Abstract
The Copper transporter 1, Ctr1, is part of a major pathway for cellular copper (Cu) uptake in the intestinal epithelium, in hepatic and cardiac tissue, and likely in many other mammalian cells and tissues. Here we summarize what is currently known about how extracellular Cu travels across the plasma membrane to enter the cytoplasm for intracellular distribution and for use by proteins and enzymes, the physiological roles of Ctr1 and its regulation. As a critical Cu importer, Ctr1 occupies a strategic position to exert a strong modifying influence on diseases and pathophysiological states caused by imbalances in Cu homeostasis. A more thorough understanding of the mechanisms that regulate Ctr1 abundance, trafficking and function will provide new insights and opportunities for disease therapies.
Keywords: copper trafficking, regulation, structure, platinum, copper homeostasis, chaperones
The Need for Copper
As life evolved on earth, metals became bioavailable from the geosphere and were incorporated as biochemical co-factors in life-sustaining reactions. Although our understanding of the mechanisms by which metals such as copper (Cu) are harnessed and used in biological systems is recent, the use of Cu has been a crucial factor for the development of human civilization for centuries. It was not until the past century or so that it has been appreciated that Cu plays essential roles in human health with respect to growth, development, cognition, and beyond. Highlights of research implicating Cu in important biological functions include, but are not limited to, Hart and colleagues demonstrating Cu as a critical supplement for the maturation of hemoglobin in rats (1), Wainio and colleagues discovering that the last enzyme in the respiratory electron transport chain, cytochrome c oxidase, requires Cu for its function (2, 3), and a decade later, McCord and Fridovich demonstrating that Cu is an essential co-factor for the activity of Cu, Zn superoxide dismutase (4).
Organisms from baker’s yeast to humans have instituted sophisticated biochemical mechanisms for ensuring Cu acquisition to meet the needs of growing, developing and metabolizing cells and organs. Copper homeostasis mechanisms in eukaryotic cells have been comprehensively reviewed elsewhere (5–8), and other articles in this compendium focus on the Cu transporting pumps, Atp7A and Atp7B, the intracellular Cu chaperones and other components of the Cu homeostasis machinery (Figure 1). Here, we encapsulate what is known about how extracellular Cu travels across the plasma membrane to enter into the complex milieu of the cytoplasm en route for intracellular distribution and utilization.
Figure 1.
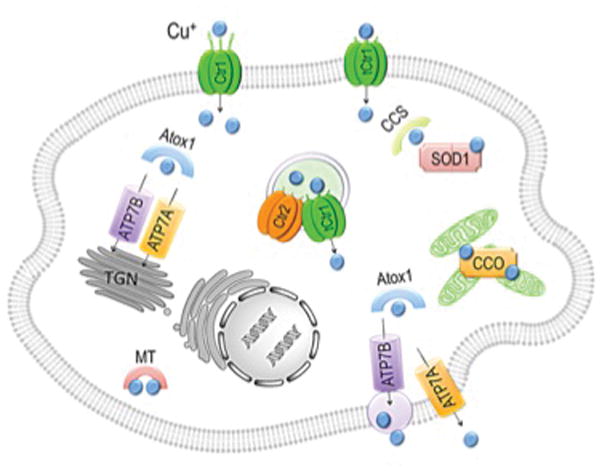
Schematic model depicting the key players involved in mammalian cellular acquisition, intracellular distribution, sensing, and mobilization of copper (Cu). ATOX1, antioxidant protein 1; Atp7A and B, copper transporting ATPaseA and B; CCO, cytochrome c oxidase; CCS, copper chaperone for SOD1; Ctr1 and 2, copper transporter 1 and 2; MT, metallothionein; SOD1, Cu/Zn- superoxide dismutase; tCtr1, truncated Ctr1.
Ctr1: an evolutionarily conserved Cu+ importer
Over many years a series of elegant physiological studies demonstrated the presence of specific import pathways that drive Cu acquisition in systems from yeast to humans. While there are likely to be multiple mechanisms for Cu acquisition, given the essentiality of this metal, a key study using the power of genetics in baker’s yeast identified the first eukaryotic gene encoding a specific Cu importer, Ctr1 (9). Based on protein sequence homology searches and functional complementation studies, additional members of the Ctr1 family have been identified across Eukaryota in fungi (10, 11), plants (12, 13), fish (14), amphibians (15, 16) and mammals (17–19), setting the stage for detailed studies of the physiological role, mechanisms of action, and regulation of Ctr1 in copper import in model systems and in humans. Some organisms, such as Saccharomyces cerevisiae and the human fungal pathogen Cryptococcus neoformans, harbor two genes encoding functionally homologous members of the Ctr1 family (9, 10, 20, 21). Moreover, the fission yeast, Schizosaccharomyces pombe, expresses both a Ctr1 family Cu+ uptake system and a structurally distinct Cu importer, Mfc1, which is a member of the Major Facilitator transporter superfamily and is largely expressed and active during meiosis (11, 22).
Therefore, while we focus here on Ctr1, Cu import is likely to be much more complex than we currently appreciate and further studies are needed in model systems to decipher additional mechanisms for Cu acquisition that may be conserved in Nature. The fact that Cu can exist in either the oxidized (Cu2+) or reduced (Cu+) states further complicates Cu acquisition in terms of bioavailability and specificity of uptake. As Ctr1 is a Cu+ transporter, there is an obligatory metallo-reduction event at the plasma membrane prior to, or concomitant with Cu+ import. In fungal model systems a number of cell surface Cu2+ reductases have been identified and characterized (23, 24), and this activity is required for normal function of the Ctr1 family-dependent Cu+ uptake transporters. Little definitive physiological evidence exists for corresponding Cu2+ reductases in mammalian systems. However, evidence suggests that members of the STEAP family of proteins may play this role (25). These proteins have been localized to the plasma membrane and to endosomal compartments and their expression is associated with Cu2+ reductase activity, making them good candidates for functionally coupling to Ctr1-mediated Cu+ import in mammalian systems.
Ctr1 structural and mechanistic insights
Ctr1 proteins are integral membrane proteins harboring three trans-membrane domains (TMD) and multiple copper-binding ligands (predominantly methionine, histidine or cysteine) within the extracellular amino-terminus, at the extracellular boundary of TMD2 and in the cytosolic carboxyl-terminus (26–29). Although Ctr1 proteins from different species are different lengths and share different degrees of homology, the presence of similarly predicted tertiary structures and region-specific highly conserved amino acid sequences allow Ctr1 proteins from mammals, amphibians, plants and insects to functionally complement the loss of high affinity Cu+ transporters in baker’s yeast cells (13, 16–18, 30).
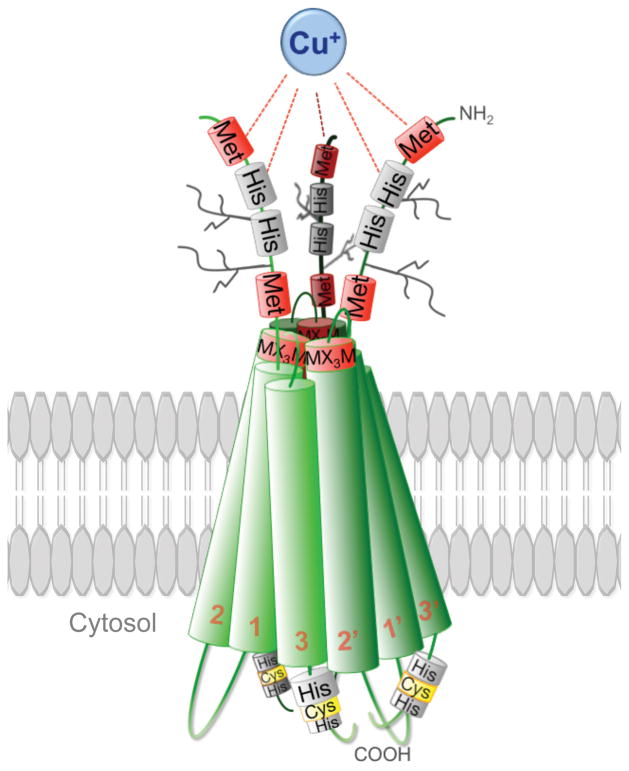
The human Ctr1 protein is 190 amino acids (aa) in length, with a ~67 aa extra-cellular amino-terminal (ecto) domain and a ~15 aa carboxyl-terminal cytosolic tail (Figure 2). Through a combination of genetic studies in yeast and Ctr1−/− mouse embryonic fibroblasts, Fluorescence Resonance Energy Transfer (FRET) experiments, structural analysis, molecular modeling and cell biology studies, an initial picture of the Ctr1 structure and mechanisms of action in Cu+ transport is beginning to emerge. Early biochemical fractionation and protease cleavage topological studies, coupled with trans-complementation experiments strongly suggested that both yeast and human Ctr1 function as a homo-multimer, most likely a homo-trimer (26, 28, 31, 32). Subsequent cryo Electron Microscopy Studies (cryoEM) on two-dimensional crystals of membrane embedded human Ctr1 clearly demonstrated its trimeric nature and revealed a pseudo-cone-shaped pore through the lipid bilayer, with a narrow extra-cellular Cu+ entry site (approximately 8 Å across, without taking sidechain contribution into account) and a wider exit (approximately 22 Å across, without taking sidechain contribution into account) on the cytosolic side (Figure 2) (33, 34). The long ecto-domain, containing an abundance of metal-coordinating methionine (Met) and histidine (His) residues, is glycosylated via both N-linked and O-linked bonds to produce a mature protein of approximately 35 kDa, when analyzed by SDS-PAGE (32, 35, 36).
Figure 2.
Structural model of three fully glycosylated Ctr1 monomers in a lipid bilayer forming a functional Ctr1 transporter. The TMD 2 of all three Ctr1 monomers lines the entrance of the pore and all nine TMDs from the three monomers line the exit of the pore. Cu+ is bound by the Met and His rich motifs in the three Ctr1 ecto-domains probably causing a conformational change of the transporter allowing Cu+ to enter the pore. Met, methionine; His, histidine; Cys, cysteine; MX3M, crucial motif for Cu transport; TMD1-3, Ctr1 monomer number 1; TMD1′-3′, Ctr1 monomer number 2.
Unlike the intracellular Cu+-transporting P-type ATPases Atp7A and Atp7B involved in Menkes and Wilson’s disease, respectively, Ctr1 lacks an identifiable ATP- hydrolysis domain to provide energy for Cu+ ion transduction across the membrane. Together with the results of studies on the energetic requirements for Ctr1-dependent Cu+ accumulation in cell culture (37), these observations suggest that Ctr1 facilitates Cu+ movement across membranes in an ATP-independent manner. However, it is not fully understood whether the passive transport of Cu+ is driven by the concentration gradient or if other forces stimulate Cu+ to traverse the Ctr1 pore. Based on substitution mutants in Cu+-coordinating residues and the Ctr1 cryo-EM structure, a model has been proposed where Cu+ moves through the Ctr1 pore by a series of ligand exchange reactions between distinct binding sites and that these reactions induce conformational changes that mediate Cu+ movement through the pore (26, 33).
The ecto-domain of Ctr1 has been reported to coordinate three Cu+ atoms via the Met rich motifs and two Cu2+ atoms through the amino-terminal copper/nickel (ATCUN) and His-rich motifs (38, 39). The ecto-domain not only plays a role in Cu import, but it can also bind Ag with a lower affinity (39), and evidence suggests that Ag traverses through the Ctr1 pore across the plasma membrane (40). Platinum-based chemotherapeutic agents also bind to the Met rich motifs within the ecto-domain (41–43). However, FRET experiments suggest that the platinum is not transferred through the actual pore of Ctr1, but instead the Ctr1 ecto-domain binds platinum-based drugs and undergoes endocytosis, thereby serving more as a receptor for delivering this class of molecules into cells (44). Circular dichroism spectroscopy data suggest that the apo-ecto-domain has a random coil conformation and that the ecto-domain shifts to a -turn when Cu+ is bound (45). These observations support the hypothesis that conformational changes in Ctr1 might be a contributing force for driving Cu+ into the pore.
Recent work has begun to clarify many questions concerning the overall structure and likely mechanism by which Cu+ navigates through the pore formed by Ctr1 at the membrane. From cryo-EM data, Ctr1 TMD 2 is modeled to line the extracellular portion of the pore and, together with the ecto-domain, create four stacked triad Met rings at the entry of the pore at the border of the plasma membrane (33, 34). These four Met rings are thought to bind Cu+ reversibly and translocate the Cu+ atoms from one Met ligand to another, uni-directionally down the pore. Whether the Met motifs in the extracellular amino-terminal domain form additional stacked rings is unknown, but it has been reported that the amino-terminal ecto-domains interact with each other and that the interaction requires both of the Met rich regions (M7 –M12 and M40 – M45) (26, 27).
It is currently thought that the His-Cys-His motif at the carboxyl-terminal tail of each Ctr1 monomer forms a sink for Cu+ to bind to before it is recruited by a Cu chaperone, glutathione, metallothioneins or other Cu-binding ligands (33). The result is that there is virtually no free cytosolic Cu in cells, making it possible for a concentration gradient to drive the import. However, recently an all-atom model of Ctr1 has suggested that the electrostatic field change along the pore facilitates the movement of Cu+ through the pore (46). This model suggests that a negative charge at the amino-terminus attracts Cu+, with neutral passage at the middle of the pore, and a dipole at the carboxyl-terminal tail forming an exit with a positive repelling charge in the middle of the pore and negative charges along the sides forcing the Cu+ to exit along the sides of the broad pore. The higher affinity between Cu and Cys compared to between Cu and Met would also contribute to an enrichment of cations in the His-Cys-His sink at the wider dipolar intracellular exit (47). Taken together, these data support a model where Cu traverses through Ctr1 by contributions of changes in the electrostatic field and conformational changes, together with the concentration gradient.
Unger and colleagues demonstrated that the conserved Gly-XXX-Gly motif (where X is not glycine) in TMD 3 of the Ctr1 family transporters is important for multimerization and for proper localization of the protein to the plasma membrane in yeast (48). Recent data generated by the use of short peptides instead of the whole Ctr1 protein have indicated that the Gly-XXX-Gly motif may not be a part of the helix structure, but instead forming a flexible linker between two shorter helixes in the third TMD (49). However, these data do not account for effects by nearby lying helixes in the whole molecule, nor the interactions with lipid bilayers, and need to be further investigated. Supporting this, the Gly-XXX-Gly motif is crucial for proper helix packing in Ctr1 homotrimer and this motif is conserved among the Ctr1 family (48).
Ctr1 in a physiological context
Mouse models with systemic or tissue-specific deletions of the Ctr1 gene have begun to illuminate fundamental aspects of its role in mammalian physiology. Mice systemically deleted for Ctr1 exhibit severe growth and developmental defects and die during mid-gestation, demonstrating the clear physiological significance for Cu and its importer in normal mammalian growth and development (50, 51). Due to this severe effect of systemic Ctr1 knockout mice, tissue specific knockouts of Ctr1 have been valuable tools. Liver, intestinal, and heart specific knockout mice models have all given essential insights into the physiological roles of the Ctr1 protein.
Intestinal knockout of Ctr1 in mice results in severe postnatal growth defects, cardiac hypertrophy, hepatic iron accumulation, fail to thrive and death around weaning (52). These data clearly demonstrate that Ctr1 is critical for dietary Cu uptake in vivo. One interesting finding in these mice is that while peripheral Cu levels are reduced, the same intestinal cells in which the Ctr1 gene has been excised, have increased levels of non-bioavailable Cu. It is very intriguing that the intestinal knockout of Ctr1 leads to accumulation of Cu inside intestinal cells. Although this phenomenon is not yet defined these results suggests that Ctr1 might play a role in intracellular transport of Cu from vesicles or other organelles, in addition to its role at the plasma membrane. This hypothesis is further supported by recent experimental data describing a functional interaction between Ctr1 and a related protein, Ctr2 (see below).
As the liver is the primary storage organ for Cu, Ctr1 ablation was carried out in mouse models to understand the role of Ctr1 in liver Cu metabolism. Surprisingly, mice lacking Ctr1 in hepatocytes show normal growth and exhibit low Cu concentrations predominantly in the liver, along with reduced activity of Cu dependent hepatic enzymes and decreased biliary excretion of Cu (53). Unanticipated findings in these mice were that they have increased urinary Cu concentrations, suggesting an interesting, and largely unstudied role for Ctr1 in urinary Cu reabsorption. Plausibly, Ctr1-dependent Cu resorption may explain why the urinary excretion of Cu under normal conditions is very low. Future studies investigating Cu homeostasis in the kidney will begin to address these interesting observations.
Given the roles for Cu in oxidative stress protection, mitochondrial cytochrome oxidase function and peptide hormone maturation, it is not surprising that dietary Cu restriction studies demonstrated that cardiac hypertrophy is an early outcome of Cu limitation in mammals (54). Indeed, while loss of Ctr1 in the intestinal epithelium also resulted in cardiac hypertrophy (55) it was not clear if this was due to a peripheral Cu deficiency or a cardiac-intrinsic requirement for Cu. Consequently, a cardiac-specific Ctr1 knock out mouse was generated to address this question. Ctr1 deletion in cardiomyocytes lead to mice with a cardiac-specific reduction in Cu levels and cardiac hypertrophy, supporting a cardiac intrinsic requirement to prevent hypertrophy. Surprisingly, these same mice exhibited increased Atp7A expression in both liver and intestinal enterocytes, and reduced Cu levels in liver, with a corresponding increase in circulating Cu in the serum (55). Together, these results suggest that loss of Ctr1 in cardiomyocytes elaborates a signal that provokes the liver, as a major Cu storage organ, to express elevated levels of ATP7a in order to mobilize Cu into the periphery. The nature of this signaling mechanism, and its role in normal systemic Cu regulation will be a fascinating area for future investigation. While Ctr1−/− mice die approximately mid-way during gestation (50, 51), Ctr1 knockout mouse embryonic fibroblasts (MEFs) are viable and accumulate approximately one third of the Cu found in wild type MEFs. Moreover, Ctr1−/− MEFs harbor a low affinity Cu uptake activity, with features that suggest Cu2+ may be a relevant substrate (37). Whether this is a carrier bound Cu uptake, e.g. ceruloplasmin mediated Cu uptake mechanism, or due to other transporters that transport Cu+ or Cu2+, remains to be elucidated. An alternative mechanism for mammalian Cu acquisition has been proposed that involves the transport of copper–chloride complexes by an anion exchanger (56). However, confirmation of this observation awaits both the identification of the anion exchanger and demonstration that its genetic ablation in cells or animals results in physiological Cu deficiency in vivo. Another possibility is that there is a mammalian counterpart to the recently identified Cu importer Mfc1 in S. pombe (22). As S. pombe mfc1 functions in Cu accumulation during meiosis, this discovery sets the stage for the identification of novel copper transporters, and Cu-dependent proteins, that may operate in the germ line or in stem cell differentiation. An additional study suggests that the human zinc importer Zip4 (Zrt- and Irt-like protein 4), expressed in Xenopus laevis oocytes, transports Cu across a wide concentration range (57), raising the exciting possibility for a role of Zip4 in mammalian Cu uptake.
Ctr1 and metallochaperones
The Cu homeostasis network invokes a series of Cu ligands, importers, carriers, recipient proteins and exporters to achieve a harmonious balance (Figure 1). Many of the nodes in this network have been identified over the past twenty years, but the precise molecular mechanisms underlying their function, regulation and interactions are still poorly understood. Once Cu has been transferred through the Ctr1 channel it has been hypothesized that a recipient protein will bind Cu+ immediately when the Cu is still bound by the His and Cys ligands at the cytosolic carboxyl-terminus of Ctr1 to ensure that no free copper exist in the cell.
The Cu chaperone Atox1 transfers copper to Atp7A and Atp7B and in yeast (Atx1) has been shown to bind directly to the intracellular domain of Ctr1 (58). Furthermore, both Atox1 and CCS possess the capacity to interact with lipid bilayer membrane, somewhat in line with this hypothesis (59, 60). Hatori et al. (61) recently proposed that the production of reactive oxygen species (ROS) and the state of the redox environment plays an important role for recruiting Atox1 to the carboxyl-terminal tail of Ctr1. Atox1 binds Cu with a CXXC domain (62) and reversible Cys oxidation has been implicated in the function of Atox1 (61). Given the critical role of Atox1 in Cu excretion via Atp7A and Atp7B, the redox modulation of Atox1 is likely to affect the overall Cu distribution and homeostasis in cells and in organisms. Reversible Cys oxidation has also been demonstrated to be involved in the function of another Cu chaperone, CCS (copper chaperone for superoxide dismutase) that requires Cys oxidation for the maturation of Cu, Zn SOD (63). These modifications of Cu chaperones might partially explain why Cu homeostasis is influenced by the cellular redox environment and might help us to further understand how the Cu homeostasis is sensed and communicated in cells.
Tightly linked to the redox environment, a recent study also demonstrated that glutathione might be a recipient peptide able to receive Cu from Ctr1 as an intermediate step before delivering Cu to Atox1 and CCS (64). The intracellular glutathione levels then influence the Ctr1 dependent Cu uptake in cultured cells. The binding affinity of Cu to glutathione is lower than to Atox1 and CCS, but the ubiquitous abundance of intracellular glutathione molecules makes this a potential scenario. Furthermore, high glutathione levels reduce the CXXC binding site of Atox1 and low glutathione levels keep the binding site in an oxidative state, leading to formation of a disulfide bond, inhibiting interactions between Atox1 and Cu (61). Based on these data one might hypothesize that while Atox1 directly interacts with Ctr1, intracellular glutathione levels are able to modulate the affinity of Atox1 for Cu and in an indirect manner affect the cellular uptake of Cu and Atox1 delivery of Cu to the two ATP-ases. Further work must be carried out in both in vitro models as well as in whole animals to advance the understanding of the roles glutathione may play on Cu uptake and homeostasis in higher Eukaryotes.
Post-translational modifications of Ctr1
Current information suggests that the regulation of Ctr1 occurs predominantly at the level of transporter localization and abundance (35, 65). Ctr1 is present at the plasma membrane at times of cellular demand for Cu and on the membrane of intracellular vesicles, partially as a result of Ctr1 clathrin-mediated endocytosis in response to elevated exogenous copper (29, 35, 66, 67). Between cultured cell lines the cellular localization of Ctr1 differs substantially, possibly reflecting differences in the overall cellular Cu status of different cell types or differences in the abundance or activity of the trafficking machinery (28, 32). Moreover, elevated Cu levels have been shown in mouse intestinal epithelium and cultured cell models to increase Ctr1 protein degradation (35, 65).
Although Ctr1 is a relatively small protein, the regulation of its abundance, activity, trafficking and interactions with other molecules could, in principle, be tuned by post-translational modifications. This is particularly relevant since, although transcription of the Ctr1 gene in vertebrates has been reported to be regulated both by cellular Cu levels (68) and the Sp1 transcription factor (69, 70), transcriptional control appears to be relatively modest in most cells and tissues evaluated to date. In contrast, there is a growing body of evidence demonstrating that Ctr1 is post-translationally modified, with potential functional consequences (35, 71).
Ctr1 possesses two glycosylation sites within the ecto-domain, one N-linked modification at Asn15 and one O-linked glycosylation at Thr27 (Figure 2) (32, 36). Mutational studies have shown that the O-linked glycosylation protects Ctr1 against proteolysis of the ecto-domain (36). When the O-linked glycosylation is prevented, the Ctr1 ecto-domain is cleaved more frequently, generating a truncated protein with an approximately two-fold reduction in Cu+ transport activity (36). This cleavage event has been shown to occur largely in Rab9-positive vesicles (72), which travel from the late endosome to the golgi compartment and are involved in the recycling of mannose 6-phosphate receptors. It will be fascinating to understand the role the ecto-domain cleavage plays in Ctr1 function specifically and copper homeostasis in general. Moreover, the identification of the proteases involved in Ctr1 ecto-domain cleavage, and the potential regulation of cleavage by glycosylation, could lead to important insights into new mechanisms for the regulation of Cu acquisition. Patients having deficiency in the protease responsible for cleaving the ecto-domain might also have disturbed Cu homeostasis.
The homologous protein Ctr2
In mammalian cells the Cu transporter 2 (Ctr2) protein was identified by homology to Ctr1 (18). Based on transfection and RNAi-mediated knock down studies in cultured cells, Ctr2 has been suggested to function as a low affinity copper importer (73), a lysosomal copper exporter (74), and as a regulator of macropinocytosis (75). Recent experimental results, based on the generation of a systemic knock out of the mouse Ctr2 gene, suggest an additional function for this protein. Surprisingly, Ctr2−/− mice, and their corresponding mouse embryonic fibroblasts, accumulate Cu well above that of the wild type littermates and cells, with Cu concentrated in endosomal or lysosomal compartments (76). This phenotype, and other biochemical data, suggest that Ctr2 plays a role in the biogenesis of a form of Ctr1 in which a large portion of the metal-binding ecto-domain has been cleaved (Figure 1). As a truncated form of Ctr1 has been shown to import Cu less efficiently than wild type protein at the plasma membrane, the strong diminution in the levels of truncated Ctr1 would explain why the Ctr2−/− animals have increased cellular uptake of Cu. Additionally, the entrapment of Cu in endosomal compartments in Ctr2−/− MEFs, and its mobilization by ectopic expression of truncated Ctr1, suggest that the truncated form of Ctr1 plays a role in the mobilization of endosomal Cu into the cytosol.
Conclusions and perspectives
Although Menkes and Wilson’s diseases are caused by mutations in the ATP7A and ATP7B genes, respectively, there is clearly broad variability in the clinical severity and biochemical phenotypes of these patients. While some of this variability is due to specific disease gene-associated mutations and the functional roles of specific amino acid residues or mRNA splice sites, it is also likely that alterations in the expression, function, subcellular trafficking, or regulation of other proteins intimately involved in Cu homeostasis are critical disease modifiers. As a major pathway for cellular Cu acquisition in the intestinal epithelium, hepatic cells, cardiac tissue, and likely in most mammalian cells and tissues, Ctr1 occupies a strategic position to exert a strong modifying influence on disease, and other pathophysiological states caused by, or perturbed by, imbalances in Cu homeostasis. Moreover, it is likely that amino acid substitutions on the Ctr1 coding region that perturb, but do not abrogate function, may be linked to human disease. A more thorough understanding of the mechanisms that regulate Ctr1 abundance, trafficking and function will provide new insights and opportunities for understanding how Ctr1 may both cause and modify human Cu-related diseases.
Acknowledgments
We thank members of the Thiele laboratory for suggestions and critical comments. This work was supported by NIH grants DK074192 and GM041840 (DJT), Postdoctoral Fellowships K2012-77PK-21938-01-2 from the Swedish Research Council and The Throne-Holst Foundation (HÖ).
References
- 1.Hart EB, Steenbock H, Wadell J, Elvehjem CA. Iron in nutrition. VII. Copper as a supplement to iron for hemoglobin building in the rat. J Biol Chem. 1928;77:797. [PubMed] [Google Scholar]
- 2.Eichel B, Wainio P, Person P, Cooperstein SJ. A Partial Separation and Characterization of cytochrome Oxidase and Cytochrome b. J Biol Chem. 1950;183:89–103. [Google Scholar]
- 3.Vander Wende C, Wainio WW. The state of the copper in cytochrome c oxidase. J Biol Chem. 1960;235:PC11–12. [PubMed] [Google Scholar]
- 4.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 5.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4(3):176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 6.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol. 2010;14(2):211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim Biophys Acta. 2012;1823(9):1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Hodgkinson V, Zhu S, Weisman GA, Petris MJ. Advances in the understanding of mammalian copper transporters. Adv Nutr. 2011;2(2):129–137. doi: 10.3945/an.110.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dancis A, et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 10.Knight SA, Labbe S, Kwon LF, Kosman DJ, Thiele DJ. A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev. 1996;10(15):1917–1929. doi: 10.1101/gad.10.15.1917. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Thiele DJ. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 2001;276(23):20529–20535. doi: 10.1074/jbc.M102004200. [DOI] [PubMed] [Google Scholar]
- 12.Sancenon V, Puig S, Mira H, Thiele DJ, Penarrubia L. Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol. 2003;51(4):577–587. doi: 10.1023/a:1022345507112. [DOI] [PubMed] [Google Scholar]
- 13.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van Montagu M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J Biol Chem. 1995;270(47):28479–28486. doi: 10.1074/jbc.270.47.28479. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie NC, Brito M, Reyes AE, Allende ML. Cloning, expression pattern and essentiality of the high-affinity copper transporter 1 (ctr1) gene in zebrafish. Gene. 2004;328:113–120. doi: 10.1016/j.gene.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Haremaki T, Weinstein DC. Xmc mediates Xctr1-independent morphogenesis in Xenopus laevis. Dev Dyn. 2009;238(9):2382–2387. doi: 10.1002/dvdy.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggio M, et al. High affinity copper transport protein in the lizard Podarcis sicula: molecular cloning, functional characterization and expression in somatic tissues, follicular oocytes and eggs. Biochim Biophys Acta. 2002;1576(1–2):127–135. doi: 10.1016/s0167-4781(02)00337-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254(1–2):87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A. 1997;94(14):7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller LB, Petersen C, Lund C, Horn N. Characterization of the hCTR1 gene: genomic organization, functional expression, and identification of a highly homologous processed gene. Gene. 2000;257(1):13–22. doi: 10.1016/s0378-1119(00)00394-2. [DOI] [PubMed] [Google Scholar]
- 20.Waterman SR, et al. Role of a CUF1/CTR4 copper regulatory axis in the4 virulence of Cryptococcus neoformans. J Clin Invest. 2007;117(3):794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding C, et al. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Molecular microbiology. 2011;81(6):1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaudoin J, et al. Mfc1 is a novel forespore membrane copper transporter in meiotic and sporulating cells. J Biol Chem. 2011;286(39):34356–34372. doi: 10.1074/jbc.M111.280396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassett R, Kosman DJ. Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem. 1995;270(1):128–134. doi: 10.1074/jbc.270.1.128. [DOI] [PubMed] [Google Scholar]
- 24.Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem. 1997;272(21):13786–13792. doi: 10.1074/jbc.272.21.13786. [DOI] [PubMed] [Google Scholar]
- 25.Ohgami RS, Campagna DR, McDonald A, Fleming MD. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277(29):26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 27.Klomp AE, et al. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J. 2003;370(Pt 3):881–889. doi: 10.1042/BJ20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277(32):29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279(17):17428–17433. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Cadigan KM, Thiele DJ. A copper-regulated transporter required for copper acquisition, pigmentation, and specific stages of development in Drosophila melanogaster. J Biol Chem. 2003;278(48):48210–48218. doi: 10.1074/jbc.M309820200. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Pena MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277(6):4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 32.Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1) Biochem J. 2002;364(Pt 2):497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci U S A. 2009;106(11):4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A. 2006;103(10):3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nose Y, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285(42):32385–32392. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maryon EB, Molloy SA, Kaplan JH. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J Biol Chem. 2007;282(28):20376–20387. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem. 2002;277(43):40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 38.Haas KL, Putterman AB, White DR, Thiele DJ, Franz KJ. Model peptides provide new insights into the role of histidine residues as potential ligands in human cellular copper acquisitiosn via Ctr1. J Am Chem Soc. 2011;133(12):4427–4437. doi: 10.1021/ja108890c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du X, et al. Kinetics and thermodynamics of metal binding to the N-terminus of a human copper transporter, hCTR1. Chem Commun (Camb) 2013;49(80):9134–9136. doi: 10.1039/c3cc45360j. [DOI] [PubMed] [Google Scholar]
- 40.Bertinato J, Cheung L, Hoque R, Plouffe LJ. Ctr1 transports silver into mammalian cells. J Trace Elem Med Biol. 2010;24(3):178–184. doi: 10.1016/j.jtemb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Crider SE, Holbrook RJ, Franz KJ. Coordination of platinum therapeutic agents to met-rich motifs of human copper transport protein1. Metallomics. 2010;2(1):74–83. doi: 10.1039/b916899k. [DOI] [PubMed] [Google Scholar]
- 42.Larson CA, Adams PL, Blair BG, Safaei R, Howell SB. The role of the methionines and histidines in the transmembrane domain of mammalian copper transporter 1 in the cellular accumulation of cisplatin. Mol Pharmacol. 2010;78(3):333–339. doi: 10.1124/mol.110.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Smith K, Petris MJ. Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem. 2004;279(45):46393–46399. doi: 10.1074/jbc.M407777200. [DOI] [PubMed] [Google Scholar]
- 44.Sinani D, Adle DJ, Kim H, Lee J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem. 2007;282(37):26775–26785. doi: 10.1074/jbc.M703973200. [DOI] [PubMed] [Google Scholar]
- 45.Rubino JT, Riggs-Gelasco P, Franz KJ. Methionine motifs of copper transport proteins provide general and flexible thioether-only binding sites for Cu(I) and Ag(I) J Biol Inorg Chem. 2010;15(7):1033–1049. doi: 10.1007/s00775-010-0663-9. [DOI] [PubMed] [Google Scholar]
- 46.Tsigelny IF, et al. An all-atom model of the structure of human copper transporter 1. Cell Biochem Biophys. 2012;63(3):223–234. doi: 10.1007/s12013-012-9358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubino JT, Chenkin MP, Keller M, Riggs-Gelasco P, Franz KJ. A comparison of methionine, histidine and cysteine in copper(I)-binding peptides reveals differences relevant to copper uptake by organisms in diverse environments. Metallomics. 2011;3(1):61–73. [PubMed] [Google Scholar]
- 48.Aller SG, Eng ET, De Feo CJ, Unger VM. Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem. 2004;279(51):53435–53441. doi: 10.1074/jbc.M409421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Huang Z, Li F. Structural insights into the transmembrane domains of human copper transporter 1. J Pept Sci. 2012;18(7):449–455. doi: 10.1002/psc.2415. [DOI] [PubMed] [Google Scholar]
- 50.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A. 2001;98(12):6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A. 2001;98(12):6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4(3):235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. American journal of physiology Gastrointestinal and liver physiology. 2009;296(2):G356–364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kopp SJ, Klevay LM, Feliksik JM. Physiological and metabolic characterization of a cardiomyopathy induced by chronic copper deficiency. Am J Physiol. 1983;245(5 Pt 1):H855–866. doi: 10.1152/ajpheart.1983.245.5.H855. [DOI] [PubMed] [Google Scholar]
- 55.Kim BE, et al. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11(5):353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimnicka AM, Ivy K, Kaplan JH. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol. 2011;300(3):C588–599. doi: 10.1152/ajpcell.00054.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antala S, Dempski RE. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry. 2012;51(5):963–973. doi: 10.1021/bi201553p. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Z, Wedd AG. A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun (Camb) 2002;(6):588–589. doi: 10.1039/b111180a. [DOI] [PubMed] [Google Scholar]
- 59.Flores AG, Unger VM. Atox1 Contains Positive Residues that Mediate Membrane Association and Aid Subsequent Copper Loading. J Membr Biol. 2013 doi: 10.1007/s00232-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pope CR, De Feo CJ, Unger VM. Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1309820110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatori Y, Clasen S, Hasan NM, Barry AN, Lutsenko S. Functional partnership of the copper export machinery and glutathione balance in human cells. J Biol Chem. 2012;287(32):26678–26687. doi: 10.1074/jbc.M112.381178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnesano F, et al. Metallochaperones and metal-transporting ATPases: a comparative analysis of sequences and structures. Genome Res. 2002;12(2):255–271. doi: 10.1101/gr.196802. [DOI] [PubMed] [Google Scholar]
- 63.Furukawa Y, Torres AS, O’Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. Embo J. 2004;23(14):2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maryon EB, Molloy SA, Kaplan JH. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am J Physiol Cell Physiol. 2013;304(8):C768–779. doi: 10.1152/ajpcell.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278(11):9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 66.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136(1):21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem. 2009;284(43):29704–29713. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minghetti M, Leaver MJ, Carpene E, George SG. Copper transporter 1, metallothionein and glutathione reductase genes are differentially expressed in tissues of sea bream (Sparus aurata) after exposure to dietary or waterborne copper. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147(4):450–459. doi: 10.1016/j.cbpc.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 69.Song IS, et al. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008;74(3):705–713. doi: 10.1124/mol.108.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang ZD, Tsai WB, Lee MY, Savaraj N, Kuo MT. Specificity protein 1 (sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol Pharmacol. 2012;81(3):455–464. doi: 10.1124/mol.111.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136(1):21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maryon EB, Zhang J, Jellison JW, Kaplan JH. Human copper transporter 1 lacking O-linked glycosylation is proteolytically cleaved in a Rab9-positive endosomal compartment. J Biol Chem. 2009;284(41):28104–28114. doi: 10.1074/jbc.M109.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409(3):731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 74.van den Berghe PV, et al. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407(1):49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blair BG, et al. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol Pharmacol. 2011;79(1):157–166. doi: 10.1124/mol.110.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohrvik H, et al. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]