Abstract
We report here the full characterization of the metal binding abilities of CnMT1 and CnMT2, two Cryptococcus neoformans proteins recently identified as metallothioneins (MTs), which have been shown to perform a crucial role for the virulence and pathogenicity of this human-infecting fungus. In this work, we first performed a thorough in silico study of the CnMT1 and CnMT2 genes, cDNAs and corresponding encoded products. Subsequently, the Zn(II)-, Cd(II)- and Cu(I) binding abilities of both proteins were fully determined through the analysis of the metal-to-protein stoichiometries and the structural features (determined by ESI-MS, CD, ICP-AES and UV-vis spectroscopies) of the corresponding recombinant Zn-, Cd- and Cu-MT preparations synthesized in metal-enriched media. Finally, the analysis of the Zn/Cd and Zn/Cu replacement processes undertaken by the respective Zn-MT complexes when allowed to react with Cd(II) or Cu(I) aqueous solutions completed the analysis. Comprehensive consideration of all gathered results allow us to consider both isoforms as genuine copper-thioneins, and led to the identification of unprecedented Cu5-core clusters in MTs. CnMT1 and CnMT2 polypeptides appear as evolutionary related to the small fungal MTs, probably by ancient tandem-duplication events responding to a high selective pressure to chelate copper, and far from the properties of Zn- and Cd-thioneins. Finally, we propose a modular structure of the Cu-CnMT1 and Cu-CnMT2 complexes basically built on the three and five-fold presence of 7-Cys units, each one yielding a Cu5-core cluster.
Keywords: Metallothionein, Cryptococcus neoformans, CnMT1, CnMT2, copper, zinc, pathogenic fungi
1. Introduction
Metallothioneins (MTs) are a superfamily of ubiquitous small Cys-rich proteins that have been identified in all eukaryotes and most prokaryotes so far analyzed.1 They constitute polymorphic systems in almost all organisms, so that diversification of MT isoforms may underlie their ability to play versatile biological roles. MTs coordinate closed-shell metal ions, such as Zn(II), Cd(II) or Cu(I) and they have been associated with several physiological processes, among which homeostasis and/or protection against metal ions appear to be the most relevant.2,3 Recent studies have revealed that metallothioneins of Cryptococcus neoformans can be considered as pathogenicity and virulence determinants.4 Cryptococcus neoformans is a dimorphic basidiomicet, responsible of cryptococcosis in both immunodeficient and immunocompetent individuals, establishing a first infection in lungs, and later on developing lethal meningitis.5 Precisely, it has been shown that the two MTs of Cryptococcus neoformans (CnMT1 and CnMT2) play a critical role in the virulence and the resistance in front of the host immune response of this opportunist fungus, because they are directly involved in the detoxification of the high copper concentrations produced by the infection-fighting macrophages.4 CnMT1 and CnMT2 genes are induced in a Cu-specific manner, and consequently they are part of the Cu-responsive C. neoformans set of genes essential for its virulence known as the C. neoformans copper regulon. This regulon is under the transcriptional control of the Cuf1 factor and, besides CnMT1 and CnMT2, it comprises genes encoding for Cu importers (namely Ctr1 and Ctr4). Paradoxically Ctr1 and Ctr4 respond to Cu limitation conditions through the same Cuf1 transcription factor, so that both Cu acquisition and Cu detoxification appear as virulence determinants in C. neoformans infections.6
Regarding the CnMT1 and CnMT2 proteins, it has been shown that their detoxifying function come from their high Cu-binding capacity,4 so that they exhibit all the features to be considered typical Cu-thioneins.3,7 However, unlike the paradigmatic Cu-thioneins such as the yeast (Saccharomyces cerevisiae) Cup1 protein and the fungus Neurospora crassa MT,8 which are very small proteins of 41 and 26 amino acids respectively, C. neoformans MTs are surprisingly longer: CnMT1 is a 122-residues long and CnMT2 a 183-residues long polypeptide (cf. Fig. 1).6 The first study of C. neoformans CnMT1 and CnMT2 proteins, besides allowing their unambiguous classification as metallothioneins, served to suggest the formation of unusual Cu5-building blocks,4 different to the Cu-clusters commonly reported in MTs until the moment. This applies both to taxonomically close yeast and fungal MTs: Cu8 for S. cerevisiae Cup19 and Cu6 for Neuropora crassa MT10 respectively, and Cu4- and Cu6-clusters in the more distant mammalian MTs.11,12,13 The presence of several Cu5Cys7 clusters in both C. neoformans MT isoforms and the high similarity, at amino acid sequence level, between these two proteins was hypothesized to account for the high specificity and capacity of CnMT1 and CnMT2 for Cu-binding. Strikingly, a modular structure has been also proposed for the five MT isoforms of several species of the ciliate Tetrahymena (T. pigmentosa and T. pyriformis) with lengths ranging between 96 to 181 amino acids.14 Therefore, unicellular eukaryotes of different taxa (ciliate, fungi) may have followed the same strategy to enhance the metal binding capacity of their MTs, by tandemly repeating a basic, Cys-containing, building block.
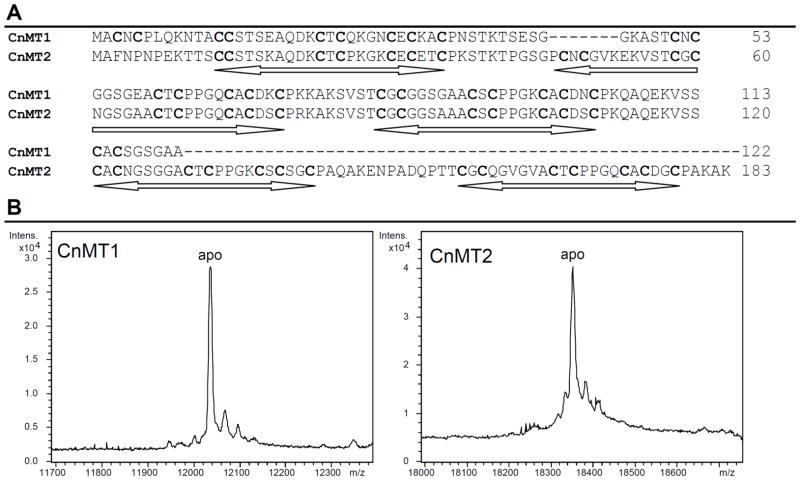
Figure 1.
(A) Alignment of the amino acid sequences of CnMT1 (25 Cys, 122 aa) and CnMT2 (37 Cys, 183 aa). Conserved Cys are in bold and the Cys-rich stretches are underlined. (B) Deconvoluted ESI-MS spectra of the Zn-CnMT1 and Zn-CnMT2 preparations recorded at acidic pH, showing the corresponding recombinant apo-forms. These peptides included the Gly-Ser (CnMT1) and Gly-Ser-Pro-Glu-Phe (CnMT2) residues added at their N-term due to GST-cloning requirements.
In this scenario it was relevant to fully determine the metal binding abilities of the C. neoformans MTs. To this end, we characterized the Zn-, Cd- and Cu-species formed in vivo (by recombinant synthesis in E. coli) and in vitro (by Zn/Cd and Zn/Cu replacement on the corresponding recombinant Zn-CnMT species) by spectroscopic and spectrometric techniques. This information confirms a modular organization of the long CnMT1 and CnMT2 peptides regarding metal cluster formation. Furthermore, the correct annotation of their encoding genes and corresponding transcripts in the C. neoformans genome, highlights the evolutionary relationship that may link these MTs with the other well-studied fungal copper-thioneins (Neurospora and Agaricus).
2. Experimental
2.1. In silico tools for genome, DNA and protein sequence analysis
The last annotated C. neoformans var. grubii H99 genome version in the Broad Institute database (www.broadinstitute.org) was used for in silico searches of the MT-encoding genes, through the Blast facility accessible in the same site. The CnMT1 and CnMT2 cDNAs had been previously obtained and sequenced in D. Thiele’s lab,6 through rtPCR on mRNA isolated from copper induced cells. These CnMT1 and CnMT2 cDNA sequences were used as queries for genomic searches. Sequences were aligned with the ClustalW facility available at the EBI website (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
2.2. Cloning and recombinant expression of the CnMT1 and CnMT2 cDNAs
The CnMT1 and CnMT2 cDNAs were obtained from D. Thiele’s lab6 as p426GPD clones. From there, they were subcloned into the BamHI/XhoI sites for CnMT1, and EcoRI/XhoI sites for CnMT2 (owing to the presence of an internal BamHI site in CnMT2) of the pGEX-4T1 expression vector (GE Healthcare), in order to obtain a GST-MT fusion protein. The two restriction sites were added to the cDNA sequences by PCR amplification, using the following oligonucleotides as primers: 5′-AAAAGGATCCATGGCTTGCAACTGCCCTCCCCAGA-3′ (forward) and 5′-AAAACTCGAGTCAGGCAGCGCCAG-3′ (reverse) for CnMT1; and 5′-GGGAGAATTCATGGCTTTCAACCC-3′ (forward) and 5′-GGGGCTCGAGTTATTTAGCCTTGGCCG-3′ (reverse) for CnMT2. The 30-cycle amplification reactions were performed with the thermo resistant Hotstar Taq DNA polymerase (Qiagen) under the following conditions: 15 min at 95 °C (activation of the DNA polymerase), 30 s at 94 °C (denaturation), 30 s at 55 °C (annealing) and 1 min at 72 °C (elongation). The final products were analyzed by 0.8% agarose gel electrophoresis and the expected bands were excised (Genelute™ Gel Extraction Kit, Sigma Aldrich). pGEX-4T1 and the amplified inserts were digested with BamHI/XhoI for CnMT1 and EcoRI/XhoI for CnMT2, followed, in each case, by a ligation reaction (DNA Ligation Kit 2.1, Takara Bio Inc.). The recombinant plasmids were transformed into the E. coli MachI strain for DNA sequencing, using the Big Dye Terminator 3.1 Cycle Sequencing Kit in an ABIPRISM 310 Automatic Sequencer (Applied Biosystems). Positive clones were transformed into the E. coli BL21 protease deficient strain for protein synthesis.
2.3. Synthesis and purification of recombinant Zn- and Cu-CnMT complexes and preparation of in vitro-substituted complexes
The corresponding GST-CnMT fusion proteins were biosynthesized in 5-L cultures of transformed E. coli cells. Expression was induced with 100 μM (final concentration) isopropyl β-D-thiogalactopyranoside (IPTG) and cultures were supplemented with 300 μM ZnCl2, 300 μM CdCl2 or 500 μM CuSO4 (final concentrations), and they were allowed to grow for further 3 h. Cu-supplemented cultures were grown either under normal aeration conditions (1 L of media in a 2 L Erlenmeyer flask, at 250 rpm) or under low oxygen conditions (1.5 L of media in a 2 L Erlenmeyer flask, at 150 rpm), since different results may be achieved depending on the culture aeration conditions owing to the fact that these determine the amount of intracellular copper in the host cells.15 The total protein extract was prepared from bacterial cultures as fully described before for other MT peptides.16 Briefly, metal-CnMT complexes were recovered from the CnMT-GST fusion constructs by thrombin cleavage and batch-affinity chromatography using the Glutathione-Sepharose 4B matrix (GE Healthcare). After concentration using Centriprep Microcon 3 (Amicon), samples were finally purified through FPLC in a Superdex75 column (GE Healthcare) equilibrated with 50 mM Tris-HCl, pH 7.0. Selected fractions were confirmed by 12% SDS-PAGE and kept at −80 °C until further use. All procedures were performed using Ar (pure grade 5.6) saturated buffers, and all syntheses were performed at least twice to ensure reproducibility. As a consequence of the cloning requirements, the dipeptide Gly-Ser in case of CnMT1, and the pentapeptide Gly-Ser-Pro-Glu-Phe in case of CnMT2 were present at the N-term of the CnMTs polypeptides; but this had previously been shown not to alter the MT metal-binding features.17 In vitro-substituted Cd(II)-CnMT and Cu(I)-CnMT complexes were obtained by titration at pH 7 of the corresponding Zn(II)-CnMT preparations with CdCl2 in water (MERCK AAS Cd2+ standard of 1000 ppm) or [Cu(CH3CN)4]ClO4 solutions as described,18 respectively. During all the experiments strict oxygen-free conditions were maintained by saturating all the solutions with Ar.
2.4. Spectroscopic analyses (ICP-AES and CD) of the Zn-, Cd- and Cu-CnMT complexes
The S, Zn, Cd and Cu content of the Zn-, Cd- and Cu-CnMT preparations was analyzed by means of Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) in a Polyscan 61E (Thermo Jarrell Ash) spectrometer, measuring S at 182.040 nm, Zn at 213.856 nm, Cd at 228.802 nm and Cu at 324.803 nm. Samples were routinely treated as in19, but they were also alternatively incubated in 1 M HCl at 65 °C for 15 min prior to measurements in order to eliminate possible traces of labile sulfide ions.20 Protein concentrations were calculated from the acid ICP-AES sulfur measure, assuming that all S atoms were contributed by the CnMT peptides. A Jasco spectropolarimeter (Model J-715) interfaced to a computer (J700 software) was used for CD measurements at a constant temperatures of 25 °C maintained by a Peltier PTC-351S apparatus. Electronic absorptions measurements were performed on an HP-8453 Diode array UV-visible spectrophotometer. All spectra were recorded with 1-cm capped quartz cuvettes, corrected for the dilution effects and processed using the GRAMS 32 program.
2.5. Electrospray Ionization Time-of-Flight Mass Spectrometry (ESI-TOF MS) of the Zn- and Cu-CnMT complexes
MW determinations were performed by electrospray ionization time-of-flight mass spectrometry (ESI-TOF MS) on a Micro TOF-Q instrument (Bruker) interfaced with a Series 1200 HPLC Agilent pump, equipped with an autosampler, all of which were controlled by the Compass Software. Calibration was attained with ESI-L Low Concentration Tuning Mix (Agilent Technologies). Samples containing CnMT complexes with divalent metal ions were analyzed under the following conditions: 20 μL of protein solution injected through a PEEK (polyether heteroketone) tubing (1.5 m x 0.18 mm i.d.) at 40 μL min-1; capillary counter-electrode voltage 5 kV; desolvation temperature 90–110 °C; dry gas 6 L min-1; spectra collection range 800–2500 m/z. The carrier buffer was a 5:95 mixture of acetonitrile:ammonium acetate (15 mM, pH 7.0). Alternatively, the Cu-CnMT samples were analyzed as follows: 20 μL of protein solution injected at 40 μL min-1; capillary counter-electrode voltage 3.5 kV; lens counter-electrode voltage 4 kV; dry temperature 80 °C; dry gas 6 L min-1. Here, the carrier was a 10:90 mixture of acetonitrile:ammonium acetate, 15 mM, pH 7.0. For the analysis of apo-CnMT and Cu-CnMT preparations at acidic pH, 20 μL of the corresponding sample were injected under the same conditions described previously, but using a 5:95 mixture of acetonitrile:formic acid pH 2.4, as liquid carrier, which caused the complete demetalation of the peptides loaded with Zn(II) but kept the Cu(I) ions bound to the protein. Under all the conditions assayed, the error associated with the mass measurements was always lower than 0.1 %. Masses for the holo-species were calculated as previously described.21
3. Results and Discussion
3.1. CnMT1 and CnMT2 gene structure and annotation in C. neoformans genome
The Blast search using CnMT1 cDNA sequence retrieved CNAG_05449 as the most probable clone containing the desired sequence. However, this exhibited clear sequence differences, also leading to a hypothesized protein 5-residue longer at its C-term end than CnMT1. In order to solve this ambiguity, the genome sequence was searched for the corresponding CnMT1 gene, which also allowed us to define its exon/intron structure. We were able to assign the CnMT1 cDNA sequence to a putative CnMT1 gene, located in C. neoformans chromosome 14, between nucleotides 341340 and 342169 at the + strand (Genebank entry CP003833.1). Using the CnMT1 cDNA sequence as a guide, the possible exon/intron boundaries, according to the GT/AG rule, were manually searched. The result was a perfect match between the CnMT1 cDNA sequence and the proposed exons of the gene, in number of four (Fig. S1A). Therefore, we concluded that the CNAG_05449T0 and CNAG_05449.2 hypothetical protein sequences were incorrectly annotated, and that the reported CnMT1 cDNA and protein sequences represented the real expression products of the CNAG_05449 gene. When performing a parallel quest for the CnMT2 coding sequences, we also realized that the CNAG_00306 transcript and hypothetical protein, identified through the blast with the CnMT2 cDNA sequence, were wrongly annotated. Hence, we also defined the correct gene structure (as shown in Fig. S1B), and furthermore, we added the final coding stretch by identification in the GenBank AACO02000005.1 entry, since the CNAG_00306 entry appears truncated at its 3′ end. The CnMT2 gene is located between 787948 bp and at 789021 according the current Genbank entry CP003820.1, at the + strain of C. neoformans chromosome 1 and it includes seven intron sequences. No further sequences were retrieved by Blast searches using as a query MT sequences of the following isoforms: human MT1 and MT2, Mytilus edulis MT10Ia, Scylla serrata MT1, D. melanogaster MtnA and MtnD, Candida glabrata MT1, Yarrowia lipolytica MT1, Saccharomyces cerevisiae Cup1 and CRS5, Neurospora crassa MT and Arabidopsis thaliana MT1A. This strongly suggests that CnMT1 and CnMT2 are the only MT encoding genes in the C. neoformans genome, although the existence of some highly divergent isoform cannot be completely ruled out. The cDNAs and genes are respectively 86.38 % and 79.85 % similar (excluding gaps). This clearly suggests that they arose by gene duplication (and further expansion of CnMT2, as discussed later) of an ancestral gene, and they may have been subsequently separated to different genome locations by some chromosomal rearrangement event.
3.2. CnMT1 and CnMT2 peptide identity
DNA sequencing confirmed that both CnMT cDNAs were cloned in pGEX in the appropriate frame after the GST encoding moiety and that they included no undesired nucleotide substitutions. Consequently, recombinant syntheses yielded CnMT1 and CnMT2 peptides (Fig. 1A), the identity, purity and integrity of which were confirmed by acid ESI-MS (pH 2.4) of the respective Zn-MT complexes. Hence, for each MT, a unique peak was detected, whose MW was consistent with that calculated for the respective apo-forms (Fig. 1B), including the N-terminal residues derived from the GST-fusion construct prior to the initiator Met. Experimental molecular masses detected were 12034.1 Da for CnMT1 and 18350.29 Da for CnMT2 vs. the respective theoretical values of 12034.56 and 18349.91 Da calculated from the amino acid sequence.
3.3. Zn-CnMT1 and Zn-CnMT2 complexes
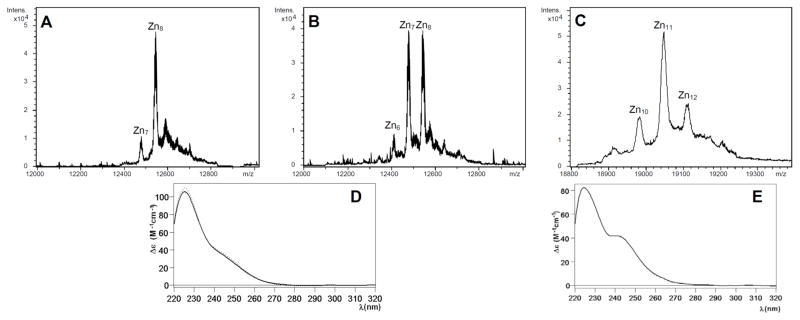
The repeated synthesis of CnMT1 by Zn(II)-enriched bacteria yielded two types of results regarding the stoichiometry of the recovered Zn-CnMT1 complexes, being always characterized by a rather low protein yield (concentrations ca. 0.1 mg/L of culture). ICP-AES analyses indicated a mean content of 8–9 Zn(II) per MT, which was highly consistent with ESI-MS spectra showing either only major Zn8-CnMT1 complexes or almost equimolar amounts of Zn7-CnMT1 and Zn8-CnMT1, among other minor species (Fig. 2A,B). Despite this disparity, both types of Zn-CnMT1 preparations exhibited identical CD spectra, which can be interpreted as a Gaussian band centered at ca. 240 nm, typical of the Zn-SCys chromophores superimposed to the 220–230 nm absorption contributed by the peptídic bonds (Fig. 2D). Contrarily to CnMT1, the synthesis of the Zn-CnMT2 complexes yielded invariable results. These consisted in also rather diluted preparations (0.5 mg/L of culture), including a mixture of major Zn11-CnMT2 and minor Zn12- and Zn10-CnMT2 species (Fig. 2C), nicely matching the average content of 11 Zn(II) per MT shown by ICP-AES. The CD spectrum of this sample (Fig. 2E) was quite similar to that of Zn-CnMT1, but for a more pronounced shoulder at ca. 245 nm. In this case, it can be interpreted as resulting from two overlapping Gaussian band, centered at 225 and 240 nm. At this point of the analysis, it became evident that both isoforms were far from presenting the features typical of MTs optimized for Zn(II) binding (i.e. Zn-thioneins),7 in view of the multiplicity and variability of the recovered species in Zn-supplemented recombinant cultures and the absence of derivative-like curves in their CD fingerprints.
Figure 2.
(A and B) Deconvoluted ESI-MS spectra of two Zn-CnMT1 preparations and (D) the CD fingerprints corresponding to (A), solid line, and (B), dotted line. (C) Deconvoluted ESI-MS spectrum of the Zn-CnMT2 preparation and (E) its corresponding CD spectrum.
3.4. Cd-CnMT1 and Cd-CnMT2 complexes
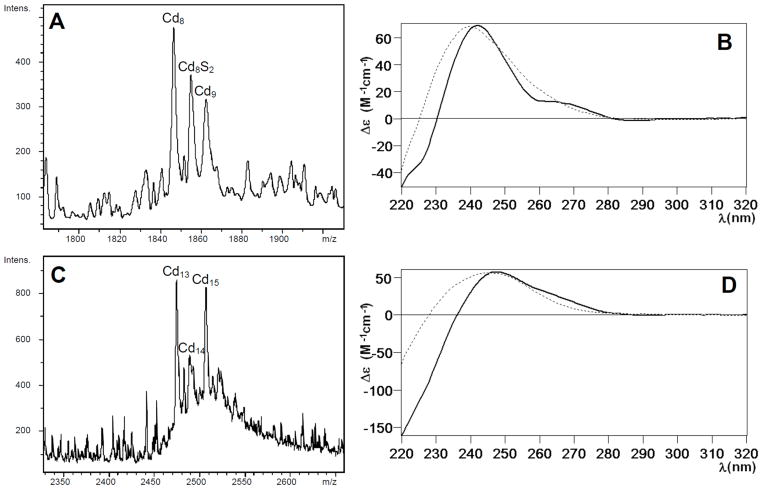
The Cd(II) binding abilities of CnMT1 and CnMT2 were studied through the characterization of their recombinant complexes, as well as those obtained by Zn/Cd replacement on Zn-CnMT1 and Zn-CnMT2. The synthesis of CnMT1 and CnMT2 in Cd(II)-supplemented cultures rendered even more diluted (0.010 and 0.011 mg/L of culture, respectively) and more heterogeneous preparations than for Zn(II). Hence, the recovered Cd-CnMT1 complexes contained 8–9 Cd(II) per MT (according to the ICP-AES results), and consisted of major Cd8-CnMT1 complexes together with minor Cd9-CnMT1 and significantly, Cd8S2-CnMT1 (Fig. 3A). The CD fingerprint of this preparation suggests an overlapping of two Gaussian bands, centered at 245 nm (contributed by the Cd-SCys chromophores) and 270 nm (contributed by the Cd-sulfide ligands) (Fig 3B).20 The analysis of the Cd(II) titration of Zn-CnMT1 showed that the maximum CD absorbance at 250 nm was reached at 11 Cd(II) eq added (Fig. S2). This point defined also the closest resemblance to the speciation found in the in vivo preparation (cf. Fig. 3A and S3B), as well as a practical coincidence of the corresponding CD spectra (Fig. 3B). Furthermore, the UV-vis spectra indicated that there was no substantial Cd(II) entry beyond this point. A detailed description of the Cd(II) titration of Zn-CnMT1 can be found as Supplementary Material, including the full set of ESI-MS (Fig. S3) and CD and UV-vis spectra (Fig. S2) of its successive steps.
Figure 3.
(A) ESI-MS spectrum corresponding to the recombinant production of CnMT1 in Cd-enriched media, at the +7 charge state. (B) CD spectra of the Cd-CnMT1 preparation (solid line) and that recorded after the addition of 11 Cd(II) eq to the Zn-CnMT1 preparation (dotted). (C) ESI-MS spectrum corresponding to the recombinant production of CnMT2 in Cd-enriched media, at the +8 charge state. (D) CD spectra of the Cd-CnMT2 preparation (solid line) and that recorded after the addition of 12 Cd(II) eq to the Zn-CnMT2 preparation (dotted). Full CD and ESI-MS spectra corresponding to the Zn/Cd titration of both isoforms can be found in the Supplementary Material.
Owing to its higher Cys content, the purified Cd-CnMT2 complexes exhibited a mean content of 12–13 Cd(II) per MT, contributed by two major Cd13- and Cd15-CnMT2 complexes, among multiple minor species (Fig. 3C). The CD spectrum of this sample resembled that of Cd-CnMT1, compatible with the presence in the sample of complexes including sulfide ligands (Fig. 3D). The Cd(II) titration of Zn-CnMT2, was analyzed by the same rationale than that of Zn-CnMT1 (full data in Supplementary Material, Fig. S4 and S5). In this case, the maximum similarity with the in vivo Cd-CnMT2 preparation was reached for 12 Cd(II) eq added (Fig. 3C), although this titration caused the generation of a high number of almost inextricable Cd-containing species (Fig. S5).
In summary, and as stated before for Zn(II), the results for Cd(II) coordination definitively suggest that both CnMTs are far from exhibiting any binding preference for Cd(II) ions. Precisely, (i) the mixtures of species obtained from the recombinant syntheses, (ii) the variability of the relative intensity of the peaks (relative abundance) of the different species obtained in different productions; and (iii) the presence of sulfide-containing Cd-CnMT1 complexes, indicates a poor ability of both CnMT1 and CnMT2 for divalent metal ion binding.
3.5. Cu-CnMT1 and Cu-CnMT2 complexes
The behavior of CnMTs when binding Cu(I) was studied in great detail, since both our results for divalent metal coordination (explained in the previous sections) and the observed role of these MT isoforms in C. neoformans copper metabolism4 already pointed to their imperative Cu-thionein character. Therefore, Cu(II)-enriched recombinant E. coli cultures were grown both at normal and at low oxygenation conditions, since low oxygenation leads to higher Cu content in the host cells.15 Additionally, Cu(I) binding to CnMTs was studied by analyzing the corresponding Zn/Cu displacement reactions on the respective Zn-CnMTs.
3.5.1. Cu(I)-binding by CnMT1: recombinant Cu-CnMT1
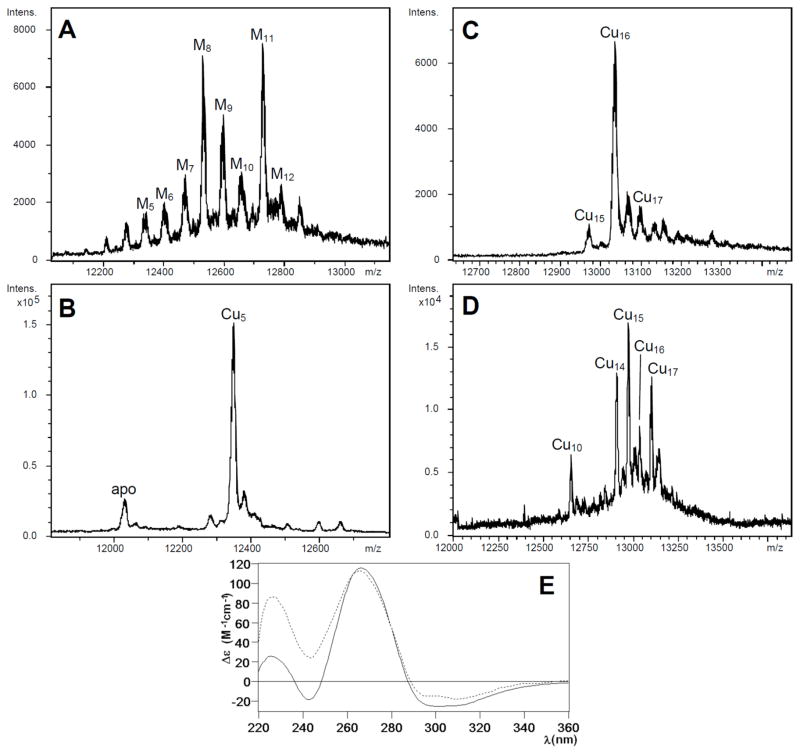
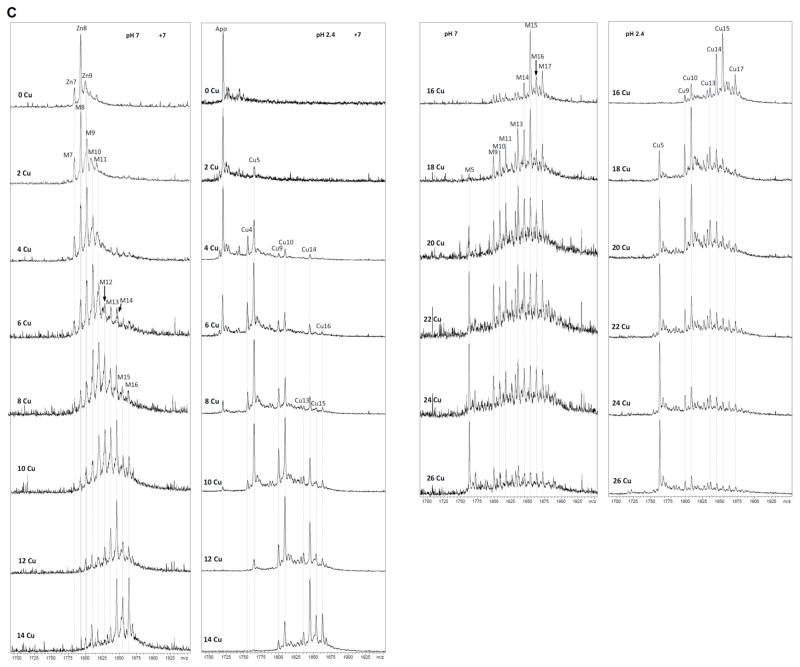
CnMT1 expression in bacteria grown under copper supplementation in normally aerated copper-supplemented cultures rendered 0.4 mg/L of MT protein, containing both Zn(II) and Cu(I), as indicated by its ICP-AES analysis: average ratio of 4 Zn: 5 Cu per CnMT1 molecule. The neutral ESI-MS spectrum of this sample revealed a mixture of heteronuclear species, with major, almost equimolar, M11- and M8-CnMT1 followed by M9-CnMT1 and other minor peaks (M=Zn(II) or Cu(I)) (Fig. 4A). Strikingly, the ESI-MS of the same sample at pH 2.4 yielded a very predominant, almost unique Cu5-CnMT1 peak (Fig. 4B). This suggests that all the Mx-CnMT1 (x= 5 to 12) complexes are basically constituted by a Cu5-cluster that complete the observed metal content with Zn(II) ions, i.e. M11 = Cu5Zn6, M8 = Cu5Zn3, M9 = Cu5Zn5. Instead, when CnMT1 was produced in a Cu-richer environment, it yielded homometallic copper complexes, since ICP-AES results ruled out any trace of Zn presence. The ESI-MS spectrum at neutral pH identified a major Cu16-CnMT1 species, accompanied by very minor Cu15- and Cu17-CnMT1 complexes (Fig. 4C). Interestingly, this preparation was resolved into a different speciation by acidic ESI-MS analysis (Fig. 4D), which indicates that some of the CnMT1-bound Cu(I) would be extremely sensitive to pH changes.
Figure 4.
Deconvoluted ESI-MS spectra corresponding to the production of CnMT1 in Cu-enriched media under (A and B) normal and (C and D) low aeration conditions, recorded at (A and C) neutral and (B and D) acidic pH. (E) CD spectra of the Cu-CnMT1 production under normal (solid line) and low (dotted) aeration conditions.
Although the CD spectrum of the Cu-CnMT1 preparation at normal aeration cannot be representative of any species due to the mixture recovered, it clearly shows interesting features, such as the absorptions between 300 and 340 nm that reveal special binding sites for Cu(I) only observed previously for MT isoforms of a prevalent Cu-thionein character.22 These same CD absorptions, together with the 265-nm centered Gaussian band typical of the fingerprint of Cu-MT complexes, are observed for the Cu-CnMT1 preparation obtained at low oxygenation. In fact, comparison of the normalized spectra of both Cu-CnMT1 preparations highlights their elevated similarity, despite the presence or absence of Zn(II) ions, which would contribute only to the 220–240 nm region (Fig. 4E).
3.5.2. Cu(I)-binding by CnMT1: Zn/Cu displacement on Zn-CnMT1
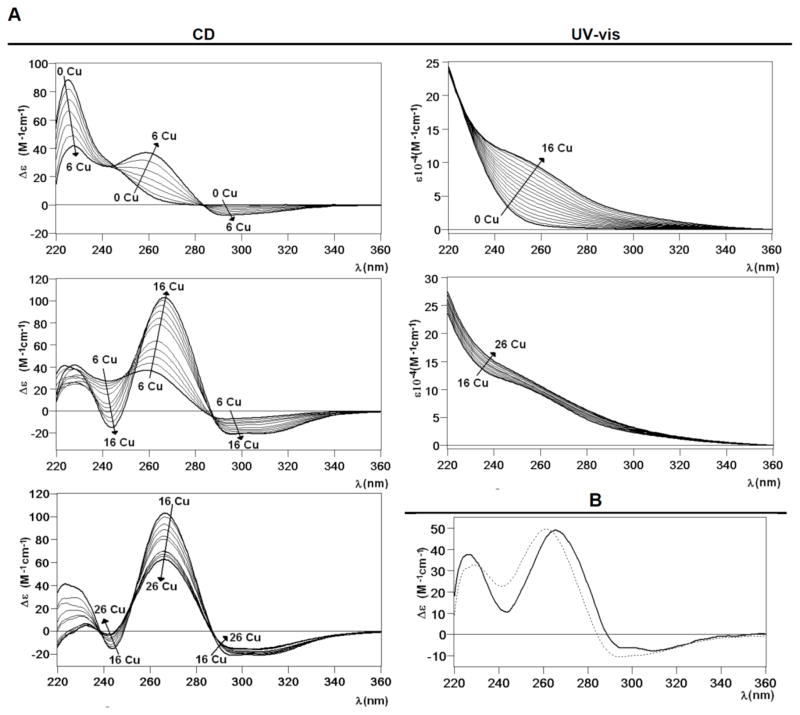
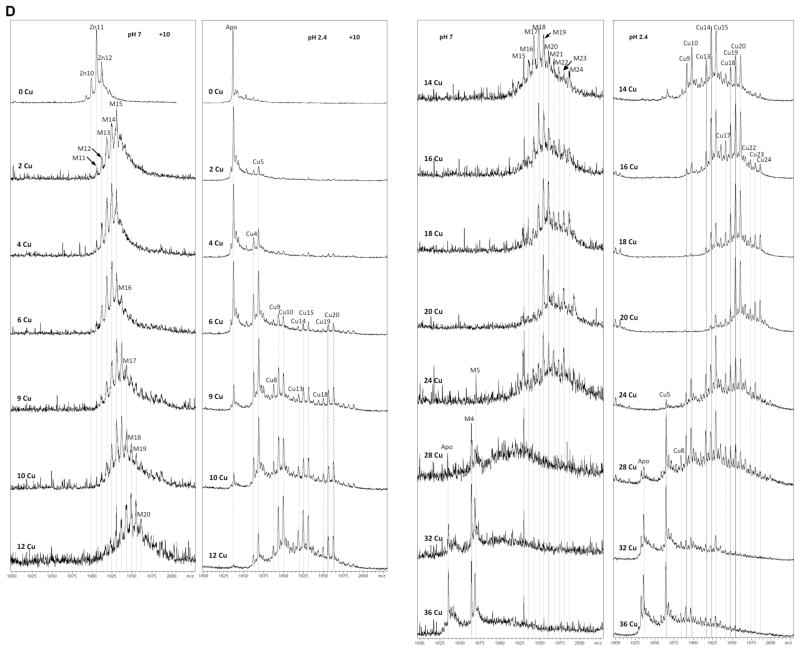
Most informative results about the Cu(I) binding abilities of CnMT1 were obtained from the addition of Cu(I) to Zn-CnMT1 (i.e. the study of the species constituted in vitro by Zn/Cu exchange). The metal-substitution process was followed by CD, UV-vis (Fig. 5A) and ESI-MS analysis (Fig. 5C) of aliquots retrieved every 2 Cu(I) eq, from 0 to 26 Cu(I) equivalents added to Zn-CnMT1. From the start results appeared highly promising, since the CD spectra of the full process show very nice isodichroic, although not isosbestic, points, at three different stages: from 0 to 6, from 6 to 16 and from 16 to 26 Cu(I) eq added (cf. Fig. 5A). This strongly suggested a cooperative copper loading and zinc displacement process in CnMT1. In the first step, between 0 and 6 Cu(I) eq added, two isodichroic points can be clearly observed, which arise by the decrease of the 225 nm band and the increase of the 260-nm CD absorption, owing to the creation of Cu-SCys chromophores, accompanied by the emergence of a new CD absorption at ca. 290 nm. For the second step, between 6 and 16 Cu(I) eq added, the 265(+) and 300–310(−)-nm CD absorptions reach their maxima at 16 Cu(I) eq, defining an isodichroic point at 290 nm. At the third step, UV-vis spectra still show Cu(I) entry, although more less important than for the previous steps, and the Gaussian CD band centered at 265 nm decreases in intensity while the absorption at higher wavelengths show no significant variation.
Figure 5.
(A) CD and UV-vis spectra recorded after the addition of Cu(I) to the Zn-CnMT1 preparation. (B) CD spectra corresponding to the Cu-CnMT1 preparation under normal aeration (solid line) and that recorded after the addition of 8 Cu(I) eq to the Zn-CnMT1 preparation (dotted). (C) ESI-MS spectra recorded after the addition of Cu(I) to the Zn-CnMT1 preparation, recorded at neutral and acidic pH, at the +7 charge state.
It is evident that from the beginning, the addition of Cu(I) increases the complexity of the sample in terms of the number of species present, as the ESI-MS analysis reveals (Fig. 5C). Only after the addition of 12 eq, 16 eq, and an excess of Cu(I), a clear major species is found (respectively, M14-, M15- and M5-CnMT1. However, the most remarkable features are revealed by the acid ESI-MS spectra, because they made patently clear that CnMT1 builds its copper aggregates on the basis of Cu5-clusters. Hence, the addition of just 2 Cu(I) eq at the beginning of the experiment already gave rise to the appearance of a Cu5 cluster, which remained very abundant while a Cu10-cluster gained in importance. These Cu5- and Cu10-clusters were predominant until 12 Cu(I) eq added to Zn-CnMT1, when Cu14 and Cu15 became the major peaks detected. In the presence of an excess of copper, beyond 16 Cu(I) eq added, the metal-MT complexes became unstable, so that only highly stable Cu5-core remained in solution. It is important to note here that apo-CnMT1 is never detected at the end of the reaction (Cu overload conditions), which is an outcome quite common for other MTs.23,24 It is also highly fascinating that the two landmarks of this Zn(II)/Cu(I) exchange reaction are the addition of 6–8 and 16 Cu(I) eq, because, besides being the spectroscopical crucial steps (Fig. 5A), they also are the moments of predominance of the Cu5- and Cu15-cores, respectively, as showed by the acidic ESI-MS analysis (Fig. 5C). Most importantly, they also represent the steps when in vitro preparations most closely reproduce the results of recombinant CnMT1 synthesis in Cu-enriched cultures (cf. Fig. 5C and Fig. 4B,D). Hence, precisely, addition of 8 Cu(I) eq to Zn-CnMT1 yields a sample with spectroscopic (Fig. 5B) and spectrometric features very close to those of the Cu-CnMT1 complexes obtained from normally-aerated cultures (cf. Fig. 4A,B vs. Fig. 5C at 8 Cu(I) eq), while those corresponding to the addition of 16 Cu(I) eq are practically identical to those of the synthesis at low aeration (i.e. high intracellular Cu) (cf. Fig. 4C,D vs. Fig. 5C at 16 Cu(I) eq), with the unique exception that M15 is the major species instead of Cu16 at pH 7.0, although both ESI-MS spectra coincide at acid pH, with Cu15 as the most abundant complex. Therefore, although a strict cooperative process for the Zn/Cu displacement in Zn-CnMT1 can be ruled out, owing to the many different species coexisting during all the experiment, it is true that it can be assumed for the Cu5-cores, since Cu(I) is loaded in CnMT1 by sets of 5 Cu(I) ions. The high stability and/or preference for Cu5-containing clusters were already suggested from the in vivo productions (vide supra). Besides, the possibility of reproducing the in vivo obtained samples at some steps of the Cu(I) addition to Zn-CnMT1 is remarkable, as in fact was the case of the yeast Crs5 MT.15 It is worth noting that the main peaks representing one or multiple Cu5-units that emerge during the Zn-CnMT1 titration are always accompanied by a minor peak lacking one of the final number of Cu(I) ions (Fig. 5C). Hence, for example, at 4 Cu(I) eq added both the Cu5/Cu4 and Cu10/Cu9 composition are visible, while at 16 Cu(I) eq, it is the Cu15/Cu14 pair the one that becomes predominant. This suggests that at least one Cu4-cluster has enough stability to persist during this Zn/Cu replacement reaction. Finally, the more than probable involvement on Cu(I) coordination to the Nterm and Cterm Cys doublets present in the CnMT1 sequence (cf. Fig. 1) would feasibly explain the origin of the Cu-CnMT1 species with more than 15 Cu(I) ions (i.e. Cu17- and Cu16-CnMT1), which are not only observable along the corresponding Zn/Cu displacement reaction, but are even the major species resulting from the Cu-MT1 recombinant synthesis in poorly-oxygenated cultures (Fig. 4 C,D).
3.5.3. Cu(I)-binding by CnMT2: recombinant Cu-CnMT2
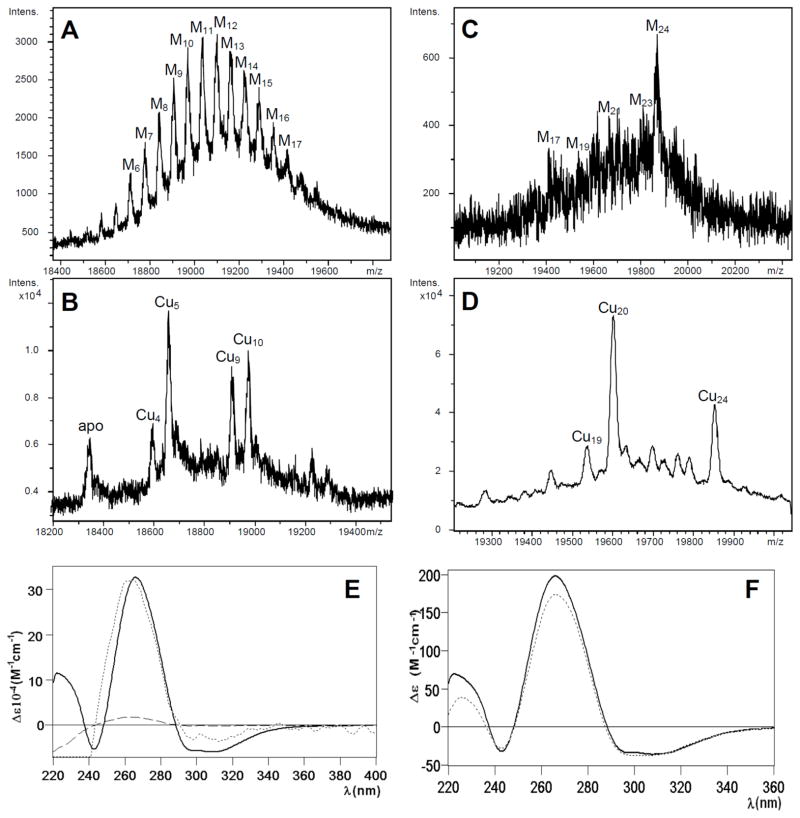
The synthesis of CnMT2 in Cu(II)-enriched cultures, performed both at normal and at low oxygenation conditions, repeated the trends described for CnMT1, but obviously with higher metal ion contents, accordingly to its increased size. Thus, normal oxygenation of copper-supplemented cultures rendered a yield of 0.2 mg MT/L, containing 6 Zn: 5 Cu per CnMT2 molecule, according to the ICP results. The ESI-MS spectrum of this preparation reflected a Gaussian-like distribution of heteronuclear complexes ranging from M6- to M17 -CnMT2 (M=Zn(II) or Cu(I)) (Fig. 6A), while its acid ESI-MS analysis revealed that these complexes predominantly contained 5 Cu(I) ions (Cu5 cores) like the Cu-CnMT1 species, although here the presence of Cu9- and Cu10-containing CnMT2 complexes was also very important (Fig. 6B). Probably due to the extraordinary length of CnMT2, and differing from CnMT1, the syntheses of this polypeptide under low aeration conditions rendered heteronuclear Zn,Cu-MT preparations (mean content according to ICP-AES results of 2 Zn : 21 Cu per CnMT2), with major M24-CnMT2 and a myriad of minor species (Fig. 6C). Since acid ESI-MS resolved most of these as 20 Cu(I)-, and 24 Cu(I)-containing species, it is likely that most, if not all, M24-CnMT2 complexes could be homonuclear species (Fig. 6D). Regarding the spectroscopic characterization of Cu-CnMT2 preparations, and despite their CD spectra not being obviously representative of unique species, it is clearly shown that the observed fingerprints fully coincide with those of Cu-CnMT1 (Fig. 6E, F). They are contributed by the Cu-MT thiolate absorptions, including the 300–340 nm signals corresponding to the special binding sites for Cu(I) characteristic of the Cu-thioneins.
Figure 6.
Deconvoluted ESI-MS spectra corresponding to the production of CnMT2 in Cu-enriched media under (A and B) normal and (C and D) low aeration conditions, recorded at (A and C) neutral and (B and D) acidic pH. CD spectra of (E) the Cu-CnMT2 production under low (solid line) and normal (dashed) aeration conditions-for comparative purposed, the dashed curve has been normalized (dotted)-; and of (F) Cu-CnMT1 (dotted) and Cu-CnMT2 (solid) productions under low aeration conditions.
3.5.4. Cu(I)-binding by CnMT2: Zn/Cu displacement on Zn-CnMT2
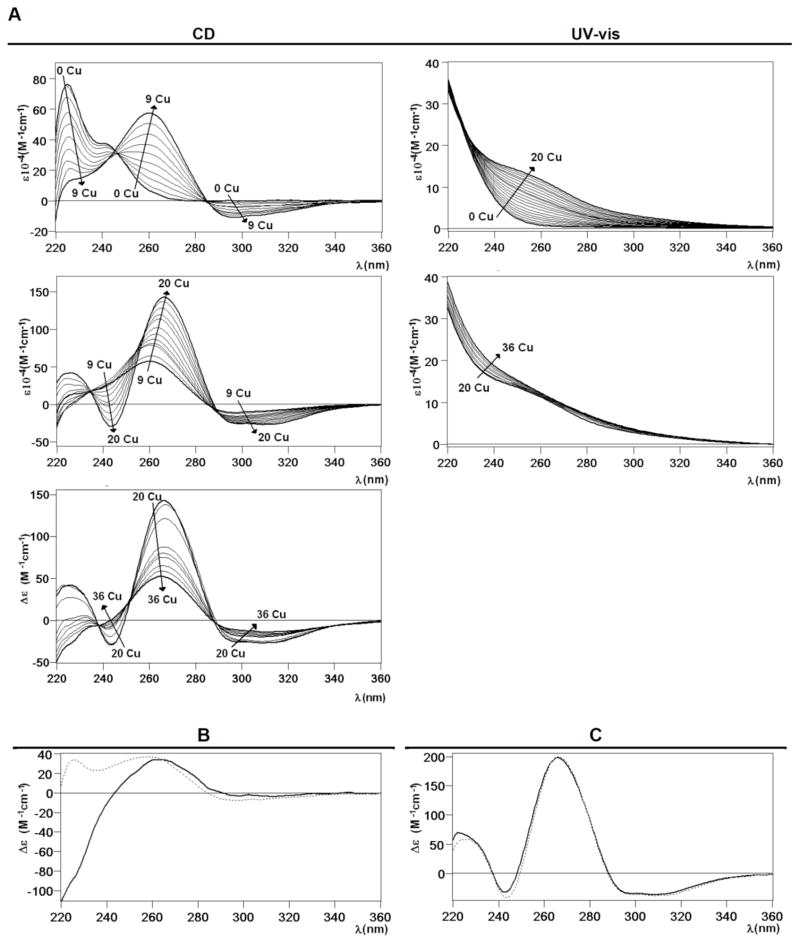
As for CnMT1, revealing data on the Cu(I) binding abilities of CnMT2 came from the deep analysis of its Zn/Cu exchange reaction, performed until 36 Cu(I) eq added to Zn-CnMT2. Hence, analogously, the corresponding CD spectra (Fig. 7) exhibited very nice isodichroic points from 0 to 9, 9 to 20 and 20 to 36 Cu(I) eq added, which suggested cooperative copper loading and zinc displacement processes. These stages were not isosbestic, and the variation of the CD absorbance at 230 and 260 nm clearly indicated that there are important changes around 9 and 20 Cu(I) eq added (Fig. 7A). In the first period, two isodichroic points were observed, with a neat increase of the Gaussian band centered at 260 nm, and the negative absorption at ca. 300 nm. In the second step, between 9 and 20 Cu(I) eq added, the spectra follow a similar pattern than in the first phase, with two nice isodichroic points at 235 and 287 nm and the CD absorption at ca. 270 reaching its highest intensity. Finally, in the last titration stage, for more than 20 eq Cu(I) added, the CD fingerprint decreases its intensity, probably as a result of the MT cluster unfolding (Fig. 7A). ESI-MS monitoring of the process revealed that although no cooperativity can be claimed, Cu4-Cu5, Cu9-Cu10, Cu14-Cu15, Cu19-Cu20, and Cu23-Cu24 pairs of complexes were favored when increasing amounts of Cu(I) were added to the sample (Fig. 7D). The addition of Cu(I) increases the complexity of the initial Zn11-CnMT2 sample in terms of the number of species present. But contrasting with this heterogeneity, acid MS spectra revealed that CnMT2 also builds its metallic complexes on the basis of Cu5-building blocks, here until a total of five Cu5 clusters. Interestingly, it also seems that just one of the Cu(I) ions in one of the Cu5 clusters exhibits a certain instability that leads to the observed perfect series of duplets of ESI MS peaks. These duplets of mass peaks gain in intensity during all the process until 20 Cu(I) eq added. Afterwards, their abundance and nuclearity diminishes until the end of the reaction (32–36 Cu(I) eq added) when only the very stable Cu5-core, and in this case also apo-CnMT2 remain in solution. Also like for CnMT1, two different steps of the titration (here, after 6 and 20 Cu(I) eq added to Zn-CnMT2) nicely reproduced the features of the in vivo samples obtained from normal and low aerated bacterial cultures conditions, respectively. The CD spectra at these two points of the titrations also reproduce the CD fingerprints of the normal and low aerated Cu-CnMT2 recombinant preparations (Fig. 7B and 7C, respectively), as described for CnMT1. Of note, the presence of complexes containing a (5n-1) number of Cu(I) ions (being n the number of the hypothetical Cu5-units) accompanying the Cu5n-CnMT2 peaks is also constant during all the Zn/Cu substitution reaction (Fig. 7D), as before commented for CnMT1, so that a parallel interpretation of this result is envisaged. Most significantly, in the case of CnMT2 this applies to the highest Cu(I) stoichiometry detected both in vivo and in vitro (Cu24-CnMT2) because a Cu25-CnMT2 species has been observed in no case. Finally, the observation that there are no CnMT2 complexes containing supernumerary Cu(I) ions beyond 5n values is concordant with the lack of flanking Cys doublets in this isoform, contrarily to the case observed for the CnMT1 polypeptide
Figure 7.
(A) CD and UV-vis spectra recorded after the addition of Cu(I) to the Zn-CnMT2 preparation. (B) CD spectra corresponding to the Cu-CnMT2 preparation under normal aeration (solid line) and that recorded after the addition of 6 Cu(I) eq to the Zn-CnMT2 preparation (dotted). (C) CD spectra corresponding to the Cu-CnMT2 preparation under low aeration (solid line) and that recorded after the addition of 20 Cu(I) eq to the Zn-CnMT2 preparation (dotted). (D) ESI-MS spectra recorded after the addition of Cu(I) to the Zn-CnMT2 preparation, recorded at neutral and acidic pH, at the +10 charge state.
4. Conclusions
The C. neoformans CnMT1 and CnMT2 metallothioneins exhibit all the coordination features typical of genuine Cu-thioneins, this including an optimal Cu-binding behavior, while suboptimal divalent metal ion binding characteristics.7 The C. neoformans MT system constitutes another outstanding example of how different criteria for considering MT as either divalent-metal-ion thioneins (Zn- or Cd-thioneins) or Cu-thioneins, precisely their gene response pattern or the specificity of their protein function, converge to a same classification. Hence, the detailed study of the metal-binding preferences of both CnMT1 and CnMT2 presented in this work, which attributes to these proteins an unambiguous character of Cu-thioneins perfectly matches the fact of being encoded by genes belonging to the C. neoformans copper regulon.6 This confirms that gene transcription induction by a given effector and optimized function of the corresponding protein towards this effector are strongly correlated in a gene/protein system, as we had previously shown for the Drosophila melanogaster Cu-thionein family.25
Recombinant synthesis both in Zn(II)- and Cd(II)-enriched E. coli cultures rendered a low yield of complexes, exhibiting variable stoichiometries. Besides, The S2--containing Cd-MT species that are invariably rendered by Cu-thioneins when synthesized in Cd-rich media, were clearly identified for both isoforms. Finally, the Zn(II)/Cd(II) displacement process gave rise to a myriad of Zn, Cd-mixed species, present even at the end of the metal replacement reaction, in full agreement with a poor divalent metal ion binding ability. On the contrary, the characterization of their Cu(I) coordination properties clearly showed how the CnMT1 and CnMT2 polypeptides are optimized to yield well-folded, high Cu(I)-containing complexes. The nature of these complexes reflects the copper concentration of the surrounding medium, as shown before for the S. cerevisiae Crs5 MT,15 so that at high-Cu conditions both isoforms would exert their maximum detoxification abilities, rendering Cu16-CnMT1 and Cu24-CnMT2 homometallic species. Zn(II)/Cu(I) replacement reactions proceed by discrete steps of 5 Cu(I) ion incorporations, and in full correspondence with the recombinantly synthesized complexes. This prompted us to propose that the Cu(I)-CnMT species are built on the basis of Cu5-clusters, a copper-thiolate cluster structure unprecedented in the literature of copper-aggregates in MTs. Significantly, the modular structure of the CnMT1 and CnMT2 polypeptides, constituted by three and five Cys-rich regions separated by spacer stretches, further supports this hypothesis, if assuming that the final stoichiometries (Cu16- and Cu24-) of the homometallic Cu(I)-CnMT species folded in vivo at high Cu concentrations represent the respective three- and five-fold amplification of the basic Cu5-core unit identified in the Cu titration reaction.
Comparison of the CnMT1 and CnMT2 gene and protein sequence features strongly coincide in supporting the emergence of the long C. neoformans MTs by ancient tandem repetitions of a primeval fungal MT unit, currently represented by the Neurospora26 and Agaricus27 MT proteins. These are the smallest known MTs (27-amino acid long), characterized by a -X3-[CXC]-X5-[CXC]-X3-[CXC]-X2-CX3- signature, and encoded by a gene including one single intron.28 This Cys pattern almost exactly coincides with the Cys-boxes hypothesized in this work to be the building blocks of CnMT1 and CnMT2 (cf. Fig. 1), with the only difference that both Neurospora and Agaricus MTs were described to yield Cu6-complexes,29,30,31 instead of the Cu5-clusters here reported for the CnMTs. Further work is being carried out in our laboratories to characterize the coordination features of these CnMT building regions and their additive capacity in order to understand how the natural selection pressure to cope with high copper concentrations may have conditioned this amplification of a primeval fungal copper-chelating small peptide.
Conclusively, this study contributes to the characterization of the high Cu(I) binding capacity of C. neoformans MTs, which act as a microbial pathogenicity and virulence determinant. The consideration of copper as an active agent used by the immune system (i.e. macrophages) of infected organisms against the invading microbes, and the study of the consequent counteracting mechanisms developed by the pathogens to thrive in this adverse surrounding, have lately gathered high research efforts (cf. excellent recent reviews32,33). Both bacterial (Enterococcus hirae, Salmonella typhimurium and Mycobacterium tuberculosis) and fungal (Cryptococcus neoformans) pathogens exhibit either cytoplasmic copper export or/and copper sequestration strategies to tolerate high copper. Therefore, the characterization of the regulation and function of these gene/protein systems attains significant importance in the context of understanding the copper homeostasis at the host-pathogen interface. CnMT1 and CnMT2 play an essential role in C. neoformans copper resistance, since MT proteins are the main mechanism of metal homeostasis in eukaryotic cells, in contrast to metal ion export that predominates in prokaryotes. Nevertheless, it is significant that MTs or MT-like proteins such as MymT in M. tuberculosis, 34 CusF in E. coli,35 and CueP in S. typhimurium,36,37 have been recently identified in bacteria, which promisingly points to a putative role as virulence factors also in pathogenic bacteria.
Supplementary Material
Acknowledgments
This work was supported by the Spanish Ministerio de Economía y Competitividad, grants BIO2012-39682-C02-01 (to SA) and 02 (to MC), which are co-financed by the European Union through the FEDER program, and NIH grant GM41840 to DJT. Authors from both Barcelona universities are members of the 2009SGR-1457 Grup de Recerca de la Generalitat de Catalunya. We thank the Centres Científics i Tecnològics (CCiT) de la Universitat de Barcelona (ICP-AES, DNA sequencing) and the Servei d’Anàlisi Química (SAQ) de la Universitat Autònoma de Barcelona (CD, UV-vis, ESI-MS) for allocating instrument time.
Abbreviations
- MT
metallothionein
- CnMT
Cryptococcus neoformans metallothionein
References
- 1.Capdevila M, Atrian S. J Biol Inorg Chem. 2011;16:977–989. doi: 10.1007/s00775-011-0798-3. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila M, Bofill R, Palacios O, Atrian S. Coord Chem Rev. 2012;256:46–62. [Google Scholar]
- 3.Palacios O, Atrian S, Capdevila M. J Biol Inorg Chem. 2011;16:991–1009. doi: 10.1007/s00775-011-0827-2. [DOI] [PubMed] [Google Scholar]
- 4.Ding C, Festa RA, Chen YL, Espart A, Palacios O, Espin J, Capdevila M, Atrian S, Heitman J, Thiele D. Cell Host & Microbe. 2013;13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronstad JW, Attarian R, Cadieux B, Choi J, D’Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M, Saikia S, Wang J. Nat Rev Microbiol. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. Mol Microbiol. 2011;81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bofill R, Capdevila M, Atrian S. Metallomics. 2009;1:229–34. doi: 10.1039/b904953c. [DOI] [PubMed] [Google Scholar]
- 8.Dolderer B, Hartmann HJ, Weser U. In: Metal Ions in Life Sciences: Metallothioneins and Related Chelators. Sigel A, Sigel H, Sigel RKO, editors. Vol. 5. RSC Publishing; Cambridge, U.K: 2009. pp. 83–106. [Google Scholar]
- 9.Calderone V, Dolderer B, Hartmann HJ, Echner H, Luchinat C, del Bianco C, Mangani S, Weser U. Proc Natl Acad Sci USA. 2005;102:51–56. doi: 10.1073/pnas.0408254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobine PA, McKay RT, Zangger K, Dameron CT, Armitage IM. Eur J Biochem. 2004;271:4213–4221. doi: 10.1111/j.1432-1033.2004.04361.x. [DOI] [PubMed] [Google Scholar]
- 11.Nielson KB, Atkin CL, Winge DR. J Biol Chem. 1985;260:5342–5350. [PubMed] [Google Scholar]
- 12.Jensen LT, Peltier JM, Winge DR. J Biol Inorg Chem. 1998;3:627–631. [Google Scholar]
- 13.Bofill R, Palacios O, Capdevila M, Cols N, Gonzalez-Duarte R, Atrian S, Gonzalez-Duarte P. J Inorg Biochem. 1999;73:57–64. doi: 10.1016/S0162-0134(98)10091-0. [DOI] [PubMed] [Google Scholar]
- 14.Díaz S, Amaro F, Rico D, Campos V, Benítez L, Martin-Gonzalez A, Hamilton EP, Orías E, Gutierrez JC. PLoS One. 2007;3:e291. doi: 10.1371/journal.pone.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagani A, Villarreal L, Capdevila M, Atrian S. Mol Microbiol. 2007;63:256–269. doi: 10.1111/j.1365-2958.2006.05510.x. [DOI] [PubMed] [Google Scholar]
- 16.Capdevila M, Cols N, Romero-Isart N, Gonzalez-Duarte R, Atrian S, Gonzalez-Duarte P. Cell Mol Life Sci. 1997;53:681–688. doi: 10.1007/s000180050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cols N, Romero-Isart N, Capdevila M, Oliva B, Gonzalez-Duarte P, Gonzalez-Duarte R, Atrian S. J Inorg Biochem. 1997;68:157–166. doi: 10.1016/s0162-0134(97)00085-8. [DOI] [PubMed] [Google Scholar]
- 18.Bofill R, Palacios O, Capdevila M, Cols N, Gonzalez-Duarte R, Atrian S, Gonzalez-Duarte P. J Inorg Biochem. 1999;73:57–64. doi: 10.1016/S0162-0134(98)10091-0. [DOI] [PubMed] [Google Scholar]
- 19.Bongers J, Walton CD, Richardson DE, Bell JU. Anal Chem. 1988;60:2683–2686. doi: 10.1021/ac00175a008. [DOI] [PubMed] [Google Scholar]
- 20.Capdevila M, Domenech J, Pagani A, Tio L, Villarreal L, Atrian S. Angew Chem Int Ed Engl. 2005;44:4618–4622. doi: 10.1002/anie.200501183. [DOI] [PubMed] [Google Scholar]
- 21.Fabris D, Zaia J, Hathout Y, Fenselau C. J Am Chem Soc. 1996;118:12242–12243. [Google Scholar]
- 22.Tío L, Villarreal L, Atrian S, Capdevila M. J Biol Chem. 2004;279:24403–24413. doi: 10.1074/jbc.M401346200. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Rafael S, Kurz A, Guirola M, Capdevila M, Palacios O, Atrian S. Metallomics. 2012;4:342–349. doi: 10.1039/c2mt00182a. [DOI] [PubMed] [Google Scholar]
- 24.Artells E, Palacios O, Capdevila M, Atrian S. Metallomics. 2013;5:1397–1410. doi: 10.1039/c3mt00123g. [DOI] [PubMed] [Google Scholar]
- 25.Egli D, Domenech J, Selvaraj A, Balamurugan K, Hua H, Capdevila M, Georgiev O, Schaffner W, Atrian S. Genes Cells. 2006;11:647–658. doi: 10.1111/j.1365-2443.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 26.Lerch K. Nature. 1980;284:368–370. doi: 10.1038/284368a0. [DOI] [PubMed] [Google Scholar]
- 27.Münger K, Lerch K. Biochemistry. 1985;24:6751–6756. [Google Scholar]
- 28.Münger K, German UA, Lerch K. EMBO J. 1985;4:2665–2668. doi: 10.1002/j.1460-2075.1985.tb03985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltramini M, Lerch K. Biochemistry. 1983;22:2043–2048. doi: 10.1021/bi00278a002. [DOI] [PubMed] [Google Scholar]
- 30.Smith TA, Lerch K, Hodgson KO. Inorg Chem. 1986;25:4677–4680. [Google Scholar]
- 31.Beltramini M, Lerch K, Vasak M. Biochemistry. 1984;23:3422–3427. doi: 10.1021/bi00310a007. [DOI] [PubMed] [Google Scholar]
- 32.Samanovic MI, Ding C, Thiele D, Darwin HK. Cell Host & Microbe. 2012;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgkinson V, Petris MJ. J Biol Chem. 2012;287:13549–23555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C. Nat Chem Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanke S, Grass G, Resing C, Nies DH. J Bacteriol. 2003;185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pontel LB, Soncini FC. Mol Microbiol. 2009;73:212–225. doi: 10.1111/j.1365-2958.2009.06763.x. [DOI] [PubMed] [Google Scholar]
- 37.Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. J Biol Chem. 2010;285:25259–25268. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.