Abstract
Galactose α1-3 galactose (Gal) trisaccharides are present on the surface of wild-type pig cells, as well as on viruses particles produced from such cells. The recognition of Gal sugars by natural anti-Gal antibodies (NAb) in human and Old World primate serum can cause the lysis of the particles via complement-dependent mechanisms and has therefore been proposed as an important antiviral mechanism. Recently, pigs have been generated that possess disrupted galactosyl-transferase (GGTA1) genes. The cells of these pigs do not express Gal sugars on their surface, i.e., are Gal null. Concerns have been raised that the risk of virus transmission from such pigs may be increased due to the absence of the Gal sugars. We investigated the sensitivity of porcine endogenous retrovirus (PERV) produced from Gal-null and Gal-positive pig cells to inactivation by purified NAb and human serum. PERV produced in Gal-null pig cells was resistant to inactivation by either NAb or human serum. In contrast, although Gal-positive PERV particles were sensitive to inactivation by NAb and human serum, they required markedly higher concentrations of NAb for inactivation compared to the Gal-positive cells from which they were produced. Complete inactivation of Gal-positive PERV particles was not achievable despite the use of high levels of NAb, indicating that NAb-mediated inactivation of cell-free PERV particles is an inefficient process.
A serious donor organ shortage limits human organ and cell transplantation as a therapy for end-stage organ disease and diabetes mellitus. Organs and tissues from pigs have the potential to relieve this shortage, since they are available in essentially unlimited quantities, and porcine grafts survive with life-supporting function in nonhuman primate (NHP) models (22). To date, however, graft survival in NHP models has been relatively limited, principally due to the chronic cell damage from natural antibodies (NAb) present in human serum that recognize galactose α1-3 galactose (Gal) epitopes present on pig cells (22). In the absence of appropriate conditioning immunosuppression in NHP recipients prior to transplantation, these antibodies can cause the almost immediate rejection of solid organ transplants via activation of the complement system, an effect called hyperacute rejection (HAR) (2).
In response, several therapeutic approaches have been investigated as a means to overcome HAR. Most recently, the production of Gal knockout pigs via the targeting of galactosyl-transferase (GGTA1) genes and nuclear transfer technologies has been reported (11, 18). While the production of Gal-null pigs is encouraging from the perspective of transplantation efficacy, it has raised certain safety concerns. In particular, the risk due to porcine endogenous retrovirus (PERV) has been the focus of significant attention (1, 27).
Complement-mediated lysis mechanisms have been proposed as an important antiviral defense against certain virus infections in vivo. Although initially the innate antiviral properties of human serum against gammaretroviruses (such as PERV and murine leukemia virus [MLV]) were thought to be due to activation of the complement system via the alternative pathway, it was later shown that this activity was mediated by the classical pathway, i.e., involving antibodies (3, 29). Subsequently, Takeuchi and others established that activity against gammaretroviruses was mediated via the recognition of Gal epitopes present on viral Env proteins by NAb that were present in the human serum (20, 21, 25). Recently, concerns have been raised that PERV-null particles produced by Gal-null pig cells will be resistant to NAb-mediated inactivation (1, 28). Therefore, while Gal-null pig organs may be more immunologically acceptable to their host, an elevated microbiological risk may be associated with the use of organs from Gall-null donor animals (1, 28).
The production of PERV that is infectious for certain human cells in vitro has been described for several breeds of pig, including micropigs, minipigs, miniature swine, and multiple land breeds (13, 31). Although the potential to infect human cells is widespread, animals have been identified that do not transmit human-tropic PERV (14). To date, all human-tropic PERV belong to either the PERV-A or PERV-B subgroup of virus (24). The third infectious subgroup, PERV-C, is not capable of infecting human cells but can participate in recombination events with PERV-A that generate PERV with increased infectious titers for human cells (14, 24, 30, 31). Although there is no evidence that PERV has been transmitted to humans exposed to porcine tissues or organs (6, 9, 15, 16, 19), concerns persist about this possibility (27).
In this report we address the consequences of GTTA1 gene knockout with respect to the biology of PERV. We address the capacity of Gal-null pig cells to produce PERV, the sensitivity of PERV to inactivation by human serum and purified NAb, and the susceptibility of Gal-null pig cells to infection with PERV. Our results demonstrate that Gal-null cells can produce replication-competent PERV and that the removal of Gal sugars renders PERV insensitive to inactivation by NAb. However, since NAb manifested only limited antiviral activity against Gal-positive virus particles, it is questionable whether loss of Gal epitopes is of practical consequence in prevention of PERV transmission in vivo.
MATERIALS AND METHODS
Cell culture.
All cell lines were obtained from the American Tissue Culture Collection and maintained in tissue culture medium (TCM): Dulbecco's modified Eagle's medium (Invitrogen Life Sciences) supplemented with 10% fetal bovine serum (Sigma) plus 100 U of penicillin/ml and 100 μg of streptomycin/ml.
The sensitivity of ST-IOWA (ATCC CRL-1746) and miniature swine PED cells (23) to G418 selection was determined by exposure of subconfluent cultures to G418 with subculture as necessary in order to maintain subconfluence.
PERV infection assays.
Cell-free infectivity assays were performed using standard methodologies as described previously (4). Briefly, viral supernatants were filtered (pore size, 0.45 μm), supplemented with 8 μg of polybrene/ml, and incubated with subconfluent target cell monolayers for 4 to 6 h at 37°C. As appropriate, infection was monitored either by β-galactosidase (β-Gal) staining or by an enzyme-linked immunosorbent assay for reverse transcriptase (RT) activity. Typically, three independent repeats were performed in each experiment.
The following viruses were used. (i) Replication-competent PERV-A. Subconfluent ST-IOWA cells were infected with PERV-A (14/220 isolate) grown in HEK293 cells (ATCC CRL-1573). PERV-A 14/220 was isolated during 293-cell in vitro transmission assays using peripheral blood mononuclear cells of an inbred miniature swine (14). It possesses the tropism of prototype PERV-A and grows to high titers in 293 cells in vitro (7). (ii) Replication-competent PERV-C. The minipig kidney cell line MPK (ATCC CCL-166) and ST-IOWA cells infected with the supernatant of MPK cells were used as sources of replication-competent PERV-C. (iii) Replication-competent PERV-A pseudotypes. Replication-competent PERV-A pseudotypes encoding LacZ activity were produced in the 293 cell line that had been infected with PERV-A 14/220 and transduced with the LacZ reporter vector MFGnlsLacZ (4). (iv) Replication-defective pseudotypes. The production of MLV-derived replication-defective retrovirus pseudotypes of PERV-A, -B, and -C, RD114, and gibbon ape leukemia virus (GALV) has been described previously (4, 24). (v) The miniature swine endothelial cell line PED was used as a source of PERV. This cell line was derived from the miniature swine primary aortic endothelial cells of an inbred animal of the SLAd/d haplotype and produces ecotropic, but not human-tropic, PERV (23).
Targeting of GGTA1 genes.
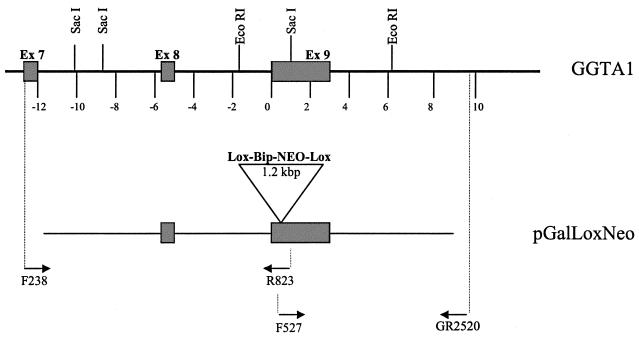
Targeting of GGTA1 loci has been described previously (11). Briefly, the GGTA1 alleles of PED and ST-IOWA cells were disrupted via homologous recombination using the gene-targeting vectors pGalGTΔS-Neo and pGalGTΔS-loxNeo, respectively. The pGalGTΔS-loxNeo vector is a modified pGalGTΔS-Neo vector (11) in which lox-P sites flank the G418 resistance sequence (see Fig. 1). Vector transfections were performed using the Dosper transfection agent (Roche, Chicago Ill.) according to the manufacturer's guidelines. Following transfection, cells were plated in a 96-well format (104 cells per well) and subjected to G418 selection until colonies developed. Targeting of the GGTA1 gene was assessed by RT-PCR as described previously (11), and targeted clones were expanded into 24-well plates prior to the isolation of genomic DNA. Analysis of the targeting events was done by using two PCR assays (11), each incorporating a primer outside of the vector region. The upstream genomic structure was assessed with primers F238 (exon 7, upstream of the 5′ end of the targeting vector) and R823 (exon 9, downstream of the selection cassette insertion site). Upon digestion with EcoRI, fragments of 2.0 (wild-type locus), 3.1 (targeted locus), and 10.4 kbp (either locus) were produced. The downstream genomic structure was assessed with primers F527 (exon 9, upstream of the selection cassette insertion site) and GR2520 (downstream of the 3′ end of the targeting vector). Upon digestion with SacI, fragments of 1.2 (wild-type locus), 2.3 (targeted locus), and 8.1 kbp (either locus) were produced.
FIG. 1.
Schematic of the galactosyltransferase gene GGTA1 and replacement vector pGalGTΔS-loxNeo. The genomic organization of the region of the genomic GTTA1 allele targeted by the GTTA1 replacement vector pGalLoxNeo is shown. Primer locations used for assessment of the 5′ and 3′ targeting events as well as the diagnostic restriction sites are presented. Note that integration-specific PCRs are achieved by amplification across the genomic DNA-to-vector boundaries.
Purification and quantification of NAbs.
Naive baboon plasma was adsorbed of NAb using a Gal-matrix column (Alberta Research Council, Alberta, Canada) as follows. The bound antibody was eluted from the column by low pH (0.25% acetic acid) into a Tris-based salt buffer (0.2 M Tris, 0.6 M NaCl) and dialyzed against phosphate-buffered saline (PBS). NAb was concentrated in accordance with the manufacturer's recommendations using Centripreps (Amicon) with a cutoff weight of 30 kDa followed by Centriplus Concentrators (Amicon) with a 50-kDa cutoff weight. The concentrated antibody was dialyzed against PBS, and the concentration was determined by UV absorbance and Bradford protein assay. Final stock solutions were diluted to 5 mg/ml and frozen in small aliquots at −80°C. Concentrations of NAb were determined by enzyme-linked immunosorbent assay as described previously (32).
Complement-NAb selection.
Approximately 2 × 106 cells were resuspended in 1 ml of TCM supplemented with 50 μg of baboon NAb/ml and incubated for 30 min at room temperature on a rotating mixer. The cells were then pelleted, washed with 10 ml of TCM, and resuspended in a total 1 ml of TCM containing 12.5% baby rabbit serum (as a source of supplementary complement) and 10 μg of DNase I (Sigma)/ml for 45 min at room temperature on a rotating mixer. The cells were pelleted, washed once with 10 ml of TCM, and resuspended in 0.5 ml of TCM, and surviving cells were counted using trypan blue exclusion. Cells were plated in a T25 flask, and the fresh TCM was added to the cells after 24 h.
FACS analysis.
For fluorescence-activated cell sorting (FACS) analysis of Gal expression, 105 cells were washed with 2 ml of FACS buffer (PBS containing Ca and Mg, 0.5% bovine serum albumin, 0.1% NaN3). After centrifugation, the cells were resuspended in approximately 20 μl of FACS buffer and fluorescein isothiocyanate-conjugated IB4-lectin (Sigma) added to give a final concentration of 8 μg/ml. The samples were incubated for 5 min at 37°C prior to washing the cells with 4 ml of FACS buffer, after which the cells were resuspended in 300 μl of FACS buffer and analyzed using a FACSort (Becton Dickinson).
Serum sensitivity assays.
To determine the complement sensitivity of PERV particles, infected cell culture supernatants were incubated with either purified baboon NAb or pooled human AB serum (Sigma and Mediatech Inc.) supplemented with 12.5% baby rabbit complement (serum) for 1 h at 37°C. These supernatants were then supplemented with 8 μg of polybrene/ml and incubated with target cell lines for 4 to 6 h at 37°C. The human 293 cell line or a single-cell clone of Gal-null ST-IOWA−/− cells were used as target cells.
To determine the complement sensitivity of wild-type and Gal-null ST-IOWA−/− cells, 5 × 105 cells were incubated for 45 min at 25°C in 1 ml of TCM supplemented with either purified NAb or pooled human AB serum supplemented with 12.5% baby rabbit serum. Cells were washed and plated in TCM, after which cell viability was assessed.
RESULTS
Production of Gal-null pig cells.
We produced two GGTA1 knockout cell lines. First, Gal-null ST-IOWA cells were produced because wild-type ST-IOWA cells are susceptible to infection by all subgroups of PERV (14, 17, 24). Therefore, assuming that the susceptibility of the cells would not be changed by the disruption of their GGTA1 genes, Gal-null ST-IOWA cells should have the potential to be used as a source of Gal-null PERV as well as a Gal-null target cell line. Secondly, Gal-null miniature swine PED cells were produced. The PERV transmission characteristics of this endothelial cell line are the same as those of the blood mononuclear cells of the majority of inbred miniature swine, i.e., the production of pig-tropic PERV-C, but not human-tropic PERV-A or -B. As such, Gal-null PED cells are an appropriate model for the analysis of PERV produced from Gal-null miniature swine xenografts.
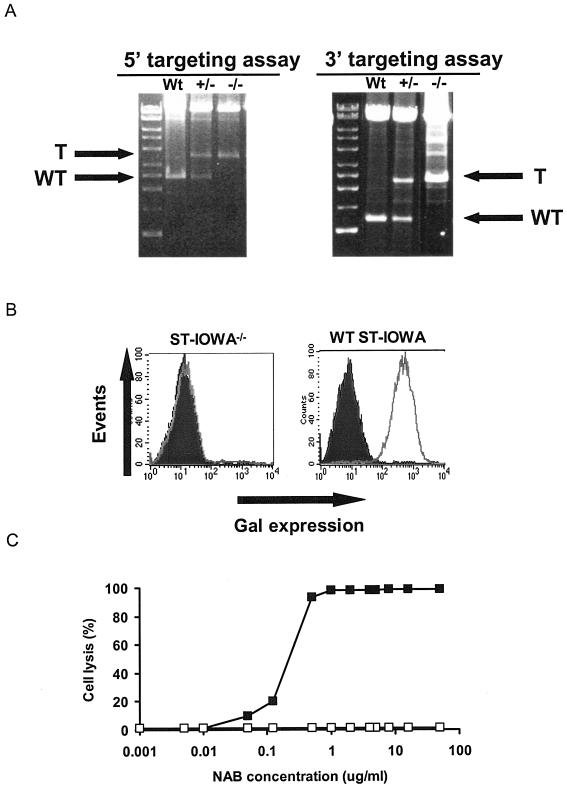
The first GTTA1 allele was disrupted via transfection of the pGalGTΔS-Neo and pGalGTΔS-loxNeo vectors, which disrupt the GTTA1 open reading frame through homologous replacement of a section of the GGTA1 allele (Fig. 1). The presence of the GGTA1 targeting event was verified by 3′ and 5′ genomic PCRs (Fig. 1 and 2A). The resultant cell populations were designated PED+/− and ST-IOWA+/−, reflecting the genotype of the GGTA1 alleles. FACS analysis indicated that the Gal expression of the cells was not affected by the targeting of one GTTA1 allele (data not shown).
FIG. 2.
Production of Gal-null porcine cells. (A) Targeting of the GGTA1 allele of PED cells using the pGalGTΔS-loxNeo vector. Arrows indicate PCR bands derived from targeted (T) and wild-type (WT) amplicons for the 5′ (primers F238 and R823) and 3′ (F527 and GR2520) genomic-DNA targeting assays. Similar results were obtained with single cell clones of Gal-null ST-IOWA cells. (B) FACS analysis of GGTA1-targeted ST-IOWA cells. Following targeting of the first allele, Gal-null cells were selected using three rounds of baboon NAb (50 μg/ml) and rabbit complement selection. The Gal status was analyzed by FACS analysis with IB4 lectin. Dark profile, unstained cells; light profile, IB4-stained cells. Similar results were obtained with PED cells. (C) Gal-null ST-IOWA cells are resistant to NAb-mediated lysis. ST-IOWA cells were exposed to purified NAb in the presence of supplementary complement, and cell lysis was determined by cell counting. ▪, Gal-positive ST-IOWA cells; □, Gal-null ST-IOWA cells.
Because of the stable insertion of the pGalGTΔS-loxNeo and pGalGTΔS-Neo targeting vectors, PED+/− and ST-IOWA+/− cell populations are resistant to G418. Consequently, these vectors are not useful for selectively targeting the second GGTA1 allele. Therefore, because mutations in the second wild-type GGTA1 allele should render the cells Gal− null, we used a complement-baboon NAb selection technique to isolate cells with spontaneous mutations in the second GGTA1 allele. FACS analysis indicated that three rounds of complement-baboon NAb treatment produced an essentially pure Gal-null cell population (Fig. 2B). The Gal-null phenotype of these ST-IOWA−/− cells conferred resistance to lysis by human serum containing high levels of NAb (Fig. 2C).
Susceptibility of Gal-null ST-IOWA cells to retroviral infection.
Alteration of the glycosylation pattern of cells can affect their susceptibility to infection by some retroviruses (5, 10, 12). We therefore investigated the susceptibility of ST-IOWA cells to retroviral infection using LacZ pseudotypes of PERV, RD114, GALV, and MLV-A. The infectious titer of each of these pseudotypes was comparable on wild-type ST-IOWA+/+ cells to that on Gal-null ST-IOWA−/− cells (Table 1), indicating that the Gal status of the ST-IOWA cells has little, if any, influence on the susceptibility of the cells. Thus, the susceptibility of Gal-null ST-IOWA−/− cells to infection (Table 1), together with their resistance to NAb-mediated complement lysis (Fig. 2C), made them a useful target cell line for infectivity assays involving complement and NAb.
TABLE 1.
Gal expression does not affect susceptibility of ST-IOWA cells to retrovirus infectiona
| Target cell line | Infectivity of retrovirus pseudotype (IU/ml)
|
|||||
|---|---|---|---|---|---|---|
| PERV-A | PERV-A 14/220 | PERV-B | PERV-C | RD114 | GALV | |
| ST-IOWA+/+ | 3 × 103 | 8 × 103 | 3 × 102 | 2 × 103 | 2 × 104 | 3 × 104 |
| ST-IOWA−/− | 3 × 103 | 6 × 103 | 4 × 102 | 3 × 103 | 2 × 104 | 4 × 104 |
Titers of cell-free retrovirus LacZ pseudotypes were determined on wild-type and GTTA1-null ST-IOWA cells. Quantitative analysis of susceptibility to MLV-A was not possible due to the low susceptibility of ST-IOWA cells (not shown). However, despite the low titers observed for MLV-A on porcine cells, the results suggested that Gal also had no effect on the susceptibility to this virus.
Infectivity and tropism of Gal-null PERV-A and PERV-C.
We used pseudotype assays to investigate the tropism of Gal-null PERV, i.e., PERV produced from Gall-null ST-IOWA cells. Replication-competent pseudotypes of PERV-A and PERV-C were produced by the transduction of wild-type ST-IOWA+/+, ST-IOWA+/−, and Gal-null ST-IOWA−/−cells with the packagable retroviral LacZ reporter vector MFGnlsLacZ, followed by infection with replication-competent PERV-A 14/220 or PERV-C (i.e., MPK cell supernatant). Using stocks of comparable RT activities, we detected similar infectious titers when titrating stocks of Gal-null and Gal-positive PERV on 293 (330 and 240 IU/ml, respectively) and ST-IOWA−/− (240 and 170 IU/ml, respectively) cells. These results indicate that the relative infectivity of the PERV particles had not been altered due to the absence of Gal molecules.
Sensitivity of PERV to inactivation by human serum and NAb.
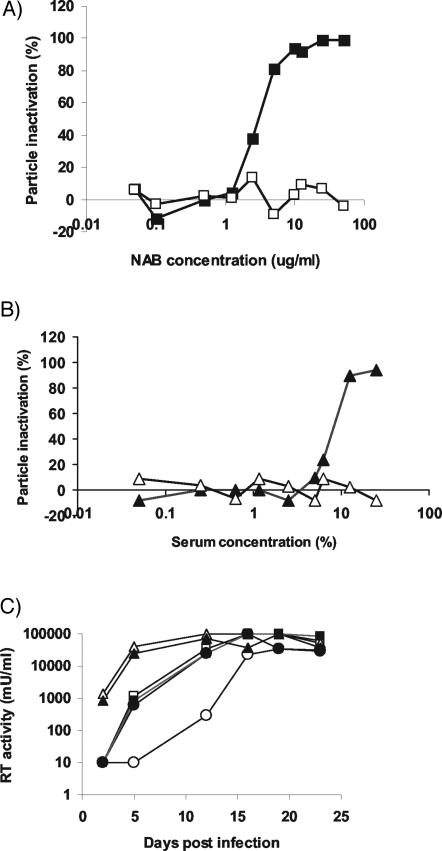
Infectivity studies with the LacZ pseudotypes reported above were used to determine the sensitivity of PERV particles to inactivation by purified NAb and pooled human AB serum. As shown in Table 2, inactivation of PERV was dependent on complement and anti-Gal antibodies. Titration studies indicated that the 50% inhibitory concentration (IC50) of NAb against Gal-positive PERV-A was approximately 3 μg/ml (Fig. 3A). The IC50 of human serum was 10% (Fig. 3B). Maximum particle inactivation via either source of NAb was approximately 95%, which, for purified NAb, was achieved at approximately 10 to 30 μg/ml (Fig. 3A). Treatment of PERV particles either with concentrations of NAb above 10 μg/ml or by increasing the complement supplement in the cultures to 50% in the presence of 30 μg of NAb/ml did not significantly increase the maximum antiviral activity (data not shown). Similarly, although a delay in PERV replication kinetics could be achieved by the treatment of Gal-positive PERV with 50 μg of NAb/ml, complete inactivation of PERV infectivity was not achievable (Fig. 3C).
TABLE 2.
The Gal status of PERV determines its sensitivity to NAb-mediated inactivationa
| Treatment | Titer of PER V-A as % of control (titer of control [IU/ml]) for producer cell line
|
|||||
|---|---|---|---|---|---|---|
| Gal-positive ST-IOWA+/+/PERV-A | Gal-null ST-IOWA−/−/PERV-A | Gal-null 293/PERV-A | Gal-positive ST-IOWA+/+/PERV-C | Gal-null ST-IOWA−/−/PERV-C | Gal-positive MPK | |
| FBS | 100 (170) | 100 (240) | 100 (170) | |||
| FBS + C′ | 135 | 79 | 100 | 100 (510) | 100 (890) | 100 (15) |
| NAb | 82 | 108 | 124 | |||
| NAb + C′ | 12 | 104 | 118 | 3 | 93 | 12 |
Relative titers of virus supernatants of PERV-A or PERV-C pseudotypes following treatment with 50 μg of NAb/ml and 12.5% rabbit serum (as a source of additional complement). Actual titers of the FBS-treated control are shown in parentheses. FBS, fetal bovine serum; C′, supplemental baby rabbit complement.
FIG. 3.
Gal-positive PERV is sensitive to inactivation by purified NAb (A) and human serum (B). The sensitivities of Gal-positive (filled symbols) and Gal-null (open symbols) PERV were assessed using cell-free LacZ pseudotype infection of Gal-null ST-IOWA−/− cells. (C) The infectivity of Gal-positive PERV-A is reduced, but not eliminated, following exposure to NAb. Replication-competent PERV-A was produced in either wild-type ST-IOWA+/+ cells (○, •), Gal-null ST-IOWA−/− cells (▪, □), or 293 cells (▴, ▵) and treated with either NAb at 50 μg/ml (open symbols) or fetal bovine serum (filled symbols). The treated supernatant was used to infect 293 cells, and infection was monitored by RT assay.
In contrast to the sensitivity of Gal-positive PERV, treatment of Gal-null PERV-A particles with NAb at concentrations up to 50 μg/ml had no significant effect on the infectious titer of the virus as measured by either pseudotype infectivity assays (Table 2 and Fig. 3A and B) or growth curves (Fig. 3C).
PERV transmission characteristics of Gal-null miniature swine cells.
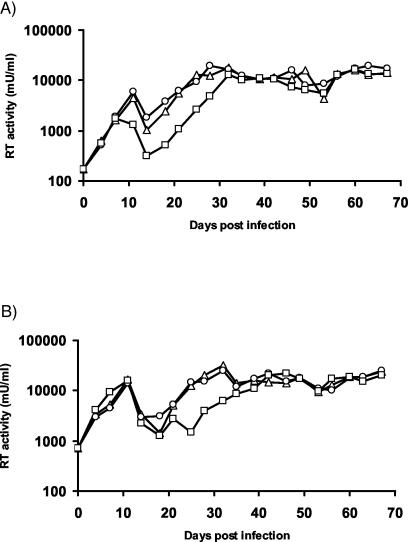
In order to investigate the biology of PERV in Gal-null miniature swine, we performed transmission studies using Gal-null and Gal-positive PED cells. Both cell types did not produce human-tropic PERV but did produce ecotropic PERV that infected Gal-null and Gal-positive ST-IOWA target cells (Fig. 4). These results indicate that virus released from Gal-null miniature swine cells can be replication competent but that engineering of the miniature swine cell line had not caused the production of replication-competent human-tropic PERV. Minor differences in the replicative capacity of the virus released from the PED cells were detected. While the replication competence of PERV released from PED+/+ cells and that with PED+/− cells were indistinguishable from each other, the growth kinetics of virus released from Gal-null PED−/− cells was somewhat reduced. This reduction was not linked to the Gal status of the ST-IOWA target cells, since Gal-null and Gal-positive ST-IOWA cells were equally able to support the replication of the ecotropic PERV (Fig. 4).
FIG. 4.
Replication competence of PERV released from engineered miniature swine PED cells. The replication competence of the ecotropic virus produced by miniature swine PED cells was assessed by using PERV infectivity assays in Gal-positive wild-type ST-IOWA+/+ (A) and Gal-null ST-IOWA−/− (B) target cells. The inoculating virus was produced from either PED+/+ (▵), PED+/− (○), or PED−/− (□) cells, and infection was monitored by RT assay.
Also, the growth curves for PERV were reproducibly characterized by two distinct phases (Fig. 4). The first phase of virus production peaked at approximately day 10. This peak was followed by a drop in RT activity until days 14 and 21 and then a rise in virus production to persistent and high levels of PERV by day 30.
DISCUSSION
The generation of Gal-null pigs is considered a major advance toward clinical xenotransplantation. It has been hypothesized, however, that this progress may come at the cost of an increased safety risk associated with PERV. These concerns prompted us to study the effects that gene targeting of GGTA1 in porcine cells may have on the biology of PERV.
Via the use of affinity-purified NAb, we demonstrate that inactivation of Gal-positive PERV particles is mediated via Gal epitopes. The IC50 of NAb against Gal-positive PERV was somewhat higher than that of the Gal-positive cells from which they were produced (3 μg/ml versus 0.3 μg/ml, respectively). The mechanism(s) responsible for this variation remains to be determined, but it is possible that the insensitivity of particles may be a result of lower-level Gal expression or alternatively may be the result of slower inactivation kinetics. In this regard, it is noteworthy that despite the use of high concentrations of NAb and human serum, complete inactivation of relatively low-titer stocks of PERV was not achieved. The incomplete inactivation of PERV by NAb is consistent with the incomplete inactivation of human immunodeficiency virus and MLV-based pseudotypes by human serum studies reported by Reed and Takeuchi (20, 25). In contrast, the potentially complete inactivation of Gal-positive PERV by NAb has been shown by others (8, 17). A number of factors could be responsible for the discrepancy between the above in vitro studies, such as the NAb content of the human serum used in the studies, the levels of Gal expression of the virus producer cells, the Gal epitope density on the virus particles, and the duration of exposure of the virus particles to human serum prior to addition to the target cells.
Much of the discussion regarding the safety consequences of the generation of Gal-null pigs refers to the loss of Gal-mediated antiviral mechanisms in vivo. In nonhuman primate models, Gal-positive porcine organs undergo hyperacute rejection unless intense immunotherapies are administered that interfere with the activity of NAb and/or the complement system (i.e., the administration of soluble Gal sugars, inactivation of complement, as well as the use of donors that are transgenic for complement regulatory proteins). Because these technologies act by disrupting the biological activities of the NAb-complement pathway, they will most likely also reduce or negate the associated antiviral properties. Therefore, the development of appropriate animal models is warranted to validate whether NAb represents an effective antiviral barrier against Gal-positive PERV produced from wild-type porcine cells or organs. The recent identification of receptors for PERV (7) enables the development of such models in receptor-transgenic Gal-null mice (26).
In summary, this study presents the first detailed assessment of the impact of the removal of Gal from porcine cells by recombinant DNA technology for the safety risk of a xenotransplantation product. We demonstrate that PERV released from Gal-null cells is insensitive to NAb inactivation and that PERV released from cells that express Gal can be inactivated via NAb-mediated mechanisms, albeit incompletely. Given this observation, we conclude that the replication competence of PERV proviruses that an individual donor pig possesses, rather than the pig's Gal status, remains the most influential determinant of PERV transmission in vivo.
REFERENCES
- 1.Chapman, L. E., and C. A. Wilson. 2003. Implications of the advent of homozygous alpha l, 3-galactosyltransferase gene-deficient pigs on transmission of infectious agents. Xenotransplantation 10:287-288. [DOI] [PubMed] [Google Scholar]
- 2.Cooper, D. K., A. H. Good, E. Koren, R. Oriol, A. J. Malcolm, R. M. Ippolito, F. A. Neethling, Y. Ye, E. Romano, and N. Zuhdi. 1993. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl. Immunol. 1:198-205. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, N. R., F. C. Jensen, R. M. Welsh, Jr., and M. B. Oldstone. 1976. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J. Exp. Med. 144:970-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delos, S. E., M. J. Burdick, and J. M. White. 2002. A single glycosylation site within the receptor-binding domain of the avian sarcoma/leukosis virus glycoprotein is critical for receptor binding. Virology 294:354-363. [DOI] [PubMed] [Google Scholar]
- 6.Dinsmore, J. H., C. Manhart, R. Raineri, D. B. Jacoby, and A. Moore. 2000. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation 70:1382-1389. [DOI] [PubMed] [Google Scholar]
- 7.Ericsson, T. A., Y. Takeuchi, C. Templin, G. Quinn, S. F. Farhadian, J. C. Wood, B. A. Oldmixon, K. M. Suling, J. K. Ishii, Y. Kitagawa, T. Miyazawa, D. R. Salomon, R. A. Weiss, and C. Patience. 2003. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. USA 100:6759-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita, F., I. Yamashita-Futsuki, S. Eguchi, Y. Kamohara, H. Fujioka, K. Yanaga, J. Furui, R. Moriuchi, T. Kanematsu, and S. Katamine. 2003. Inactivation of porcine endogenous retrovirus by human serum as a function of complement activated through the classical pathway. Hepatol. Res. 26:106-113. [DOI] [PubMed] [Google Scholar]
- 9.Heneine, W., A. Tibell, W. M. Switzer, P. Sandstrom, G. V. Rosales, A. Mathews, O. Korsgren, L. E. Chapman, T. M. Folks, and C. G. Groth. 1998. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet 352:695-699. [DOI] [PubMed] [Google Scholar]
- 10.Kubo, Y., T. Ono, M. Ogura, A. Ishimoto, and H. Amanuma. 2002. A glycosylation-defective variant of the ecotropic murine retrovirus receptor is expressed in rat XC cells. Virology 303:338-344. [DOI] [PubMed] [Google Scholar]
- 11.Lai, L., D. Kolber-Simonds, K. W. Park, H. T. Cheong, J. L. Greenstein, G. S. Im, M. Samuel, A. Bonk, A. Rieke, B. N. Day, C. N. Murphy, D. B. Carter, R. J. Hawley, and R. S. Prather. 2002. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089-1092. [DOI] [PubMed] [Google Scholar]
- 12.Marin, M., D. Lavillette, S. M. Kelly, and D. Kabat. 2003. N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions. J. Virol. 77:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, U., V. Kiessig, J. H. Blusch, A. Haverich, K. von der Helm, T. Herden, and G. Steinhoff. 1998. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet 352:692-694. [DOI] [PubMed] [Google Scholar]
- 14.Oldmixon, B. A., J. C. Wood, T. A. Ericsson, C. A. Wilson, M. E. White-Scharf, G. Andersson, J. L. Greenstein, H. J. Schuurman, and C. Patience. 2002. Porcine endogenous retrovirus transmission characteristics of an inbred herd of miniature swine. J. Virol. 76:3045-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paradis, K., G. Langford, Z. Long, W. Heneine, P. Sandstrom, W. M. Switzer, L. E. Chapman, C. Lockey, D. Onions, E. Otto, et al. 1999. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science 285:1236-1241. [DOI] [PubMed] [Google Scholar]
- 16.Patience, C., G. S. Patton, Y. Takeuchi, R. A. Weiss, M. O. McClure, L. Rydberg, and M. E. Breimer. 1998. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet 352:699-701. [DOI] [PubMed] [Google Scholar]
- 17.Patience, C., Y. Takeuchi, and R. A. Weiss. 1997. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3:282-286. [DOI] [PubMed] [Google Scholar]
- 18.Phelps, C. J., C. Koike, T. D. Vaught, J. Boone, K. D. Wells, S. H. Chen, S. Ball, S. M. Specht, I. A. Polejaeva, J. A. Monahan, P. M. Jobst, S. B. Sharma, A. E. Lamborn, A. S. Garst, M. Moore, A. J. Demetris, W. A. Rudert, R. Bottino, S. Bertera, M. Trucco, T. E. Starzl, Y. Dai, and D. L. Ayares. 2003. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitkin, Z., and C. Mullon. 1999. Evidence of absence of porcine endogenous retrovirus (PERV) infection in patients treated with a bioartificial liver support system. Artif. Organs 23:829-833. [DOI] [PubMed] [Google Scholar]
- 20.Reed, D. J., X. Lin, T. D. Thomas, C. W. Birks, J. Tang, and R. P. Rother. 1997. Alteration of glycosylation renders HIV sensitive to inactivation by normal human serum. J. Immunol. 159:4356-4361. [PubMed] [Google Scholar]
- 21.Rother, R. P., and S. P. Squinto. 1996. The alpha-galactosyl epitope: a sugar coating that makes viruses and cells unpalatable. Cell 86:185-188. [DOI] [PubMed] [Google Scholar]
- 22.Sachs, D. H., M. Sykes, S. C. Robson, and D. K. Cooper. 2001. Xenotransplantation. Adv. Immunol. 79:129-223. [DOI] [PubMed] [Google Scholar]
- 23.Seebach, J. D., M. K. Schneider, C. A. Comrack, A. LeGuern, S. A. Kolb, P. A. Knolle, S. Germana, H. DerSimonian, C. LeGuern, and D. H. Sachs. 2001. Immortalized bone-marrow derived pig endothelial cells. Xenotransplantation 8:48-61. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi, Y., C. Patience, S. Magre, R. A. Weiss, P. T. Banerjee, P. Le Tissier, and J. P. Stoye. 1998. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 72:9986-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi, Y., C. D. Porter, K. M. Strahan, A. F. Preece, K. Gustafsson, F. L. Cosset, R. A. Weiss, and M. K. Collins. 1996. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature 379:85-88. [DOI] [PubMed] [Google Scholar]
- 26.Thall, A. D. 1999. Generation of alpha 1,3galactosyltransferase deficient mice. Subcell. Biochem. 32:259-279. [DOI] [PubMed] [Google Scholar]
- 27.Weiss, R. A. 1998. Transgenic pigs and virus adaptation. Nature 391:327-328. [DOI] [PubMed] [Google Scholar]
- 28.Weiss, R. A., S. Magre, and Y. Takeuchi. 2000. Infection hazards of xenotransplantation. J. Infect. 40:21-25. [DOI] [PubMed] [Google Scholar]
- 29.Welsh, R. M., Jr., N. R. Cooper, F. C. Jensen, and M. B. Oldstone. 1975. Human serum lyses RNA tumour viruses. Nature 257:612-614. [DOI] [PubMed] [Google Scholar]
- 30.Wilson, C. A., S. Wong, J. Muller, C. E. Davidson, T. M. Rose, and P. Burd. 1998. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J. Virol. 72:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson, C. A., S. Wong, M. VanBrocklin, and M. J. Federspiel. 2000. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J. Virol. 74:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu, Y., T. Lorf, T. Sablinski, P. Gianello, M. Bailin, R. Monroy, T. Kozlowski, M. Awwad, D. K. Cooper, and D. H. Sachs. 1998. Removal of anti-porcine NAbs from human and nonhuman primate plasma in vitro and in vivo by a Galα1-3Galβ1-4βGlc-X immunoaffinity column. Transplantation 65:172-179. [DOI] [PubMed] [Google Scholar]