 gp42 mutants, the gp42 mutants localized to the hydrophobic pocket dramatically reduced membrane fusion when compared to wild-type gp42 (LI193, LI195, LI206, and F210A [Fig. 3]). All the remaining mutants, with the exception of LI134, were greatly reduced in fusion (LI118, LI122, LI179, LI216, and W125G [Fig. 3]). LI134 had a defect leading to a roughly fivefold reduction in membrane fusion. These mutations, which do not localize to the gp42 hydrophobic pocket or the HLA class II binding site, can be classified as “core/distant” mutations, since they are localized distant from these two gp42 functional domains. They likely interrupt important structural determinants of gp42, as they either are buried in the central part of the protein or are located in key structural determinants, and they most likely contribute to the overall structural integrity of gp42. They will be more fully discussed later. A summary of the results of the fusion assay is listed in Table 2.
FIG. 3.
Mutant gp42s vary in ability to mediate cell-cell fusion. CHO cells transiently transfected to express EBV gH, gL, gB, wild-type or mutant gp42, and luciferase driven by a T7 promoter were overlaid with Daudi cells stably transfected to express T7 RNA polymerase. Mutants are in order of location from the amino terminus to carboxyl terminus, linker insertion mutants are followed by point mutants, and readings are averages of two samples.
TABLE 2.
Summary of assays reveals four classes of gp42 mutations
| Classificationa | Mutant | Functionb | Fusionc | Bindingd |
|---|---|---|---|---|
| Unaffected | LI12 | TM domain | + | + |
| Unaffected | LI27 | N terminus | + | + |
| Unaffected | LI82 | N terminus | + | + |
| Unaffected | LI93 | N terminus | + | + |
| Unaffected | W44A | N terminus | + | + |
| Unaffected | Q89A | N terminus | + | + |
| Unaffected | T104A | Class II | + | + |
| Unaffected | R154A | Class II | + | + |
| Unaffected | Y185F | + | + | |
| Core/distant | LI118 | Structural | − | − |
| Core/distant | LI122 | Structural | − | − |
| Core/distant | LI134 | Structural | +/− | +/− |
| Core/distant | LI179 | Structural | − | − |
| Core/distant | LI195 | − | − | |
| Core/distant | LI216 | Structural | − | − |
| Core/distant | W125G | Structural | − | − |
| Class II | LI104 | Class II | − | − |
| Class II | LI112 | Class II | − | − |
| Class II | LI148 | Class II | − | − |
| Class II | LI149 | Class II | − | − |
| Class II | Y107A | Class II | − | − |
| Class II | E160A | Class II | − | − |
| Class II | R220A | Class II | +/− | − |
| LI193 | − | + | ||
| LI206 | − | + | ||
| LI210 | +/− | + | ||
| F210A | − | + |
Classification based on location of mutation and results of cell-cell fusion and class II binding assays.
Proposed or known functional domain where mutation is located. TM, transmembrane.
Mutants were tested for ability to mediate membrane fusion in cell-cell fusion assays as described in the text and for Fig. 3. Relative fusion levels were scored as follows: +, greater than 50%; +/−, roughly between 10 and 50%; and −, no fusion.
Mutants were tested for ability of soluble form to bind HLA class II-expressing human B cells as described in the text and for Fig. 4.
Binding of gp42 mutants to HLA class II-positive B cells.
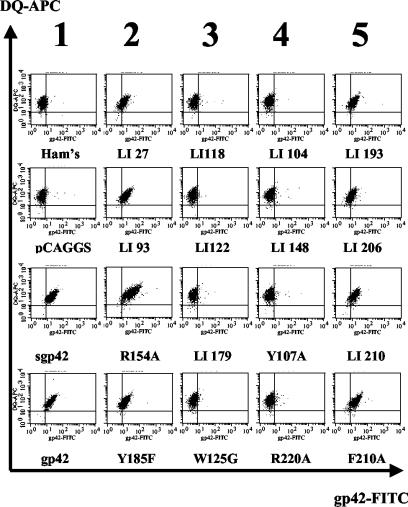
In order to further characterize gp42 mutants, we assayed their ability to bind HLA class II. We reasoned that it was unlikely that all mutants unable to mediate membrane fusion would also be defective in HLA class II binding and that the results might reveal another requirement for membrane fusion. Similar to previous studies in which the binding of a baculovirus-expressed gp42 was assayed (26), the Daudi human B-cell line, which expresses abundant levels of all alleles of HLA class II, was used (18). In this assay, supernatants of transfected CHO cells were collected, and the amount of secreted gp42 was quantified by ELISA (data not shown) and diluted so that similar amounts of the soluble gp42 were contained in each sample. The concentration-adjusted gp42 was then added to Daudi cells at 4°C to allow binding of gp42 to HLA class II on the cell surface. Binding of gp42 to HLA class II was detected by using rabbit serum directed against gp42 followed by FITC-conjugated secondary antibody. HLA class II expression was monitored by using a biotinylated anti-HLA class II antibody followed by allophycocyanin-conjugated streptavidin antibody. The cells were then analyzed by FACS. Controls are shown in the first column of Fig. 4. No FITC-positive cells were detected when media alone (Ham's) or cell supernatants from vector control-transfected cells (pCAGGS) were tested, despite the abundance of HLA class II-positive cells. When either the baculovirus-expressed gp42 or supernatants from wild-type gp42 were tested, binding was readily detected (Fig. 4, sgp42 [baculovirus] and gp42 [CHO cells]). Previous studies have shown that gp42 binding to target cells is dependent on HLA class II expression (23, 35).
FIG. 4.
FACS analysis reveals differences among mutants in binding to HLA class II and mediating fusion. Daudi B lymphocytes (5 × 105) expressing HLA class II were incubated with 25 μl of supernatant from transfected CHO cells expressing wild-type or mutant gp42. Cells were stained for gp42 and class II HLA-DQ and analyzed by flow cytometry using a Becton-Dickinson FACS-Sort. Columns represent five groups. Column 1 shows controls; the top two are negative media and vector controls, the bottom are baculovirus-produced sgp42 and transfected CHO cell supernatant gp42 (wild type). Column 2 reveals the mutants that are unaffected in binding to HLA class II and are able to mediate fusion. Column 3 shows those that cannot mediate binding to HLA class II or fusion, but the locations are not in HLA class II contact sites. Column 4 contains mutants unable to bind HLA class II and mediate fusion, but the mutations are localized to HLA class II contact sites. Column 5 contains mutants that are able to bind to HLA class II but are unable to mediate fusion and localize to the hydrophobic pocket.
Binding of a subset of gp42 mutants to HLA class II is shown in Fig. 4, and the remaining data with all of the gp42 mutants are summarized in Table 2. From this analysis and our previous analysis of the gp42 mutants in fusion, the mutants can be subdivided according to characteristics of binding to HLA class II and fusion competence, resulting in four categories of gp42 mutants. In the second column of Fig. 4, examples of the first category of mutants are shown. These mutants are unaffected in both binding to HLA class II and fusion. The mutants are LI27, LI93, R154A, Y185F (all shown in Fig. 4), LI12, LI82, W44A, Q89A, and T104 (data not shown). The second group consists of the so-called core/distant mutants (column 3). These mutations do not localize to HLA class II contact sites or the hydrophobic pocket and are generally negative in binding to HLA class II (LI118, LI122, LI179, W125G [all shown in Fig. 4], LI134, LI195, and LI216 [summarized in Table 2]). None of these mutants is able to efficiently induce membrane fusion, likely as a consequence of an inability to bind HLA class II. The inclusion of linker insertion mutant LI195 in this category is somewhat of a surprise. Although the mutation localizes to the gp42 hydrophobic pocket, it appears to be close enough to the α-helix region that mediates HLA class II binding to disrupt the correct conformation, rendering the mutant unable to bind HLA class II. In the fourth column, representative mutants are shown which have mutations that localize to HLA class II binding sites, are unable to bind HLA class II, and as a consequence are unable to mediate fusion (LI104, LI148, Y107A, and R220A [Fig. 4]). Other mutants tested that were also unable to bind HLA class II are summarized in Table 2 (LI112, LI149, and E160A). The T104A and R154A mutants, which localize to HLA class II binding sites, are able to bind HLA class II and mediate fusion, indicating that these sites tolerate mutation without affecting HLA class II binding. The final group consists of gp42 mutants that localize to the hydrophobic pocket and is represented in the fifth column. These mutants generally are not affected in binding to HLA class II (with the exception of LI195, as described above), yet these mutants cannot mediate fusion (LI193, LI206, LI210, and F210A [Fig. 4]). Y185F is the only mutant in the hydrophobic pocket able to mediate fusion similar to the wild type (summarized in Table 2).
DISCUSSION
We wished to confirm crystallization studies of EBV gp42 bound to HLA class II and also ascertain if domains not binding HLA class II were involved in membrane fusion. The analyses of gp42 mutants provide additional clues as to how gp42 may be involved in the initiation of membrane fusion, an essential process for the entry of EBV into B lymphocytes. From these studies, four general categories of gp42 mutants were identified and are summarized in Table 2. Figure 5 shows three-dimensional ribbon models with the locations of the mutations based on these categories that are explained further below. The four categories define mutants that (i) are unaffected for binding and fusion, (ii) likely induce folding defects in the gp42 structure, disrupting HLA class II binding and fusion, (iii) are located in the HLA class II binding site, do not bind to HLA class II, and also do not mediate fusion, or (iv) are located in the hydrophobic pocket and are able to bind HLA class II but unable to mediate fusion. Interestingly, for each of these categories we were able to identify a single point mutant.
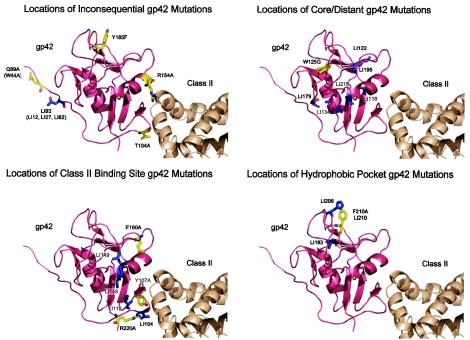
FIG. 5.
Ribbon models identifying locations of all gp42 mutants based on classes identified from Table 2. The classes are inconsequential, core/distant, HLA class II binding, or hydrophobic pocket. In each panel, the sites of the linker insertion (LI) mutants are indicated in blue and the site-specific mutants are indicated in yellow. Abbreviations and color scheme follow those in previous figures.
Mutations in the transmembrane domain and amino-terminal domain located near the membrane-spanning domain are unaffected in HLA class II binding and fusion (Fig. 5, upper left).
LI12, LI27, LI82, LI93, W44A, and Q89A, in all of which the mutations located within the amino-terminal domain of gp42, were unaffected in their ability to bind HLA class II and mediate fusion. These results are compatible with results from our structural studies of gp42 bound to HLA class II that indicate that this region is not involved in the contact of gp42 with HLA class II. Interestingly, this region may be important for the dimerization of gp42, as observed in our previous structural studies, and the interaction of gp42 with the gH and gL complex. Previous studies have shown by deletion that a region in the first 58 amino acids of gp42 is critical for the interaction of gp42 with gH and gL (38). In addition, this gp42 deletion mutant was unable to complement a gp42-deficient recombinant virus, indicating that complex formation between gp42 and gH/gL is likely essential for EBV infectivity. None of the present mutants within this region of gp42, the HLA class II interface, or the hydrophobic pocket had any defect in the association of gp42 with gH/gL (data not shown), indicating that additional mutants should be isolated to determine the functional significance of the interaction of gp42 with gH/gL and its importance in fusion. Although gp42 was observed as dimers in the crystal structure, formation of gp42 dimers has not been detected by other analyses; thus, specific mutants within the dimerization region of gp42 may directly test the functional significance of gp42 dimer formation.
Mutations that localize to neither the HLA class II binding site nor the hydrophobic pocket are unable to mediate fusion and binding (Fig. 5, upper right).
Surprisingly, several mutants with linker insertions in regions that neither make contact with HLA class II nor are located in the hydrophobic pocket do not bind class II HLA-DQ and are unable to mediate fusion. These include LI118, LI122, LI134, LI179, and LI216, although LI134 seems to allow low levels of binding and fusion. Since each of the mutations is distant to the HLA class II binding site but blocks HLA class II binding, with the possible exception of LI134, it is likely that these mutants alter the overall structure of gp42. Compatible with this idea, many of these mutants fall within major structural features of gp42. LI118 and LI216 fall within two antiparallel β-sheets, for which the insertion of the 5-amino-acid linker would be expected to have dramatic structural consequences. LI134 is located at the base of one of two α-helices within the gp42 structure. Although not within the core, this mutation may also be expected to alter the gp42 conformation. Compatible with this, LI134 binds weakly to HLA class II and does not mediate fusion at wild-type levels.
The linker insertion site for the mutants LI122 and LI179 is contained within exterior loops that connect structural determinants of the gp42 core. Since both insertion sites are fairly distant from the HLA class II binding site and are not near cysteines forming gp42 disulfide bonds, these results may indicate that the overall gp42 structure is easily perturbed, as indicated by the inability to bind HLA class II, but still sufficiently folded to result in expression and secretion of gp42, as was observed with all of the mutants. The point mutant W125G would also appear to globally alter the gp42 structure, as demonstrated by lack of binding to HLA class II and fusion competence. The tryptophan residue is within the core of gp42 and may act as a platform for the gp42 hydrophobic pocket to rest on. The dramatic substitution to a glycine residue, if the tryptophan does serve as a major structural determinant, may alter the stability of the protein. Consistent with this idea, of all of the mutations described in the present study, the W125G mutant exhibited both a decrease in cell surface expression and in the supernatant of transfected cells.
Not all mutants localized to HLA class II binding domains disrupt gp42 function (Fig. 5, lower left).
Y107A, E160A, and R220A mutants all lost their ability to bind HLA class II and are unable to mediate fusion, highlighting the requirement of gp42 binding to HLA class II in fusion. Surprisingly, low levels of fusion are consistently detected with the R220A mutant, which does not detectably bind to HLA class II expressed on Daudi cells. One possible explanation is that perhaps the salt bridge is not as critical as the hydrogen bonds and some low-level binding may occur that is not readily apparent in the binding assay, allowing for some measurable fusion to occur. All of the linker insertion mutants that are within or close to HLA class II contact sites are unable to bind HLA class II and are also unable to mediate fusion (LI104, LI112, LI148, and LI149). A 5-amino-acid insert clearly eliminates all binding activity in this region. Although T104A and R154A localize to HLA class II binding sites, they are able to bind to HLA class II as well as mediate fusion. Previous structural studies of the gp42 HLA class II complex indicated that the carbonyl oxygens from the main chains of T104 and Y107 of gp42 form hydrogen bonds with Rβ72 of HLA class II (28). Mutational analysis of Rβ72 of HLA class II indicated that this residue is essential for gp42 binding to HLA class II and EBV entry (26). The T104A mutation, which would be predicted not to disrupt the hydrogen bond between T104 and Rβ72, has no effect on gp42 binding to HLA class II or EBV-induced membrane fusion, as might be expected. However, mutation of Y107 blocks both, probably due to multiple effects by potentially changing the local conformation of the region that binds HLA class II as well as blocking a hydrogen bond with Eβ46. In regard to the mutation of R154 of gp42, previous mutational studies of HLA class II Sβ63, which hydrogen bonds with gp42 R154, indicated that this interaction of R154 with Sβ63 is not important for gp42 binding to HLA class II or EBV entry since mutation of Sβ63 to either an alanine or lysine did not block gp42 binding to HLA class II or EBV entry (26). This result is further supported by the present results indicating that R154 of gp42 is not important for gp42 binding to HLA class II or EBV-mediated fusion.
Though gp42 is structurally similar to some other C-type lectin superfamily members, the gp42/HLA class II interaction is unique compared to other natural killer (NK) receptor complexes, namely, Ly49A/MHC class I and NKG2D/MHC class I homolog (MICA) (28). The interaction is also different from the HSV gD/HveA binding complex. McShane et al. clearly showed that a single residue mutation of HLA-DQ could eliminate sgp42 binding and EBV infection, whereas single mutations of HveA did not affect HSV entry, suggesting that most contact residues contribute to receptor function collectively rather than individually (26).
Many mutations that localize to the hydrophobic pocket are unaffected in HLA class II binding but do not mediate fusion (Fig. 5, lower right).
LI193, LI206, LI210, Y185F, and F210A are all able to bind to class II-expressing cells. However, of these, only Y185F is able to efficiently mediate fusion similarly to the wild type. In the hydrophobic pocket, it is interesting to see that a single point mutant can distinguish HLA class II binding from the ability to mediate membrane fusion. It is perhaps not surprising that the relatively conservative mutation of tyrosine to phenylalanine at amino acid 185 does not alter fusion activity. In contrast, the more drastic change of phenylalanine to alanine at amino acid 210 does disrupt fusion significantly. It is likely, as with the case of HLA class II binding, that some of these hydrophobic residues are more critical than others for generating a strong interaction with a potential unknown binding partner. Interestingly, all of the linker insertion mutants within the gp42 hydrophobic pocket have greatly reduced fusion when compared to that of the wild type, LI210 clearly being positive for fusion but at reduced levels, whereas LI193 and LI206 have fusion consistently above background but not nearly as high as that of LI210. Of the linker insertions, only LI195 appears to be entirely negative for fusion. Importantly, the site of this insertion is close to the α-helix HLA class II binding site, resulting in a mutant gp42 that is unable to bind HLA class II. This may be the primary defect in this mutant and not due to the gp42 hydrophobic pocket. It is likely that proximity of the mutation to the HLA class II binding site coupled with the insertion of five amino acids disrupts the HLA class II binding function of gp42 in the LI195 mutant. Therefore, this mutant has been classified as a core/distant mutant, as it does not show the hydrophobic pocket mutant phenotype, that is, the ability to bind HLA class II but not mediate fusion.
The mutants described in this report have helped to define functional domains required for fusion. We have demonstrated that, in addition to a functional gp42/HLA class II binding site, a structurally intact hydrophobic pocket is required to initiate membrane fusion. The discovery of this hydrophobic pocket is interesting for two reasons: the functional requirement (i) adds to our current model of EBV-induced membrane fusion and (ii) provides a potential target for antiviral strategies. Like the HSV gD/HveA interface, only a handful of the EBV gp42/HLA class II contact residues that comprise the interface are actually required for binding (5). The structural studies of gD by Carfí et al. suggested that the N-terminal gD hairpin was conformationally flexible and that a conformational change accompanying binding might be part of the viral entry mechanism (4). Although structurally distinct from gD, gp42 could have a similar functional mechanism, and such a conformational change might also be required for membrane fusion. Binding of HLA class II might induce a conformational change that reveals the hydrophobic pocket, which is then able to bind its viral or cellular ligand. If the pocket is occupied before binding to HLA class II, the structure could reposition the viral ligand to come in contact with the host membrane. The functional requirement of the hydrophobic pocket makes it an excellent target for antiviral inhibitors. Modis et al. discovered a ligand-binding hydrophobic pocket in the dengue virus envelope glycoprotein E that is briefly exposed as fusion-competent trimers form, implicating a multiconformational mechanism of membrane fusion (27). This hydrophobic pocket was discovered to bind a single molecule of the detergent n-octyl-β-d-glucoside, indicating a potential site for small-molecule inhibitors. The hydrophobic pocket of the human immunodeficiency virus gp120/41 trimer has already proven fruitful in both identifying synthetic peptides and screening small molecules to inhibit binding and fusion (7, 8). Such results are encouraging for future studies of potential inhibitors of gp42-mediated membrane fusion. The mutants generated from these experiments will be used to supplement our knowledge of binding requirements with the gH/gL complex and with gB if there is a direct interaction. We will next use these mutants to identify the ligand of this hydrophobic pocket, which could be viral or cellular, which would further characterize requirements and mechanisms involved with initiating membrane fusion and provide a target to block viral entry.
Acknowledgments
We thank Gant Luxton, George Stanton, and Asheera Alvarez for helping generate mutants; Charu Deshpande for help in generating stably transfected Daudi cells; and members of the Longnecker and Spear laboratories for a surplus of technical advice, especially Cheryl Jogger, Alina Fridberg, Keith Haan, and Marisa McShane.
R.L. is a Stohlman Scholar of the Leukemia and Lymphoma Society of America and supported by the Public Health Service grants CA62234, CA73507, and CA93444 from the National Cancer Institute and DE13127 from the National Institute of Dental and Craniofacial Research. T.S.J. is a Scholar of the Leukemia and Lymphoma Society of America and supported by the Public Health Service grants GM61050, AI38972, and CA93444 from the National Cancer Institute. A.L.S. is supported by the training program in the Cellular and Molecular Basis of Disease (T32 GM08061) from the National Institutes of Health.
REFERENCES
- 1.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 2.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 3.Burkitt, D. 1962. A children's cancer dependent on climatic factors. Nauchni. Tr. Vissh. Med. Inst. Sofiia 194:232-234. [DOI] [PubMed] [Google Scholar]
- 4.Carfí, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 5.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J. Virol. 77:8127-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J. Virol. 76:10894-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debnath, A. K., L. Radigan, and S. Jiang. 1999. Structure-based identification of small molecule antiviral compounds targeted to the gp41 core structure of the human immunodeficiency virus type 1. J. Med. Chem. 42:3203-3209. [DOI] [PubMed] [Google Scholar]
- 8.Eckert, D. M., V. N. Malashkevich, L. H. Hong, P. A. Carr, and P. S. Kim. 1999. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell 99:103-115. [DOI] [PubMed] [Google Scholar]
- 9.Epstein, M. A., B. G. Achong, and Y. M. Barr. 1964. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 15:702-703. [DOI] [PubMed] [Google Scholar]
- 10.Fingeroth, J. D., J. J. Weis, T. F. Tedder, J. L. Strominger, P. A. Biro, and D. T. Fearon. 1984. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA 81:4510-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haan, K. M., W. W. Kwok, R. Longnecker, and P. Speck. 2000. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J. Virol. 74:2451-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 13.Haan, K. M., and R. Longnecker. 2000. Coreceptor restriction within the HLA-DQ locus for Epstein-Barr virus infection. Proc. Natl. Acad. Sci. USA 97:9252-9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heineman, T., M. Gong, J. Sample, and E. Kieff. 1988. Identification of the Epstein-Barr virus gp85 gene. J. Virol. 62:1101-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrold, R. E., A. Marchini, S. Fruehling, and R. Longnecker. 1996. Glycoprotein 110, the Epstein-Barr virus homolog of herpes simplex virus glycoprotein B, is essential for Epstein-Barr virus replication in vivo. J. Virol. 70:2049-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutt-Fletcher, L. M., and C. M. Lake. 2001. Two Epstein-Barr virus glycoprotein complexes. Curr. Top. Microbiol. Immunol. 258:51-64. [DOI] [PubMed] [Google Scholar]
- 17.Janz, A., M. Oezel, C. Kurzeder, J. Mautner, D. Pich, M. Kost, W. Hammerschmidt, and H. J. Delecluse. 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74:10142-10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein, G., G. Clements, J. Zeuthen, and A. Westman. 1976. Somatic cell hybrids between human lymphoma lines. II. Spontaneous and induced patterns of the Epstein-Barr virus (EBV) cycle. Int. J. Cancer 17:715-724. [DOI] [PubMed] [Google Scholar]
- 19.Kobasa, D., M. Rodgers, K. Wells, and Y. Kawaoka. 1997. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J. Virol. 71:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langeland, N., A. M. Oyan, H. S. Marsden, A. Cross, J. C. Glorioso, L. J. Moore, and L. Haarr. 1990. Localization on the herpes simplex virus type 1 genome of a region encoding proteins involved in adsorption to the cellular receptor. J. Virol. 64:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, S. K., T. Compton, and R. Longnecker. 1997. Failure to complement infectivity of EBV and HSV-1 glycoprotein B (gB) deletion mutants with gBs from different human herpesvirus subfamilies. Virology 237:170-181. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. K., and R. Longnecker. 1997. The Epstein-Barr virus glycoprotein 110 carboxy-terminal tail domain is essential for lytic virus replication. J. Virol. 71:4092-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longnecker, R. 1998. Molecular biology of Epstein-Barr virus, p. 133-174. In D. J. McCance (ed.), Human tumor viruses. American Society for Microbiology, Washington, D.C.
- 26.McShane, M. P., M. M. Mullen, K. M. Haan, T. S. Jardetzky, and R. Longnecker. 2003. Mutational analysis of the HLA class II interaction with Epstein-Barr virus glycoprotein 42. J. Virol. 77:7655-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullen, M. M., K. M. Haan, R. Longnecker, and T. S. Jardetzky. 2002. Structure of the Epstein-Barr virus gp42 protein bound to the MHC class II receptor HLA-DR1. Mol. Cell 9:375-385. [DOI] [PubMed] [Google Scholar]
- 29.Nemerow, G. R., C. Mold, V. K. Schwend, V. Tollefson, and N. R. Cooper. 1987. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J. Virol. 61:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuhierl, B., R. Feederle, W. Hammerschmidt, and H. J. Delecluse. 2002. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. USA 99:15036-15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 32.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed. Raven Press, New York, N.Y.
- 33.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speck, P., K. M. Haan, and R. Longnecker. 2000. Epstein-Barr virus entry into cells. Virology 277:1-5. [DOI] [PubMed] [Google Scholar]
- 35.Spriggs, M. K., R. J. Armitage, M. R. Comeau, L. Strockbine, T. Farrah, B. Macduff, D. Ulrich, M. R. Alderson, J. Mullberg, and J. I. Cohen. 1996. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR β chain and inhibits antigen presentation. J. Virol. 70:5557-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanner, J., J. Weis, D. Fearon, Y. Whang, and E. Kieff. 1987. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50:203-213. [DOI] [PubMed] [Google Scholar]
- 37.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whetter, L. E., S. P. Day, E. A. Brown, O. Elroy-Stein, and S. M. Lemon. 1994. Analysis of hepatitis A virus translation in a T7 polymerase-expressing cell line. Arch. Virol. Suppl. 9:291-298. [DOI] [PubMed] [Google Scholar]
- 40.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]