Abstract
Human ectocervical cells, following retroviral transduction with the human papillomavirus type 16 E6/E7 oncogenes, are altered in their array of transcribed cellular genes, including increased mRNA for the insulin-like growth factor binding protein 3 (IGFBP-3). IGFBP-3 expression is associated with cellular senescence, and its addition to many cell types inhibits growth or induces apoptosis. By immunoblotting and enzyme-linked immunosorbent assay methods, we demonstrate that late-passage, immortalized E6/E7-transduced cells secrete high levels of IGFBP-3 (25 ng/ml), which represent a 500-fold increase compared to levels in early-passage, nonimmortalized transduced cells (<0.05 ng/ml). Concomitantly, these late-passage cervical cells exhibit an increase in sensitivity to IGF-1, including enhanced phosphorylation of the IGF receptor (IGF-R) and insulin receptor substrate as well as increased DNA synthesis (5-fold) and cell proliferation (3.7-fold). However, there was no change in the level of IGF-R in these cells (surface or total), and the cells did not synthesize IGF-1, indicating that these arms of the IGF pathway were independently regulated and not responsible for the augmented signaling. Consistent with a causal relationship between IGFBP-3 expression and enhanced IGF-1 responses, we found that early-passage cells could be converted to the late-passage, IGF-1-responsive phenotype by preincubation with IGFBP-3. Thus, in contrast to findings with some cell types, IGFBP-3 expression in cervical cells is associated with augmented IGF-1 signaling and cell proliferation and correlates with the timing of cellular immortalization.
Infection with high-risk human papillomaviruses (HPVs) such as HPV type 16 (HPV-16) has been identified as an etiologic factor in more than 95% of cervical cancers (50). The HPV E6 and E7 genes are retained and expressed in cervical cancers and can stimulate cell proliferation as well as alter cell differentiation (51). Two key targets of the E6 and E7 oncoproteins are the tumor suppressor p53 and retinoblastoma (pRb) proteins, respectively. The E6 protein promotes the ubiquitin-dependent degradation of p53 (29, 45), and the E7 protein associates with and degrades pRb (19, 38), thereby stimulating the E2F signaling pathway. As a consequence of these and other activities attributed to the E6/E7 proteins, epithelial cells exhibit impaired cell cycle control (51).
In several epidemiological studies, changes in the balance of insulin-like growth factor (IGF) and insulin-like growth factor binding proteins (IGFBPs) have been associated with increased risk for some of the most prevalent human cancers (22, 35, 42, 47). IGF can regulate the proliferation of normal and tumorigenic cells via autocrine or paracrine regulatory mechanisms (7, 11) and potentially via endocrine pathways as well (18, 41). Perturbations in each level of the IGF signaling pathway have been implicated in carcinogenesis (4, 5). However, the effects of IGF and the corresponding IGFBPs on cell growth and differentiation are variable and dependent upon cell type. For example, IGF-1 has been shown to exhibit both stimulatory and inhibitory effects on cell growth (8, 11, 34). While epithelial cells display receptors for IGF-1 and respond to the ligand, they do not synthesize the growth factor (3). Rather, underlying fibroblasts synthesize and secrete IGF-1, which then tends to accumulate in epithelial cell layers.
IGFBP-3, the most abundant IGFBP in human serum, is synthesized mainly by hepatic Kupffer cells and binds over 90% of circulating IGF, resulting in a prolonged half-life (6). IGFBP-3 is also produced locally by a variety of normal and tumor cells, suggesting that the cellular microenvironment may directly affect the action of IGF-1 (11). The biological functions of IGFBP-3, aside from being the major binding protein for IGF-1, are complex and remain poorly understood. However, IGFBP-3 is known to inhibit cell proliferation by interfering with the interaction of IGF-1 and its receptor (34, 37). Interestingly, IGFBP-3 can also modulate cell growth and survival independently of IGF (7), presumably via interactions with cellular proteins such as the Alzheimer's survival protein, humanin (31), and nuclear targets such as the retinoid X receptor alpha (7, 8, 36).
Our laboratory recently reported that human ectocervical cells transduced with HPV-16 E6/E7 oncogenes maintain stable oncoprotein expression but exhibit progressively increasing levels of IGFBP-3 mRNA coincident with immortalization (9). Furthermore, our investigators documented overexpression of IGFBP-3 mRNA in high-grade squamous intraepithelial neoplasias by in situ hybridization, demonstrating a correlation with neoplastic progression (9). In this study, we quantified the secretion of IGFBP-3 by E6/E7-transduced cells and analyzed its effects on the proliferation of cervical cells and their response to IGF-1. In contrast to early-passage cells, late-passage cells secreted IGFBP-3 and showed an increased response to IGF-1 as determined by IGF-1 receptor (IGF-1R) and insulin receptor substrate (IRS) phosphorylation. Preincubation of early-passage cells with recombinant human IGFBP-3 and subsequent treatment with IGF-1 induced a dose-dependent activation of IGF-1R and IRS as well as an increase in cell proliferation. Thus, the increased responsiveness of HPV-immortalized cells to IGF-1 could potentially contribute to their in vivo growth, where IGF-1 is produced by surrounding stromal cells.
MATERIALS AND METHODS
Cell culture.
Primary human ectocervical epithelial cells were derived from fresh cervical tissues obtained after hysterectomy for benign uterine diseases. Standard trypsinization procedures were used to isolate the epithelial cells, which were maintained in serum-free keratinocyte growth medium supplemented with 50 μg of bovine pituitary extract/ml and 2.6 ng of recombinant epidermal growth factor/ml (supplemented KSFM; Invitrogen, Carlsbad, Calif.) (39). Primary cultures were infected at passage 4 with high-titer LXSN retroviruses expressing HPV-16 E6/E7 genes, with the HPV-16 E6 gene containing an AU1 epitope at the 3′ terminus to facilitate immunodetection (2). Control LXSN retroviruses expressed only the neomycin resistance gene. After infection, cells were selected with 50 μg of G418/ml for 5 days and were subcultured at least once before initiating experiments. All subsequent passages were performed at a split ratio of 1:4. Cells that were passaged fewer than 10 times after transduction were designated early passage, cells passaged 10 to 30 times were designated intermediate passage, and those passaged more than 40 times were considered late passage.
Conditioned cell culture medium was prepared by plating 105 cells per well in 12-well plates. Cells were incubated for 6 days in 300 μl of supplemented KSFM, and medium was collected for further experiments.
To analyze total IGF-1R levels, cells were plated in 100-mm-diameter tissue culture dishes and grown in supplemented KSFM to 80 to 90% confluency before harvesting.
For antiphosphotyrosine and IRS immunoprecipitations, 1.5 × 106 cells were plated in 150-mm tissue culture dishes in supplemented KSFM. After allowing cells to attach overnight, the medium was changed to 15 ml of serum-free keratinocyte medium per dish without supplements (basal KSFM). The cells were starved for 3 days, the medium was reduced to 12 ml per dish, and different concentrations of human recombinant IGFBP-3 (Upstate Biotechnology, Lake Placid, N.Y.) were added as indicated in the figures. After 3 days, recombinant human IGF-1 (Invitrogen) was added at a final concentration of 25 ng/ml, and cells were incubated for 10 min at 37°C before lysis.
Immunoprecipitation and Western blotting.
Secreted IGFBP-3 was analyzed by Western blotting of conditioned cell culture medium. Aliquots of medium (35 μl) were supplemented with 15 μl of sodium dodecyl sulfate (SDS)-extraction buffer (0.8% SDS, 20% glycerol, 0.125 M Tris-HCl; pH 6.8), reduced with 10% 2-mercaptoethanol, and heated for 7 min at 95°C. Proteins were electrophoretically separated on a 1.5-mm 10% Tris-glycine minigel (Invitrogen), transferred to an Immobilon-P membrane (Millipore, Bedford, Mass.), and incubated overnight at 4°C with anti-IGFBP-3 mouse immunoglobulin G1 (IgG1) antibody at 1 μg/ml (BD Transduction Laboratories, Lexington, Ky.) in phosphate-buffered saline (PBS) containing 0.5% polyoxyethylene (20) sorbitan monolaurate (Tween 20) and 5% natural nonfat dry milk. Following reaction with 0.3 μl of alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Tropix, Foster City, Calif.)/ml for 90 min, IGFBP-3 was visualized with a chemiluminescent substrate (Tropix).
Total IGF-1R levels were determined by Western blotting of cell lysates. A 50-μg aliquot of total protein was separated in an 8% Tris-glycine gel (Invitrogen), transferred to an Immobilon-P membrane (Millipore), and labeled for 90 min with 0.2 μg of anti-IGF-1Rβ rabbit polyclonal IgG antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.)/ml diluted in washing buffer (0.5% Triton X-100, 140 mM NaCl, 10 mM Na3PO4) in the presence of 2% bovine serum albumin (ICN Biomedicals, Inc., Aurora, Ohio). Following reaction with 0.3 μg of alkaline phosphatase-conjugated goat anti-rabbit IgG antibody (Tropix)/ml, total IGF-1R levels were visualized with chemiluminescent substrate (Tropix).
To immunoprecipitate tyrosine-phosphorylated proteins, cells were scraped into 0.9 ml of SDS-lysis buffer (0.4% SDS, 100 mM NaCl, 2 mM EDTA, 50 mM HEPES-NaOH; pH 7.4), immediately heated for 10 min at 95°C, and disrupted by sonication. Prior to determination of protein concentration (Bio-Rad Laboratories), 100 μl of 20% Triton X-100 was added. A 700-μg aliquot of total protein was mixed with 30 μl of protein A-agarose beads (Pierce, Rockford, Ill.) and 4 μg of 5H1 anti-phosphotyrosine monoclonal antibody (a gift of A. Burkhardt and J. Bolen) and rotated overnight at 4°C. Subsequently, beads were washed and bound proteins were eluted in 1× SDS gel sample buffer (3% SDS, 20 mM dithiothreitol, 1.5 mM EDTA, 11% sucrose, 0.008% bromphenol blue, 112 mM morpholinepropanesulfonic acid [MOPS]-NaOH; pH 7.0). Samples were alkylated by addition of 80 mM iodoacetamide followed by electrophoresis and transferred to an Immobilon-P membrane as outlined above. Western blotting was performed by labeling with 0.5 μg of anti IGF-1Rβ rabbit polyclonal antibody (Santa Cruz) and goat anti-rabbit IgG antibody (Tropix)/ml as described above.
Immunoprecipitations for IRS-1 were carried out on 700 μg of protein prepared as described above, using 1 μg of anti-IRS-1 rabbit polyclonal antibody (Upstate Biotechnology). For this experiment, cells were lysed in 1.0 ml of modified radioimmune precipitation assay buffer (150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 20 mM MOPS-NaOH; pH 7.0) containing 0.57 mM phenylmethanesulfonyl fluoride, 1 μM leupeptin, and 0.3 μM aprotinin, and a protein assay was performed. After incubation of cell lysates with protein A-agarose beads and subsequent washes, bound proteins were eluted by boiling samples for 7 min in 45 μl of SDS-extraction buffer containing 10% 2-mercaptoethanol. Samples were fractionated by SDS-polyacrylamide gel electrophoresis (Invitrogen) and transferred to an Immobilon-P membrane (Millipore) which was labeled with 1 μg of antiphosphotyrosine mouse monoclonal antibody (Upstate Biotechnology)/ml diluted in Tris-buffered saline (150 mM NaCl, 10 mM Tris-HCl; pH 8.0) containing 5% bovine serum albumin (Sigma, St. Louis, Mo.) and 1% ovalbumin (Sigma). Goat anti-mouse IgG antibody (0.3 μg/ml; Tropix) was used as secondary antibody. After visualization of tyrosine-phosphorylated IRS, the blot was stripped for 30 min at 70°C in stripping solution containing 12% 0.5 M Tris-HCl (pH 6.8), 2% SDS, and 0.34% 2-mercaptoethanol diluted in distilled H2O. To determine the total abundance of IRS protein, the membrane was relabeled with 0.5 μg of anti-IRS-1 rabbit polyclonal antibody (Upstate Biotechnology) and goat anti-mouse IgG antibody (0.3 μg/ml; Tropix).
ELISA.
Secretion of IGFBP-3 and IGF-1 in conditioned cell culture medium was quantified by an enzyme-linked immunosorbent assay (ELISA). IGFBP-3 concentrations were evaluated utilizing a two-step sandwich-type ELISA kit (Diagnostic Systems Laboratories, Inc., Webster, Tex.) according to the manufacturer's instructions. Briefly, standards (protein-based buffer containing five different concentrations of synthetic IGFBP-3), controls (low and high concentrations of IGFBP-3), and samples were incubated with agitation for 2 h at room temperature in microtitration wells coated with anti-IGFBP-3 polyclonal antibody. After incubation and washing, the wells were treated with a horseradish peroxidase-labeled anti-IGFBP-3 polyclonal antibody for 1 h with agitation at room temperature. Following several washes, the wells were labeled with tetramethylbenzidine for 10 min. The reaction was terminated by the addition of an acidic stopping solution, and the enzymatic product was determined by 450-nm wavelength absorbance measurements using a Dynatech Micro-Elisa reader. Based on a plotted standard curve, the IGFBP-3 concentration could be directly calculated. Determination of IGF-1 secretion was performed applying a similar ELISA kit (Diagnostic Systems Laboratories, Inc.) and following the above protocol with slight alterations. The assay included an extraction step before labeling, in which IGF-1 was separated from its binding proteins. Standards, controls, and samples were then in one step incubated with anti-IGF-1 antibody labeled with horseradish peroxidase in anti-IGF-1 antibody-coated microtitration wells.
FACS.
The level of IGF-1R on the cell surface was determined by flow cytometry. Cells were harvested from 100-mm tissue culture dishes by trypsinization and washed once in PBS and fluorescence-activated cell sorter (FACS) buffer (PBS containing 2% bovine serum albumin [ICN Biomedicals, Inc.]). A total of 106 cells were then incubated for 30 min in the dark on ice in 20 μl of IGF-1Rα R-phycoerythrin-conjugated mouse anti-human monoclonal antibody (Becton Dickinson Pharmingen, San Diego, Calif.) diluted in 500 μl of FACS buffer and subsequently washed three times in FACS buffer. A total of 10,000 cells were collected and analyzed for fluorescence using a FACStar Plus flow cytometer (Becton Dickinson, San Diego, Calif.). Single parameter fluorescence histogram data were generated and evaluated by FCS Express (De Novo Software, Thornhill, Ontario, Canada).
Cell proliferation assays.
To investigate the effects of IGFBP-3 and IGF-1 on cell proliferation, 2.5 × 105 cells were seeded into 60-mm tissue culture dishes in supplemented KSFM. After overnight incubation the dishes were washed twice with PBS and the medium was changed to 2.5 ml of basal KSFM. Various concentrations of IGFBP-3 (Upstate Biotechnology) were then added. After 3 days, the cell number was quantitated (in triplicate), after which the cultures were supplemented with IGF-1 (25 ng/ml) for an additional 3 days. The cells were harvested and counted to determine final cell numbers. All experimental groups were performed in triplicate and expressed as the mean.
[3H]thymidine incorporation.
Cells at early and late passages were subcultured in 24-well tissue culture plates at a density of 20,000 cells/well in supplemented KSFM. After allowing cells to attach overnight, medium was changed to basal KSFM for 3 days. To assay an IGFBP-3-induced increase in DNA synthesis, the medium was supplemented with different concentrations of human recombinant IGFBP-3 and the cells were incubated for an additional 3 days. IGF-1 was then added (final concentration of 25 ng/ml), and 2 hours later 2.5 μCi of [3H]thymidine (ICN Biomedicals, Inc.) was added for 22 h. Cells were washed twice with cold PBS, fixed for 10 min with cold 100% ethanol and for 20 min with cold 5% trichloroacetic acid, and finally washed twice with trichloroacetic acid and twice with distilled water. Cells were solubilized, and DNA was extracted in 0.5 ml of 1 N NaOH, followed by neutralization with 0.5 ml of 1 N HCl. [3H]thymidine incorporation into DNA was determined by liquid scintillation counting (1209 RackBeta liquid scintillation counter; Pharmacia, Turku, Finland). All experiments were performed in triplicate.
RESULTS
IGFBP-3 secretion increases during in vitro passaging of E6/E7-transduced cervical epithelial cells.
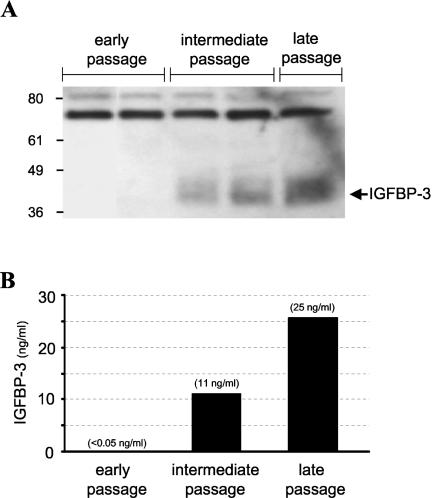
Primary cultures of human ectocervical epithelial cells were isolated from fresh cervical tissue, transduced with either an empty LXSN retrovirus or one encoding E6/E7 of HPV-16, and then selected in G418 antibiotic. The E6/E7-transduced cells have been passaged more than 200 population doublings without a detectable crisis period, whereas the control cells ceased proliferating after 16 population doublings. Stable expression of the HPV-16 E6/E7 oncoproteins in these cells has already been confirmed by immunoprecipitation and immunoblotting analysis (2). To monitor levels of secreted IGFBP-3 throughout cell passaging, we performed both Western immunoblotting (Fig. 1A) and ELISA (Fig. 1B) of conditioned cell culture medium. Western blotting demonstrated that the E6/E7-transduced cells did not secrete detectable IGFBP-3, whereas intermediate- and late-passage cells did (Fig. 1A). Proteolytic fragments of IGFBP-3, which have been implicated in altering IGF-binding activity (11), were not observed. Even with the use of a sensitive ELISA that can detect IGFBP-3 levels of ≥0.04 ng/ml, there was no detectable secreted IGFBP-3 protein at early passages (<passage 10) (Fig. 1B). However, at intermediate passages corresponding to cell immortalization (passage 20 to 30), IGFBP-3 was detectable at 11 ng/ml, which represents at least a 200-fold increase over early-passage cells. There was even a larger increase in IGFBP-3 secretion (>500-fold) in late-passage cells (25 ng/ml) (Fig. 1B). The results in Fig. 1 are representative of three separate experiments and correlate with the previous observation that mRNA levels are elevated 85-fold (9). The observation that IGFBP-3 secretion correlates temporally with cell immortalization is also reinforced by our laboratory's findings that the nonimmortalizing E6/E7 genes of HPV-6 do not induce IGFBP-3 (9).
FIG. 1.
IGFBP-3 secretion increases during in vitro passaging of E6/E7-transduced cervical epithelial cells. (A) Detection of secreted IGFBP-3 protein in cell culture medium. Culture medium supernatants were assayed for the presence of IGFBP-3 by immunoblotting as described in Materials and Methods. Measurements were performed at different passages after retroviral transduction (early, <passage 10; intermediate, passage 10 to 30; late, >passage 40). The arrow indicates the position of the IGFBP-3 protein. (B) Quantification of secreted IGFBP-3 protein. The levels of secreted IGFBP-3 were determined by ELISA of conditioned cell culture medium at the indicated passages. Early-passage, E6/E7-transduced cells did not secrete detectable protein levels of IGFBP-3, whereas intermediate- and late-passage cultures expressed the binding protein at 11 and 25 ng/ml, respectively. The sensitivity of the ELISA was 0.04 ng/ml.
IGFBP-3 augments ligand activation of the IGF-1R.
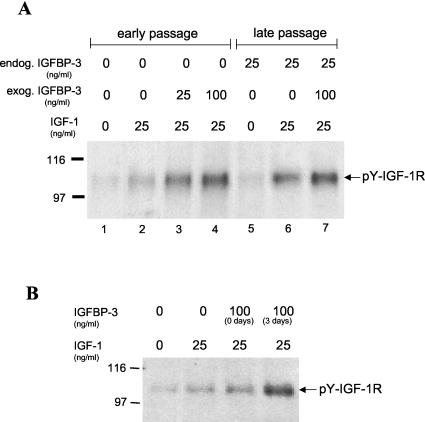
Since IGFBP-3 can bind IGF-1, it was possible that the high levels of IGFBP-3 secreted by the late-passage cells might modulate their response to either autocrine or paracrine sources of IGF-1. We therefore first investigated whether the transduced cervical cells might also be secreting IGF-1 and thereby initiating an active autocrine loop. We screened supernatant medium from the late-passage cervical cells by ELISA but could find no detectable IGF-1 protein (assay sensitivity of 0.03 ng/ml), suggesting that an autocrine source of IGF-1 was unlikely. However, it was possible that IGFBP-3 might modulate paracrine responses to exogenous IGF-1, as normally observed in vivo where IGF-1 is synthesized by mesenchymal cells and acts on epithelial cells. To evaluate this possibility, we performed a series of experiments on early- and late-passage cervical cells stimulated with exogenous IGF-1. Cervical cells were starved 3 days in basal KSFM, treated with 25 ng of human recombinant IGF-1/ml for 10 min, and then examined for activation of the IGF-1R. As shown in Fig. 2A, the IGF-1R was more highly phosphorylated on tyrosine in late-passage cells than early-passage cells (compare lanes 2 and 6), demonstrating that late-passage cells are more sensitive to IGF-1 in the presence of secreted IGFBP-3. There was only a low level of IGF-1R phosphorylation in the absence of IGF-1 in either the early- or late-passage cells (lanes 1 and 5), consistent with our previous finding that these cells do not synthesize detectable amounts of IGF-1. If the production of IGFBP-3 by late-passage cells (25 ng/ml) was responsible for this differential activation of IGF-1R by IGF-1, we postulated that we should be able to convert early-passage cells to this higher level of activation by incubation with equivalent amounts of IGFBP-3. To test this hypothesis, we added 25 ng of IGFBP-3/ml to early-passage cells (the level found in late-passage cells) and examined the level of receptor activation. IGFBP-3 enhanced receptor activation in early-passage cells to levels found in the late-passage cells (compare lanes 3 and 6). Addition of more IGFBP-3 further increased this activation (compare lanes 2, 3, and 4), suggesting that 25 ng of IGFBP-3/ml is not saturating for this enhancement activity. It appears, therefore, that equivalent IGF-1R activation can be observed in cervical cells if they are stimulated with 25 ng of IGFBP-3/ml or they produce IGFBP-3 themselves at that same level (compare lanes 3 and 6).
FIG. 2.
IGFBP-3 augments ligand activation of the IGF-1R. (A) Dose response of IGFBP-3 effects on IGF-1R activation. Cultured cervical cells were maintained for 3 days in basal medium containing various concentrations of IGFBP-3. The cells were then treated with 25 ng of IGF-1/ml for 10 min, lysed, and analyzed for tyrosine phosphorylation of the IGF-1R by immunoblotting as described in the text. The arrow indicates the position of the IGF-1R β-subunit. (B) Effects of immediate and prolonged IGFBP-3 exposure on IGF-1R activation. Early-passage cervical cells were exposed to IGFBP-3 at the same time as they received IGF-1 for 0 days or, alternatively, they were exposed to IGFBP-3 for 3 days prior to addition of IGF-1 (3 days). Late-passage cells responded to exogenous IGF-1 with enhanced IGF-1R phosphorylation. In early-passage cells, a similar increase in tyrosine-phosphorylated (pY) IGF-1R was observed after preincubation with IGFBP-3, indicating their conversion to the IGF-1-responsive phenotype of late-passage cells. Simultaneous IGFBP-3-IGF-1 incubation of early-passage cells diminished this effect, suggesting a necessity for preincubation with IGFBP-3.
Previous studies have suggested that a 3-day preincubation with IGFBP-3 is optimal for observing some of its biological activities. To determine whether acute or delayed effects were responsible for the effects we observed on IGF-1R activation, we analyzed cells which were exposed simultaneously to IGFBP-3 and IGF-1 or were preincubated for 3 days with IGFBP-3 prior to IGF-1 addition. In Fig. 2B, it is apparent that the best activation of IGF1-R was observed following 3 days of incubation with the binding protein. This implies that IGFBP-3 is not simply binding to IGF-1 and altering its receptor affinity or effect on target cells. Similarly, the results also indicate that the binding protein is probably not acting directly on the receptor to alter either its ligand affinity or signaling responses. The requirement for a prolonged or chronic exposure to IGFBP-3 suggests that a more indirect effect is responsible for this change in growth factor sensitivity, possibly due to the induction of cellular genes.
Total and cell surface IGF-1R levels remain stable during passaging of E6/E7-transduced cervical cells.
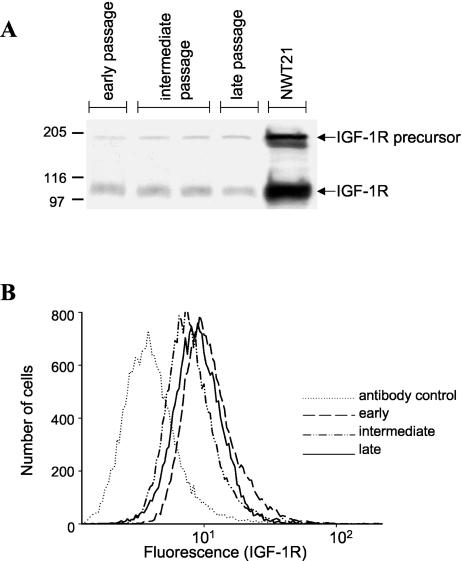
The above studies indicate that there is enhanced phosphorylation of IGF-1R in cells preincubated with IGFBP-3. This normally derives from an increase in the percentage of receptors that are activated. However, increased phosphorylation could potentially result from an increase in the amount of phosphorylation per receptor or in the absolute number of receptors. Since we determined receptor phosphorylation by first immunoprecipitating all tyrosine-phosphorylated proteins and then probing with antireceptor antibodies, this procedure would quantify receptor phosphorylation independent of the number of phosphorylation sites. That is, the increase in observed IGF-1R phosphorylation is not due to an increase in the amount of phosphorylation per receptor. To evaluate whether receptor overexpression accounted for this phenotype, we quantified total IGF-1R. Total IGF-1R levels were determined by Western immunoblot analysis using an antibody that specifically binds to the IGF-1R β-chain (Fig. 3A). Cellular levels of IGF-1R showed little change from early to late passage.
FIG. 3.
Total and cell surface IGF-1R levels remain stable during cell passaging. (A) Detection of total cell IGF-1R. Total IGF-1R protein levels were detected by Western blotting at the indicated passages using an antibody that specifically binds to the IGF-1R β-chain. NWT21 cells (a mouse 3T3 fibroblast cell line overexpressing the IGF-1R) served as a positive control. The positions of the IGF-1R β-subunit and the IGF-1R precursor are indicated. (B) Detection of cell surface IGF-1R. Cell surface IGF-1R levels were evaluated on live cervical cells by flow cytometry using the indicated antibody. The number of cells versus relative fluorescence intensity is plotted. The levels of total and surface IGF-1R showed no significant changes during cell passaging.
While changes in receptor number are not responsible for the increase in IGF-1R phosphorylation, it was possible that late-passage cells might have a redistribution of receptor, with more receptor being on the surface and accessible to ligand. We therefore analyzed the amount of surface IGF-1R by FACS using a phycoerythrin-conjugated IGF-1R antibody (Fig. 3B). There was no significant difference in cell surface IGF-1R numbers during passaging. Thus, the increased phosphorylation of the IGF-1R in late-passage cervical cells was not due to gross alterations in receptor number or distribution. In parallel, the incubation of cervical cells with various concentrations of human recombinant IGFBP-3 for 3 days did not cause significant changes in cell surface IGF-1R number (data not shown). These results indicate, therefore, that the increase in IGF-1R phosphorylation is due to an increase in the percentage of receptors that are phosphorylated.
IGFBP-3 enhances IGF-1R downstream signaling.
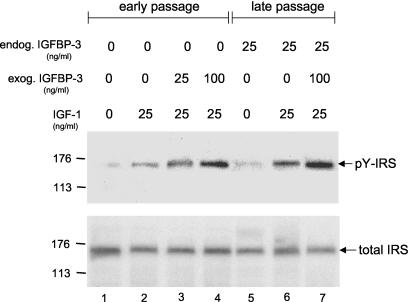
Having established that IGFBP-3 enhanced IGF-1R phosphorylation, we further examined whether this apparent activation resulted in the phosphorylation of one of its downstream targets, IRS. Cervical cells were treated with IGF-1 and IGFBP-3 as described for Fig. 2A, after which they were immunoprecipitated and immunoblotted for tyrosine-phosphorylated IRS (Fig. 4). The phosphorylation of IRS closely paralleled the results with the phosphorylation of IGF-1R, suggesting that there was successful signaling by the activated receptor. The proportional increase between IGF-1R and IRS phosphorylation also suggested that IGFBP-3 does not activate IRS independently of IGF-1R. As in Fig. 2A, the activation of IRS was enhanced in late-passage cells (Fig. 4, compare lanes 2 and 6), and it was possible to induce equivalent levels of IRS phosphorylation in early-passage cells by adding IGFBP-3 at levels found in late-passage cells (compare lanes 3 and 6). Again, this suggests that IGFBP-3 appears to be a key mediator of elevated receptor activation. The levels of IRS in both early- and late-passage cells were very similar (Fig. 4, total IRS).
FIG. 4.
IGFBP-3 enhances IGF-1R downstream signaling. Cells were incubated for 3 days in basal medium containing various concentrations of IGFBP-3. The cultures were then treated with IGF-1 for 10 min, and cell lysates were analyzed by immunoprecipitation and immunoblotting for the amount of tyrosine-phosphorylated (pY) IRS. After detection of (pY) IRS levels, the same membrane was stripped and reprobed for total IRS levels as described in the text.
IGFBP-3 enhances IGF-induced DNA synthesis and cell division.
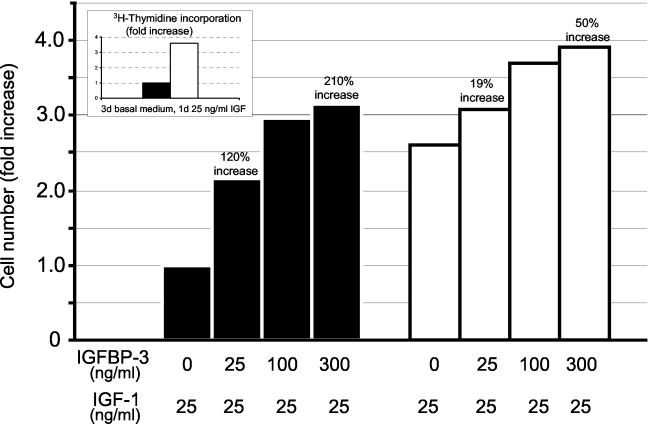
Stimulation of the IGF-1 pathway can result in cell proliferation, and we therefore evaluated whether this pathway was functionally activated in the cervical cells by IGFBP-3. First we determined the level of DNA synthesis induced by IGF-1 in early- and late-passage cells by IGF-1. Cells were cultured for 3 days in basal KSFM and then stimulated with 25 ng of IGF-1/ml. [3H]thymidine was added for 22 h, and its incorporation into DNA was measured as described in Materials and Methods. As shown in the insert of Fig. 5, late-passage cells were 3.5-fold more sensitive to IGF-1 than early-passage cells, consistent with their production of 25 ng of IGFBP-3/ml. To evaluate whether exogenous IGFBP-3 augmented this induction of DNA synthesis by IGF-1 and whether cell division resulted, we measured the cell number following treatment with 25 ng of IGF-1/ml and various concentrations of IGFBP-3 (Fig. 5). IGFBP-3 at 25 ng/ml induced a 120% increase in the number of early-passage cells. At 300 ng/ml, a concentration at which IGFBP-3 is in excess compared to IGF-1, IGFBP-3 induced a 210% increase in these cells. In contrast, there was only a 19% increase in the number of late-passage cells treated with 25 ng of IGFBP-3/ml. This limited mitogenic response was most likely due to the presence of 25 ng of IGFBP-3/ml in the medium (as a consequence of cell synthesis). Even at the highest amounts of IGFBP-3 (300 ng/ml), the late-passage cells showed only a 50% increase in cell number. When early- and late-passage cells were grown in supplemented KSFM (with all growth factors), there was no difference in cell proliferation, suggesting that the differences in growth rate were indeed the consequence of the response to IGFBP-3 and not an inherent difference in their growth rate (data not shown). Thus, IGFBP-3 augments IGF-1 signaling and cell division in early-passage cells which do not synthesize IGFBP-3. In late-passage cells which already produce IGFBP-3, there is only a limited mitogenic response to the binding protein.
FIG. 5.
IGFBP-3 enhances IGF-induced DNA synthesis and cell division. (Inset) Cellular DNA synthesis (measured by [H3]thymidine incorporation) was enhanced 3.5-fold in late-passage cells, consistent with their production of 25 ng of IGFBP-3/ml. Early-passage (black bars) and late-passage (white bars) cells (2.5 × 105 per plate) were maintained for 3 days in basal growth medium followed by a 3-day period in the same medium supplemented with 25 ng of IGF-1/ml and increasing amounts of IGFBP-3. The number of cells per plate was quantified with a Coulter Counter, and the results are expressed as the fold increase in the number of cells. IGFBP-3 at 25 and 300 ng/ml induced a 120 and 210% increase in cell number, respectively. In contrast, there was only a 19 and 50% increase in the number of late-passage cells treated with the same concentrations of IGFBP-3.
DISCUSSION
IGFBP-3 is emerging as an important modulator of IGF-regulated cellular growth (11, 25, 34). Many cell types display altered production of IGFBP-3 in response to hormonal (15, 30, 33) and metabolic (25, 27) factors as well as to viral and cellular oncogenes (9). Alterations in IGFBP-3 levels appear to induce complex effects, observed as either inhibiting or potentiating IGF-1 action (11, 34). In the present study, we further defined the functional role of IGFBP-3 in high-risk HPV-16 E6/E7-infected human ectocervical epithelial cells. We investigated the expression and effects of IGFBP-3 on the IGF-1 signaling pathway, as well as cell proliferation during the process of cell passaging and immortalization after retroviral transduction with HPV-16 E6/E7 oncogenes. While both oncoproteins are expressed stably during successive cell passaging (2), IGFBP-3 gene expression increases markedly during immortalization (9). Here, we used sensitive immunoblotting and ELISA techniques to quantify secreted IGFBP-3 levels.
To date, the majority of studies examining the role of IGFBP-3 in IGF-1R signaling have used immortalized cell lines (1, 24, 27). We have performed studies on cervical cells as they transition from the nonimmortal to immortal state. We demonstrate that IGFBP-3 secretion initiates during cell immortalization (passage 20 to 30) and increases somewhat thereafter (>passage 40). In contrast, cervical cells do not secrete detectable levels of IGFBP-3 early after retroviral transduction (<passage 10). These results indicate that besides the presence of the viral E6 and E7 proteins (which are at equivalent levels before, during, and after immortalization), there must be additional cellular changes required for IGFBP-3 induction, or that there is a very small population of immortalized cells at early times which synthesize IGFBP-3 and that these cells are selected for by the protocol of in vitro passaging.
IGFBP-3 is an important regulator of cell responsiveness to IGF-1 (11), and increased serum levels have been correlated with an increased risk for breast (26, 47), prostate (34), and colorectal cancers (42). We have utilized our human cervical epithelial cell model to study the functional role of IGFBP-3 in modulating IGF-1 bioactivity. We were able to verify that cervical cells, like other epithelial cells (3), do not produce detectable levels of IGF-1. Nevertheless, it is important to note that in vivo IGF-1 is synthesized and released by fibroblasts and stimulates the growth of surrounding epithelial cells via a paracrine mechanism (3). Furthermore, IGF-1 has been suggested to contribute to malignant progression (25). Therefore, changes in local IGFBP-3 concentrations could be critical for modulating the mitogenic activity of IGF-1 and might potentiate the development of cervical tumors after infection with high-risk HPVs. Our data indicating that E6/E7-immortalized cervical cells secrete IGFBP-3 and display increased mitogenic sensitivity to IGF-1 are compatible with several other reports in which IGFBP-3 was shown to potentiate the mitogenic effects of IGF-1 (10, 12, 14, 17, 24). However, there are also reports that IGFBP-3 is responsible for growth inhibition and the induction of senescence (23, 28, 32) and that a loss of IGFBP-3 is observed in both prostate and lung cancer (46, 48). These contradictory findings most likely derive from differences in cell type and/or culture conditions. A previous study has documented by in situ hybridization that IGFBP-3 mRNA was undetectable in normal ectocervical tissue but overexpressed in high-grade squamous intraepithelial neoplasias (9), suggesting that our in vitro studies have direct applicability to cervical dysplasia and cancer.
Several studies indicate that direct interactions with IGF-1 are responsible for the biological activity of IGFBP-3. For example, IGFBP-3 prolongs the IGF-1 half-life dramatically (11), and it serves as a principle carrier and reservoir of IGF-1, delivering and presenting IGF-1 to target cells. However, our results appear to be independent of direct IGF-1-IGFBP-3 interactions. IGFBP-3 requires chronic preexposure (3 days) in order to induce the IGF-sensitized state. Since our experimental protocols for IGF-1R phosphorylation employ brief (10-min) exposures to IGF-1, it is also highly unlikely that alterations in IGF-1 stability are a factor. In addition, we see very little effect of IGFBP-3 when it is added concomitantly with IGF-1.
It is possible that IGFBP-3 sensitizes cells to IGF-1 via the induction of cell proteins that regulate this signaling pathway. This hypothesis is strengthened by the observation that it requires 72 h for IGFBP-3 to fully sensitize cells to IGF-1, whereas it associates with the cell surface within 24 h, indicating a significant delay between cell binding and signal sensitization. Despite uncertainty about the precise mechanism involved, IGFBP-3 enhancement of IGF action has been observed in various cell types, including fibroblasts, osteoblasts, and breast cancer cells (13, 20, 40). Interestingly, it has been revealed very recently that IGFBP-3 can bind and enhance the antiapoptotic activity of the cellular protein humanin (31). Whether this direct interaction may contribute to the stimulatory effects of IGFBP-3 that we observe in our cervical cells is currently unknown. Furthermore, there are sequences within the carboxyl terminus of IGFBP-3 which regulate cell association (21), possibly indicating the presence of specific cell surface receptors that bind IGFBP-3 (16, 40, 49). The observation that IGFBP-3 also encodes a nuclear transport signal and can be observed to translocate into the cell nucleus provides additional support for this binding protein having its own biological signaling mechanisms (43, 44).
The primary observation of this study is that IGFBP-3 augments ligand activation of the IGF-1R in cervical epithelial cells and that immortalization of these cells is accompanied by a dramatic (500-fold) increase in the expression of this binding protein. In addition, our laboratory has also demonstrated that IGFBP-3 can increase IGF-1-induced cell proliferation, suggesting that the in vivo expression of this binding protein in cervical dysplasia (9) may contribute to a selective growth advantage for HPV-immortalized cells.
Acknowledgments
We thank Frank Suprynowicz for helpful discussions of experimental procedures and Carl Baker for reviewing the manuscript.
This research was supported by a grant from the National Cancer Institute, R0153371.
REFERENCES
- 1.Andreatta-Van Leyen, S., J. R. Hembree, and R. L. Eckert. 1994. Regulation of insulin-like growth factor 1 binding protein 3 levels by epidermal growth factor and retinoic acid in cervical epithelial cells. J. Cell. Physiol. 160:265-274. [DOI] [PubMed] [Google Scholar]
- 2.Baege, A. C., A. Berger, R. Schlegel, T. Veldman, and R. Schlegel. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreca, A., M. De Luca, P. Del Monte, S. Bondanza, G. Damonte, G. Cariola, E. Di Marco, G. Giordano, R. Cancedda, and F. Minuto. 1992. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J. Cell. Physiol. 151:262-268. [DOI] [PubMed] [Google Scholar]
- 4.Baserga, R. 1995. The insulin-like growth factor 1 receptor: a key to tumor growth? Cancer Res. 55:249-252. [PubMed] [Google Scholar]
- 5.Baserga, R., A. Hongo, M. Rubin, M. Prisco, and B. Valentinis. 1997. The IGF-1 receptor in cell growth, transformation and apoptosis. Biochem. Biophys. Acta 1332:105-126. [DOI] [PubMed] [Google Scholar]
- 6.Baxter, R. C., and J. L. Martin. 1989. Binding proteins for the insulin-like growth factors: structure, regulation and function. Prog. Growth Factor Res. 1:49-68. [DOI] [PubMed] [Google Scholar]
- 7.Baxter, R. C. 2000. Insulin-like growth factor (IGF)-binding proteins: interactions with IGF's and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 278:967-976. [DOI] [PubMed] [Google Scholar]
- 8.Baxter, R. C. 2001. Signaling pathways involved in antiproliferative effects of IGFBP-3: a review. Mol. Pathol. 54:145-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger, A. J., A. Baege, T. Guillemette, J. Deeds, R. Meyer, G. Disbrow, R. Schlegel, and R. Schlegel. 2002. Insulin-like growth factor-binding protein 3 expression increases during immortalization of cervical keratinocytes by human papillomavirus type 16 E6 and E7 proteins. Am. J. Pathol. 161:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, J.-C., Z. M. Shao, M. S. Sheikh, A. Hussain, D. LeRoith, C. T. Roberts, and J. A. Fontana. 1994. Insulin-like growth factor-binding protein enhancement of insulin-like growth factor-1 (IGF-1)-mediated DNA synthesis and IGF-1 binding in a human breast carcinoma cell line. J. Cell. Physiol. 158:69-78. [DOI] [PubMed] [Google Scholar]
- 11.Clemmons, D. R. 1997. Insulin-like growth factor binding proteins and their role in controlling IGF actions. Cytokine Growth Factor Rev. 8:45-62. [DOI] [PubMed] [Google Scholar]
- 12.Conover, C. A., M. Ronk, F. Lombana, and D. R. Powell. 1990. Structural and biological characterization of bovine insulin-like growth factor binding protein-3. Endocrinology 127:2795-2803. [DOI] [PubMed] [Google Scholar]
- 13.Conover, C. A., and D. R. Powell. 1991. Insulin-like growth factor (IGF)-binding protein-3 blocks IGF-1 induced receptor down regulation and cell desensitization in cultured bovine fibroblasts. Endocrinology 129:710-716. [DOI] [PubMed] [Google Scholar]
- 14.Conover, C. A. 1992. Potentiation of insulin like growth factor (IGF) action by IGF-binding protein-3: studies of underlying mechanism. Endocrinology 130:3191-3199. [DOI] [PubMed] [Google Scholar]
- 15.Conover, C. A., J. T. Clarkson, and L. K. Bale. 1995. Effects of glucocorticoids on insulin-like growth factor (IGF) regulation of IGF-binding protein expression in fibroblasts. Endocrinology 136:1403-1410. [DOI] [PubMed] [Google Scholar]
- 16.Conover, C. A., J. T. Clarkson, and L. K. Bale. 1996. Factors regulating insulin-like growth factor-binding protein-3 binding, processing, and potentiation of insulin-like growth factor action. Endocrinology 137:2286-2292. [DOI] [PubMed] [Google Scholar]
- 17.DeMellow, J. S. M., and R. C. Baxter. 1988. Growth hormone-dependent insulin like growth factor (IGF) binding protein both inhibits and potentiates IGF-1 stimulated DNA synthesis in human skin fibroblasts. Biochem. Biophys. Res. Commun. 156:199-204. [DOI] [PubMed] [Google Scholar]
- 18.D'Ercole, A. J., and A. S. Calikoglu. 2001. The case of local versus endocrine IGF-1 actions: the jury is still out. Growth Hormone IGF Res. 11:261-265. [DOI] [PubMed] [Google Scholar]
- 19.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 20.Ernst, M., and G. A. Rodan. 1990. Increased activity of insulin-like growth factor (IGF) in osteoblastic cells in the presence of growth hormone (GH): positive correlation with the presence of the GH-induced IGF-binding protein BP-3. Endocrinology 127:8070-8814. [DOI] [PubMed] [Google Scholar]
- 21.Firth, S. M., U. Ganeshprasad, and R. C. Baxter. 1998. Structural determinants of ligand and cell surface binding of insulin-like growth factor-binding protein-3. J. Biol. Chem. 273:2631-2638. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci, E. 1999. Insulin-like growth factor-1 and binding protein-3 and risk of cancer. Hormone Res. 3:34-41. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein, S., E. J. Moerman, R. A. Jones, and R. C. Baxter. 1991. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc. Natl. Acad. Sci. USA 88:9680-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grill, C. J., and W. S. Cohick. 2000. Insulin-like growth factor binding protein-3 mediates IGF-1 action in a bovine mammary epithelial cell line independent of an IGF interaction. J. Cell. Physiol. 183:273-283. [DOI] [PubMed] [Google Scholar]
- 25.Grimberg, A., and P. Cohen. 2000. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 183:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hankinson, S. E., W. C. Willett, G. A. Colditz, D. J. Hunter, D. S. Michaud, B. Deroo, B. Rosner, F. E. Speizer, and M. Pollak. 1998. Circulating concentrations of insulin-like growth factor-1 and risk of breast cancer. Lancet 351:1393-1396. [DOI] [PubMed] [Google Scholar]
- 27.Hembree, J. R., C. Agarwal, and R. L. Eckert. 1994. Epidermal growth factor suppresses insulin-like growth factor binding protein 3 levels in human papillomavirus type 16 immortalized cervical epithelial cells and thereby potentiates the effects of insulin-like growth factor 1. Cancer Res. 54:3160-3166. [PubMed] [Google Scholar]
- 28.Hochscheid, R., G. Jaques, and B. Wegmann. 2000. Transfection of human insulin-like growth factor-binding protein 3 gene inhibits cell growth and tumorigenicity: a cell culture model for lung cancer. J. Endocrinol. 166:553-563. [DOI] [PubMed] [Google Scholar]
- 29.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 and 18. EMBO J. 10:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huynh, H., Y. F. Yang, and M. Pollak. 1996. Estradiol and antiestrogens regulate a growth inhibitory insulin-like growth factor binding protein-3 autocrine loop in human breast cancer cells. J. Biol. Chem. 271:1016-1021. [DOI] [PubMed] [Google Scholar]
- 31.Ikonen, M., B. Liu, Y. Hashimoto, L. Ma, K.-W. Lee, T. Niikura, I. Nishimoto, and P. Cohen. 2003. Interaction between Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein-3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. USA 100:13042-13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karas, M., M. Danilenko, D. Fishman, D. LeRoith, J. Levy, and Y. Sharoni. 1997. Membrane-associated insulin-like growth factor-binding protein-3 inhibits insulin-like growth factor-1-induced insulin-like growth factor-1 receptor signaling in Ishikawa endometrial cancer cells. J. Biol. Chem. 26:16514-16520. [DOI] [PubMed] [Google Scholar]
- 33.Katz, L. E. L., R. G. Rosenfeld, and P. Cohen. 1995. Clinical significance of insulin-like growth factor binding proteins (IGFBP's). Endocrinologist 5:36-43. [Google Scholar]
- 34.Kelley, K. M., Y. Oh, S. E. Gargosky, Z. Gucev, T. Matsumoto, V. Hwa, L. Ng, D. M. Simpson, and R. G. Rosenfeld. 1996. Insulin-like growth factor binding proteins (IGFBPs) and their regulatory dynamics. Int. J. Biochem. Cell Biol. 28:619-637. [DOI] [PubMed] [Google Scholar]
- 35.Khosravi, J., A. Diamandi, J. Mistry, and A. Scorilas. 2001. Insulin-like growth factor I (IGF-1) and IGF-binding protein-3 in benign prostatic hyperplasia and prostatic cancer. J. Clin. Endocrinol. Metab. 86:694-699. [DOI] [PubMed] [Google Scholar]
- 36.Liu, B., H. Y. Lee, S. A. Weinzimer, D. R. Powell, J. L. Clifford, J. M. Kurie, and P. Cohe. 2000. Direct functional interactions between insulin-like growth factor binding protein-3 and retinoid-X receptor-alpha regulate transcriptional signaling and apoptosis. J. Biol. Chem. 275:33607-33613. [DOI] [PubMed] [Google Scholar]
- 37.Mohensi-Zadeh, S., and M. Binoux. 1997. Insulin-like growth factor (IGF) binding protein-3 interacts with the type 1 IGF receptor, reducing the affinity of the receptor for its ligand: an alternative mechanism in the regulation of IGF action. Endocrinology 38:5645-5648. [DOI] [PubMed] [Google Scholar]
- 38.Münger, K., B. A. Werness, N. Dyson, W. C. Phlebs, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nees, M., J. M. Geoghegan, T. Hyman, S. Frank, L. Miller, and C. Woodworth. 2001. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappa-responsive genes in cervical keratinocytes. J. Virol. 75:4283-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh, Y., H. L. Muller, H. Pham, and R. G. Rosenfeld. 1993. Demonstration of receptors of insulin-like growth factor binding protein-3 on HS578T breast cancer cells. J. Biol. Chem. 268:26045-26048. [PubMed] [Google Scholar]
- 41.Ohlsson, C., K. Sjögren, J.-O. Jansson, and O. G. P. Isaksson. 2000. The relative importance of endocrine versus autocrine/paracrine insulin-like growth factor-1 in the regulation of body growth. Pediatr. Nephrol. 14:541-543. [DOI] [PubMed] [Google Scholar]
- 42.Probst-Hensch, N. M., J.-M. Yuan, F. Z. Stanczyk, Y.-T. Gao, R. K. Ross, and M. C. Yu. 2001. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br. J. Cancer 85:1695-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radulescu, R. T. 1994. Nuclear localization signal in insulin-like growth factor-binding protein type 3. Trends Biochem. Sci. 19:278. [DOI] [PubMed] [Google Scholar]
- 44.Schedlich, L. J., T. F. Young, S. M. Firth, and R. C. Baxter. 1998. Insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J. Biol. Chem. 273:18347-18352. [DOI] [PubMed] [Google Scholar]
- 45.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 46.Schwarze, R. S., S. E. DePrimo, L. M. Grabert, V. X. Fu, J. D. Brooks, and D. F. Jarrard. 2002. Novel pathways associated with bypassing cellular senescence in human epithelial cells. J. Biol. Chem. 277:14877-14883. [DOI] [PubMed] [Google Scholar]
- 47.Vadgama, J. V., Y. Wu, G. Datta, H. Khan, and R. Chillar. 1999. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology 57:330-340. [DOI] [PubMed] [Google Scholar]
- 48.Yu, H., M. R. Spitz, J. Mistry, J. Gu, G. K. Hong, and X. Wu. 1999. Plasma levels of insulin-like growth factor-1 and lung cancer risk: a case-control analysis. J. Natl. Cancer Inst. 91:151-156. [DOI] [PubMed] [Google Scholar]
- 49.Zheng, B., and D. R. Clemmons. 1998. Blocking ligand occupancy of the αVβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 95:11217-11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochem. Biophys. Acta 1288:55-78. [DOI] [PubMed] [Google Scholar]
- 51.zur Hausen, H. 2000. Papillomavirus causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 92:690-798. [DOI] [PubMed] [Google Scholar]