Abstract
Preterm birth is a risk factor for respiratory syncytial virus (RSV) bronchiolitis and hospitalization. The pathogenesis underlying this is not fully understood, and in vivo studies are needed to better clarify essential cellular features and molecular mechanisms. Such studies include analysis of lung tissue from affected human infants and various animal models. The preterm and newborn lamb lung has developmental, structural, cellular, physiologic, and immunologic features similar to that of human infants. Also, the lamb lung is susceptible to various strains of RSV that infect infants and cause similar bronchiolar lesions. Studies in lambs suggest that viral replication in airways (especially bronchioles) is extensive by 4 days after infection, along with bronchiolitis characterized by degeneration and necrosis of epithelial cells, syncytial cell formation, neutrophil infiltration, epithelial cell hypertrophy and hyperplasia, and innate and adaptive immune responses. RSV bronchiolitis greatly affects airflow and gaseous exchange. RSV disease severity is increased in preterm lambs compared with full-term lambs; similar to human infants. The lamb is conducive to experimental assessment of novel, mechanistic therapeutic interventions such as delivery of vascular endothelial growth factor and enhancement of airway epithelial oxidative responses, Club (Clara) cell protein 10, and synthesized compounds such as nanobodies. In contrast, exposure of the fetal ovine lung in vivo to ethanol, a risk factor for preterm birth, reduces pulmonary alveolar development and surfactant protein A expression. Because the formalin-inactivated RSV vaccination enhances some inflammatory responses to RSV infection in lambs, this model has the potential to assess mechanisms of formalin-inactivated RSV enhanced disease as well as newly developed vaccines.
Keywords: bronchiolitis, infants, lambs, pneumonia, preterm, respiratory syncytial virus (RSV)
Introduction
Respiratory syncytial virus (RSV) is a major cause of acute lower respiratory infection in infants and young children worldwide and a leading cause of infantile bronchiolitis that can result in hospitalization and occasionally death (Aujard and Faurox 2002; Blanken et al. 2013; Collins and Graham 2008; Collins and Melero 2011; Hon et al. 2012; Welliver et al. 2010). Such RSV infections can be worsened by secondary bacterial pathogens (Stark et al. 2006; van den Bergh et al. 2012). In industrialized countries, RSV accounts for up to 70% of hospitalized bronchiolitis cases (Aujard and Faurox 2002; Collins and Melero 2011; Garcia et al. 2010; Hall et al. 2013; Nair et al. 2010; Sommer et al. 2012). However, the mechanistic basis by which lung immaturity contributes to increased RSV disease severity is not fully understood. Key elements affecting susceptibility of the newborn lung to RSV may include bronchiolar epithelial cell immaturity or alterations in the types of cells lining bronchioles, such as nonciliated cells (including Club [Clara] cells) and type II cells. The ovine lung has much similarity to the lung in human infants in terms of alveologenesis, airway branching patterns, percentage of Club (Clara) cells, and the presence of submucosal glands. This allows studies in lambs demonstrating effects of RSV on airway epithelial cell degeneration and necrosis, inflammatory responses, and innate (e.g., surfactant proteins, antimicrobial peptides, cytokines, chemokines, dendritic cells) and adaptive (e.g., Th1, Th2) immune responses. Also, the lamb model has been used to assess the effects of drugs such as ethanol, which can alter bronchiolar epithelial cell differentiation/maturation in utero and alter innate and adaptive immune responses.
Although palivizumab (Synagis; MedImmune) has potent protective properties and ribavirin has been used in some clinical situations, there are no fully satisfactory vaccines or therapeutic regimens (Abed and Boivin 2006; Anderson 2013; Empey et al. 2010; Hon et al. 2012). Thus, there is a need to develop new therapeutic approaches that could be used to prevent or treat RSV infections, and these need to be tested in relevant animal models. Several therapies that have reduced severity of RSV infection have been tested in lambs and include delivery of vascular endothelial growth factor (VEGF) and enhancement of airway oxidative responses, Club (Clara) cell protein 10 (CC10), and nanobody (ALX-0171).
RSV Disease in Humans
RSV is an enveloped, negative strand RNA virus of the family Paramyxoviridae, subfamily Pneumovirinae, and genus Pneumovirus first discovered in 1952 as a lower respiratory tract pathogen of children in their first year of life. RSV most commonly causes mild respiratory tract disease; however, severe bronchiolitis and pneumonia can occur in 25–50% of children, resulting in intensive care unit admission, supplemental oxygen, mechanical ventilation, impaired learning, and death (Aujard and Faurox 2002; Collins and Melero 2011; Espinoza et al. 2013; Garcia et al. 2010; Hall et al. 2013; Nair et al. 2010; Sommer et al. 2012). Worldwide, RSV is estimated to cause 199,000 deaths annually in children aged less than 5 years (Garcia et al. 2010; Hall et al. 2013; Nair et al. 2010). Reinfections can occur especially if RSV-specific neutralizing antibody titers are low. In the 1960s a formalin-inactivated RSV (FI-RSV) vaccine was associated with enhanced RSV disease severity and two deaths in infants that were subsequently infected with RSV (Castilow et al. 2007; Collins and Graham 2008; Collins and Melero 2011). Today, a licensed RSV vaccine is not available. Streptococcus pneumoniae and Haemophilus influenzaare both common respiratory bacterial pathogens that can complicate primary RSV and other pulmonary viral infections (Stark et al. 2006; van den Bergh et al. 2012).
Therapies and Vaccine Strategies
Numerous therapeutic compounds against RSV have been and are being developed and assessed. Of, these, ribavirin and palivizumab have been used in hospital settings. Ribavirin has toxicity issues, and Palivizumab is not used universally because of set criteria as well as availability and cost issues (Abed and Boivin 2006; Empey et al. 2010; Hon et al. 2012; Wu et al. 2008). Many other innovative and promising therapies are under development and testing and include antibodies and small molecules against the various RSV envelope proteins (F, G, SH), nucleocapsid proteins (N, P, L), nucleocapsid-associated proteins (M2-1, M2-2), matrix protein (M1), and nonstructural proteins (NS1, NS2) (Empey et al. 2010). These therapies inhibit aspects of RSV replication such as fusion and entry, attachment, virus transcription, assembly, and cytokine/immune responses.
The FI-RSV vaccine-related deaths of the 1960s greatly affected RSV vaccine development. Since that event, the mechanistic basis underlying FI-RSV–enhanced response has been studied extensively (Castillow et al. 2007; Collins and Melero 2011; Delgado et al. 2009). During this same time, numerous new approaches to RSV vaccination have been developed, with close attention and care to avoid vaccine-enhanced responses. Vaccine approaches include: live virus, vectored (replicating or nonreplicating) virus, subunit vaccines (Empey et al. 2010) that use attenuated or inactivated RSV, RSV protein(s) adjuvanted or incorporated into micro/nanoparticles, epitope scaffolds, virosomes, virus-like particles, virus or bacteria or plant-based vectors, and prime boost vaccination with heterologous vectors (Costello et al. 2012; Rudraraju et al. 2013). The variety of approaches both enhances the understanding of effective responses to RSV and increases the likelihood that a safe and effective vaccine (or vaccines) will be developed. Difficulties in developing an RSV vaccine include safety issues that are incumbent for any vaccine and the avoidance of vaccine-induced enhanced disease with subsequent RSV infection. Recently, a novel approach has focused on RSV fusion F glycoprotein antigenic site Ø, a metastable site specific to the prefusion state that is potent in inducing RSV-neutralizing antibodies (McLellan et al. 2013). Immunization with site Ø-stabilized variants of RSV F in mice and macaques induced RSV-specific neutralizing activity that protected against RSF infection. This approach is a break-through for RSV and also a conceptual platform for development of vaccines against other viruses.
Animal Models of RSV Infection
A number of animal models of RSV infection have been developed. As reviewed by Bem et al. (2011), RSV models include heterologous host-virus models, in which an animal is infected by human strains of RSV, and cognate host-virus models, in which an animal is infected by a virus closely related to human RSV. Heterologous host-virus models include chimpanzees, sheep, cotton rat, and mice, all of which can be infected with human strains of RSV. Cognate host-virus models include cattle, which can be infected with bovine strains of RSV, and mice, which can be infected with pneumonia virus of mice (Bem et al. 2011, Gershwin 2012; Sacco et al. 2013). In addition to those, other animals models of human RSV infection include other types of subhuman primates (baboons, African green monkey, and rhesus macaques), ferrets, and chinchillas (Bem et al. 2011; Eyles et al. 2013). Of these models, each has some type of advantage and disadvantage depending on the clinical, immunological, cellular, or pathogenic aspect of RSV disease being investigated. Other considerations for all animal models include costs, availability, and ethics.
Lamb Model of RSV Infection and Insights into RSV Pathogenesis
Despite the numerous animal models of RSV infection, our laboratory and others have shown that the ovine respiratory tract, particularly that of lambs, has features well suited for comparison with human infants in terms of development, structure, susceptibility to human RSV strains, lesion development, immune response, and clinical features (Derscheid and Ackermann 2012). In addition, the ovine lung is susceptible to respiratory bacterial pathogens that can occur secondary to an initial RSV infection, as in humans. Alveolar development in humans and lambs occurs prenatally, in contrast with the postnatal alveolar development that occurs in rodents (Alcorn et al. 1981; Derscheid and Ackermann 2012; Flecknoe et al. 2003; McGowan and Snyder 2004; Plopper et al. 1983). Lamb and infant lungs have similarities in airway branching patterns, relative numbers of Club (Clara) cells, type II cell development, and the presence of airway submucosal glands, which express lactoperoxidase (LPO). Airway branching in lambs and infants is less truncated than in rodents (Scheerlinck et al. 2008). The percentage of Club (Clara) cells in bronchioles of lambs and infants is 18–22%, whereas airways of mice are roughly 50% Club (Clara) cells (Barth et al. 1994; Derscheid and Ackermann 2013; McGowan and Snyder 2004; Plopper et al. 1983). We have shown that Club (Clara) cells and type II cells progressively differentiate with gestational age and have increased surfactant protein (SP) A and SP-D expression with maturation; both SP-A and SP-D bind and aggregate RSV, and reduced expression may underlie susceptibility to RSV infection in infants born preterm (Derscheid and Ackermann 2013; Meyerholz, DeGraff et al. 2006). Submucosal glands are present in airways of lambs and infants but absent or at insufficient amounts in rodents to produce LPO levels required for an increasingly recognized epithelial oxidative system (Derscheid and Ackermann 2012; Derscheid and Ackermann 2013; Derscheid, van Geelen, Gallup et al. 2013; Gerson et al. 2000; Mestas and Hughes 2004; Pack et al. 1980; Widdicombe et al. 2001; Wijkstrom-Frei et al. 2003).
RSV Pathogenesis and Bronchiolitis
The similarity of lung RSV lesions in sheep and ruminants to human RSV pathology has been well documented by us and others (Derscheid and Ackermann 2012; Johnson et al. 2007; Lehmkuhl and Cutlip 1979a; Lehmkuhl and Cutlip 1979b). Lambs are susceptible to bovine and human strains of RSV (Derscheid and Ackermann 2012; Derscheid et al. 2013; Meehan et al. 1994; Meyerholz, Grubor, Gallup et al. 2004; Meyerholz, Grubor, Fach et al. 2004; Meyerholz et al. 2007; Olivier et al. 2009; Olivier et al. 2011). We have shown that human RSV A2 strain infects lambs, and others have shown that the Long strain of RSV infects lambs (Figure 1) (Derscheid and Ackermann 2012; Olivier et al. 2009; Olivier et al. 2011). More recently, we have shown that RSV Memphis strain 37 infects lambs robustly, which has compelling comparative relevance because the Memphis Strain 37 is used in human clinical trials (Derscheid and Ackermann 2012; Derscheid, van Geelen, McGill et al. 2013; Derscheid et al. 2013; DeVincenzo et al. 2010). A preterm lamb model of RSV infection has been developed that has features such as enhanced bronchiolitis, neutrophil infiltration, syncytial cell formation, myeloperoxidase (MPO) production, and enhanced RSV replication levels, all of which are similar to that seen in preterm human infants (Meyerholz, Grubor, Fach et al. 2004; Sow et al. 2010). This model is significant because lambs can be born preterm (90% gestation) and survive for such studies, whereas rodents do not survive preterm birth. Although 90% gestation is not consistent with premature birth in human infants; studies increasingly show that even preterm birth of infants at 95% gestation (“early term”; 37–38 weeks) have a degree of clinical alterations. Finally, lambs (and other ruminants as well as swine) receive maternal immunoglobulin only through the ingestion of colostrum at birth. This biological feature allows lambs to be deprived of colostrum and thereby lack maternal antibodies to RSV and other pathogens, thus eliminating any confounding questions of the effects of maternal antibody on clearance of RSV or other pathogens (lambs do not receive maternal antibodies transplacentally). This feature is especially useful for vaccination studies, and recently it has been shown that lambs receiving FI-RSV vaccine produced responses that enhanced disease with subsequent RSV infection (Derscheid et al. 2014). Clinical parameters in lambs mirror those of human infants in terms of temperature (which is variable in both lambs and infants) and enhanced expiratory effort. Dendritic cell activity and immune and inflammatory responses in lambs also have consistencies with RSV infection in human infants, as reviewed previously (Derscheid and Ackermann 2012; Derscheid and Ackermann 2013; Fach, Brockmeier, et al. 2007; Fach, Meyerholz, et al. 2007; Fach et al. 2010; Sow, Gallup, Meyerholz et al. 2009; Sow et al. 2010; Sow et al. 2011; Sow et al. 2012).
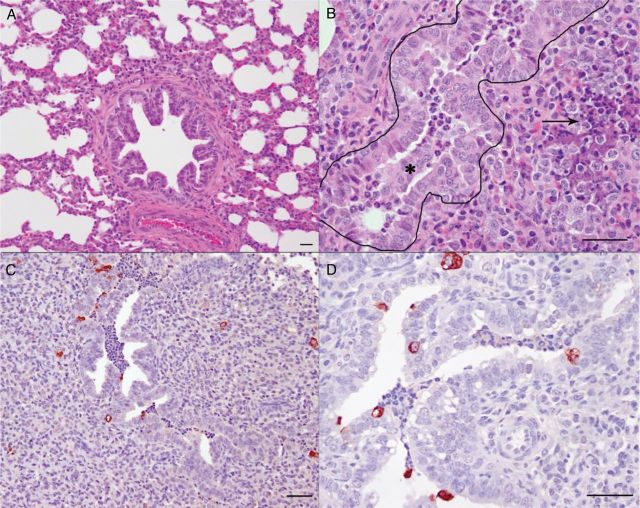
Figure 1.
Respiratory syncytial virus (RSV) strain A2 causes bronchiolitis in lambs (Olivier et al. 2009). (A) Lung from a control lamb not infected with RSV that contains a bronchiole and alveoli. (B) Lung from a lamb 6 days after inoculation with RSV with a bronchiole (outlined) containing epithelial cells admixed with neutrophils (*). Alveoli around the bronchiole are collapsed with accumulation of degenerate neutrophils and areas of necrosis (arrow). (C and D) Immunohistochemical detection of RSV antigen in lung from a lamb 6 days after inoculation with RSV, in which RSV viral antigen is present within the bronchi, epithelial cells lining the bronchi, and the syncytial cells in areas of alveolar consolidation. Bars = 25 µm.
Because of the similarities between lambs and infants outlined herein, studies in lambs provide insight to RSV pathogenesis that cannot be assessed or inferred in studies from human infants, especially preterm/premature infants, because of ethical considerations. Many RSV studies of human RSV infection focus on nasal or bronchoalveolar wash fluids, ciliated cells isolated from the upper respiratory tract, or continuous cells lines originally generated from the upper respiratory tract (Guo-Parke et al. 2013; Pickles 2013; Villenave et al. 2013). These fluids and cells are more readily available than bronchiolar fluids and cells. In lambs and from reports of human infants with severe RSV disease, lesions are especially intense in the distal bronchi, bronchioles, and terminal airways/alveoli. Clinically, this is significant because infants often do not become hospitalized or die from RSV rhinitis, tracheitis, or upper bronchitis; severe RSV is more often associated with RSV–induced bronchiolitis. This is due, at least in part, to ( 1) the tropism of RSV to the bronchiolar epithelium, (2) the narrow lumen of the bronciholes and distal airway, (3) low airflow pressure at this level, and (4) alterations in airway dilation due to inflammation (Tayyari et al. 2011). In lambs and human infants with severe RSV, RSV-infected bronchiolar epithelial cells undergo apoptosis/necrosis, and subsequent cell debris from the degenerate/necrotic epithelial cells, along with infiltrating neutrophils and macrophages, mucin, and seroproteinaceous fluid, partially occlude the lumen of these airways. In addition, bronchiolar epithelial cells adjacent to the RSV-infected epithelial cells undergo hyperplasia to repair the areas damaged by RSV-induced degeneration and necrosis, and this hyperplasia further narrows airway lumens. In addition to having a narrow and partially occluded lumen, the bronchiole has a relatively weak level of airflow compared with the upper respiratory tract, and thus it is difficult to expel intraluminal exudates by coughing and enhanced expiration. Also, peribronchiolar infiltrates of lymphocytes and plasma cells present in the airway adventitia can have physical effects on alter airway dilation. Moreover, the cytokines, chemokines, and other inflammatory mediators present further affect lung physiology at this location. Thus, the bronchiole is, in a way, an unfortunate location for RSV to replicate because it is an essential conduit for airflow through a narrow lumen with poor airflow pressures. Thus gaseous exchange in the alveoli immediately adjacent is profoundly affected. In preterm infants and preterm/newborn lambs, many of these issues are exacerbated by an increased level of RSV replication. The increased RSV replication that occurs triggers enhanced bronchiolar lesions and exudate accumulation, which obstructs airflow.
Why does RSV replicate at higher levels preterm and in newborns? This is poorly understood, but considerations include the cell types present in bronchiole (Club [Clara] cells and other nonciliated cells), the extent of maturation of these cells at preterm and in newborns, RSV receptor expression, and extent/intensity of innate and adaptive immunity responses in this location. The bronchiole is a difficult microenvironment to study because the cells are difficult to isolate and grow in culture and the airflow, blood flow, and other physiologic conditions are difficult to replicate in vitro. Thus, in vivo models are essential for shedding new light on the mechanistic basis of RSV bronchiolitis and lesion development. For example, using laser capture microdissection, bronchiolar epithelial cells from preterm and term lambs had significant differences in SP-A and other innate immune responses in both preterm and term lambs with and without RSV infection (Gallup et al. 2005; Kawashima et al. 2006; Meyerholz, DeGraaff et al. 2006; Sow, Gallup, Meyerholz et al. 2009). In recent studies in RSV-infected lambs, we have determined that syncytial cell formation occurs by 3 days after RSV inoculation and epithelial hyperplasia is present at 4 days after inoculation; viral titers appear to peak at day 4, but bronchiolar lesions develop a bit further and peak at 5 and 6 days after RSV infection when RSV titers are beginning to decrease. By days 8 and 14, RSV titers are minimal and lesions are resolving. A more in-depth understanding of the kinetics of RSV bronchiolitis is needed in animal models to identify features not readily accessible in human infants.
Assessment of Therapies in Lambs
Vascular Endothelial Growth Factor
VEGF is essential for fetal lung development and maturation and also has numerous other activities, including endothelial cell proliferation, enhancement of SP-A by lung epithelial cells, and monocyte chemotaxis (Brown et al. 2001; Meyerholz, Kawashima et al. 2006; Voelkel et al. 2006). Because of these activities, we developed the hypothesis that VEGF may reduce RSV disease severity in the newborn lung. We discovered that pretreatment of lambs with VEGF protects lambs from RSV infection (Figure 2) (Meyerholz et al. 2007; Olivier et al. 2011). VEGF reduced disease severity against both a bovine strain of RSV and a human strain (hRSV A2) of RSV. The mechanistic basis by which VEGF protects is not known but could be related to VEGF effects on epithelial cell differentiation, maturation, and/or expression of SP-A. Alternatively or in addition, VEGF protection may be mediated by recruitment of alveolar macrophages. Low doses of VEGF do not alter the number of alveolar macrophages within the ovine lung; however, higher doses induce infiltration of a relatively uniform population of alveolar macrophages into alveoli and distal airways (Meyerholz, Kawashima et al. 2006). VEGF can affect many additional inflammatory and immune responses that could mediate protection against RSV infection.
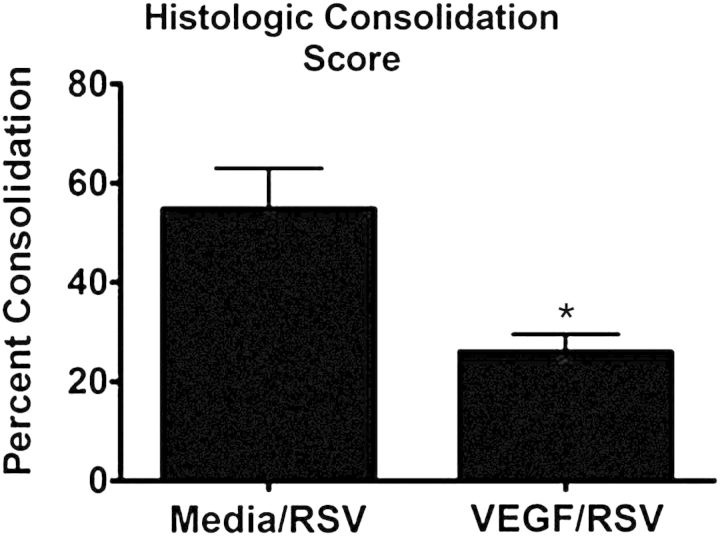
Figure 2.
Pretreatment with exogenous recombinant human vascular endothelial growth factor (rhVEGF) decreases histologic consolidation score of lambs infected with human respiratory syncytial virus (RSV) A2 strain (Olivier et al. 2011). Lambs received either media followed by RSV A2 (no vascular endothelial growth factor [VEGF]) or rhVEGF followed by RSV A2 and were assessed for clinical signs, viral lesions, and mRNA levels. At 6 days after RSV A2 infection, VEGF-treated lambs had reduced histologic consolidation scores (an overall score of bronchiolitis and lung inflammation), indicating a decreased in alveolar consolidation and inflammatory cell infiltration (*p < 0.05). Exogenous rhVEGF treatment also reduced RSV mRNA levels at 4 and 6 days after RSV inoculation (not shown). Previous studies have demonstrated that VEGF treatment also reduces the severity of bovine RSV in newborn lambs (not shown).
Dual-Functioning Oxidase/LPO Defense
Accumulating data suggest that an oxidative host defense system contributes to airway sterility (Bae et al. 2010; Banfi 2007; Fischer 2009; Lorentzen et al. 2011; Moskwa et al. 2007). We and others have shown that the oxidative system kills bacteria by producing hypothiocyanite (OSCN−) in an LPO-catalyzed reaction: hydrogen peroxide (H2O2) + thiocyanate (SCN−) → OSCN− (Banfi 2007; Derscheid, van Geelen, Gallup et al. 2013; Fischer 2009; Fischer et al. 2011). OSCN− production requires three processes working in concert: (1) LPO secretion by submucosal glands, (2) H2O2 generation by dual oxidases (Duox) of airway epithelia, and (3) SCN− secretion (Banfi 2007; Belding et al. 1970; Conner et al. 2002; Conner et al. 2007; Dohán et al. 2003; Fischer et al. 2007; Forteza et al. 2005; Fragoso et al. 2004; Furtmüller et al. 2002; Harper et al. 2005). The mechanism(s) by which OSCN− eliminates microbes is not known, but OSCN− can oxidize thiol groups in surface proteins (Fischer et al. 2011; Klebanoff 1967). Importantly, OSCN− is not toxic to eukaryotic cells. LPO has high affinity for SCN− and also iodide (I−) (Fragoso et al. 2004; Furtmüller et al. 2002). LPO can catalyze the oxidation of I− to hypoiodite (OI−) in the presence of H2O2 (Furtmüller et al. 2002; Gattas et al. 2009). Although I− is not a physiologic component of the airway surface liquid (ASL), when present, I− allows hypoiodous acid (HOI) generation by the LPO/Duox enzymes. There is some evidence that OSCN− and OI− have slightly different spectrums of antimicrobial activity. For example, although OSCN− lacks activity against RSV in vitro, OI− has anti-RSV activity in vitro and in vivo (Derscheid and Ackermann 2012; Fischer et al. 2011). The lung also has other oxidative systems with antimicrobial activity, including the phagocytic nicotinamide adenine dinucleotide phosphate oxidase, MPO, and nitric oxide synthase. Of these, MPO, like LPO, can convert potassium iodide (KI) and sodium thiocyanate (NaSCN) to a halide in the presence of H2O2, and neutrophils expressing MPO enter lung airways during RSV infection (Nauseef 2013); however, the amount of MPO in ASL and the extent to which MPO in ASL contributes to halide formation in ASL are not known.
The Duox/LPO defense system has activity against both bacteria and multiple respiratory viruses, including RSV and adenovirus in vitro (Banfi 2007; Chang et al. 2013; Fischer et al. 2011; Gerson et al. 2000). To test the potential antiviral activity of this system in vivo, large animal models of viral infections are essential because mouse airways lack sufficient LPO and rat airways contain only a few LPO-secreting cells. The ovine respiratory tract is similar to that of humans in terms of LPO and Duox expression and other physiologic and immunologic features. After in vitro studies and pilot work in lambs, we developed the hypothesis that the antimicrobial activity of Duox/LPO can be optimized in vivo through the supplementation of KI or NaSCN to treat RSV and possibly a number of other respiratory infections. Amelioration of respiratory viral infections by enhanced oxidative responses is significant because the therapy is relatively inexpensive, available, and easily distributed and administered.
The following study assessed the extent to which KI administration (for generation of HOI) reduces RSV disease in vivo using lambs (Derscheid, Van Geelen, Gallup et al. 2013). NaSCN was not assessed because its oxidative product, OSCN−, lacks anti-RSV activity in vitro (Fischer et al. 2011). The study included four groups of lambs: (1) control group (no KI and no RSV), (2) M37 group (no KI and challenged with nebulized M37 strain of RSV), (3) KI + M37 (KI administered daily with nebulized M37 strain of RSV), and (4) dapsone plus KI and M37 strain of RSV). KI administration significantly reduced RSV disease severity, RSV gross lesions, RSV mRNA levels, RSV viral antigen distribution in lung, and RSV viral titers (Figure 3). This experimental design was repeated in older lambs (3-week-old lambs) with similar significance (not shown). One group also received dapsone, and the lack of anti-RSV activity in this group that received dapsone, as reflected by markedly increased RSV lung lesions, RNA levels, RSV antigen, and RSV titers (not shown), confirmed/validated that the anti-RSV effect(s) of KI is mediated by oxidative responses and not by KI itself. This is because dapsone inhibits LPO activity, and without LPO activity, OI− (the oxidative product of KI in the Duox/LPO reaction) cannot be formed (Bozeman et al. 1992; Derscheid, Van Geelen, Gallup et al. 2013). The findings of anti-RSV activity mediated by the Duox/LPO oxidative defense system are consistent with those in sheep by Gerson et al. (2000), in which dapsone administration significantly enhanced colonization of Pasteurella haemolytica (renamed to Mannheimia haemolytica) a Gram-negative pathogen of ruminant respiratory tract. P. haemolytica (M. haemolytica) bears much phenotypic and genotypic similarity to Haemophilus sp., including Haemophilus influenzae. In contrast with the anti-RSV activity mediated by the Duox/LPO system, RSV can inhibit the antioxidant enzymes of lung epithelia (Hosakote et al. 2012).
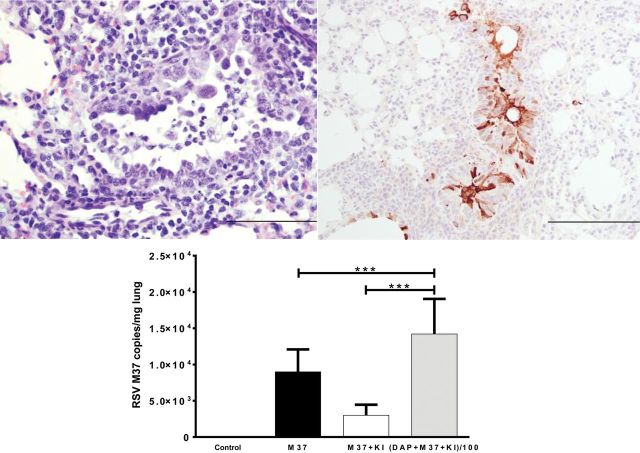
Figure 3.
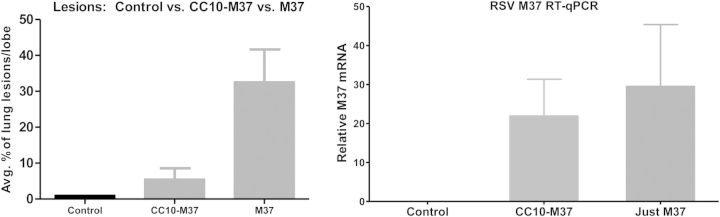
Club (Clara) cell protein 10 (CC10) reduces respiratory syncytial virus (RSV) gross lesions and RSV mRNA levels. Two lambs received recombinant human CC10 (rhCC10) intravenously (1.5 mg/kg) twice daily 1 day before RSV inoculation (6 ml; 107 PFU/ml by nebulizer) and each day thereafter; another group of lambs (n = 6) received RSV alone and no rhCC10; a third group lacked rhCC10 and were nebulized with media (control). rhCC10-treated lambs had reduced gross lesions and decreased RSV mRNA levels by quantitative polymerase chain reaction (qPCR). ***p < 0.05.
Club (Clara) Cell Protein
Club (Clara) cells are nonciliated bronchiolar epithelial cells that biometabolize xenobiotics, secrete immunomodulatory substances, and are progenitor cells for Club (Clara) and type II cells (Wang et al. 2003). The Club (Clara) cell proliferation pool is vulnerable to exhaustion, especially in neonates and chronic smokers (chronic toxin exposure) (Barth et al. 1994; Derscheid and Ackermann 2013). With Club (Clara) cell injury or loss, a proinflammatory environment can form because of the loss of immunomodulatory secretions by Club (Clara) cells (Wang et al. 2003). Club (Clara) cells secrete large amounts of Club (Clara) cell secretory protein (also known as CC10 and as CC16), secretoglobin, and uteroglobin. CC10 is increased in bronchoalveolar lavage fluid and serum during acute injury such as smoke inhalation or application of pneumotoxicants (naphthalene, 4-ipomeanol, chloroethylene). In contrast, CC10 is decreased in chronic or dysplastic airway dysfunction (asthma, chronic obstructive pulmonary disease, or bronchopulmonary dysplasia) (Barth et al. 1994; Elizur et al. 2007; Wang et al. 2003). With RSV infection, CC10-deficient mice have increased inflammatory responses and RSV persistence. With CC10 expression, there is a reduction of inflammatory responses and RSV persistence (Derscheid and Ackermann 2013). CC10 may not only have a protective effect on Club (Clara) cells but may also stimulate development of Club (Clara) cells. Club (Clara) (and type II) cells also produce substances with known anti-RSV activity, including SP-A, SP-D (which bind and opsonize RSV), beta-defensins, beta-galactoside-binding protein, and RSV receptors such as retinoic acid inducible gene-1, which triggers epithelial responses as well as inflammatory/immunomodulatory substances (Derscheid and Ackermann 2013; Elizur et al. 2007). RSV infection enhanced production of secretoglobin (CC10) family proteins in nasal mucus secretions from calves infected with bovine RSV (Sacco et al. 2013).
The extent of CC10 expression in developing fetal lamb lung was measured by quantitative polymerase chain reaction (qPCR) at 50%, 75%, and 90% gestation and at full term and in adults. CC10 levels were low preterm and increased with age after birth (Figure 4); these findings are consistent with the degree of epithelial cell maturation as determined with periodic acid-Schiff stain, which detects carbohydrate-like molecules (e.g., glycogen). Epithelial cells with increased periodic acid-Schiff staining were present during gestation and were less mature and produced less SP-A and SP-D than full-term lambs and adult sheep. The expression of CD208+ (type II cells) and CD208− cells Club (Clara) cells were reduced preterm, which is also consistent with immaturity (Meyerholz, DeGraff et al. 2006).
Figure 4.
Respiratory syncytial virus (RSV) Memphis 37 (M37) strain causes bronchiolitis in lambs that is reduced with enhanced airway oxidative responses. (A) Lung from a lamb infected with RSV M37 strain 4 days after inoculation in which there is erosion of the epithelium with accumulation of cell debris in the airway lumen and formation of a syncytial cell. (B) Lung from a lamb 6 days after inoculation with RSV M37 strain in which viral antigen is detected by immunohistochemistry and viral antigen is present in epithelial cells, macrophages, and in cell debris of the airway lumen. There is moderate thickening of the epithelium due to hyperplasia. (C) Enhancement of the airway oxidative response (dual oxidases/lactoperoxidase oxidative system) by administration of potassium iodide (KI) reduces RSV M37 mRNA levels (as well as viral titers, antigen, and lesions; not shown) in RSV M37–inoculated lambs treated with KI (M37 + KI) compared with lambs inoculated with RSV M37 but not receiving KI (M37). Inhibition of the oxidative defense system with dapsone (DAP) in a group of lambs resulted in significantly enhanced RSV M37 RNA levels (DAP + M37 + KI) despite KI treatment (Derscheid, Van Geelen, Gallup et al. 2013).
CC10 Reduces RSV M37 Severity
In a pilot study, we assessed the effects of recombinant human CC10 (rhCC10) in preventing RSV M37 pneumonia. Briefly, two lambs received rhCC10 intravenously (1.5 mg/kg) twice daily 1 day before RSV inoculation and each day thereafter until lungs were collected on day 6 after RSV inoculation (6 ml 107 PFU/ml by nebulizer); another group of lambs (n = 6) received RSV alone and no rhCC10; a third group lacked rhCC10 and were nebulized with media (control). Lambs receiving rhCC10 had no side effects from inoculation. The rhCC10-treated lambs had reduced gross lesions and decreased RSV mRNA levels by reverse-transcription qPCR (Figure 5).
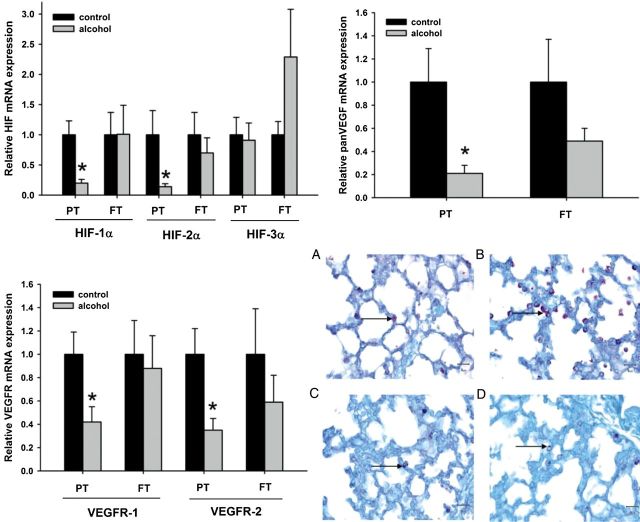
Figure 5.
Maternal ethanol consumption reduces lung maturation through hyxpoxia inducible factor (HIF) and its downstream gene vascular endothelial growth factor (VEGF) (Lazic et al. 2011). (A) Relative mRNA expression of HIF-1α, -2α, and -3α in the neonatal ovine lung. Exposure to ethanol in utero reduced mRNA expression of HIF-1α and HIF-2α in the lungs of preterm (PT) lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of full-term (FT) lambs exposed to ethanol in utero. No significant changes are seen with HIF-3α mRNA expression levels in both PT and FT lambs. (*p < 0.10; significance was established at 90% confidence interval). (B) Relative mRNA expression of vascular endothelial growth factor (panVEGF) in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of panVEGF in the lungs of PT lambs when compared with control lambs of the same age group. Such changes are not observed in the lungs of FT lambs exposed to ethanol in utero. (*p < 0.10; significance was established at 90% confidence interval). (C) Relative mRNA expression of vascular endothelial growth factor receptor 1 and 2 (VEGFR-1 and VEGFR-2, respectively) in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of VEGFR-1 and VEGFR-2 in the lungs of PT lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of FT lambs exposed to ethanol in utero. (*p < 0.10; significance was established at 90% confidence interval). (D) Periodic acid-Schiff (PAS) stain for glycogen granules in the type II pneumocytes (ATII). Glycogen granules in the ATII are more abundant in the PT lambs exposed to ethanol in utero (B) when compared with control lambs of the same age group (A). Glycogen content in the ATII is similar in the FT lambs exposed to ethanol in utero (D) and the control lambs of the same age group (C).
ALX-0171
Ablynx NV has developed a therapeutic protein based on a camelid variable heavy chain only (VH/H) immunoglobin backbone with strong binding affinity to the F (fusion) protein of the RSV virus, thereby inhibiting RSV fusion to target cells. Briefly, ALX-0171 is composed of VH/H chains linked to form a trivalent binding structure with a 2000-fold increase in potency compared with monovalent structures. ALX-0171 is delivered through nebulization, and it is highly stable. ALX-0171 has demonstrated antiviral efficacy in both in vitro and in vivo studies, the latter being performed in the cotton rat therapeutic model for RSV. To further assess the extent to which ALX-0171 may treat RSV infections in vivo, ALX-0171 is currently being assessed in neonatal lambs infected with a human RSV strain (hRSV M37). The lamb studies demonstrate the capacity of the lamb model to characterize delivery, distribution and efficacy of a nebulized drug formulated to treat RSV infection in infants.
RSV Vaccination in Lambs
Recently, we showed that the newborn lamb develops enhanced RSV lesions after vaccination with an FI-RSV vaccine (Derscheid et al. 2014). This work was completed in lambs deprived of colostrum, which eliminated the potential influence of maternal antibodies. This study sets the stage for additional work in lambs in regards to understanding FI-RSV pathogenesis and for assessment of vaccines.
Refining RSV Models
Experimental RSV infection in lambs and other models requires constant assessment and validation of basic methods. As indicated, lambs are susceptible to various human (Long, A2, Memphis 37 strain) and bovine strains of RSV, as well as ovine parainfluenzavirus 3 and a human strain of parainfluenzavirus 3 (Derscheid and Ackermann 2012; DeVincenzo et al. 2010). All of these are enveloped viruses and must be administered with suitable physiologic conditions. RSV, ovine parainfluenzavirus 3, and the human strain of parainfluenzavirus 3 can be delivered by intranasal inoculation with a syringe or atomizer, intrabronchially with a fiberoptic bronchoscope, intratracheally with a syringe and needle, and with whisper jet nebulizers (Derscheid and Ackermann 2012; Derscheid, van Geelen, McGill et al. 2013; Meyerholz et al. 2007; Olivier et al. 2009). For nebulization, we have shown that the addition of sucrose at 15–20% reduces RSV loss during nebulization and enhances RSV disease severity (manuscript under review). Work by others demonstrated that RSV grown in Vero cells has truncated G glycoprotein, which altered infectivity in vitro. Similarly, our recent work in lambs demonstrated that RSV grown in Hep2 cells has increased virulence compared with RSV grown in Vero cells (Derscheid, van Geelen, McGill et al. 2013).
Effects of Ethanol on Fetal Lung Development and Innate Immunity
Maternal ethanol consumption is a risk factor for preterm birth, and the enhanced severity of RSV in preterm infants is well known (Albertsen and Gronbaek 2004; Aujard and Fauroux 2002; Garcia et al. 2010; Sommer et al. 2012). It is possible that ethanol increases RSV disease severity beyond that of preterm birth alone because of Club (Clara) cell damage or alteration in development/function. If true, other toxins or unknown substances reaching the fetal lung in utero may affect Club (Clara) cells and, thus, susceptibility to RSV infection (Lazic et al. 2007; Lazic et al. 2010; Lazic et al. 2011). Club (Clara) cells biometabolize toxins and other substances knowingly or unknowingly consumed by pregnant mothers (e.g., ethanol, nicotine) (Lazic et al. 2007; Lazic et al. 2010; Lazic et al. 2011). Ethanol and other toxins can injure Club (Clara) cells and nearby type II cells, resulting in reduced innate immunity and increased susceptibility for RSV infection (Derscheid and Ackermann 2012; Derscheid and Ackermann 2013). Despite the fact that ethanol has detrimental effects on the developing fetus, one out of 29 pregnant women consumes alcohol during pregnancy, and another survey found that 5 to 10 out of 1000 pregnant women consume 7 or more drinks per week (Ethen et al. 2009; Jaddoe et al. 2007). Although ethanol has effects on the nervous system and liver, the detrimental effects of ethanol on developing fetal lung are of increasing concern. Ethanol decreases surfactant synthesis and proinflammatory cytokines and impairs response to bacterial challenge. Club (Clara) Nicotinamide adenine dinucleotide phosphate cells produce cytochrome CYP2E1, which biometabolizes ethanol to acetaldehyde and malondialdehyde. These form protein adducts, invoke inflammatory responses, and reduce repair of bronchiolar epithelium.
The effects of ethanol on prenatal lamb lung development has been developed by delivering ethanol to gestating ewes, resulting in blood levels consistent with a moderate drinker, which is the most common form of ethanol consumption by pregnant women (Ethen et al. 2009; Jaddoe et al. 2007; Lazic et al. 2007; Lazic et al. 2011; Sozo et al. 2009). Our work shows that ethanol-exposed lambs have reduced epithelial maturation, ciliary function, and innate immune gene expression of SP-A and SP-D, tumor necrosis factor alpha, interleukin 10, and monocyte chemotactic protein by lung epithelia, along with striking reductions in hypoxia-inducible factor 1α and 2α, VEGF, and VEGF receptors (VEGFRs) (Lazic et al. 2007; Lazic et al. 2011). SP-A and SP-D have antimicrobial activity against RSV and enhance macrophage activation (Lazic et al. 2007; Lazic et al. 2011). Loss of these innate immune and inflammatory responses increases susceptibility to infection (Derscheid and Ackermann 2013; Krishnan et al. 2003; Starner et al. 2005; Stokes et al. 2013). Ethanol-induced changes in lung development and immunity in lambs have been confirmed by the Harding laboratory, and sheep are used for other types of ethanol studies (Sozo et al. 2009). The precise effect of ethanol on fetal Club (Clara) cells is not known but is being assessed.
Maternal ethanol consumption alters transcriptomes of developing ovine lung (Derscheid and Ackermann 2012). RNA expression levels in RNA from the lungs of four lambs were determined by next-generation sequencing with Illumina next-generation sequencing on GAIIx (1 × 40 cycles/bases single read) and HiSeq 2000 (2 × 100 cycles/bases, paired-end). Two lambs were preterm and two were term; of these, one preterm and one term lamb were exposed to ethanol in utero (maternal consumption), and the other two lambs were not exposed to ethanol at any time. Data analysis was with SCS/RTA, TopHat workflow, and Cufflinks, Cuffcompare, and Cuffdiff. Transcripts with increased expression in preterm lung exposed to ethanol included genes related to (1) inflammation, immunity, and growth factors: lysozyme, secreted frizzled-related protein 2, which affects Wnt signaling; interleukin 12A; CXCL10; insulin-like growth factor 1; IGF-1; Fos; IGF binding protein 5; early growth response protein 1; EGR1; CD28; and CD4; (2) metabolism/stress: leptin receptor and serotonin transporter; (3) cell proliferation inhibition: CDKN1C; p57, a Kip2 cell proliferation inhibitor; and (4) angiogenesis/vascularization: SERPINF1. Genes downregulated by ethanol but upregulated by RSV include IL-8, TNFα, and ICAM-1.
Conclusions
The various animal models of RSV infection contribute to the broad understanding of the RSV pathogenesis. The lamb model has several features consistent with RSV in infants, which allows insight into epithelial cell infection, pathogenesis, development of bronchiolitis, and its effect on airflow and gaseous exchange, inflammatory responses, and innate and adaptive immune responses. The ability to study lambs born preterm is one of several unique features of the lamb model. Also, the lamb model is conducive to therapeutic interventions, especially those that are mechanistic and at a fundamental level. Although VEGF and CC10 can enhance a wide variety of immunomodulators that mediate resistance to RSV infection, ALX-0171 is a very precise inhibitor of RSV. Enhancement of oxidative defense (Duox/LPO) through KI administration invokes oxidative radical defense against RSV and potentially other viruses and microbial agents. Lambs develop enhanced RSV disease if vaccinated with FI-RSV, which opens up the model to studies of FI-RSV–enhanced disease and assessment of vaccines. The ability to deprive lambs of maternal antibodies is an advantageous feature of the lamb model, which is particularly useful in assessment of vaccines. The findings that maternal ethanol consumption alters development and innate immunity of the lung in utero may explain, at least in part, why ethanol is a risk factor for preterm birth and also predisposes the infant lung to increased RSV disease severity.
Acknowledgments
I acknowledge and thank all of those who have contributed to the work and the funding agencies, which include the National Institutes of Health (NIAID R56AI09100), MedImmune, Inc., Gilead, Inc., and Ablynx NV.
References
- Abed Y, Boivin G. Treatment of respiratory virus infections. Antiviral Res. 2006;70:1–16. doi: 10.1016/j.antiviral.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen K, Gronbaek M. Alcohol consumption during pregnancy and the risk of preterm delivery. Am J Epidemiol. 2004;159:155–161. doi: 10.1093/aje/kwh034. [DOI] [PubMed] [Google Scholar]
- Alcorn DG, Adamson TM, Maloney JE, Robinson PM. A morphologic and morphometric analysis of fetal lung development in the sheep. Anat Rec. 1981;201:655–667. doi: 10.1002/ar.1092010410. [DOI] [PubMed] [Google Scholar]
- Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25:160–171. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Aujard Y, Fauroux B. Risk factors for severe respiratory syncytial virus infection in infants. Resp Med. 2002;96 S9–S14. [PubMed] [Google Scholar]
- Bae YS, Choi MK, Lee W-J. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Banfi B. A novel host defense system of airways is defective in cystic fibrosis: Update. Am J Resp Crit Care Med. 2007;175:967–976. doi: 10.1164/ajrccm.175.9.967. [DOI] [PubMed] [Google Scholar]
- Barth PJ, Wolf M, Ramaswamy A. Distribution and number of Clara cells in the normal and disturbed development of the human fetal lung. Pediatr Pathol. 1994;14:637–651. doi: 10.3109/15513819409023338. [DOI] [PubMed] [Google Scholar]
- Belding ME, Klebanoff SJ, Ray CG. Peroxidase-mediated virucidal systems. Science. 1970;167:195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301 doi: 10.1152/ajplung.00065.2011. L148–L156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken MO, Rovers MM, Bont L; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze. N Engl J Med. 2013;369:782–783. doi: 10.1056/NEJMc1307429. [DOI] [PubMed] [Google Scholar]
- Bozeman PM, Learn DB, Thomas EL. Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem Pharm. 1992;434:553–563. doi: 10.1016/0006-2952(92)90449-s. [DOI] [PubMed] [Google Scholar]
- Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol. 2001;281 doi: 10.1152/ajplung.2001.281.4.L1001. L1001–L1001. [DOI] [PubMed] [Google Scholar]
- Castilow EM, Olson MR, Varga SM. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39:225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. Dual oxidase regulates recruitment in allergic airways. Free Radic Biol Med. 2013;65C:38–46. doi: 10.1016/j.freeradbiomed.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med. 2002;166 doi: 10.1164/rccm.2206018. S57–S61. [DOI] [PubMed] [Google Scholar]
- Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello HM, Ray WC, Chaisatpongsakron S, Peeples ME. Targeting RSV with vaccines and small molecule drugs. Infect Disord Drug Targets. 2012;12:110–128. doi: 10.2174/187152612800100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derscheid RJ, Ackermann MR. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses. 2012;4:2359–2378. doi: 10.3390/v4102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derscheid RJ, Ackermann MR. The innate immune system of the perinatal lung and responses to respiratory syncytial virus infection. Vet Pathol. 2013;50:827–841. doi: 10.1177/0300985813480216. [DOI] [PubMed] [Google Scholar]
- Derscheid RJ, Gallup JM, Knudson CJ, Varga SM, Grosz DD, van Geelen A, Hostetter J, Ackermann MR. Effects of formalin-inactivated respiratory syncytial virus (FI-RSV) in the perinatal lamb model of RSV. PLOS One. 2014 doi: 10.1371/journal.pone.0081472. 8:e81472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derscheid RJ, van Geelen A, Gallup JM, Hostetter SJ, Banfi B, McCray PB, Jr, Ackermann MR. Increased concentration of iodide in airway secretions is associated with reduced RSV disease severity. Am J Resp Cell Mol Biol. 2013;50:389–397. doi: 10.1165/rcmb.2012-0529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derscheid RJ, van Geelen A, McGill JL, Gallup JM, Cihlar T, Sacco RE, Ackermann MR. Human respiratory syncytial virus Memphis 37 grown in HEp-2 cells causes more severe disease in lambs than virus grown in Vero cells. Viruses. 2013;22:2881–2897. doi: 10.3390/v5112881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- Elizur A, Adair-Kirk TL, Kelley DG, Griffin GL, deMello DE, Senior RM. Clara cells impact the pulmonary innate immune response to lps. Am J Physiol Lung Cell Mol Physiol. 2007;293 doi: 10.1152/ajplung.00024.2007. L383–L392. [DOI] [PubMed] [Google Scholar]
- Empey KM, Peebles RS, Jr, Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50:1258–1267. doi: 10.1086/651603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza JA, Bohmwald K, Céspedes PF, Gómez RS, Riquelme SA, Cortés CM, Valenzuela JA, Sandoval RA, Pancetti FC, Bueno SM, Riedel CA, Kalergis AM. Impaired learning resulting from respiratory syncytial virus infection. Proc Natl Acad Sci U S A. 2013;110:9112–9117. doi: 10.1073/pnas.1217508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles JE, Johnson JE, Megati S, Roopchand V, Cockle PJ, Weeratna R, Makinen S, Brown TP, Lang S, Witko SE, Kotash CS, Li J, West K, Maldonado O, Falconer DJ, Lees C, Smith GJ, White P, Wright P, Loudon PT, Merson JR, Jansen KU, Sidhu MK. Nonreplicating vaccines can protect african green monkeys from the memphis 37 strain of respiratory syncytial virus. J Infect Dis. 2013;208:319–329. doi: 10.1093/infdis/jit169. [DOI] [PubMed] [Google Scholar]
- Fach SJ, Brockmeier SL, Hobbs LA, Lehmkuhl HD, Sacco SE. Pulmonary dendritic cells isolated from neonatal and adult ovine lung tissue. Vet Immunol Immun Pathol. 2007;112:171–182. doi: 10.1016/j.vetimm.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Fach SJ, Meyerholz DK, Gallup JM, Ackermann MR, Lehmkuhl HD, Sacco RE. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 and IL-10 gene transcripts. Viral Immunol. 2007;20:119–130. doi: 10.1089/vim.2006.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fach SJ, Olivier A, Gallup JM, Waters TE, Ackermann MR, Lehmkuhl HD, Sacco RE. Differential expression of cytokine transcripts in neonatal and adult ovine alveolar macrophages in response to respiratory syncytial virus or toll-like receptor ligation. Vet Immunol Immunopathol. 2010;136:55–64. doi: 10.1016/j.vetimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Lennemann NJ, Krishnamurthy S, Pocza P, Durairaj L, Launspack JL, Rhein BA, Wohlford-Lenane C, Lorentzen D, Banfi B, McCray PB., Jr. Enhancement of respiratory mucosal antiviral defenses by the oxidation of iodide. Am J Respir Cell Mol Biol. 2011;45:874–881. doi: 10.1165/rcmb.2010-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H Mechanism and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11:2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. Developmental regulation of Duox1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292 doi: 10.1152/ajplung.00029.2007. L1506–L1514. [DOI] [PubMed] [Google Scholar]
- Flecknoe SL, Wallace MJ, Cock ML, Harding R, Hooper SB. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285 doi: 10.1152/ajplung.00306.2002. L664–L679. [DOI] [PubMed] [Google Scholar]
- Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol. 2004;561:183–194. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtmüller PG, Jantschko W, Regelsberger G, Jakopitsch C, Arnhold J, Obinger C. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry. 2002;41:11895–11900. doi: 10.1021/bi026326x. [DOI] [PubMed] [Google Scholar]
- Gallup JM, Kawashima K, Lucero G, Ackermann MR. New quick method for isolating RNA from laser captured cells stained by immunofluorescent immunochemistry; RNA suitable for direct use in fluorogenic TaqMan one-step real-time RT-PCR. Biol Proced Online. 2005;7:70–92. doi: 10.1251/bpo107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CG, Bhore R, Soriano-Fallas A, Trost M, Chason R, Ramilo O, Mejias A. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126 doi: 10.1542/peds.2010-0507. e1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, Conner GE. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med. 2009;47:1450–1458. doi: 10.1016/j.freeradbiomed.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin LJ. Immunology of bovine respiratory syncytial virus infection of cattle. Comp Immunol Microbiol Infect Dis. 2012;35:253–257. doi: 10.1016/j.cimid.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, Abraham WM, Conner GE. The lactoperoxidase system function in bacterial clearance of airways. Am J Resir Cell Mol Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- Guo-Parke H, Canning P, Douglas I, Villenave R, Heaney LG, Coyle PV, Lyons JD, Shields MD, Power UF. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am J Respir Crit Care Med. 2013;188:842–851. doi: 10.1164/rccm.201304-0750OC. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, Zhu Y, Grijalva CG, Prill MM, Iwane MK. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132 doi: 10.1542/peds.2013-0303. e341–348. [DOI] [PubMed] [Google Scholar]
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hon KL, Leung TF, Cheng WY, Ko NM, Tang WK, Wong WW, Yeung WH, Chan PK. Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis. J Crit Care. 2012;27:464–468. doi: 10.1016/j.jcrc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Hosakote YM, Komaravelli N, Mautemps N, Liu T, Garafalo RP, Casola A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Resp Lung Cell Mol Physiol. 2012;303 doi: 10.1152/ajplung.00192.2012. L991–L1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, Bakker R, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The Generation R study. Ann Epidemiol. 2007;17:834–840. doi: 10.1016/j.annepidem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Meyerholz DK, Gallup JM, Grubor B, Lazic T, Lehmkuhl HD, Ackermann MR. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Craven M, Welliver RC, Ahmad N, Halonen M. Differences in participation of innate and adaptive immunity to respiratory syncytial virus in adults and neonates. J Infect Dis. 2003;188:433–439. doi: 10.1086/376530. [DOI] [PubMed] [Google Scholar]
- Lazic T, Matic M, Gallup JM, Van Geelen A, Meyerholz DK, Grubor B, Imerman PM, Almeida de Macedo MM, Ackermann MR. Effects of nicotine on pulmonary surfactant proteins A and D in ovine lung epithelia. Pediatr Pulmonol. 2010;45:255–262. doi: 10.1002/ppul.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic T, Sow FB, Van Geelen A, Meyerholz DK, Gallup JM, Ackermann MR. Exposure to ethanol during the last trimester of pregnancy alters the maturation and immunity of fetal lung. Alcohol. 2011;45:673–680. doi: 10.1016/j.alcohol.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic T, Wyatt TA, Matic M, Meyerholz DK, Grubor B, Gallup JM, Kersting KW, Imerman PM, Almeida-De-Macedo M, Ackermann MR. Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol. 2007;41:347–355. doi: 10.1016/j.alcohol.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl HD, Cutlip RC. Experimental respiratory syncytial virus infection in feeder-age lambs. Am J Vet Res. 1979a;40:1729–1730. [PubMed] [Google Scholar]
- Lehmkuhl HD, Cutlip RC. Experimentally induced respiratory syncytial virus infection in lambs. Am J Vet Res. 1979b;40:512–544. [PubMed] [Google Scholar]
- Lorentzen D, Durairaj L, Pezzulo AA, Nakano Y, Launspach J, Stoltz DA, Zamba G, McCray PB, Jr, Zabner J, Welsh MJ, Nauseef WM, Bánfi B. Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic Biol Med. 2011;50:1144–1150. doi: 10.1016/j.freeradbiomed.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan SE, Snyder JM. Development of alveoli, In. In: Harding R, Pinkerton KE, Plopper CG, editors. Waltham, MA: Elsevier Academic Press. p; 2004. pp. 55–73. The Lung Development, Aging and the Environment. [Google Scholar]
- McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan JT, Cutlip RC, Lehmkuhl HD, Kluge JP, Ackermann MR. Infected cell types in ovine lung following exposure to bovine respiratory syncytial virus. Vet Pathol. 1994;31:229–236. doi: 10.1177/030098589403100210. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, DeGraaff JA, Gallup JM, Ackermann MR. Depletion of alveolar glycogen corresponds with immunohistochemical development of CD208 antigen expression in perinatal lamb lung. J Histochem Cytochem. 2006;54:1247–1253. doi: 10.1369/jhc.6A7002.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Gallup JM, Lazic T, de Macedo MMA, Lehmkuhl HD, Ackermann MR. Pretreatment with recombinant human vascular endothelial growth factor reduces virus replication and inflammation in a perinatal lamb model of RSV infection. Viral Immunol. 2007;20:188–196. doi: 10.1089/vim.2006.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Fach SJ, Sacco RE, Lehmkuhl HD, Gallup JM, Ackermann MR. Reduced clearance of respiratory syncytial virus in a preterm lamb model. Microbes Infection. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Gallup JM, Lehmkuhl HD, Anderson RD, Lazic T, Ackermann MR. Adenovirus-mediated gene therapy enhances parainfluenza virus 3 infection in neonatal lambs. J Clin Micro. 2004;42:4780–4787. doi: 10.1128/JCM.42.10.4780-4787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Grubor B, Lazic T, Gallup JM, de Macedo MMA, McCray PB, Jr., Ackermann MR. Monocytic/macrophagic pneumonitis following intrabronchial deposition of vascular endothelial growth factor in neonatal lambs. Vet Pathol. 2006;43:689–694. doi: 10.1354/vp.43-5-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Kawashima K, Gallup J, Grubor B, Ackermann MR. Expression of innate immune genes (SP-AD, SBD-1, TLR4) by respiratory epithelia at preterm gestation is less than full-term. Dev Comp Immunol. 2006;30:1060–1069. doi: 10.1016/j.dci.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskwa P, Lorentzen D, Excoffon KJDA, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A, Ope M, Lindblade KA, Carosone-Link P, Lucero M, Ochieng W, Kamimoto L, Dueger E, Bhat N, Vong S, Theodoratou E, Chittaganpitch M, Chimah O, Balmaseda A, Buchy P, Harris E, Evans V, Katayose M, Gaur B, O'Callaghan-Gordo C, Goswami D, Arvelo W, Venter M, Briese T, Tokarz R, Widdowson MA, Mounts AW, Breiman RF, Feikin DR, Klugman KP, Olsen SJ, Gessner BD, Wright PF, Rudan I, Broor S, Simões EA, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochimica Biophysica Acta. 2013;1840:757–767. doi: 10.1016/j.bbagen.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A, Gallup JM, de Macedo MM, Varga SM, Ackermann MR. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int J Exp Pathol. 2009;90:431–438. doi: 10.1111/j.1365-2613.2009.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A, Gallup JM, van Geelen A, Ackermann MR. Exogenous administration of vascular endothelial growth factor prior to human respiratory syncytial virus A2 infection reduces pulmonary pathology in neonatal lambs and alters epithelial innate immune responses. Exp Lung Res. 2011;37:131–143. doi: 10.3109/01902148.2010.484518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res. 1980;208:65–84. doi: 10.1007/BF00234174. [DOI] [PubMed] [Google Scholar]
- Pickles RJ. Human airway epithelial cell cultures for modeling respiratory syncytial virus infection. Curr Top Microbiol Immunol. 2013;372:371–387. doi: 10.1007/978-3-642-38919-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Mariassy AT, Lollini LO. Structure as revealed by airway dissection. A comparison of mammalian lungs. Am Rev Respir Dis. 1983;128 doi: 10.1164/arrd.1983.128.2P2.S4. S4–S7. [DOI] [PubMed] [Google Scholar]
- Rudraraju R, Jones BG, Sealy R, Surman SL, Hurwitz JL. Respiratory syncytial virus: Current progress in vaccine development. Viruses. 2013;5:577–594. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RE, McGill JL, Pillatzki AE, Palmer MV, Ackermann MR. Respiratory syncytial virus infection in cattle. Vet Pathol. 2013;51:427–436. doi: 10.1177/0300985813501341. [DOI] [PubMed] [Google Scholar]
- Scheerlinck J-P Y, Snibson KJ, Bowles VM, Sutton P. Biomedical applications of sheep models: from asthma to vaccines. Trends Biotech. 2008;26:259–266. doi: 10.1016/j.tibtech.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Sommer C, Resch B, Simoes EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. J Open Microbiol. 2012;5:144–154. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow FB, Gallup JM, Derscheid R, Krishnan S, Ackermann MR. Ontogeny of the immune response in the ovine lung. Immunological Investigations. 2012;41:304–316. doi: 10.3109/08820139.2011.631657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow FB, Gallup JM, Krishnan S, Patera AC, Suzich J, Ackermann MR. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro-inflammatory response in the lungs of preterm lambs. Respir Res. 2010;12 doi: 10.1186/1465-9921-12-106. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhanced effects of VEGF on the immune response. Dev Comp Immunol. 2009;33:761–771. doi: 10.1016/j.dci.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow FB, Gallup JM, Olivier A, Krishnan S, Patera AC, Suzich J, Ackermann MR. Respiratory syncytial virus is associated with an inflammatory response in lungs and architectural remodeling of lung-draining lymph nodes of newborn lambs. Am J Physiol Lung Cell Mol Physiol. 2011;300 doi: 10.1152/ajplung.00169.2010. L12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow FB, Gallup JM, Sacco R, Ackermann MR. Laser capture microdissection revisited as a tool for transcriptomic analysis: application of an Excel-based qPCR preparation software (PREXCEL-Q) Int J Biomed Sci. 2009;5:105–124. [PMC free article] [PubMed] [Google Scholar]
- Sozo F, O'Day L, Maritz G, Kenna K, Stacy V, Brew N, Walker D, Bocking A, Brien J, Harding R. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2009;296 doi: 10.1152/ajplung.90532.2008. L510–L518. [DOI] [PubMed] [Google Scholar]
- Stark JM, Stark MA, Colasurdo GN, LeVine AM. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. J Med Viol. 2006;78:829–838. doi: 10.1002/jmv.20631. [DOI] [PubMed] [Google Scholar]
- Starner TD, Agerberth B, Gudmundsson GH, McCray PB., Jr. Expression and activity of beta defensins and LL-37 in the developing human lung. J Immunol. 2005;174:1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–1136. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, Wang X, Boonacker CW, Veenhoven RH, Bruin JP, Bogaert D, Sanders EA. Associations between pathogens in the upper respiratory tract of young children: Interplay between viruses and bacteria. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047711. e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villenave R, Shields MD, Power UF. Respiratory syncytial virus interaction with human airway epithelium. Trends Microbiol. 2013;21:238–244. doi: 10.1016/j.tim.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290 doi: 10.1152/ajplung.00185.2005. L209–L221. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- Welliver RC, Checchia PA, Bauman JH, Fernandes AW, Mahadevia PJ, Hall CB. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin. 2010;26:2175–2181. doi: 10.1185/03007995.2010.505126. [DOI] [PubMed] [Google Scholar]
- Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 2001;198:207–221. doi: 10.1046/j.1469-7580.2001.19820207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29:206–212. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- Wu H, Pfrarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol. 2008;317:103–123. doi: 10.1007/978-3-540-72146-8_4. [DOI] [PubMed] [Google Scholar]