Abstract
The increasing use of medical imaging as an investigative tool is leading to the incidental and frequent finding of renal cysts in the general population. The presence of a solitary or multiple renal cysts has been generally considered benign in the absence of a family history of renal cystic disease or evidence of chronic kidney disease. Nonetheless, a number of recent studies have questioned this consensus by reported associations with the development of hypertension or malignant change. For these reasons, some clinicians consider the presence of renal cysts to be a contraindication to kidney donation. The situation is complicated by the different usage of the term ‘simple’ by some radiologists (to indicate non-complex lesions) or nephrologists (to indicate age-related non-hereditary lesions). We propose that the term ‘simple’ be replaced with the morphological description, Stage I renal cyst (Bosniak Classification). The presence of a Stage I renal cyst should not preclude kidney donation. However, occult renal disease should be excluded and appropriate donor assessment performed.
Keywords: acquired cystic disease, ADPKD, renal cell carcinoma, renal transplantation, simple renal cysts
INTRODUCTION
Renal cysts are composed of enclosed liquid or semisolid fluid [1] and are commonly identified on abdominal imaging [2]. Understanding the clinical significance of renal cysts has evolved from a pathological classification in 1964 [3], to the current distinction between the various inherited and acquired cystic kidney diseases (ACKD) [4], fuelled by clinical and radiological observations. While comprehending the pathogenesis of the commonest inherited cystic kidney disease, autosomal dominant polycystic kidney disease (ADPKD) has advanced to the current era of therapeutic clinical trials [5], the importance of solitary, Stage I renal cysts (often described as ‘simple renal cysts’) and their potentially pathological associations requires further consideration [6]. This review will discuss the definition, differential diagnosis, epidemiology and natural history of Stage I renal cysts including the evidence for their acceptability in appropriately assessed prospective kidney donors.
DEFINITION
Stage I renal cysts are usually asymptomatic, solitary and unilateral structures located in the renal cortex [6]. They have a smooth wall lined by a single layer of epithelial cells [4]. The typical ultrasound criteria for defining a Stage I renal cyst include round/oval shape; thin, smooth walls; posterior enhancement; no internal debris or septa [2]. If there is diagnostic uncertainty, a computerized tomography (CT) scan is typically recommended for further clarification [7]. However, contrast-enhanced ultrasound (CEUS) [8] is increasingly recognized as a reliable alternative modality to CT for classifying renal cysts and avoids the use of potentially nephrotoxic contrast agents and ionizing radiation [9]. The updated Bosniak renal cyst classification system [10] (Table 1), accepted by urologists and radiologists, was devised using the morphological appearance and enhancement of renal cysts on CT to determine their diagnosis and provide recommendations for their management. Some now recommend only using the morphological Bosniak classification [10] to describe renal cysts and in particular to abstain from using the term ‘simple’, a potential misnomer, since as discussed below they may be associated with risk factors and complications. However, currently this practice is not universally accepted by radiologists reporting renal cystic lesions incidentally detected on ultrasound, (non-renal protocol) CT and magnetic resonance imaging (MRI) as the term ‘simple renal cyst’ is considered to be widely understood by non-specialist physicians and surgeons.
Table 1.
The Bosniak renal cyst classification [10]
| Stage | Cyst wall | Septa | Calcification | Enhancement | Management |
|---|---|---|---|---|---|
| I | Hairline thin | No | No | No | No follow-up |
| II | Minimal regular thickening | Few, hairline thin | Smooth, hairline thin | No | No follow-up |
| IIFa | Minimal regular thickening | Multiple, minimal smooth thickening | Thick, nodular | No | CT: 3, 6, 12 monthly then annual |
| IIIb | Irregular thickening | Measurably thick, irregular | Thick, nodular, irregular | Yes | As IIF or surgical |
| IV | Gross, irregular thickening | Irregular gross thickening | Thick, nodular, irregular | Yes, tissue and cyst | Surgical |
aIIF F denotes follow-up. Cyst size of diameter of 3 cm is also an indication for follow-up.
bIII: indeterminate Stage III should be managed as IIF, while definitive Stage III should be managed surgically [6].
The average size of Stage I renal cysts are 5–10 mm in diameter, though they can be larger [4]. While the original definition of a Stage I renal cyst does not include size, the revised Bosniak renal cyst classification system highlights a diameter of ≥3 cm as worthy of follow-up and utilizes this size among the features to distinguish between a Stage II or IIF cyst [10]. A Stage I cyst now describes the former ‘simple’ renal cyst [10] and has a <1% risk of malignancy [2], Stage II cysts are considered to be minimally complicated [7] with a <3% risk of malignancy [2].
In terms of management, the importance of the definition of a Stage I renal cyst (Bosniak classification) is that it is considered to have low malignant potential and does not require follow-up [2]. It is paramount to distinguish Stage I renal cysts from ADPKD or a complex cyst including cystic renal cell cancer [11] as prognosis and management are very different. Conventionally, Stage I renal cysts do not require any treatment. However, if they cause symptoms or if a complication arises such as infection, haemorrhage or rupture, intervention may be required [7], which will be discussed later.
DIFFERENTIAL DIAGNOSIS
To differentiate Stage I renal cysts from ADPKD or ACKD, age, family history, number of cysts and possibly kidney size on ultrasound imaging, the presence of impaired kidney function or other associated clinical features may be helpful. Ultrasound diagnostic criteria [12] exist for individuals ‘at risk’ of ADPKD, in terms of the presence of a positive family history. The revised Ravine criteria propose the diagnostic probability of ADPKD in families of unknown genotype (the usual clinical scenario) based on age-banded criteria of the number of kidney cysts detected on ultrasound [12]. In ‘at risk’ adults aged ≥40 years, ADPKD can be excluded if <2 renal cysts are detected on ultrasound (negative predictive value of 100%) [12]. If the alternative and more sensitive imaging modalities of CT/MRI are used for diagnosis, recent recommendations advise only counting cysts if >1 cm in size [13]. In individuals with no family history, the diagnosis or exclusion of ADPKD is more challenging in patients who are young (<40 years) or older with equivocal imaging based on a few cysts and kidneys which are not enlarged. In these individuals, genetic testing should be considered [14]. A further diagnostic challenge is in differentiating Stage I renal cysts from ‘mild ADPKD’ which has been reported to be associated with hypomorphic or incompletely penetrant alleles and mosaicism [14]; a careful review of the apparently ‘negative’ family history could be informative.
An alternative diagnosis of ACKD should be considered in patients with chronic kidney disease or established renal failure (not caused by inherited cystic kidney disease) who develop three or more Stage I cysts [15]. Stage I cysts are observed in 8–13% of patients with chronic kidney disease and this frequency rises in those with established renal failure with duration on dialysis therapy, from 50% after 6 years to 100% after 10 years on dialysis [16]. ACKD is recognized to be a risk factor for renal cell carcinoma and has been reported in patients who remain on dialysis long term or who have received a renal transplant [17]. An ultrasound study of the native kidneys of 561 renal transplant recipients reported that 23% developed ACKD and 19.4% of this group was found to have a renal cell carcinoma [17]. ACKD is a powerful risk factor for renal cell carcinoma and screening of native kidneys by CT/MRI is recommended in renal transplant recipients with ACKD [17], since ultrasound may be insufficiently sensitive. However, a large study of patients on long-term dialysis registered on the United States Renal Data System (USRDS) only diagnosed renal cell carcinoma in 12.1% of patients with ACKD and found that the majority of patients with renal cell carcinoma did not have co-existent renal cysts. The authors therefore did not recommend screening patients on dialysis with acquired renal cysts [18]. Renal cell carcinomas associated with ACKD are considered to behave differently and to have distinct histological and genetic associations [19].
EPIDEMIOLOGY
The prevalence of Stage I renal cysts depends on the population studied, age and modality of imaging, with CT and MRI having increased sensitivity to detect smaller cystic lesions, down to 1 mm [13]. Following comparison with pathology specimens in patients with ADPKD, this limit of detection of even CT/MRI imaging modalities to identify small kidney cysts has been eloquently described as an iceberg effect, since as many as 62 times more cysts were detected (<1 mm) in nephrectomy specimens [20]. This study has not been performed in patients with ‘normal’ kidneys and Stage I renal cysts and is unlikely to be ethically feasible. Hence the prevalence of Stage I renal cysts may be significantly greater than reported herein.
Age
Stage I cysts have been detected, with an incidence of 1 in 1100 (0.09%) in fetuses during early pregnancy (14–16 weeks gestation), although the majority were noted to be transient [21]. In the same cohort of 29 984 babies with ultrasound repeated at birth, the incidence of Stage I renal cysts was only 0.007% [21]. The development of Stage I renal cysts is extremely rare between birth and 20 years. Thereafter, their prevalence increases progressively with age [6].
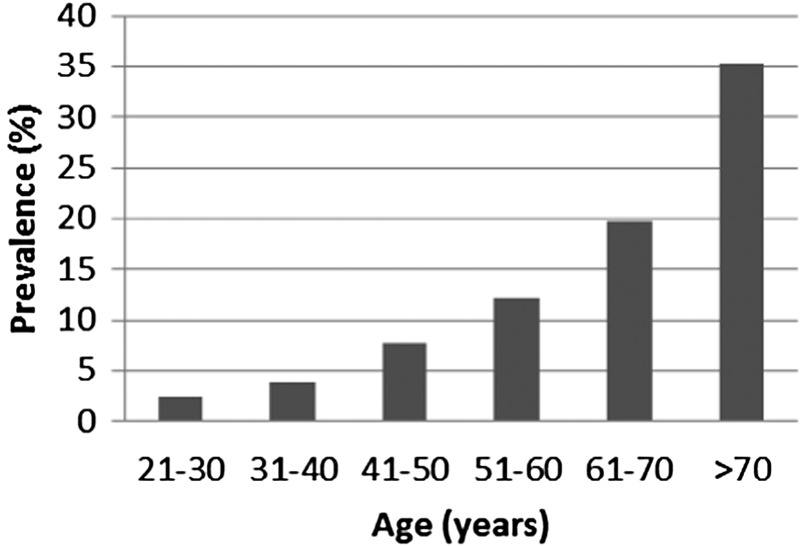
A large ultrasound study in an asymptomatic Australian population (729 individuals) with normal renal function reported an age-related prevalence of ≥1 Stage I renal cysts of 0% (15–29 years); 1.7% (30–49 years); 11.5% (50–70 years) and 22.1% (over 70 years) [22]. A more recent ultrasound study in 577 healthy individuals (aged 20–94 years) from Taiwan reported an increased prevalence of Stage I renal cysts associated with increasing age by decade (Figure 1) [23].
FIGURE 1:
Prevalence of Stage I renal cysts diagnosed on ultrasound in a healthy ageing population in Taiwan. Modified from [23].
Two large studies have reported the prevalence of Stage I renal cysts detected by CT scanning in populations in the UK [24] and the USA [11]. In a study from Edinburgh, Stage I renal cysts were incidentally detected on CT in 254 of 617 patients (295 females and 322 males) aged between 17–92 years, giving an overall population prevalence of 41% [24]. The age-related prevalence of Stage I renal cysts was confirmed on CT: 8.2% (17–39 years); 27.5% (40–59 years); 49% (60–80 years) and 60.6% (over 80 years) [24]. In the USA, the prevalence of Stage I renal cysts in 1957 healthy potential kidney donors was reported as 28% in 18–49 year olds and 43% in 50–75 year olds [11].
Gender
In all studies reported, Stage I renal cysts were more prevalent in men with reported male-to-female ratios of 1.4 [24] and 2.8 [23]. A large study of 29 523 healthy adults (aged 40–88 years) who participated in a general health screening programme (including abdominal ultrasound or CT imaging) from South Korea confirmed a male-to-female ratio of 1.7–2.0 across each decade for the prevalence of Stage I renal cysts [25]. This gender difference has been further evaluated regarding cyst location and confirmed for cortical cysts. However, the gender difference appeared less clear for medullary cysts [11].
PATHOGENESIS
Stage I renal cysts were postulated to originate from diverticulae in the distal convoluted tubule or collecting ducts [26] due to weakening of the tubular basement membrane precipitated by obstruction-related back pressure or age [4, 26]. This theory has since been refuted [27]. The currently accepted hypothesis is that renal ischaemia or injury prompts an aberrant hypertrophic response leading to cyst growth and leads to further nephron loss due to compensatory hyperfiltration [4, 28]. Given their slow rates of growth, by the time a Stage I renal cyst can be detected, many years will have elapsed since the initial injury [29]. The risk of exposure to subclinical renal injury likely increases with age and ageing is clearly associated with both a decline in glomerular filtration rate [30] and an increasing prevalence of renal cysts.
NATURAL HISTORY
A number of longitudinal studies have reported the natural history of Stage I renal cysts in various populations [23, 31, 32] to be benign. The natural history of Stage I renal cysts appears to be different in fetuses compared to children and adults. When detected in fetuses during pregnancy, Stage I renal cysts tend to be transient, with the majority resolving by birth. In babies with cysts persisting at birth, their appearance remained ‘simple’ in the majority, with only one baby from a study population of 29 984 proceeding to develop a unilateral multicystic dysplastic kidney [21].
Children
In a retrospective Turkish study [31], Stage I renal cysts were detected in a cohort of 45 children undergoing ultrasound for a clinical indication (abdominal pain, urinary tract infection, obesity or haematuria). The mean age of diagnosis of a Stage I renal cyst was 7.4 years and the mean follow-up period was 2.9 years during which the size of cysts increased in 49%, remained unchanged in 31% and decreased in 10%. In children with an increase in cyst size, the average size increase was 5.7 mm with an annual growth rate of 3.8%. There was no increase in the number of Stage I cysts. Two patients in this cohort were reported to suffer severe complications: one required a nephrectomy for a haemorrhagic cyst with a diameter of 170 mm compressing the renal vasculature; the other suffered severe loin pain associated with a 73 mm cyst [31]. We query the appropriateness of associating these complications with the natural history of ‘simple (Stage I) renal cysts’. Although the Bosniak classification has not been validated in children, it has been recognized as a useful guideline in children with renal cysts [33]. From the description of the two children suffering complications in this cohort, we question whether these lesions were ever ‘simple (Stage I) renal cysts’, particularly since their size was reported to be >3 cm (at least Bosniak Stage IIF). Indeed, the authors acknowledge that the patient requiring nephrectomy had a ‘Stage IV cyst’ [31], which by definition, is not simple. The only other paediatric study evaluating the natural history of Stage I renal cysts identified a frequency of 0.2% with no change in cyst size in 74% [34].
Adults
Despite the availability of studies with long-term follow-up describing the natural history of Stage I renal cysts in adults, an overall consensus as to the major risk factors and clinical associations is lacking. This incongruity may be due to the variability in the modalities of diagnostic imaging utilized, small cohorts and combination of data for Stage I and complex renal cysts.
It is generally accepted that most Stage I cysts enlarge slowly [6], although they may increase in size more quickly in younger patients and then stabilize [32]. A Japanese study involving 57 adult patients followed up by annual renal ultrasound for a mean duration of 9.9 years detected an average annual increase in cyst size of 1.4 mm and annual growth rate of 3.2% [32]. Most cysts did not grow larger than twice their original size during the 10-year follow-up period. Multivariate analyses determined that age was the most significant determinant of increasing cyst size [32].
Of concern, considering the benign perception of ‘Stage I renal cysts’, renal neoplasms were identified in 2 of 61 patients with renal cysts during a mean follow-up of 9.9 years [32]. Interestingly, the rate of growth of these subsequently malignant cystic lesions was similar to that of age matched patients with benign lesions. The distinguishing pathological feature identified on serial renal ultrasound in both patients was the development of an irregular or solid mass within the cyst wall, prompting further investigation and diagnosis of a tumour. In spite of these two cases, the authors concluded that follow-up of asymptomatic Stage I renal cysts remains unnecessary as imaging undertaken incidentally during the study did not enable earlier diagnosis [32].
Worldwide, only a handful of case reports describe the rare occurrence of Stage I renal cysts evolving into neoplasms [35–38]. In all cases, there was a change in the characteristics of the cyst wall, emphasizing the essential need for CT imaging to further evaluate any complexity of cysts [6]. CEUS is an emerging technique for assessing complex renal cysts and there are cases where it has provided additional definitive diagnostic information to CT, resulting in correct classification of malignant lesions [39].
The Japanese study also reported that the number of cysts increased with advancing age in the majority from a mean of 2.3 to 3.2 cysts over 10 years [32]. This is contrary to data from the Taiwanese study, of a similar cohort size, in which Stage I renal cysts were found to remain solitary in 82% of the study population [23].
RISK FACTORS AND ASSOCIATIONS
Several studies report advancing age [23, 32, 40], male gender [23, 40], renal dysfunction [23, 40], hypertension [40] and smoking [23] as associated with renal cysts on multivariate analysis. One study has reported the ipsilateral coexistence of renal stones with Stage I renal cysts on multivariate analysis, with an odds ratio of 2.15 [23]. There are several limitations to these studies including the definition of hypertension; failure to analyse Stage I renal cysts separately from more complex cysts or inherited cystic kidney disease and failure to exclude confounding factors. Whether the apparent associations between Stage I renal cysts and the aforementioned factors particularly renal dysfunction and hypertension are coincidental or genuine remains controversial and will be discussed below.
A possible pathogenic link between smoking and Stage I renal cysts has been proposed: smoking could be either directly toxic or induce renovascular disease [24] causing relative renal ischaemia. However, there is conflicting evidence from studies from South East Asia either supporting [23] or refuting [40] smoking as an independent risk factor for the development of Stage I renal cysts.
Since the initial report in 1942 of an association between a renal cyst inducing renal compression in a teenager with hypertension [41], numerous studies have reported that renal cysts may cause hypertension, which frequently resolved following cyst removal [42] or aspiration [43]. One surgical study involving treatment of patients with large renal cysts (mean cyst size of 7.45 cm) suggested a beneficial effect on blood pressure (overall decrease or antihypertensive therapy reduction) in 62% [42]. The mechanism of cyst-associated hypertension has been postulated to be renin related with epithelial cells lining the cyst-releasing renin [44]. Supporting evidence for the role of renin comes from other reports that smaller cysts (which in some studies are more associated with hypertension) [1, 45], are embedded within renal parenchyma leading to high hydrostatic pressure, compression of surrounding tissue and renal ischaemia [45]. Increased renin secretion has also been detected in the renal veins of kidneys with very large or perihilar renal cysts [40, 44].
A large (29 523 adults) retrospective study [25] aimed to assess whether there was an association between Stage I renal cysts and hypertension in a healthy South Korean population. While comprehensively reporting cyst number, size and location, the investigators did not use the standard accepted definition of hypertension (≥140/90 mmHg) [46], instead using a level of ≥130/85 mmHg. However, within this population, the presence of a Stage I renal cyst was associated with a 60% higher odds ratio of hypertension.
A second large retrospective South Korean study [47] evaluated the association between the presence of Stage I renal cysts and hypertension. Although they report statistically significant differences between the mean systolic and diastolic blood pressure of patients with (125/80 mmHg) and without (122/77 mmHg) renal cysts, neither group were hypertensive as defined by various international committees [46, 48, 49] and the differences between the groups were small and of doubtful clinical significance. The authors emphasize the importance of cyst size with larger cysts (>5 cm) being more frequently associated with hypertension. This study was undertaken between 2003 and 2004 prior to the revision of the Bosniak classification [10], where cysts >3 cm require follow-up thus contradicting the original definition of ‘simple’ Stage I cysts. Therefore, we do not consider this study [47] as evidence of an association between Stage I renal cysts and hypertension.
When evaluating whether there is an association between Stage I renal cysts and hypertension, it is important to acknowledge that the definition of hypertension may be an arbitrary endpoint, since increasing blood pressure is a continuous risk factor [48]. Although guidelines exist for the definition of hypertension [46, 48, 49] the risk of adverse events may be associated with lower levels of high blood pressure, defined as pre-hypertension (120–139/80–89 mmHg) [46]. Indeed a recent large cross-sectional study conducted in China identified an increasing association between the occurrence, number and size of Stage I renal cysts and pre-hypertension and hypertension [1].
Table 2 summarizes the key studies which have evaluated the association between renal cysts and blood pressure or hypertension in adults during the past 20 years. Although all of the studies do show an association between higher mean blood pressure and (‘simple’) Stage I renal cysts, the clinical significance of the difference is currently unclear. Two of six studies provide evidence of an association between Stage I renal cysts and the current definition of hypertension. Most studies do not support an association between the presence of Stage I renal cysts and renal dysfunction [47]. The authors of a study who identified impaired renal function [creatinine >1.5 mg/dL (132.6 μmol/L)] as a possible risk factor for the development of Stage I renal cysts, emphasized in their conclusion that Stage I renal cysts were not detrimental to renal function [23].
Table 2.
Studies evaluating the potential association between renal cysts and high BP or hypertension
| Country year reference | Cyst type | Study method | Number of cyst/total | Result/comments |
|---|---|---|---|---|
| Japan 2000 [40] | Include all (+ADPKD) unless query malignant | Population health screen, BP, U/S, blood | 45/17 914 | Higher mean SBP with (123 mmHga) than without (118 mmHg) cysts |
| Turkey 2010 [50] | ‘SRC’, excluded >3 cm or ADPKD | BP, blood, U/S, urinalysis in newly diagnosed hypertensive cohort | 92/190 | Higher mean ambulatory BP with (134 mmHga) than without (128 mmHg) cysts multivariate analysis: ‘SRC’ associated nocturnal non-dipping of BP |
| South Korea 2006 [25] | ‘SRC’, excluded congenital cystic kidney disease/agenesis, single kidney | Cohort of population health screen with U/S ± CT, correlated clinical features | 5674/29 523 | Higher mean BP ± SD with (121 ± 15/76 ± 9 mmHg) than without (118 ± 15/75 ± 9 mmHg) cysts. Not standard definition of hypertension |
| South Korea 2003–4 [47] | ‘SRC’, excluded congenital cystic kidney disease | Cohort of population health screen with SRC on U/S, correlated clinical features | 436/6603 | Higher mean BP ± SD with (125 ± 16/80 ± 11 mmHg) than without (122 ± 15/77 ± 10 mmHg) cysts. BP associated with more cysts. Confounding factors (male gender, age) |
| USA 2000–8 [11] | Cystic + solid renal lesions | Cohort of potential kidney donors with CT, BP, blood | 629/1948 | ‘SRC’ >10 mm associated with OR 1.4 of hypertension. Confounding factors (albuminuria, hyperfiltration) |
| China 2013 [1] | ‘SRC’, excluded ADPKD, VHL, TS, medullary sponge kidney, duplex kidney, renal tumour, pregnancy | Cohort of population health screen with SRC on U/S, BP, blood, correlated clinical features | 1694/14 995 | Pre-hypertension and hypertension associated with ‘SRC’ <2 cm |
BP, blood pressure; CT, computed tomography; OR, odds ratio; SD, standard deviation; SRC, simple renal cyst; TS, tuberous sclerosis; U/S, ultrasound; VHL, von Hippel-Lindau.
Numbers represent patients with cysts of the total study population.
aNot hypertension. Nocturnal dipping >10% fall in nocturnal BP.
DONOR KIDNEYS
A key goal of assessment of kidneys donated for transplantation is to ensure the kidney is suitable, ideally without any anomalies and if from a live donor, to minimize the risk for donation. Guidelines exist for the recommended assessment of potential living kidney donors [51, 52]. Despite an increase in living kidney donation, overall there remains a significant shortage of kidney donors world-wide and the waiting list for transplantation continues to grow [53]. Strategies to increase the donor pool have included using kidneys from expanded criteria donors and older donors. The use of marginal kidneys in transplantation must balance the risk of complications including inferior renal outcomes with the advantage of improved survival compared with continuing on dialysis [54]. Recently the short-term follow-up (mean of 36 months) of renal transplant recipients from expanded criteria donors and older living donors (over 60 years) revealed similar renal outcomes, deemed satisfactory although acknowledged to be lower than standard criteria donors [55]. Clearly longer-term follow-up is required to further understand the utility of expanded criteria donor kidneys and to appropriately match them with recipients. There is limited data on the long-term follow-up of kidney donors. However, a recent study conducted over a median of 7.6 years, identified they had an increased (90 per 10 000) lifetime risk of developing end-stage renal disease (ESRD) compared with matched healthy non-donors (14 per 10 000) [56].
The presence of stage I renal cysts in a potential kidney donor is not a barrier to renal transplantation. However when detected, this should prompt careful evaluation including consideration of size, number and other features of the donor which may identify occult renal disease or propose an increased risk. Guidelines for assessment of kidney donors with renal cysts recommend that a multidisciplinary team including a radiologist should review the donor history and imaging [51]. A contrary view is that in the absence of a family history, ‘abnormal renal imaging’ including ‘the presence of two or three cysts in each kidney’ is a contraindication to kidney donation [57]. However, there was no discussion of the evidence base for this recommendation. Although Stage I renal cysts can be associated with higher blood pressure and increasing age, short-term follow-up studies support the safe use of donor kidneys with Stage I renal cysts for transplantation [58]. A retrospective German study reported outcome data on donors and their recipients from 25 prospective donors (mean age 49.5 years) with normal renal function but who were found to have incidental unilateral or bilateral renal cysts during assessment. CT scan reports detailed a single cyst (<5–26 mm) in 19 donor kidneys and multiple cysts (<5–52 mm) in 6 donor kidneys, all of which were ‘simple cysts, Bosniak category I’— however it is notable that the size of some cysts were >3 cm. All of the donor kidneys were utilized for transplantation and at nephrectomy there were no concerns on macroscopic inspection. There were no major complications postoperatively and during follow-up of recipients at 1 and 2 years, mean serum creatinine was 1.88 mg/dL (166 μmol/L) and 2.02 mg/dL (179 μmol/L) respectively. Follow-up ultrasound imaging revealed small mean increases in the diameters (8.25 and 11.5 mm) of the transplanted cysts in 25% of kidneys at 1 and 2 years, respectively. Only one cyst had suspicious features; however, further evaluation by CT revealed that it was haemorrhagic and no further treatment was required. No recipient developed complications related to renal cysts and importantly, transplant function was comparable to recipients of non-cyst bearing kidneys. During follow-up of the donors, 2 of 14 who originally had no cysts in their remaining kidney developed Stage I cysts. In donors who had bilateral renal cysts at the time of donation, only 2 of 11 cysts in the remaining kidney increased in size. After 2 years of follow-up, donors retained almost preserved renal function and no donor developed complications related to cysts or their nephrectomy [58].
These results provide reassurance as to the short-term feasibility and safety of utilizing donor kidneys with Stage I cysts, an approach which may be particularly useful in expanding the donor pool for older recipients. However, careful long-term follow-up of recipients and donors following the use of kidneys with Stage I renal cysts is required.
COMPLICATIONS
Approximately 2–4% of Stage I renal cysts may become symptomatic (abdominal pain or haematuria) due to increasing size (6–8 cm) or a complication such as infection, haemorrhage or rupture [7]. During assessment, it is important to exclude a malignancy as neoplasms are more frequently symptomatic [6]. The radiological [7] and surgical [42] management options for Stage I renal cysts have been discussed in a helpful recent review [7] and are not further considered here. Treatment is only recommended for symptomatic patients.
CONCLUSION
The most important principle in appropriately managing Stage I renal cysts is to establish the correct diagnosis. Current data suggest that Stage I renal cysts are rarely detrimental. However, there is an association with higher mean blood pressure and overt hypertension particularly where large cysts are present. Potential kidney donors with Stage I renal cysts require careful evaluation but successful kidney transplantation can occur. Longer-term follow-up outcomes are awaited. If a Stage I renal cyst causes symptoms, careful diagnostic imaging is advised to exclude the rare occurrence of malignancy.
FUNDING
R.J.S. was supported by a Clinical Lectureship from the National Institute of Health Research (NIHR) UK.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Lee CT, Yang YC, Wu JS, et al. Multiple and large simple renal cysts are associated with prehypertension and hypertension. Kidney Int. 2013;83:924–930. doi: 10.1038/ki.2012.481. [DOI] [PubMed] [Google Scholar]
- 2.Whelan TF. Guidelines on the management of renal cyst disease. Can Urol Assoc J. 2010;4:98–99. doi: 10.5489/cuaj.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osathanondh V, Potter EL. Pathogenesis of polycystic kidneys. Historical survey. Arch Pathol. 1964;77:459–465. [PubMed] [Google Scholar]
- 4.Floege J, Johnson RJ, Feehally J. Comprehensive Clinical Nephrology. 4th ed. St. Louis, MO. London: Elsevier Mosby; 2010. [Google Scholar]
- 5.Chang MY, Ong AC. New treatments for autosomal dominant polycystic kidney disease. Br J Clin Pharmacol. 2013;76:524–535. doi: 10.1111/bcp.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eknoyan G. A clinical view of simple and complex renal cysts. J Am Soc Nephrol. 2009;20:1874–1876. doi: 10.1681/ASN.2008040441. [DOI] [PubMed] [Google Scholar]
- 7.Skolarikos A, Laguna MP, de la Rosette JJ. Conservative and radiological management of simple renal cysts: a comprehensive review. BJU Int. 2012;110:170–178. doi: 10.1111/j.1464-410X.2011.10847.x. [DOI] [PubMed] [Google Scholar]
- 8.Nicolau C, Bunesch L, Sebastia C. Renal complex cysts in adults: contrast-enhanced ultrasound. Abdom Imaging. 2011;36:742–752. doi: 10.1007/s00261-011-9727-8. [DOI] [PubMed] [Google Scholar]
- 9.Park BK, Kim B, Kim SH, et al. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. Eur J Radiol. 2007;61:310–314. doi: 10.1016/j.ejrad.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Rule AD, Sasiwimonphan K, Lieske JC, et al. Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis. 2012;59:611–618. doi: 10.1053/j.ajkd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahbari-Oskoui F, Mittal A, Mittal P, et al. Renal relevant radiology: radiologic imaging in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014;9:406–415. doi: 10.2215/CJN.08940813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katabathina VS, Kota G, Dasyam AK, et al. Adult renal cystic disease: a genetic, biological, and developmental primer. Radiographics. 2010;30:1509–1523. doi: 10.1148/rg.306105513. [DOI] [PubMed] [Google Scholar]
- 16.Truong LD, Choi YJ, Shen SS, et al. Renal cystic neoplasms and renal neoplasms associated with cystic renal diseases: pathogenetic and molecular links. Adv Anat Pathol. 2003;10:135–159. doi: 10.1097/00125480-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz A, Vatandaslar S, Merkel S, et al. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007;2:750–756. doi: 10.2215/CJN.03661106. [DOI] [PubMed] [Google Scholar]
- 18.Hurst FP, Jindal RM, Fletcher J, et al. Incidence, predictors and associated outcomes of renal cell carcinoma in long-term dialysis patients. Urology. 2011;77:1271–1276. doi: 10.1016/j.urology.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda N, Ohe C, Mikami S, et al. Review of acquired cystic disease-associated renal cell carcinoma with focus on pathobiological aspects. Histol Histopathol. 2011;26:1215–1218. doi: 10.14670/HH-26.1215. [DOI] [PubMed] [Google Scholar]
- 20.Grantham JJ, Mulamalla S, Grantham CJ, et al. Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol. 2012;7:1087–1093. doi: 10.2215/CJN.00900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blazer S, Zimmer EZ, Blumenfeld Z, et al. Natural history of fetal simple renal cysts detected in early pregnancy. J Urol. 1999;162(3 Pt 1):812–814. doi: 10.1097/00005392-199909010-00066. [DOI] [PubMed] [Google Scholar]
- 22.Ravine D, Gibson RN, Donlan J, et al. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis. 1993;22:803–807. doi: 10.1016/s0272-6386(12)70338-4. [DOI] [PubMed] [Google Scholar]
- 23.Chang CC, Kuo JY, Chan WL, et al. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc. 2007;70:486–491. doi: 10.1016/S1726-4901(08)70046-7. [DOI] [PubMed] [Google Scholar]
- 24.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol. 2003;58:626–629. doi: 10.1016/s0009-9260(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Lim JH, Jeong I, et al. What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens. 2013;27:539–544. doi: 10.1038/jhh.2013.12. [DOI] [PubMed] [Google Scholar]
- 26.Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol. 1977;118:707–710. doi: 10.1016/s0022-5347(17)58167-7. [DOI] [PubMed] [Google Scholar]
- 27.Grantham JJ, Donoso VS, Evan AP, et al. Viscoelastic properties of tubule basement membranes in experimental renal cystic disease. Kidney Int. 1987;32:187–197. doi: 10.1038/ki.1987.191. [DOI] [PubMed] [Google Scholar]
- 28.Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991;40:143–152. doi: 10.1038/ki.1991.192. [DOI] [PubMed] [Google Scholar]
- 29.Al-Said J, Brumback MA, Moghazi S, et al. Reduced renal function in patients with simple renal cysts. Kidney Int. 2004;65:2303–2308. doi: 10.1111/j.1523-1755.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 30.Premaratne E, Macisaac RJ, Tsalamandris C, et al. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia. 2005;48:2486–2493. doi: 10.1007/s00125-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 31.Bayram MT, Alaygut D, Soylu A, et al. Clinical and radiological course of simple renal cysts in children. Urology. 2013 doi: 10.1016/j.urology.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 32.Terada N, Arai Y, Kinukawa N, et al. The 10-year natural history of simple renal cysts. Urology. 2008;71:7–11. doi: 10.1016/j.urology.2007.07.075. ; discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 33.Wallis MC, Lorenzo AJ, Farhat WA, et al. Risk assessment of incidentally detected complex renal cysts in children: potential role for a modification of the Bosniak classification. J Urol. 2008;180:317–321. doi: 10.1016/j.juro.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 34.McHugh K, Stringer DA, Hebert D, et al. Simple renal cysts in children: diagnosis and follow-up with US. Radiology. 1991;178:383–385. doi: 10.1148/radiology.178.2.1987597. [DOI] [PubMed] [Google Scholar]
- 35.Nishibuchi S, Suzuki Y, Okada K. A case report of renal cell carcinoma in a renal cyst. Hinyokika Kiyo. 1992;38:181–184. [PubMed] [Google Scholar]
- 36.Bowers DL, Ikeguchi EF, Sawczuk IS. Transition from renal cyst to a renal carcinoma detected by ultrasonography. Br J Urol. 1997;80:495–496. doi: 10.1046/j.1464-410x.1997.00295.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakai N, Kanda F, Kondo K, et al. Sonographically detected malignant transformation of a simple renal cyst. Int J Urol. 2001;8:23–25. doi: 10.1046/j.1442-2042.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu JM, Chuang CK, Chang YH, et al. A simple renal cyst invaded by infiltrating urothelial carcinoma mimicking a Bosniak Class IV renal cyst. Clin Nephrol. 2011;76:412–416. doi: 10.5414/cn107159. [DOI] [PubMed] [Google Scholar]
- 39.Clevert DA, Minaifar N, Weckbach S, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc, 2008;39(1–4):171–178. [PubMed] [Google Scholar]
- 40.Terada N, Arai Y, Kinukawa N, et al. Risk factors for renal cysts. BJU Int. 2004;93:1300–1302. doi: 10.1111/j.1464-410X.2004.04844.x. [DOI] [PubMed] [Google Scholar]
- 41.Farrell J, Young RH. Hypertension caused by unilateral renal compression. JAMA. 1942;118:711–712. [Google Scholar]
- 42.Bryniarski P, Kaletka Z, Życzkowski M, et al. Ten-year treatment outcomes including blood cell count disturbances in patients with simple renal cysts. Med Sci Monit. 2013;19:518–523. doi: 10.12659/MSM.889337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zerem E, Imamovic G, Omerovic S. Simple renal cysts and arterial hypertension: does their evacuation decrease the blood pressure? J Hypertens. 2009;27:2074–2078. doi: 10.1097/HJH.0b013e32832f1458. [DOI] [PubMed] [Google Scholar]
- 44.Solak A, Gür MS, Genç B, et al. Localized cystic disease of the kidney: a rare cause of hypertension in a young adult. J Clin Imaging Sci. 2013;3:33. doi: 10.4103/2156-7514.116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen JF, Emamian SA, Nielsen MB. Significant association between simple renal cysts and arterial blood pressure. Br J Urol. 1997;79:688–691. doi: 10.1046/j.1464-410x.1997.00139.x. [DOI] [PubMed] [Google Scholar]
- 46.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 47.Chin HJ, Ro H, Lee HJ, et al. The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney Int. 2006;70:1468–1473. doi: 10.1038/sj.ki.5001784. [DOI] [PubMed] [Google Scholar]
- 48.Guidance N.I.f.H.a.C.E., Hypertension. The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34 (Internet) 2011 [PubMed] [Google Scholar]
- 49.Hypertension E.E.T.F.f.t.M.o.A. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;(10):1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 50.Afsar B, Afsar RE, Sen ST, et al. Simple renal cysts and circadian blood pressure: are they related to each other in patients with hypertension? Int Urol Nephrol. 2011 doi: 10.1007/s11255-010-9734-7. [DOI] [PubMed] [Google Scholar]
- 51.Andrews PA, Burnapp L, Manas D, et al. Summary of the British Transplantation Society/Renal Association U.K. guidelines for living donor kidney transplantation. Transplantation. 2012;93:666–673. doi: 10.1097/TP.0b013e318247a7b7. [DOI] [PubMed] [Google Scholar]
- 52.Kher A, Mandelbrot DA. The living kidney donor evaluation: focus on renal issues. Clin J Am Soc Nephrol. 2012;7:366–371. doi: 10.2215/CJN.10561011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook PJ, Krawiec KD. A primer on kidney transplantation: anatomy of the shortage. Law Contemp Probl. 2014;77:1–28. [Google Scholar]
- 54.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945–951. doi: 10.1093/ndt/gfh022. [DOI] [PubMed] [Google Scholar]
- 55.Lionaki S, Kapsia H, Makropoulos I, et al. Kidney transplantation outcomes from expanded criteria donors, standard criteria donors or living donors older than 60 years. Ren Fail. 2014;36:526–533. doi: 10.3109/0886022X.2013.876348. [DOI] [PubMed] [Google Scholar]
- 56.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pham PC, Wilkinson AH, Pham PT. Evaluation of the potential living kidney donor. Am J Kidney Dis. 2007;50:1043–1051. doi: 10.1053/j.ajkd.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Grotemeyer D, Voiculescu A, Iskandar F, et al. Renal cysts in living donor kidney transplantation: long-term follow-up in 25 patients. Transplant Proc. 2009;41:4047–4051. doi: 10.1016/j.transproceed.2009.09.077. [DOI] [PubMed] [Google Scholar]