Abstract
Insulin-dependent diabetes mellitus (IDDM) is a chronic condition characterised by impaired blood sugar metabolism and autoimmunity. We report two children: a 5-year-old girl on exogenous insulin therapy of 30 IU/day and a 9-year-old boy on short-acting insulin 30 IU/day, long-acting insulin 70 IU/day, with IDDM since 4 and 7 years, respectively. We infused in vitro-generated donor bone marrow (BM)-derived haematopoietic stem cells (HSC) in patient 1 and insulin-secreting cells trans-differentiated from autologous adipose tissue-derived mesenchymal stem cells along with BM-HSC in patient 2 under non-myeloablative conditioning. Patient 1 improved during the initial 6 months, but then again lost metabolic control with increased blood sugar levels and insulin requirement of 32 IU/day; we lost her to follow-up after 18 months. Patient 2, over follow-up of 24.87 months, has stable blood sugar levels with glycosylated haemoglobin of 6.4% and present insulin requirement of 15 IU/day.
Background
Insulin-dependent diabetes mellitus (IDDM) is an organ-specific autoimmune disease resulting from the destruction of insulin-producing pancreatic β cells. Despite active research, IDDM has no definitive cure and patients with IDDM require lifelong insulin therapy. Bone marrow (BM)-derived haematopoietic stem cells (HSC) have been thought to be promising candidates for treating IDDM; and in light of recent discoveries, mesenchymal stem cells (MSC) and trans-differentiated insulin-secreting cells (ISC) have demonstrated great therapeutic potential and are aiding researchers in finding a cure for IDDM.
We report two patients with IDDM treated with stem cell therapy (SCT); one treated with BM-derived HSC and another with adipose tissue-derived MSC (ADMSC) and trans-differentiated ISC along with HSC.
Case presentation
Case 1: In January 2006, a 5-year-old girl presented with a classical triad of polyuria, polydypsia and polyphagia of IDDM along with weight loss, weakness and fatigue. She had three episodes of diabetic ketoacidosis (DKA) with uncontrolled blood sugar. She was a known case of IDDM for the past 4 years and was on 30 IU of insulin/day.
Case 2: In April 2011, a 9-year-old boy presented with the same symptoms. He was a known case of IDDM for the past 7 years; he was on short-acting insulin of 30 IU/day and long-acting insulin 70 IU/day. He had a family history of few relatives (grandmother, maternal grandmother, uncle and aunt) having IDDM.
Both the patients were on a strict vegetarian diet with routine exercise and normal lifestyle and were subjected to SCT.
Investigations
Case 1: With 22 kg body weight (BW), the patient's clinical examinations were unremarkable. Fasting and postprandial blood sugars (FBS/PPBS) were 210 and 246 mg/dL, respectively. She was admitted with glycosylated haemoglobin (HbA1c) of 10.1%, serum C-peptide 0.09 ng/mL, urine sugar +3, urinary albumin +2 and serum acetone 12 ng/mL. Her glutamic acid decarboxylase (GAD) antibody was positive, 90 IU/mL (Reference range <10 IU/mL).
Case 2: With 26 kg BW, the patient's clinical examinations were unremarkable. FBS and PPBS were 225 and 250 mg/dL, respectively. On admission, his HbA1c was 9.87%, serum C-peptide 0.06 ng/mL, urine sugar +4, urinary albumin +4 and serum acetone 19 ng/mL. GAD antibody was positive, 160 IU/mL.
Both patients’ thyroid, liver functions and lipid profiles were unremarkable.
Insulin/islet cell/adrenal antibodies were normal in both patients.
Treatment
The patients were administered bortezomib 1.3 mg/m2 body surface area, on days 1, 4, 8 and 11 to deplete autoimmune/rejecting β cells along with methylprednisone 125 mg, intravenously in 100 mL normal saline and rabbit antithymocyte globulin (r-ATG), 1.5 mg/kg BW on day 15, followed by stem cells infusion. Patient 1 was infused with in vitro-generated donor BM-derived HSC (with her mother as donor). Patient 2 was infused with a combination of in vitro-generated trans-differentiated ISC from autologous ADMSC and autologous BM-derived HSC. ISC and HSC were generated as per our previous protocol.1 In patient 1 HSC and in patient 2 ISC along with HSC were infused into portal circulation (80 and 77 mL), thymic circulation (2 mL in both) by femoral catheterisation under C-arm guidance and into abdominal subcutaneous tissue (20 mL in both). Informed patient consent forms and SC generation protocols were approved by the Institutional Review Board.
Total nucleated cells infused were 21×106/kg BW and HSC 52.1×106/kg BW in patient 1 and in patient 2 total nucleated cells were 20.4×106/kg BW, HSC 51.3×106/kg BW and ISC 1.66×104/kg BW. SC infusion was uneventful.
Outcome and follow-up
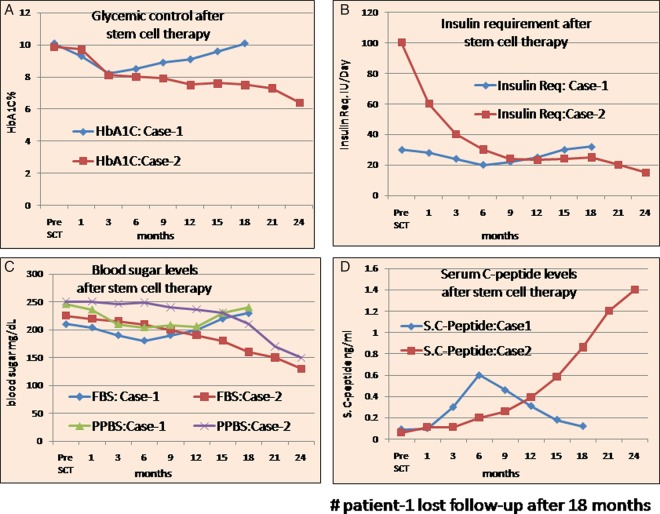
Case 1: The patient's FBS/PPBS had dropped down to 180 and 204 mg/dL, respectively, at 6 months post-SCT with HbA1c of 8.5%, serum C-peptide 0.8 ng/mL and insulin requirement 20 IU/day. After 6 months FBS/PPBS again stared rising and reached 230 and 240 mg/dL, respectively, with HbA1c of 10.1%, serum C-peptide 0.06 ng/mL with increased insulin requirement of 32 IU/day at 18 months. Her GAD antibody level was 86 IU/mL. She had become free of DKA episodes after SCT. We lost her to follow-up after 18 months, at the weight of 23 kg.
Case 2: Over follow-up of 24.87 months, the patient weighed 28.7 kg, his FBS/PPBS was 130 and 150 mg/dL, respectively, with HbA1c 6.4%. His serum C-peptide was sustained at 1.4 ng/mL with sustained present insulin requirement 15 IU/day. His GAD antibody level had dropped to 12 IU/mL. He was free of DKA episodes after SCT (figure 1).
Figure 1.
(A) Patients’ glycaemic control: pre-SCT and post-SCT; (B) Patients’ insulin requirement: pre-SCT and post-SCT; (C) Patients’ FBS and PPBS levels: pre-SCT and post-SCT; (D) Patients’ serum (S.) C-peptide levels: pre-SCT and post-SCT (FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; PPBS, postprandial blood sugar; SCT, stem cell therapy).
Discussion
IDDM is the result of the autoimmune response against pancreatic β cells. At the time of clinical diagnosis, near 70% of β-cell mass is destroyed as a consequence of the autodestruction that begins months/years before the clinical diagnosis. Patients with IDDM depend on exogenous insulin therapy for control of sugar levels.2 Consequently, interest in stem cells’ great regenerative capacity and differentiation potential as a possible cure for diabetes has surfaced.
Although few studies show the possibility of BM/BM-derived HSC transplantation as a therapeutic approach for β-cell replacement, the immunological destruction of newly regenerated β cells remains a problem. In non-obese diabetic (NOD) mice, induction of chimerism by sublethal or lethal irradiation followed by BM/BM-HSC transplantation does not result in recovery from IDDM. It is believed that HSC transplantation prevents diabetes in NOD mice but does not contribute to significant islet-cell regeneration once disease is established or in patients with long-term IDDM.3 While MSC with well-proven low immunogenic potential together with immunomodulatory effects, when transplanted under certain conditions, seem not only to keep immune destruction of islets in check, but can also trans-differentiate into pancreatic β cells.4
Other studies suggest that concomitant transplantation of HSC and MSC in NOD mice induces regeneration of ISC. In addition, MSC also inhibit β-cell-specific T-cell-mediated responses against newly formed ISC, which in turn are able to survive in an immunological milieu finally leading to reversal of metabolic control.5
In our study in case 1 with HSC infusion, we also observed improvement in glycaemic control with decrease in insulin requirement for 6 months, after which loss of glycaemic control and increased requirement of exogenous insulin followed. While in case 2 with ISC (trans-differentiated from ADMSC) and HSC infusion, sustained improvement in glycaemic control with reduced and steady insulin requirement was observed at follow-up of 24.87 months.
We conclude that SCT consisting of ADMSC trans-differentiated ISC and HSC co-infusion is feasible in patients with IDDM and/or other autoimmune disorders. However, further follow-up is necessary to confirm the duration of insulin independence and the mechanisms of action of the procedure.
Learning points.
Curative therapy for diabetes mellitus mainly implies replacement of functional insulin-producing β cells.
The regenerative potential of stem cells has promising results in view of Insulin-dependent diabetes mellitus (IDDM) treatment; haematopoietic (HSC) and mesenchymal stem cells (MSC) are highly focused candidates.
Several studies have contraindicated β-cell regeneration capacity of HSC.
The regenerative, hypoimmunogenic and immunomodulatory potential of MSC make them a promising therapeutic tool for severe refractory autoimmune diseases.
For patients with IDDM, co-infusion of adipose tissue-derived MSC trans-differentiated insulin-secreting cells along with HSC seems more promising than HSC infusion.
Footnotes
Contributors: SDD carried out stem cell generation and all other laboratory research work and wrote the manuscript. HLT designed the protocol and was responsible for patients’ care and helped in writing the manuscript. SGC and TC reviewed the literature and helped in writing the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc 2008;40:1135–9 [DOI] [PubMed] [Google Scholar]
- 2.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest 2001;108:1247–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet 2004;364:203–5 [DOI] [PubMed] [Google Scholar]
- 4.Melton DA. Reversal of type 1 diabetes in mice. N Engl J Med 2006;355:89–90 [DOI] [PubMed] [Google Scholar]
- 5.Orlando G, Gianello P, Salvatori M, et al. Cell replacement strategies aimed at reconstitution of the β-cell compartment in type 1 diabetes. Diabetes 2014;63:1433–44 [DOI] [PubMed] [Google Scholar]