Abstract
Duchenne muscular dystrophy (DMD) is an X-linked human disorder in which absence of the protein dystrophin causes degeneration of skeletal and cardiac muscle. For the sake of treatment development, over and above definitive genetic and cell-based therapies, there is considerable interest in drugs that target downstream disease mechanisms. Drug candidates have typically been chosen based on the nature of pathologic lesions and presumed underlying mechanisms and then tested in animal models. Mammalian dystrophinopathies have been characterized in mice (mdx mouse) and dogs (golden retriever muscular dystrophy [GRMD]). Despite promising results in the mdx mouse, some therapies have not shown efficacy in DMD. Although the GRMD model offers a higher hurdle for translation, dogs have primarily been used to test genetic and cellular therapies where there is greater risk. Failed translation of animal studies to DMD raises questions about the propriety of methods and models used to identify drug targets and test efficacy of pharmacologic intervention. The mdx mouse and GRMD dog are genetically homologous to DMD but not necessarily analogous. Subcellular species differences are undoubtedly magnified at the whole-body level in clinical trials. This problem is compounded by disparate cultures in clinical trials and preclinical studies, pointing to a need for greater rigor and transparency in animal experiments. Molecular assays such as mRNA arrays and genome-wide association studies allow identification of genetic drug targets more closely tied to disease pathogenesis. Genes in which polymorphisms have been directly linked to DMD disease progression, as with osteopontin, are particularly attractive targets.
Keywords: animal models, drug development, Duchenne muscular dystrophy, golden retriever muscular dystrophy, genome wide association studies, mRNA arrays, mdx mouse, preclinical studies
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder that affects approximately 1 in 5000 newborn human males (Cowan et al. 1980) in whom absence of the protein dystrophin causes degeneration of skeletal and cardiac muscle (Hoffman et al. 1987). A less severe form, with in-frame DMD gene mutations, is termed Becker muscular dystrophy (BMD) (Malhotra et al. 1988). For sake of treatment development, considerable focus has appropriately been placed on introducing the DMD gene or correcting the underlying mutation (Foster et al. 2012; Konieczny et al. 2013). Some of these therapies involve pharmacologic approaches, as with compounds that promote stop codon read-through or correct frame-shift mutations. Drugs also have been developed to upregulate surrogate proteins that could take the place of dystrophin at the muscle cell membrane (Malik et al. 2012). Still others that target downstream mechanisms of disease and associated lesions, such as muscle necrosis, inflammation, fibrosis, or regeneration, have been studied (Malik et al. 2012; Ruegg 2013).
Animal models have been used extensively to identify targets for drug therapy and assess therapeutic efficacy. Spontaneous mammalian forms of X-linked muscular dystrophy due to dystrophin deficiency have been identified in mice (Bulfield et al. 1984; Gillis 1999), cats (Carpenter et al. 1989; Gaschen et al. 1992), pigs (Hollinger et al. 2013a), and multiple dog breeds (Figure 1) (Cooper et al. 1988; Kornegay et al. 1988, 2012a; Smith et al. 2011; Walmsley et al. 2010). The disease phenotype in dystrophic dogs is more severe than in mice, suggesting that canine studies might better translate to humans. Dogs with golden retriever muscular dystrophy (GRMD) have been used increasingly to define disease mechanisms and assess potential treatments (Kornegay et al. 2012a). In the context of these studies, marked phenotypic variation has been seen at the level of individual animals and different muscles. Such variability confounds statistical analysis in preclinical trials but also provides a platform to define disease pathogenesis.
Figure 1.

The canine dystrophin protein (Ensembl protein ID ENSCAFP00000031637), along with mutation information for seven dog breeds known to exhibit DMD-linked muscular dystrophy. CH indicates calponin homology domains, which are actin-binding. WWP indicates the WW domain, which binds proline-rich polypeptides and is the primary interaction site for dystrophin and dystroglycan. EF indicates members of the EF-hand family; this domain stabilizes the dystrophin-dystroglycan complex. ZNF represents a putative zinc-binding domain, ZnF_ZZ, which is present in dystrophin-like proteins and may bind to calmodulin. All 79 exons are represented. Exons and protein domains are depicted approximately to scale. Insertion and deletion mutations are shown above the exons. Arrows at the bottom of the figure indicate point mutations. Reprinted by permission from Bentham Science Publishers: Current Genomics. Comparative genomics of X-linked muscular dystrophies: The golden retriever model, 14:330–342, © 2013.
This paper reviews the use of animals in developing pharmacologic therapies in a general sense and for DMD in particular. Emphasis is placed on drugs that either promote dystrophin or surrogate gene/protein expression or delay downstream pathogenetic events. Published preclinical studies targeting pathologic lesions or disease mechanisms are reviewed, highlighting the frequent failure of animal experiments to translate to human trials. We subsequently emphasize the potential for genetic assays, like mRNA expression profiles and genome-wide association studies (GWAS), to better identify drug targets, especially when results are correlated with phenotypic data.
Current Status and Therapeutic Goals
Glucocorticoids are the current standard of care for DMD (Manzur et al. 2008; Moxley et al. 2010). Beneficial effects of prednisone are poorly understood and cannot be explained by antiinflammatory properties alone (Kissel et al. 1993; Weller et al. 1991) (see treatments targeting Muscle Inflammation below). Despite beneficial effects of prolonged ambulation and improved cardiopulmonary function, glucocorticoids are often discontinued due to side effects ranging from weight gain to pathologic bone fractures (Connolly et al. 2002). This has prompted use of many different protocols and a search for alternative treatments, as with steroid analogues and other agents that target disease mechanisms. In this context, the ultimate goal is to identify a therapy that achieves at least the benefit of steroids, with fewer side effects.
Drug Development Regulatory Process
The Food and Drug Administration is governed by the Federal Food, Drug, and Cosmetic Act, which defines drugs as “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals” and “articles (other than food) intended to affect the structure or any function of the body of man or other animals” (http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/default.htm). Therapeutic biologics (monoclonal antibodies and small protein molecules) are included in the definition of drugs (Roberts and McCune, 2008). The FDA generally becomes involved in the drug discovery process after the drug's sponsor (usually the manufacturer or potential marketer) has screened the new molecule for pharmacological activity and acute toxicity potential in animals and wishes to test its diagnostic or therapeutic potential in humans. The process for human use entails filing an Investigational New Drug Application and testing through a series of clinical trials (Phases 1–4), extending from smaller populations to establish safety (Phase 1) to large diverse clinical groups to determine efficacy (Phase 4) (Lesko et al. 2000). There are currently 121 DMD studies of diagnostic tests and treatment trials in various stages of initial enrollment to termination on the NIH Clinical Trials.gov website (http://www.clinicaltrials.gov/ct2/results?term=Duchenne+Muscular+Dystrophy&Search=Search). An analogous agency, the European Medicines Agency (http://www.ema.europa.eu/ema/index.jsp?curl=pages/home/Home_Page.jsp&mid=), regulates drug development in Europe (Lis et al. 2012).
There is high risk in drug development. Only 16% of drugs originating collectively from the 50 largest pharmaceutical companies in the United States between 1993 and 2004 were approved (DiMasi et al. 2010). The top two reasons for failed drug development in pharmaceutical companies in the United Kingdom from 1964 to 1985 were pharmacokinetics (PK) (39%) and efficacy (29%). With regard to PK data, it is especially important to pay close attention to differences among species (Lin 1995; Tibbitts 2003) and, in the case of dogs, even breeds (Fleischer et al. 2008). With more careful analysis of the PK properties of drugs and development of tools to better predict drug absorption and clearance, drug–drug interactions, and scaling of PK parameters from animals to man (Walker 2004), PK issues accounted for only 10% of drug failures in 2000 (Kola and Landis 2004). Beyond issues related to drug PK, problems with drug targeting and efficacy have persisted. A more recent study found that success rates for Phase II human clinical trials for drugs originating from 16 companies, representing 60% of global research and development spending, had fallen from 28% in 2006–2007 to 18% for 2008–2009 (Arrowsmith 2011). Alarmingly, this same review noted that 51% of 108 reported Phase II failures occurred due to insufficient efficacy, despite the fact that most drugs proceeded through standard validation methods, with assessment in animal models (Plenge et al. 2013). Paralleling the low success rate for pharmaceutical industry drug approval, only 28 of 76 (37%) highly cited studies that investigated a preventive or therapeutic intervention in an in vivo animal model over the 1980 to 2000 period were replicated in human randomized trials (Hackam and Redelmeier 2006). Overall, most of these animal efficacy studies included dose-response gradients, clinically relevant outcomes, and long-term endpoints, but few incorporated random animal allocation, adjustment for multiple hypotheses testing, or blinded outcome assessment. Animal studies incorporating dose-response gradients were more likely to translate to humans. A follow-up study provided 55 different recommendations to improve translation of preclinical research, including power calculation to determine sample size, randomized treatment allocation, and characterization of disease phenotype in the animal model prior to experimentation (Henderson et al. 2013). Additional papers have highlighted inconsistencies in experimental design and statistical analysis, with associated challenges in reproducing results, for a broad range of preclinical studies directed at neurological diseases (Benatar 2007; Sena et al. 2007; Steward et al. 2012). This led National Institute of Neurological Disorders and Stroke (NINDS) Director Story Landis and colleagues (Landis et al. 2012) to call for greater transparency in preclinical studies and more rigorous experimental design, to include random assignment of animals to treatment groups, blinding of data assessment, greater attention to power analysis of outcome parameters used to establish benefit, and increased stringency in data handling.
The “lost in translation” problem for preclinical research has been attributed to three issues, identified as the “Butterfly Effect” (chaotic behavior whereby small differences in the animal model lead to substantial differences in clinical results); the “Princess and the Pea” problem, based in variability of effect size when progressing from biochemical findings through tissue culture and animal and human studies (the pea does not indent the mattress to the same degree as the princess); and the “Two Cultures” problem in preclinical and clinical research, as evidenced by the data collection issues summarized above (Ergorul and Levin 2013). Some guidance for increasing the rigor of preclinical studies could be drawn from the “Animal Rule” put in place by the FDA to guide drug approval in instances of chemical, biologic, radiologic, and nuclear threats “when adequate and well-controlled clinical studies in humans cannot be ethically conducted and field efficacy studies are not feasible.” In such cases, drugs may be approved for human use when four criteria are fulfilled: (1) data define pathophysiological mechanisms for the product; (2) the product effect is demonstrated in more than one species; (3) the animal study endpoint is clearly related to the desired benefit in humans; and (4) PK data in animals and humans are sufficiently well understood to allow selection of an effective dose in humans (Roberts and McCune 2008).
Animal Models
The National Research Council has defined a biomedical model as “a surrogate for a human being, or a human biologic system, that can be used to understand normal and abnormal function from gene to phenotype and to provide a basis for preventive or therapeutic intervention in human diseases” (National Research Council 1998). This same report emphasized that biomedical models can be “of many types – from animal models of human diseases to animal, in vitro, or modeling systems for studying any aspect of human biology or disease” and that “a model need not be an exact replica of a human disease or condition” (here, specifically citing the mdx mouse model of DMD/BMD). An earlier National Research Council study, conducted in 1985 before the discovery of the DMD gene, emphasized that biologic models can be divided into two broad classes, depending on whether the modelling is based on analogy or homology. Modelling by analogy implies a point-by-point relationship between one structure or process to another (National Research Council 1985). From a mathematical perspective, this is termed mapping. By definition, modelling by analogy requires that there are similarities between the structures and processes being compared in the modeling relationship. On the other hand, modelling by homology implies a shared evolutionary history and matching DNA makeup between the two structures or processes. Importantly, for models by homology to be functionally useful, they must also be good models by analogy for the phenomenon being studied. Unfortunately, as discussed further below in the context of DMD, homolog animal models are not always good analog models because of physiologic adaptions that may have occurred over time. Surrogate models may also be classified as either one-to-one, as with a disease state in humans and a particular species that share the same clinical features (mdx or GRMD compared with DMD), or many-to-many, where findings from more than one species or organ system may model particular features of the underlying process or state (multiple features of DMD potentially being modeled by different available animal models or systems) (National Research Council 1985). The many-to-many approach is probably best suited to address questions raised by complex genetic diseases such as DMD.
The regulatory process discussed above highlights the critical role that animal studies play in essentially all phases of preclinical drug development, extending from the original target identification; determination of the drug's PK, absorption, distribution, metabolism, and excretion; potential toxicity to the treated animal or person; and ultimate efficacy against the intended disease (Roberts and McCune 2008). Efficacy studies have often been completed in symptomatic, experimental animal models, where a drug's efficacy is tested on a clinical effect, as with high blood pressure, versus the underlying disease itself. With the advent of the genomic era, specific genetic disease models have become more prevalent (Hunter 2011).
Genetic models may either occur naturally (spontaneously) or be produced through genetic manipulation (genetically modified [transgenic or gene knock-down/out] animals). For sake of DMD, both spontaneous and genetically modified animal models have been used to define pathogenetic mechanisms and establish efficacy of experimental therapies. Nonmammalian models, occurring either by natural DMD gene mutation or gene knock-down/out, have been characterized in the zebrafish (Gibbs et al. 2013), Drosophila fly (Shcherbata et al. 2007), and Caenorhabditis elegans nematode (Bessou et al. 1998). These models offer tremendous value in studying disease pathogenesis and developmental therapeutics.
Spontaneous mammalian models of DMD have been identified in the mouse (Bulfield et al. 1984; Gillis 1999), cat (Carpenter et al. 1989; Gaschen et al. 1992), pig (Hollinger et al. 2013a), and multiple dog breeds (Cooper et al. 1988; Kornegay et al. 1988, 2012a; Smith et al. 2011; Walmsley et al. 2010). Cats with DMD gene mutations express a curious debilitating hypertrophic myopathy and are predisposed to a syndrome similar to malignant hyperthermia during stress or anesthesia (Gaschen et al. 1992, 1998). These features limit the feline condition's value as a model. The malignant hyperthermia-like syndrome also occurs in DMD (Gurnaney et al. 2009) and occasional GRMD dogs (JN Kornegay, unpublished data). Pigs with a spontaneous DMD gene missense mutation and an analogous stress syndrome were recently described (Hollinger et al. 2013a; Nonneman et al. 2012). In addition, transgenic/knock-out gene technology can be applied to produce pigs with specific DMD gene mutations (Klymiuk et al. 2013), providing other valuable models (see the mdx52 model below).
From an investigational standpoint, drug discovery typically starts by developing and testing hypotheses using in vitro systems, including cell culture and ex vivo explants, and, increasingly, as discussed below, genetic assays. The DMD research field is fortunate to have nonmammalian and both small (mdx mouse) and large (GRMD; potentially pigs) mammal models available for follow-up mechanistic and preclinical studies. Once hypotheses have been substantiated in vitro or in nonmammalian models, treatments should ideally first be tested in the mdx mouse and, if efficacy is shown, moved to GRMD dogs before initiating DMD trials. But, as reflected by this review, because of expense, lack of familiarity, and relative scarcity, dystrophic dogs have not been widely used in pharmacologic testing. This deficiency could be partially countered by using the GRMD model in tandem with the mdx mouse in genetic assays to better identify relevant therapeutic targets and then more consistently testing drug efficacy first in mdx mice and then dystrophic dogs, especially if compounds carry substantial risk to humans.
DMD
To understand whether an animal disease truly models its counterpart in people, one must first appreciate the nuances of the human condition. For sake of preclinical drug efficacy studies, care should be taken in identifying the age over which treatment is administered and the natural history of outcome parameters during this period. Ideally, the time course for treatment and outcome parameters used to assess efficacy should approximate those of DMD. Patients with DMD have delayed milestones, typically not walking until approximately 18 months of age or later (Bushby et al. 1999, 2010). The diagnosis is generally not made until around 5 years of age. Other early symptoms that may be seen prior to diagnosis include frequent falls and difficulty in running and climbing stairs. By definition, affected boys are wheelchair bound by 12 to 13 years, although mild and severe forms of the disease may deviate from this trajectory (Nicholson et al. 1993c). In contrast, BMD patients walk beyond 16 years of age (Bushby et al. 2010). There is variation among DMD patients in other outcome parameters, including measures of strength and joint angles/contractures (Brooke et al. 1983; McDonald et al. 1995; Ziter et al. 1977). The rate of functional decline may also vary. Brooke et al. (1983) noted that children walk and climb stairs fairly easily until 8 years, when function declines rapidly. McDonald et al. (1995) found a relatively uniform decline of muscle strength from 5 to 13 years. Results from joint angle measurements, reflecting the degree of contracture, and strength testing tend to correlate (Brooke et al. 1983). Vignos et al. (1963) indicated that quadriceps strength was the single best predictor of time to wheelchair. The 6-minute walk test (6MWT) has become the principal outcome measure used in DMD clinical trials (Mazzone et al. 2013; McDonald et al. 2013a, 2013b). Results of the 6MWT correlate with wheelchair status (Mazzone et al. 2013; McDonald et al. 2013a) and knee extensor strength (McDonald et al. 2013b). Most affected boys die due to respiratory or cardiac disease by their late teens or early twenties, although life has been extended with more aggressive supportive care (Eagle et al. 2007; Finsterer and Stöllberger 2003; Inkley et al. 1974).
Individual patients, muscles, and myofiber types are differentially affected in DMD (Ciciliot et al. 2013; McDonald et al. 1995). Variable involvement at the patient level suggests the possibility of genetic influences, as with modifier genes (see discussion below under Pharmacogenomic Drug Targeting). With regard to individual muscles, DMD is a proximal myopathy, with muscles like the quadriceps being selectively affected. In contrast, certain other muscles, such as those of the eye, are relatively spared (Karpati et al. 1988). Some muscles may even undergo paradoxical hypertrophy, as with classical calf pseudohypertrophy (Cros et al. 1989; Jones et al. 1983). Fast twitch (type IIb) myofibers are selectively affected, leading to characteristic slow twitch (type 1) predominance (Webster et al. 1988).
As discussed above and further below, GRMD dogs also demonstrate dramatic phenotypic variation. Moreover, differential muscle involvement is a prominent feature of both the GRMD and mdx models (reviewed in Kornegay et al. 2012b). Selective muscle involvement appears to occur because of mechanical factors at play during different stages of the disease. Eccentric (lengthening) contraction injury contributes to selective quadriceps involvement in DMD (Edwards et al. 1984). High usage and attendant necrosis of muscles, as with the tongue and certain flexors, early in life seems to result in a disordered regenerative response and hypertrophy. Data from the GRMD cranial sartorius (CS) muscle suggests that initial enlargement results from increased muscle mass (true hypertrophy) followed by deposition of connective tissue and fat (pseudohypertrophy) (Kornegay et al. 2003, 2012b). Individual patient/animal and muscle phenotypic variation must be considered when targeting DMD lesions or disease mechanisms.
Mdx Mouse
The mdx mouse has a spontaneous nonsense point mutation in exon 23 (Sicinski et al. 1989). Although genetically homologous to DMD, this model is not analogous, with affected mice having a relatively mild phenotype. After undergoing an acute wave of muscle necrosis at 3 to 5 weeks of age (Bulfield et al. 1984; Gillis 1999), mice largely recover and do not experience substantial clinical dysfunction until approximately 18 months of age (Lefaucheur et al. 1995). However, affected mice have progressive histopathologic changes and an approximately 20% reduction in life span (Chamberlain et al. 2007).
In keeping with this sequential disease course, Grounds (2008) has proposed a so-called “two-tier hypothesis” to explain disease progression in DMD and the mdx mouse. As discussed further below under recommendations to ensure consistency among mdx preclinical trials, emphasis has been placed on the need to develop different therapeutic strategies for the acute and chronic phases. Drawing parallels to DMD, the ages in mice and humans have been compared, with 3, 4, 6, and 8 weeks of age in mice roughly corresponding to about 6 months, 10 years, 16 to 18 years, and 20 years for humans, respectively (Grounds et al. 2008). In this sense, the acute muscle damage in mice at 3 to 5 weeks would correspond to a much more extended period in DMD boys.
With the mdx mouse and other murine models, transgenic/gene knock-out technology can be used to further modify the genotype and associated phenotype (Willmann et al. 2009). Most notably, the mdx phenotype is exaggerated when the utrophin gene is knocked-out to produce so-called double knock-out (dko) mice (Deconinck et al. 1997). Other genes can be knocked-out or silenced to study their role in disease pathogenesis. Although double mutants typically have a more severe phenotype that better models symptoms of DMD, the second mutation introduces a biochemical or biologic difference that could affect the disease course independent of the absence of dystrophin. Knock-out technology also allows specific DMD gene mutations to be produced, as with targeting exon 52, to create models to better explore therapeutic strategies like exon skipping (Aoki et al. 2012; Araki et al. 1997).
Reasons for the relatively mild mdx phenotype are not well defined. Bodor and McDonald (2013) have speculated that signs are more severe in larger species and individuals because of added stress placed on higher caliber muscle fibers. Grounds (2008) stressed that growth exacerbates necrosis in muscles lacking dystrophin and suggested that the proportionally longer life spans of humans and dogs could allow for additional bouts of necrosis and fibrosis and a more severe phenotype. Another factor might be the shorter telomere length and lower telomerase activity and associated reduced muscle regenerative capacity in humans and dogs versus mice (see mechanisms contributing to Muscle Atrophy below). Regardless of the exact cause(s), the relatively mild mdx phenotype has caused concerns about the degree to which findings will translate to humans. Additional questions have been raised because of the mouse's size and whether variables influenced by scale, such as cell migration or drug diffusion, can be appropriately modeled (Partridge 2013). As discussed further below, despite these reservations, international consensus has established the mdx mouse as the model of choice for preclinical and proof-of-concept studies, because they have the exact monogenic biochemical defect present in DMD (Willmann et al. 2009).
To increase consistency of mdx preclinical studies and improve translation to humans, efforts have been made to standardize selection of mice to be used (genetic background, gender, etc); husbandry practices (diet, stress, etc); experimental design (age at onset and route and duration of drug administration); and outcome parameters (De Luca 2012; Grounds et al. 2008; Nagaraju et al. 2009; Spurney et al. 2009; Willmann et al. 2012). A recent comprehensive review of the mdx mouse as a preclinical model (Grounds et al. 2008) put forth two major recommendations to ensure more consistent results: (1) Employ basic standard experimental regimes for preclinical testing. Building on the “two-tier hypothesis” discussed above, two potential regimes were recommended. In regime A, treatments would be started at 14 to 17 days of age before the acute wave of necrosis, with tissues sampled at 28 days and, potentially, 12 weeks or later. Regime B would utilize older (> 4 weeks) mice in which histopathologic changes have stabilized. For sake of these studies, mice would be exercised to further exacerbate phenotypic effects. (2) Employ basic methods to analyse the preclinical experiments. Longitudinal measurements of whole body function, ex vivo individual muscle strength, serum creatine kinase, and standardized histopathologic lesions should be completed. Willmann et al. (2012) made somewhat analgous recommendations in a subsequent review. To provide context for the anticipated benefit of an experimental therapeutic, they also suggested calculating a “recovery score” by comparing the differential between treated and untreated mdx and wild-type mice.
In assessing potential biomarkers to be used in the mdx mouse, Spurney et al. (2009) found that body weight, normalized grip strength, horizontal activity, rest time, cardiac function measurements, blood pressure, total central/peripheral nuclei per fiber, and serum creatine kinase were most effective for detecting drug-induced changes (Table 1). Given the mild phenotype of mdx mice, eccentric contraction and exercise are often used to exaggerate clinical signs. A forced treadmill protocol to accentuate the phenotype and increase likelihood of detecting drug effects over a 3- to 6-month trial period has been recommended (Spurney et al. 2009). Mdx and dko mice develop cardiomyopathy (Duan 2006; Spurney et al. 2011a) and respiratory disease (Huang et al. 2011), allowing for preclinical testing of drugs that target the heart (Spurney et al. 2011b, 2011c) and improve diaphragmatic function (Percival et al. 2012).
Table 1.
Outcome parameters that most effectively distinguish drug-induced changes in mdx mice
| Test | Age (wk) | Wild-type (C57BL/10) micea | Mdx micea | Significance (p value) |
|---|---|---|---|---|
| Body weight (g) | 10–12 | 22.17 ± 0.96 | 23.64 ± 1.33 | 0.0023 |
| 38–40 | 26.93 ± 2.06 | 27.30 ± 1.50 | NS | |
| Grip strength (front) (normalized KGF/kg) |
10–12 | 5.506 ± 0.274 | 4.409 ± 0.346 | < 0.0001 |
| 38–40 | 5.430 ± 0.722 | 4.054 ± 0.485 | < 0.0001 | |
| Grip strength (hind) (normalized KGF/kg) |
10–12 | 7.273 ± 0.380 | 6.244 ± 0.466 | < 0.0001 |
| 38–40 | 9.189 ± 1.207 | 8.455 ± 1.067 | NS | |
| Horizontal activity | 10–12 | 1650 ± 270.75 | 1014.10 ± 230.03 | < 0.0001 |
| 38–40 | 1394.91 ± 344.50 | 1326.62 ± 416.72 | NS | |
| Rest time(s) | 10–12 | 562.22 ± 8.45 | 576.50 ± 12.15 | 0.0012 |
| 38–40 | 569.05 ± 13.17 | 569.63 ± 20.00 | NS | |
| LVID (d) | 38–40 | 3.90 ± 0.14 | 3.70 ± 0.19 | 0.0077 |
| LV%FS | 38–40 | 30.63 ± 2.58 | 27.89 ± 1.86 | 0.0091 |
| Systolic BP | 38–40 | 79.15 ± 3.08 | 74.10 ± 8.62 | NS |
| Diastolic BP | 38–40 | 68.54 ± 6.50 | 50.60 ± 8.36 | < 0.0001 |
| Mean BP | 38–40 | 71.85 ± 4.83 | 58.20 ± 8.08 | 0.0001 |
| Peripheral nuclei/fiber | 38–40 | 1.33 ± 0.11 | 1.142 ± 0.151 | 0.0211 |
| Central nuclei/fiber | 38–40 | 0.010 ± 0.007 | 0.633 ± 0.152 | < 0.001 |
| Total nuclei/fiber | 38–40 | 1.34 ± 0.10 | 1.77 ± 0.28 | 0.0029 |
| Serum CK (U/L) | 38–40 | 85.20 ± 74.66 | 6439.66 ± 2506.59 | < 0.001 |
aData expressed as mean ± SD.
Abbreviations: BP, blood pressure; CK, creatine kinase; LVID (d), left ventricular diameter in diastole; LV%FS, left ventricular per cent shortening fraction; normalized, kg of force divided by body weight in kg; NS, not significant.
GRMD
Numerous canine breeds with dystrophin-deficient muscular dystrophy have been clinically characterized, but few have been studied at the molecular level (see Figure 1 above). We have conducted extensive studies in a dystrophin-deficient form of muscular dystrophy originally characterized in golden retrievers (GRMD) (Kornegay et al. 2012a). An mRNA processing error in GRMD dogs results from a single base change in the 3′ consensus splice site of intron 6 (Sharp et al. 1992). Exon 7 is consequently skipped during mRNA processing. The resulting transcript predicts termination of the dystrophin reading frame within its N-terminal domain in exon 8. Compared with mdx mice, dystrophic dogs more closely mimic the DMD phenotype, with signs occurring early and progressing. With this said, even some dogs stabilize and live well into adulthood (Ambrosio et al. 2008).
Dystrophic pups are often ineffectual sucklers and exhibit stunted growth. Some have a particularly fulminant form in which severe dyspnea can lead to death or necessitate euthanasia during the neonatal period. By 6 weeks of age, the pelvic limbs may be simultaneously advanced and trismus is noted. Subsequently, dogs develop stilted gait; atrophy of particularly the truncal, temporalis, and certain extensor muscles; a plantigrade stance due to hyperextension of the carpal joints and flexion at the tibiotarsal (TTJ) joints; excessive drooling, suggesting pharyngeal muscle involvement; and initial lumbar kyphosis that progresses to lordosis (Figure 2). Whereas most muscles atrophy, some, such as the CS and tongue, hypertrophy (Kornegay et al. 2003, 2012b). Respiratory and cardiac involvement occurs and can be objectively assessed (DeVanna et al. 2014; Fine et al. 2011; Su et al. 2012). To partially explain variable disease involvement, Valentine et al. (1988) suggested that homozygous females and smaller dogs might have milder signs. Analogous moderation of signs in female mdx mice has been attributed to estrogen effects (Salimena et al. 2004). Bodor and McDonald (2013) cited Valentine's work, as well as a tendency for smaller beagle crosses with the GRMD mutation to have a less severe phenotype (Shimatsu et al. 2003; Yugeta et al. 2006), in making a case that size is a major contributor to disease severity in DMD and the animal models. Nonetheless, in assessing outcome parameters in our own GRMD preclinical studies, we did not find differential disease involvement in homozygous females and larger dogs (Kornegay et al. 2012a). Still, efforts should be made to balance gender and size in treatment groups.
Figure 2.
Homozygous female GRMD dog (Jelly) at 6 years of age. Note the characteristic plantigrade stance, most noticeably carpal hyperextension and glossal hypertrophy.
The experimental design, outcome parameters, and sample size must be carefully considered to achieve significance in GRMD preclinical studies, especially in light of phenotypic variation. Clinical signs of GRMD progress particularly rapidly between the ages of 3 and 6 months. Thus, somewhat akin to the mdx mouse, the natural history of GRMD provides a relatively short window over which therapies can be assessed. Comparative longevity studies for dogs and humans suggest that the first year of a golden retriever's life roughly equates to 20 years of a human (Patronek et al. 1997). By extrapolation, the 3- to 6-month age in a GRMD dog would correspond to 5 to 10 years of a DMD boy, a period over which symptoms also progress markedly (Brooke et al. 1983; McDonald et al. 1995). A variety of outcome parameters, in many cases modeled after analogous procedures used to assess DMD patients and mdx mice, has been developed (Table 2) (Kornegay et al. 2012a). Efforts have been made to standardize methods used for these procedures in dogs in parallel with those for mice (Nagaraju et al. 2009). For our own natural history studies, we have generally collected data at 3 and 6 months of age to be in sync with the period used for preclinical studies. To counter the effects of phenotypic variation on data analysis, baseline (pretreatment) values should first be established at 3 months and compared with those at the end of treatment. In this way, the relative longitudinal effect of systemic treatments can be established. For local (intramuscular) or regional (single limb) approaches, the opposite untreated limb can serve as a control. Although independent control groups for each preclinical trial should ideally be assessed, budgetary and animal number limitations may necessitate the use of natural history controls.
Table 2.
Outcome parameters in GRMD dogs
| Test | Age (mo) | Normal dogs | GRMD dogs | Significance (p value) | Reference |
|---|---|---|---|---|---|
| Body weight (kg) | 3 | 10.65 ± 1.75 | 7.47 ± 1.21 | < 0.01 | Kornegay et al. 1999 |
| 6 | 20.24 ± 2.30 | 12.86 ± 3.08 | < 0.01 | ||
| 12 | 23.17 ± 1.70 | 18.23 ± 3.22 | < 0.01 | ||
| TTJ tetanic flexion (normalized N/kg) | 3 | 0.486 ± 0.142 | 0.200 ± 0.094 | < 0.01 | Kornegay et al. 1999 |
| 6 | 0.825 ± 0.256 | 0.469 ± 0.183 | < 0.01 | ||
| 12 | 1.10 ± 0.27 | 0.550 ± 0.200 | < 0.01 | ||
| TTJ tetanic extension (normalized N/kg) | 3 | 2.55 ± 0.28 | 1.32 ± 0.43 | < 0.01 | Kornegay et al. 1999 |
| 6 | 2.95 ± 0.53 | 0.965 ± 0.506 | < 0.01 | ||
| 12 | 2.98 ± 0.28 | 1.34 ± 0.58 | < 0.01 | ||
| Speed (m/sec) | 2 | 1.77 ± 0.29 | 1.02 ± 0.24 | 0.001 | Barthélémy et al. 2011 |
| 9 | 2.61 ± 0.18 | 0.88 ± 0.46 | <0.0001 | ||
| Stride length/height at withers | 2 | 1.97 ± 0.26 | 1.35 ± 0.27 | 0.0001 | Barthélémy et al. 2011 |
| 9 | 1.99 ± 0.06 | 0.92 ± 0.31 | <0.0001 | ||
| LVID (d) (cm)a | 3 | 3.0 ± 0.0 | 2.5 ± 0.2 | < 0.01 | Fine et al. 2011 |
| 6 | 3.6 ± 0.1 | 2.9 ± 0.1 | < 0.01 | ||
| 12 | 4.1 ± 0.2 | 3.2 ± 0.1 | < 0.01 | ||
| Fraction shortening (%)a | 3 | 36.9 ± 2.5 | 35.0 ± 1.2 | NS | Fine et al. 2011 |
| 6 | 33.7 ± 0.5 | 39.3 ± 2.6 | NS | ||
| 12 | 32.3 ± 1.7 | 38.8 ± 6.5 | NS | ||
| EKG lead 2 Q/R ratioa |
3 | 0.2 ± 0.0 | 0.4 ± 0.1 | < 0.01 | Fine et al. 2011 |
| 6 | 0.3 ± 0.0 | 0.7 ± 0.0 | < 0.01 | ||
| 12 | 0.3 ± 0.0 | 0.6 ± 0.1 | < 0.01 | ||
| Serum CKb | 2 days | 700 (200–800)c | 18,900 (2,300–39,500) | ND | Valentine et al. 1988 |
| 6 wk | 300 (200–500) | 8,200 (6,500–162,100) | ND | ||
| 6 | 400 (400–400) | 32,400 (30,300–42,100) | ND |
Listed tests were assessed longitudinally and are at least somewhat analogous to those of Mdx mice in Table 1.
Abbreviations: CK, creatine kinase; LVID (d), left ventricular diameter at diastole; ND, not determined; N/kg, Newtons/kg; TTJ, tibiotarsal joint; NS, not significant.
aCardiac data were from GRMD dogs crossbred with Labrador retrievers carrying a different DMD gene mutation.
bCK results for CXMD (GRMD) dogs were from male “small breed” dogs.
cMedian and range for CK values.
Loss of ambulation is the defining clinical feature of DMD and could logically be used to characterize disease phenotype in GRMD. However, although occasional severely affected dogs lose the ability to walk, most remain ambulatory. We have used tetanic torque/force generated by TTJ flexion/extension as a key outcome parameter in GRMD dogs (Kornegay et al. 1999). Our original natural history study suggested that measurement of flexion torque would be most useful to document therapeutic benefit in GRMD dogs. Groups of 15 and 5 were necessary to demonstrate differences of 0.2 and 0.4 in the means of treated and untreated GRMD dogs at 6 months of age, with associated powers of 0.824 and 0.856, respectively. The use of flexion as an outcome parameter is complicated by a relative recovery of strength between 3 and 6 months. Extension values in this initial study varied more markedly, necessitating larger group sizes to establish significance. Since this original study, we have more carefully monitored inbreeding coefficient in selecting sire-dam pairs. As a result, the overall phenotype has been less severe and not as variable. In a subsequent trial of prednisone in GRMD dogs, we demonstrated therapeutic benefit of approximately 60% (p < 0.05) for extension force using a group size of six GRMD dogs (Figure 3). Interestingly, there was a paradoxical decrease in flexion force, which we attributed to reduced early necrosis that would otherwise lead to functional flexor hypertrophy. Consistent with this interpretation, there were reduced numbers of fetal myosin positive myofibers, indicating reduced regeneration. Another study of GRMD dogs treated with prednisone and cyclosporine found a similar decrease in flexion force (Barthélémy et al. 2012).
Figure 3.
Tetanic force in prednisone-treated GRMD dogs. Note the reverse pattern for extension and flexion. For extension (A), a trend for increased force at 1 mg/kg becomes significant at 2 mg/kg. Flexion is similar (B) but values are decreased. *p < 0.05 compared with normal. Data are from Liu et al. 2004.
As with DMD, results of GRMD functional tests track and correlate with each other (Kornegay et al. 2011, 2012a; Nghiem et al. 2013). Dating to our early phenotypic studies, we have been intrigued with the relationship between the TTJ angle and torque generated by extension and flexion of this joint. These values have also been placed in the context of CS muscle hypertrophy. Mildly affected dogs have proportionally larger TTJ angles and tetanic extension torques and smaller CS circumferences and tetanic flexion torques at 6 months of age. The opposite pattern is seen in severely affected dogs. Given the prominent role that the 6MWT now plays in DMD clinical trials, we have begun assessing this metric in GRMD dogs and correlating results with other functional outcome parameters. Providing precedent for applying the 6MWT to canine studies, abnormalities have already been shown in dogs with heart (Boddy et al. 2004) and respiratory (Swimmer and Rozanski 2011) disease. In our experiments, dogs are first conditioned/trained for several sessions beginning at approximately 8 wks of age and then evaluated at 2-week intervals. Our preliminary data indicate that affected dogs walk shorter distances than normal/carrier dogs.
The GRMD cardiomyopathy has a later clinical onset than the skeletal muscle phenotype, with, as an example, echocardiographic changes not being seen until at least 6 months of age and often considerably later (Kornegay et al. 2012a). Thus, ideally, for determining drug effects on the dystrophic heart, dogs should be older when entered into preclinical trials, or the cardiac phenotype should be exaggerated by stressing the heart, as with dobutamine (Maruo et al. 2007; McEntee et al. 1998). A membrane-sealing poloxamer was shown to be beneficial using group sizes of four each treated and untreated 13- to 18-month–old GRMD dogs that had been challenged with dobutamine (Townsend et al. 2010). The differential between poloxamer- and saline-treated dogs varied from approximately 50% to 100% for the physiologic indices tested in these dogs. Another study of the peptide, bradykinin, showed normalization of most cardiac indices using four and five untreated and treated 11-month–old GRMD dogs (Su et al. 2012) (see Muscle Fibrosis below).
Optimization of Animal Model Use for DMD Preclinical Trials
As discussed above and in greater detail below for treatments targeting DMD, preclinical studies in animal models often have failed to translate to humans. To optimize translation of findings from the mdx mouse and GRMD models to DMD patients, the following points should be considered:
Design powered studies with sample sizes sufficient to detect drug effects with the outcome paramaters used.
Follow randomization and blinding procedures, including who is blinded and when.
Provide details of the statistical methods used for data analysis and report all the results for each analysis.
Develop reliable and sensitive primary and secondary endpoints for the animal model used.
Independently validate drug efficacy results in another laboratory.
Validate drug efficacy in two species (e.g., mdx mice and GRMD dogs for DMD) whenever possible and especially with treatments that carry substantial risk.
DMD Treatment Development
Potential treatments for DMD may be broadly categorized as genetic, cellular, or pharmacologic (Foster et al. 2012; Konieczny et al. 2013; Malik et al. 2012; Ruegg 2013). In the context of pharmacologic approaches, some drugs promote stop codon read-through, correct frame-shift mutations, or upregulate surrogate proteins. Others target specific pathogenetic mechanisms that contribute to the dystrophic phenotype.
Genetic Therapies
Genetic and cell-based therapies, in principle, offer the possibility of cure. Genetic strategies have included adeno-associated virus (AAV)-mediated insertion of truncated dystrophin transgenes, antisense oligonucleotides to induce exon skipping and reestablish the dystrophin reading frame, agents to read-through stop codon mutations, and replacement of dystrophin at the sarcolemma with surrogates such as utrophin. Animal models have been important to establish potential efficacy and safety. In the case of AAV-mediated mini-/micro-dystrophin transgene therapy, foundational studies in the mdx mouse (Wang et al. 2000; Yuasa et al. 1998) were extended to the GRMD (CXMD) model (Kornegay et al. 2010; Wang et al. 2007; Yuasa et al. 2007). Canine experiments have demonstrated long-term dystrophin protein expression and highlighted the potential for immunologic rejection of viral capsid antigens and/or dystrophin acting as a neoantigen (reviewed in Kornegay et al. 2012a).
Antisense Oligonucleotide-Induced Exon Skipping and Chimeraplasty
Although viral-mediated gene replacement (augmentation) is a logical approach to therapy, other methods rely on innate systems intended to repair mRNA or remove introns to form the transcript. The basis for applying antisense therapies to DMD resides in the observation that BMD patients have in-frame mutations that allow production of truncated, partially functional dystrophin proteins (Bushby et al. 1993; Malhotra et al. 1988). Pioneering work by Louise Nicholson demonstrated that truncated isoforms of dystrophin, apparently produced through transcript alternative splicing, occur in DMD patients (Nicholson 1993a; Nicholson et al. 1993b). Alternatively spliced dystrophin isoforms have also been demonstrated in mdx mice (Chamberlain et al. 1993) and GRMD dogs (Schatzberg et al. 1998). As a corollary, so-called revertant fibers, which express dystrophin, occur spontaneously in DMD (Arechavala-Gomeza et al. 2010) and both animal models (Kornegay et al. 2003; Pigozzo et al. 2013).
Antisense oligonucleotides are designed to complement specific pre-mRNA sequences so that targeted exons are removed (spliced, skipped) at the level of the spliceosome, reestablishing the reading frame (Chen and Cheng 2012; Summerton and Weller 1997). Strategies were developed principally in the mdx mouse (Mann et al. 2001) and then applied to dystrophic dogs (Bish et al. 2012; Vulin et al. 2012; Yokota et al. 2009, 2012) before moving to DMD. Efficacy of different oligomer sequences and chemical backbones was first established in each species using cultured muscle cells (Arechavala-Gomeza et al. 2007; Mann et al. 2001; Vulin et al. 2012; Yokota et al. 2009). Trials in DMD patients have relied on two principal antisense oligomer chemistries, 2′O-methylribooligonucleoside-phoshophorothioate and phosphorodiamidate morpholino oligomers (morpholinos), marketed, respectively, by Glaxo-Smith-Kline/Prosensa (drisapersen) (Goemans et al. 2011) and Serepta (eteplirsen) (Cirak et al. 2012). Each oligomer targets exon 51, a notable DMD gene hot spot involved in approximately 13% of cases. Despite promising initial results in DMD patients, the drisapersen trial was recently stopped because of concerns about efficacy (Hoffman and Connor 2013). The eteplirsen trial continues. Methods to improve delivery of the antisense peptides and, therefore, achieve wider skeletal muscle and cardiac effects, as well as increase the duration of efficacy, are needed (Benedetti et al. 2013; Betts and Wood 2013; Partridge 2010).
An analogous method, termed chimeraplasty, employs chimeric oligonucleotides to induce normal host cell mismatch repair mechanisms to correct single nucleotide point mutations (Graham and Dickson 2002). Chimeric oligonucleotides injected intramuscularly into a single dystrophic dog achieved correction of the GRMD mutation and expression of a normal-sized dystrophin protein for 48 months (Bartlett et al. 2000). Chimeraplast-mediated exon skipping also was demonstrated in the mdx mouse (Bertoni et al. 2003). Despite these successes, inconsistencies in chimeraplasty studies for other diseases have dampened enthusiasm for this approach (de Semir and Aran 2006).
Stop-Codon Read-Through
An alternative genetic strategy for DMD employs drugs, such as gentamicin, that selectively promote translational read-through of premature stop codons (Hirawat et al. 2007; Nudelman et al. 2009). This therapeutic strategy applies to the approximately 15% of DMD patients who have nonsense mutations (Dent et al. 2005) resulting from single nucleotide DNA polymorphisms that give rise to in-frame UAA, UAG, or UGA codons in messenger RNA coding regions. These stop codons lead to premature termination of protein translation, with resultant truncated, nonfunctional proteins. Gentamicin was initially shown to increase dystrophin read-through in the mdx mouse, first using cultured myotubes and then moving to in vivo studies, where treated mice expressed dystrophin in muscle and had functional improvement (Barton-Davis et al. 1999). Subsequent gentamicin treatment of DMD patients harboring stop codons provided mixed results, with one study failing to show full-length dystrophin protein or functional improvement (Wagner et al. 2001) and another demonstrating dystrophin protein expression (Politano et al. 2003).
Because of concerns regarding potential toxicity of higher dose or longer duration gentamicin regimens, a high-throughput, screening tool was developed to identify chemical compounds with equal efficacy and high safety profiles. This process led to the discovery of PTC124, subsequently called ataluren, which is chemically distinct from aminoglycosides. Ataluren suppressed DMD gene nonsense mutations in mdx muscle cell culture and led to dystrophin expression and functional improvement in mice treated systemically (Welch et al. 2007). These encouraging results prompted DMD trials with ataluren, starting with Phase 1 studies in healthy volunteers that showed no toxicity and extending to Phase 2 trials in DMD patients. The DMD trials have generated inconclusive results. Whereas the initial Phase 2a study demonstrated dystrophin expression in about one-third of the treated patients, the larger 2b study showed only marginal 6MWT benefit and did not include dystrophin protein expression data (Hoffman and Connor 2013; Peltz et al. 2013).
Dystrophin Surrogates (Utrophin)
A number of proteins colocalize with dystrophin at the sarcolemma and could serve as surrogates (Nghiem et al. 2013). As examples, upregulation of GalNAc transferase (Martin et al. 2009) and sarcospan (Peter et al. 2008) ameliorated the mdx phenotype. Particular interest has focused on utrophin (dystrophin-related protein), the autosomal isoform of dystrophin (Fairclough et al. 2011; Miura and Jasmin, 2006), with a number of factors suggesting that it could have therapeutic benefit in DMD. Normally expressed chiefly at the neuromuscular synapse and myotendinous junction (Khurana et al. 1991; Nguyen et al. 1991), utrophin moves extrajunctionally towards the muscle belly in the absence of dystrophin (Karpati et al. 1993; Nguyen et al. 1991) and is upregulated in DMD (Karpati et al. 1993; Mizuno et al. 1993), the mdx mouse (Law et al. 1994), and the GRMD (Nghiem et al. 2013; Wilson et al. 1994) and German shorthaired pointer (Schatzberg et al. 1999) dog models. Mdx mice, in which utrophin is knocked out (dko mice), have a more severe phenotype (Deconinck et al. 1997). Upregulation of utrophin, either genetically (Tinsley et al. 1996) or through treatment with the small molecule SMTC1100 (Tinsley et al. 2011), improves the mdx phenotype. High-throughput, cell-based screens have been used to identify drugs that upregulate utrophin (Moorwood et al. 2013). Treatment of mdx mice with recombinant human biglycan, an extracellular matrix protein associated with the dystrophin-glycoprotein complex, recruits utrophin to the sarcolemma and reduces histopathologic lesions and the degree of eccentric contraction decrement (Amenta et al. 2011). Adenovirus-mediated utrophin therapy reduced histopathologic lesions in GRMD dogs (Cerletti et al. 2003). However, other canine studies have not validated a role for utrophin in improving phenotype. Utrophin was not differentially expressed in mildly and severely affected GRMD dogs (Zucconi et al. 2010) or in the less-severely affected German shorthaired pointer model (Schatzberg et al. 1999). Although utrophin was increased in the spared/hypertrophied GRMD CS muscle, levels did not correlate with the degree of muscle hypertrophy (Nghiem et al. 2013).
Cell Therapies
As with genetic approaches, cell based-therapies provide an opportunity to replace dystrophin, achieving a relative cure. In light of the loss of muscle mass with chronic disease, cell replacement also offers the potential to reverse muscle atrophy (Meregalli et al. 2013). Studies of myoblasts and their parent satellite cells (Briggs and Morgan 2013) have been complemented by experiments utilizing stem cell populations in both the mdx (Benedetti et al. 2013) and GRMD (Rouger et al. 2011; Sampaolesi et al. 2006) models, with some approaches moving to human trials (Benedetti et al. 2013).
Drug Target Identification
There are numerous hurdles with gene and cell-based therapies. As examples, viral vector constructs or cell populations may be rejected immunologically, and older affected individuals have chronic, essentially irreversible muscle loss. Therefore, although these approaches will remain a major focus of research, additional emphasis has been placed on development of therapeutic strategies that target downstream disease mechanisms. In the early days of DMD treatment development, there were three main hypotheses for disease pathogenesis. Over and above the membrane theory that was ultimately substantiated, considerable support existed for neurogenic and vascular mechanisms (Rowland 1976). Interest in these other two theories was driven, in large part, by the nature of histopathologic changes, namely myofiber atrophy and central nuclei in keeping with denervation (McComas et al. 1970, 1988) and small group myofiber necrosis typical of muscle ischemia (Mendell et al. 1971). From these early days and extending to the present, pharmacologic targets principally have been identified based on the nature of pathologic lesions (Figure 4) and underlying pathogenetic mechanisms. Here, we discuss treatment strategies in the context of the progression of DMD lesions, extending from acute necrosis to fibrosis with atrophy.
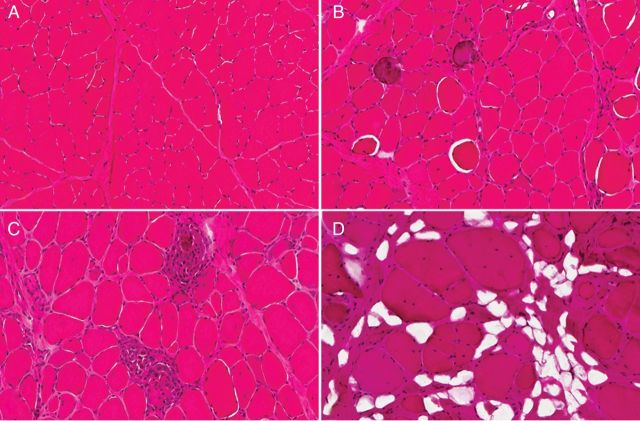
Figure 4.
The histologic appearance of normal canine muscle (A) is contrasted with characteristic progressive histopathologic lesions of GRMD (B–D). (B) Several myofibers are swollen/hypercontracted (hyalin necrosis), and two myofibers are mineralized (calcified). The endomysial space is relatively normal. (C) Two small groups of myofibers have undergone necrosis and there is an associated inflammatory cell infiltrate. The endomysial space is mildly expanded. (D) The endomysial space is markedly expanded due to both fibrosis and fatty deposition. Individual myofibers are enlarged and many have central nuclei. H&E stain and 20 × original magnification for all.
Myofiber Necrosis
Membrane lesions in DMD are generally thought to allow leakage of calcium, with activation of proteases like calpain, leading to necrosis (accidental cell death) (Miller and Girgenrath 2006). Apoptosis (programmed cell death) mediated through the ubiquitin proteasome system (UPS) also may play a role in DMD pathogenesis (Tidball et al. 1995). Accordingly, pathways associated with necrosis and apoptosis are potential drug targets in DMD (De Paepe and De Bleecker 2013) (see discussion of calpain and UPS inhibitors as potential therapies under Muscle Atrophy below). Indeed, dystrophin surrogates and poloxamer-based therapies, which have been efficacious in both the mdx mouse (Ng et al. 2008; Spurney et al. 2011c) and GRMD dog (Townsend et al. 2010), are intended to repair membrane injury.
The membrane theory for DMD pathogenesis derived, in part, from the overlapping nature of histopathologic changes like hyaline fiber necrosis (Figure 4B) seen in both DMD and nutritional myopathies resulting from reduced levels of the antioxidants vitamin E and selenium (Clark 1984; Kakulas 1975; Serafin et al. 1987). However, muscle lesions are not typically seen in vitamin E-deficient humans (Binder et al. 1965), and antioxidant treatment has not been beneficial in DMD patients (Bäckman et al. 1988; Gamstorb et al. 1986). Moreover, vitamin E supplementation did not substantially improve the mdx mouse phenotype (Hübner et al. 1996). With this said, there is continued interest in the role of oxidants in DMD pathogenesis, and oxidative stress remains a target for therapeutic intervention (Arthur et al. 2008; Lawler 2011). Pentoxifylline (Burdi et al. 2009), N-acetylcysteine (Whitehead et al. 2008), green tea extract (Nakae et al. 2012; Evans et al. 2010), and idebenone (Buyse et al. 2009) are examples of compounds with antioxidant properties shown to benefit mdx mice (see further the role that some of these compounds have in inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway under Muscle Inflammation below). In a subsequent clinical trial of idebenone, DMD patients had improved indices of respiratory (expiratory) function and a trend toward increased cardiac contractility (Buyse et al. 2011). Angiotensin-converting-enzyme (ACE) inhibitors have several potentially beneficial effects in muscle, with one being reduction of oxygen radicals (Cozzoli et al. 2011), and are a current standard of care for DMD cardiomyopathy (see further under Muscle Fibrosis below).
Mitochondrial dysfunction and associated failed energy metabolism have been implicated in DMD pathogenesis, acting in concert with reactive oxygen species and altered calcium homeostasis (Godin et al. 2012). The mitochondrial permeability transition pore (PTP), whose induction can lead to mitochondrial swelling and cell death by apoptosis, has become a key therapeutic target (Rasola and Bernardi 2007). In particular, treatments have targeted cyclophilin D, which regulates PTP opening in response to calcium and reactive oxygen species (Giorgio et al. 2010). Treatment of mdx mice with the cyclophilin inhibitor, Debio-025, reduced mitochondrial swelling and light microscopic histopathologic lesions (Millay et al. 2008). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) is another target because of its role in activities beneficial to mitochondrial function, including reduced PTP opening, increased mitochondrial mass, and improved calcium handling (Godin et al. 2012), as well as upregulation of utrophin (Angus et al. 2005). Injection of an AAV6-PGC1α construct in mdx mice led to upregulation of utrophin and improved functional and histopathological indices (Hollinger et al. 2013b). Treatment of mdx mice with two agents, AICAR and GW50156, that increase expression or are activated by PGC1α, also improved mitochondrial function and phenotype (Jahnke et al. 2012).
An additional therapeutic approach to reduce muscle necrosis harkens back to the vascular theory of DMD pathogenesis and relates to the loss of neuronal nitric oxide synthase (nNOS) due to the absence of dystrophin and instability of its associated membrane complex. Without nNOS, muscle blood flow is reduced (Chang et al. 1996; Thomas et al. 1998), likely contributing to the characteristic grouped myofiber necrosis of both ischemia and DMD. Pharmacological enhancement of nitric oxide–cyclic guanosine monophosphate signaling pathways with phosphodiesterase 5 inhibitors, as with sildenafil, enhanced nNOS activity and improved the mdx phenotype (Percival et al. 2012). Despite these promising results, a clinical trial in which sildenafil was assessed for its potential benefits in DMD and BMD cardiomyopathy has suspended patient recruitment (http://clinicaltrials.gov/ct2/show/NCT01168908?term=sildenafil&rank=32).
Myofiber Calcification
Numerous studies have focused on the role of calcium homeostasis in DMD pathogenesis. This is a logical extension of studies of mitochondrial function and oxygen reactive species given the interrelated role that these factors have in cell function. Although calcium has a nonspecific role in numerous cell injury pathways, histopathologic studies have shown that levels are increased in muscle biopsies from DMD patients more so than other myopathies (Bodensteiner and Engel 1978). Muscle injury in the mdx mouse is associated with increased entry of calcium through channels activated by reactive oxygen species (Allen et al. 2010), providing potential targets for therapy. An analogous increase in calcium is seen pathologically in GRMD muscle (Figure 4B) (Valentine et al. 1989). Creatine supplementation of mdx muscle cell cultures improved calcium handling and promoted formation and survival of myotubes (Pulido et al. 1998). Benefits of the tricyclic antidepressants, imipramine and amitriptyline, in mdx mice were explained, in part, by effects on calcium homeostasis in skeletal muscle (Carre-Pierrat et al. 2011). On the other hand, mdx mice treated with dantrolene, which inhibits the release of calcium from the sarcoplasmic reticulum, did not show benefit (Quinn et al. 2013). Perhaps most importantly, a Cochrane review of DMD clinical trials found calcium antagonists had no useful effect (Phillips and Quinlivan 2008).
Muscle Inflammation
Myofiber necrosis in DMD (McDouall et al. 1990), the mdx mouse (Carnwath et al. 1987), and GRMD dog (Figure 4C) (Kornegay et al. 1988; Valentine et al. 1990) elicits a mixed inflammatory cell response, with macrophages and lymphocytes predominating. Macrophages were originally thought to play principally a phagocytic role. They are now seen as having a dual purpose, with M1 macrophages occurring acutely to phagocytize cell debris, followed closely by M2 macrophages to drive myofiber regeneration (Kharraz et al. 2013; Tidball and Villalta 2010). A complex set of cytokines (tumor necrosis factor, interkeukins, transforming growth factor beta [TGFβ], and osteopontin [OPN]) (De Paepe and De Bleecker 2013; Evans et al. 2009) and transcription factors (NF-κB, E2F1) (Blanchet et al. 2012; Chen et al. 2005; Peterson et al. 2011) are involved in DMD pathogenesis.
The cytokine OPN is particularly intriguing in that mRNA levels are increased in DMD (Chen et al. 2000) and mdx mouse (Porter et al. 2003) muscle (also see mRNA arrays discussion below). OPN is secreted by inflammatory cells and myoblasts and serves multiple, at times seemingly contradictory, functions in diseased muscle. Expression has been linked to increased fibrosis in the mdx mouse (Vetrone et al. 2009) and enhanced muscle regeneration in a murine injury model (Uaesoontrachoon et al. 2013). Moreover, the same OPN promoter polymorphism is associated with greater disease severity in DMD (Bello et al. 2012; Pegoraro et al. 2011) and enhanced muscle mass in normal human females (Hoffman et al. 2013). Working with the Hoffman laboratory, we have demonstrated that OPN is also dramatically increased in muscle from GRMD dogs (Figure 5), with levels negatively correlating with CS muscle size (PP Nghiem, JN Kornegay, K Uaesoontrachoon, L Bello, LW Fisher, Y Yin, Z Wang, A Kesari, P Mittal, SJ Schatzberg, NH Lee, EP Hoffman, unpublished data). Multiple factors, including stage of disease and isoform types, probably contribute to these differing effects of OPN.
Figure 5.
Heat map depicting supervised hierarchical clustering in GRMD dogs. A total of 485 genes correlated with OPN expression in 72 mRNA expression profiles from normal and GRMD CS, long digital extensor (LDE), and vastus lateralis (VL) muscles (Nghiem et al. 2013) at 4 to 9 weeks and 6 months. All mRNA profiles were correlated with OPN mRNA expression, and the top correlated genes (r ≥ 0.9; p ≤ 0.001; 485 genes) are depicted here. Note that the OPN-correlated genes (1) have minimal to no expression in normal muscle profiles, (2) increase in expression with age in GRMD profiles, (3) increase in expression in the more affected GRMD muscles at the respective time point, and (4) are variably expressed even within muscles.
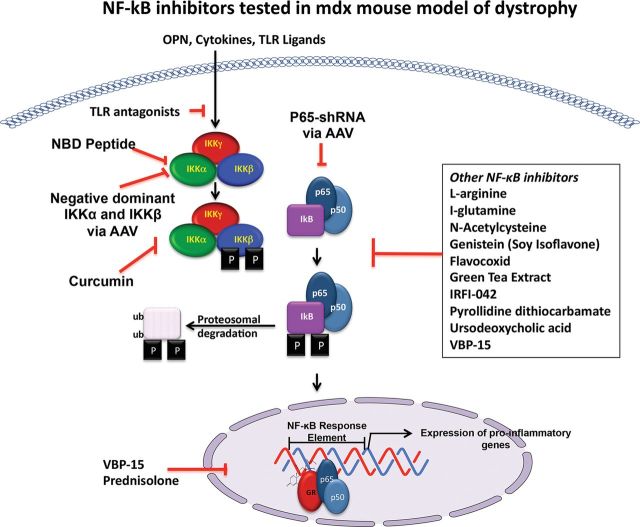
With regard to transcription factors, research has focused on the NF-κB pathway, which exacerbates muscle lesions and dysfunction in DMD and the mdx mouse (Acharyya et al. 2007; Monici et al. 2003). Treatments targeting NF-κB are of particular interest in DMD, because prednisone blocks this pathway (Auphan et al. 1995). A number of compounds that inhibit the NF-ĸB pathway have been shown to benefit the mdx mouse (Figure 6) (Table 3). As an example, inhibiting NF-κB signaling with Nemo Binding Domain (NBD) peptide alleviates dystrophic histopathologic lesions and improves muscle function in DMD mouse models (Delfin et al. 2011; Peterson et al. 2011). We have collaborated with the Guttridge laboratory at The Ohio State University on a study of NBD in GRMD dogs, employing a previously performed treatment protocol and biomarker analysis (Liu et al. 2004). Consistent with observations in mice, NBD treatment in GRMD dogs exhibited an efficacious response, providing further support for its potential use as a DMD therapeutic (JN Kornegay, DC Guttridge, unpublished data). Importantly, studies have linked NF-κB and OPN in a number of disease processes in which inflammation and fibrosis occur. For instance, OPN mediates activation of the NF-κB pathway in hepatic fibrosis (Urtasun et al. 2012) and relapsing episodes of multiple sclerosis (Steinman 2009). On the other hand, NF-κB induction of OPN may contribute to fibrosis in myocardial infarction (Zhang et al. 2010). Both molecules were increased, and levels correlated positively, in synovial fluid of osteoarthritis patients (Qin et al. 2013). These findings suggest that NF-κB and OPN may be interrelated through a feedback loop (Zhang et al. 2010). Accordingly, treatments inhibiting the NF-κB pathway (Figure 6, Table 3) likely exert their effect, in part, through reduced OPN expression. Other downstream OPN effectors would also be potential therapeutic targets (Jain et al. 2007). The statin drug, simvastatin, reduced expression of NF-κB, OPN, and collagen I in a rat model of myocardial infarction and could have therapeutic value in DMD (also see discussion of Muscle Fibrosis below). More specific OPN targeting using humanized antibodies directed against OPN isoforms involved in particular diseases also has shown promise (Dai et al. 2010; Fan et al. 2008, 2011). Further definition of OPN isoforms involved in DMD and subsequent antibody targeting warrants further investigation.
Figure 6.
NF-κB inhibitors tested in mdx mice. Inhibition of the NF-κB pathway, utilizing mechanisms illustrated here, has shown phenotypic benefit in the mdx mouse (also see Table 3).
Table 3.
NF-κB therapeutic approaches tested in the mdx mouse
| Intervention | Pathology | Function | Reference |
|---|---|---|---|
| AAV-p65-shRNA | Yes | No | Yang et al. 2012 |
| Nemo Binding Domain Peptide | Yes | Yes | Delfin et al. 2011; Peterson et al. 2011 |
| Nemo Binding Domain Peptide | Yes | Yes | Reay et al. 2011 |
| AAV-IKKa-dn or -IKKbdn | Yes | No | Tang et al. 2010 |
| Pyrollidine Dithiocarbamate | Yes | No | Carlson et al. 2005; Graham et al. 2010; Messina et al. 2006a |
| Ursodeoxycholic Acid (UDCA) | Yes | Yes | Siegel et al. 2009, 2011 |
| Curcumin | Yes | Yes | Pan et al. 2008 |
| Curcumin | No | Yes | Durham et al. 2006 |
| Flavocoxid | Yes | Yes | Messina et al. 2009 |
| IRFI-042 | Yes | Yes | Messina et al. 2006b |
| Genistein | Yes | Yes | Messina et al. 2011 |
| Green Tea Extract | Yes | No | Evans et al. 2010 |
| L-Arginine | Yes | No | Hnia et al. 2008 |
| L-Glutamine | Yes | No | Mok et al. 2008 |
| N-Acetylcysteine | Yes | No | Whitehead et al. 2008 |
| VBP-15 | Yes | Yes | Heier et al. 2013 |
| TLR-7/8 Antagonist | Yes | Yes | Henriques-Pons et al. 2014 |
Aside from macrophages, other inflammatory and immune cells play important roles in the pathogenesis of DMD and the mdx and GRMD models (Evans et al. 2009; Iannitti et al. 2010; Tidball and Villalta 2010). Mast cells are associated with areas of myofiber necrosis in all three conditions, suggesting that released proteases could contribute to cell injury (Gorospe et al. 1994). DMD patients and GRMD dogs had more persistent degranulation, perhaps allowing prolonged release of mediators (heparan sulfate, tryptase) that would enhance fibrosis. Cromolyn administration to block mast cell degranulation reduced myofiber necrosis in mdx mice (Radley and Grounds 2006). Populations of CD4 and CD8-positive lymphocytes have also been seen in DMD (Engel and Arahata 1986), mdx mice (Spencer et al. 2001), and GRMD dogs (Barthélémy et al. 2012), further highlighting involvement of the immune system in disease pathogenesis. Of particular note, dystrophin, serving as a neoantigen in revertant fibers, may induce a lymphocytic response in DMD (Flanigan et al. 2013). Depleting immune cell populations and general immunosuppressant regimens have improved the mdx phenotype (Evans et al. 2009; Iannitti et al. 2010; Vetrone et al. 2009). Recent studies by Nagaraju's laboratory demonstrated that proinflammatory innate immune receptors such as toll-like receptors (TLRs) on muscle and immune cells play an important role in dystrophic lesions in mdx mice (Henriques-Pons et al. 2014). Knockout of the central TLR adaptor protein, myd88, in mdx mice improved skeletal and cardiac muscle function. Likewise, preclinical trials in young mdx mice with a TLR7/9 antagonist significantly reduced skeletal muscle inflammation and increased muscle force, demonstrating that inhibiting this pathway may have therapeutic potential for DMD.
Given the role of inflammation in disease pathogenesis, it is tempting to speculate that prednisone's benefit in DMD and somewhat mixed effects in the mdx mouse (Guerron et al. 2010; Sali et al. 2012) and GRMD dog (Barthélémy et al. 2012; Liu et al. 2004) occur, at least partially, from reduced inflammation. In support of this, mdx mice treated with prednisone had reduced inflammatory cell numbers and adhesion while showing reduced sarcolemma damage and myofiber degeneration-regeneration (Wehling-Henricks et al. 2004). Dystrophic dogs given prednisone alone or with cyclosporine generally show an improved phenotype but have increased numbers of calcified myofibers, potentially due to a reduction of macrophage activity (Barthélémy et al. 2012; Liu et al. 2004). Interestingly, DMD patients treated with another antiinflammatory agent, azathioprine, did not have a comparable beneficial effect despite suppression of the same inflammatory cell populations, suggesting mechanisms other than immunosuppression are involved (Griggs et al. 1993; Kissel et al. 1993). Similarly, numbers of CD4 and CD8-positive cells in GRMD dogs treated with the prednisone-cyclosporine regimen were comparable with those in untreated GRMD controls (Barthélémy et al. 2012). Although prednisone has a well-established catabolic effect on protein, treated DMD patients have a net increase in muscle mass, thought to occur through decreased muscle proteolysis versus increased protein synthesis (Rifai et al. 1995). But, prednisolone also promotes myogenesis, with associated utrophin upregulation, in mdx myoblast cultures (Passaquin et al. 1993). Other compounds, like VBP15 (Heier et al. 2013) and a proprietrary drug (Compound A) (Huynh et al. 2013), which have been extensively studied in the Hoffman and Nagaraju laboratories, benefit the mdx mouse through inhibition of the NF-ĸB pathway (Figure 6) (Table 3), without side effects of glucocorticoid receptor stimulation. Importantly, OPN levels were decreased in mdx mice treated with Compound A but increased in those receiving prednisone (Huynh et al. 2013).
Muscle Fibrosis
Fibrosis is readily seen histologically in DMD (Pearce and Walton 1962) and the mdx (Stedman et al. 1991) and GRMD (Figure 4D) (Kornegay et al. 1988; Valentine et al. 1989) models. Increased endomysial connective tissue occurs early in DMD and correlates with clinical disease progression (Desguerre et al. 2009). Mediators of fibrosis, therefore, are logical therapeutic targets (Klingler et al. 2012). Attention has centered on TGFβ, which is involved in fibrosis in DMD (Bernasconi et al. 1995), the mdx mouse (Gosselin et al. 2004), and GRMD dog (Passerini et al. 2002). As discussed above under Muscle Inflammation, NF-ĸB induction of OPN also may contribute to fibrosis in myocardial infarction (Zhang et al. 2010), providing an additional potential target for therapy. Angiotensin activates or increases expression of TGFβ (Morales et al. 2012), NF-κB (Muller et al. 2000), and OPN (Remus et al. 2013). Accordingly, ACE inhibitors can provide benefit at several levels and are recommended for DMD patients at the onset of cardiac clinical dysfunction (Spurney 2011d). Studies in mdx mice have suggested that ACE inhibitors may also benefit skeletal muscle and that treatment during the preclinical stage of DMD could be indicated (Cozzoli et al. 2011). Early treatment of mdx mice with the ACE-inhibitor, lisinopril, together with the aldosterone inhibitor, spironolactone, reduced fibrosis and improved function of both cardiac and skeletal muscle (Rafael-Fortney et al. 2011). In other studies, treatment of mdx mice with the angiotensin II receptor antagonist, losartan, reduced fibrosis in the heart and skeletal muscles (Bish et al. 2011a; Cohn et al. 2007; Spurney et al. 2011b). Although the cardiac phenotype was improved, functional benefit in skeletal muscle varied. Treatment of GRMD dogs with the vasodilator peptide, bradykinin, which is degraded by ACE and increased by ACE inhibitors, improved a number of cardiac functional parameters (Su et al. 2012). TGFβ-inhibitors, acting through different mechanisms, have shown variable effects in the mdx mouse, with some demonstrating functional and/or pathologic benefit (Cohn et al. 2007; Nelson et al. 2011; Taniguti et al. 2011) and others showing only minimal effects (Gosselin et al. 2006, 2007). Thus, ACE inhibitors and angiotensin receptor blockers have a clear indication in reducing cardiac fibrosis and potential analogous benefit in skeletal muscle.
Muscle Atrophy
All of the lesions/mechanisms discussed above, extending from necrosis to fibrosis, contribute to muscle wasting and the ultimate atrophy (sarcopenia) that characterizes DMD (Shin et al. 2013). Analogous changes occur in the mdx mouse and GRMD models. However, the dramatic muscle wasting seen in DMD does not occur until late in mdx mice, when muscles are approximately one-half normal size (Lefaucheur et al. 1995). In contrast, atrophy occurs earlier in GRMD (Kornegay et al. 2003; Valentine et al. 1990). Neither mdx mice nor GRMD dogs show the same degree of fatty deposition seen in DMD. Reasons for these species differences are not clear. Anderson et al. (1993) found higher expression of basic fibroblast growth factor in the mdx mouse versus DMD patients and GRMD dogs. Given that basic fibroblast growth factor promotes proliferation of muscle precursor cells (Abdel-Salam et al. 2009), they speculated this could explain the greater regenerative response in mdx mice.
Muscle regeneration is dependent on differentiation of resident satellite cells to myoblasts, which fuse to form myotubes and, ultimately, new myofibers (Briggs and Morgan 2013). Cellular proliferation is facilitated by telomeres, regions near the ends of chromosomes that deter degradation of genes during chromosome replication (Bojesen 2013). The enzyme telomerase is necessary for addition of DNA sequence repeats to the telomere (Holysz et al. 2013). Muscle atrophy in DMD is associated with satellite cell senescence (Heslop et al. 2000; Jejurikar and Kuzon 2003; Webster and Blau 1990) and telomere shortening (Decary et al. 2000). Species variation in normal telomere length and telomerase activity could account for the more severe phenotype of DMD and GRMD versus the mdx mouse. Cells of humans and dogs have relatively short telomeres, in the 5- to 15-kb range, and low telomerase activity (Argyle and Nasir 2003; Cross et al. 1989). Mice have considerably longer telomeres, in the 20- to 70-kb range and as long as 140 kb (Kipling and Cooke 1990), and proportionally higher telomerase activity (Prowse and Greider 1995). Lending support for this mechanism of phenotypic variation is the fact that knocking out the mdx mouse telomerase gene results in more severe signs (Sacco et al. 2010).
Muscle atrophy in DMD could result from either increased protein degradation or reduced production (Griggs and Rennie 1983). An early study completed before dystrophin was identified, and drawing somewhat on protein data from nongenetic animal models available at the time, concluded that DMD resulted from reduced production versus degradation (Rennie et al. 1982) (also see effects of prednisone under Muscle Inflammation above). Of course, with DMD gene mutations, there is inherent reduced production of stable dystrophin protein. Genetic therapies are directed at restoring dystrophin and, in turn, additional members of the dystrophin-glycoprotein complex. Other approaches are less selective and involve the use of anabolic agents and hormones. Much attention has focused on the anabolic hormone, insulin-like growth factor, which promotes muscle hypertrophy by activating the phosphatidylinositol 3-kinase/Akt pathway and, in turn, mTOR and further downstream targets. Insulin-like growth factor was among a group of compounds that improved the mdx phenotype in one preclinical screen using whole-body strength as an outcome parameter (Granchelli et al. 2000) and increased force generation in miniature bioartificial mdx muscles in an ex vivo system (Vandenburgh et al. 2009). On the other hand, mdx mice made transgenic for insulin-like growth factor developed muscle hypertrophy only during periods of muscle regeneration subsequent to bouts of necrosis (Shavlakadze et al. 2010). An additional study showed benefit in mdx mice treated early but not those with more chronic involvement or in dko mice (Gehrig et al. 2012). As discussed above under Muscle Inflammation, prednisone has a net anabolic effect on muscle. A novel androgen receptor modulator, GLPG0492, improved both functional and pathologic biomarkers in mdx mice (Cozzoli et al. 2013).
Inhibition of the UPS and calpain systems, which contribute to muscle degradation in DMD, has been proposed as a treatment based largely on evidence that activities of involved enzymes are elevated in the mdx mouse and that blockade can improve their phenotype (Badalamente and Stracher 2000; Bonuccelli et al. 2007; Spencer et al. 1995; Spencer and Mellgren 2002). In support of this hypothesis, systems involved in protein degradation are misregulated in the muscular dystrophies (De Palma et al. 2012; Sandri et al. 2013). Moreover, a defect in autophagy in mdx mice, associated with abnormal Akt and mTOR signaling, was reversed by a low-protein diet, with reduced histopathologic changes and improved function (De Palma et al. 2012). Additionally, proteasome inhibition restored members of the dystrophin-glycoprotein complex in DMD muscle explants and reduced the severity of histopathologic lesions in mdx mice (Gazzerro et al. 2010). But, other mdx mouse studies failed to show efficacy for either calpain inhibition (Selsby et al. 2010) or overexpression of an antiapoptosis protein (Dominov et al. 2005). Similarly, we were unable to demonstrate benefit of a novel calpain inhibitor in GRMD dogs (Childers et al. 2012). In a separate collaboration with the Willis laboratory at the University of North Carolina-Chapel Hill, mRNA expression of UPS and calpain system components and calpain 1 and 2 and proteasome activities were measured in several GRMD muscles and the heart at 6 months of age (Wadosky et al. 2011). A majority of muscles had decreased levels of proteasome activity, and only one-half had increased calpain activity. More alarmingly, numerous UPS components were decreased in the heart. Taken together, these results do not support pharmacologic inhibition of the UPS and calpain systems in DMD and suggest that there could be unintended deleterious consequences.
Another strategy for reversing muscle atrophy involves inhibition of the myostatin gene (growth/differentiation factor 8), which serves as a key negative regulator of muscle growth (Lee 2004; McPherron et al. 1997). Mdx mice in which myostatin is knocked out (Wagner et al. 2002) or postnatally inhibited (Wagner et al. 2005) have a less severe phenotype. In collaboration with the Xiao Xiao laboratory at the University of North Carolina-Chapel Hill, we showed that regional limb AAV8-mediated overexpression of the inhibitory myostatin propeptide enhanced muscle growth in normal dogs (Qiao et al. 2009). A similar systemic approach generated analogous results in GRMD dogs evaluated by the Sweeney laboratory at the University of Pennsylvania (Bish et al. 2011b). In an effort to validate the mdx mouse-myostatin knockout studies, we crossbred dystrophin-deficient GRMD dogs with whippets carrying a spontaneous two-nucleotide myostatin gene deletion (Mosher et al. 2007). Surprisingly, rather than showing improvement, the dystrophic myostatin-heterozygous GRippet dogs were more severely affected than their dystrophic myostatin-wild type littermates (JN Kornegay, DJ Bogan, JR Bogan, JL Dow, J Wang, Z Fan, LC Warsing, RW Grange, M Ahn, Z Zhu, MA Styner, KR Wagner, unpublished data). Disproportionate enlargement and atrophy/hypoplasia of certain muscles appeared to lead to postural instability and joint contractures in the GRippets.
An antibody intended to reduce myostatin's activity was assessed in adult muscular dystrophy patients, including some with BMD (Wagner et al. 2008). While no safely issues were seen, the trial was not sufficiently powered to show clinical efficacy.
Pharmacogenomic Drug Targeting
The present low rate of translation of pharmacologic therapies between animal models and their human counterparts highlights a strong need for better, more effective ways to extract meaningful information from cross-species studies. Part of the solution, undoubtedly, lies in increasing experimental rigor and transparency in animal experiments, as outlined above. A critical paradigm shift in designing and interpreting studies requires expanding the perception of animal models to “many-to-many” rather than “one-to-one” when investigating their correlations with the human diseases they model. It's also important to note that species differences often reflect individual differences within a species. Another piece of the puzzle likely will be based on moving from the pathologic lesion/pathogenetic mechanism approach that has traditionally been used to identify drug targets to one that is based more in specific gene/protein targets that have been clearly associated with disease phenotype (Hoffman et al. 2003). Towards this end, gene expression profiling studies have been published for DMD, the mdx mouse, and, more recently, GRMD dogs. Correlations across species can be interpreted when comparing profiles built using analogous tissues and conditions. In this way, gene expression profiles from different species can illustrate the similarities and differences in species-specific responses to pharmacologic agents of interest. These comparisons give insight into the relevancy of each animal model and its applicability for investigating specific aspects of human disease pathogenesis and treatment (Yu et al. 2012). Ultimately, these studies should provide a better understanding of disease pathogenesis and identify molecular therapeutic targets, with cross-species relevance, to modify or arrest disease progression (Barrie et al. 2012).
mRNA Arrays
Remarkable phenotypic variation occurs among dystrophin-deficient species that share the same genetic lesion. Similar differences occur among individuals and muscles within a species, raising questions about primary versus secondary effects of dystrophin deficiency (Hoffman and Gorospe 1991; Porter et al. 2003). Microarray gene expression profiling and follow-up protein studies in DMD and dystrophin-deficient animals have provided insight into how genes other than dystrophin modify the disease phenotype. Porter et al. (2003) showed that differential gene expression in the mdx mouse is closely associated with the degree of individual muscle involvement and longitudinal disease progression. Differential gene expression was far more pronounced in more severely affected limb muscles compared with the relatively spared extraocular muscles. Temporal gene expression measurements in limb muscle were associated with distinct stages of disease (e.g. development) and different gene ontologies. Gene expression differences between normal and affected mice early in the disease were associated with inflammation, proteolysis, and the extracellular matrix/fibrosis pathways. Furthermore, these differences tracked with the progression of histopathologic changes. Specific reference was made to a potential role for OPN in disease pathogenesis. A later paper compared gene expression between an mdx limb muscle and the diaphragm, which shows more progressive disease (Porter et al. 2004). Overall, gene expression patterns were similar, suggesting that general disease mechanisms are shared between both tissues. With this said, evidence of differential gene expression pointed to disease processes that are potentially tissue specific. As an example, the diaphragm showed more persistent expression of genes tied to muscle regeneration, in keeping with progressive disease. Thus, again, the microarray expression data tracked well with disease phenotype.
Tseng et al. (2002) compared mRNA expression in the mdx mouse at 16 weeks of age, when the disease is relatively quiescent, with previously published array results from 6- to 9-year-old DMD boys with ongoing disease (Chen et al. 2000). They found several genes that could account for the less-severe mdx phenotype. Most notably, mRNAs for actin-related proteins and mast cell chymase were upregulated in mdx mice versus DMD patients. Actin-related proteins might stabilize the dystrophic myofiber membrane, whereas mast cell chymase activates matrix metalloproteinases involved in collagen degradation. In addition, the expression levels of certain genes involved in mitochondrial function and energy metabolism were downregulated in DMD, potentially contributing to a so-called “metabolic crisis” in affected patients, but unchanged in mdx mice, relative to control samples. Finally, mRNA for myostatin was decreased 4-fold in mdx mice (myostatin was also downregulated in DMD, but data were not discussed).
In an extension of their earlier study, Chen et al. (2005) assessed gene expression in three DMD age groups: fetal, presymptomatic infant (8–10 months), and symptomatic 5- to 12-year olds. Genes involved in inflammation and muscle regeneration were upregulated in the fetal group, and this increased gene expression persisted in the older patients. TLR and NF-ĸB pathway genes were upregulated even in presymptomatic infants, whereas expression levels of TGFβ and major histocompatibility complex (MHC) genes were higher in the 5- to 12-year-old group. On the other hand, a cluster of genes associated with metabolism was downregulated, in keeping with the proposed “metabolic crisis” of DMD. Pathways potentially involved in muscle wasting/atrophy were also queried. Although the AKT1/atrogin ubiquitin ligase pathway has been implicated in a number of muscle-wasting disorders, it was not activated in any DMD age group. Similarly, while blocking myostatin to enhance muscle regeneration has been logically proposed as a therapy for DMD, myostatin levels were already downregulated in symptomatic patients, and the myostatin inhibitor, follistatin, was increased in all three age groups.
An additional DMD array study found differential expression, mostly upregulation, of genes involved in inflammation, muscle regeneration, and cell signaling (Haslett et al. 2002). These authors commented that gene expression patterns largely paralleled histopathological lesion progression, providing credence for the traditional approach used to identify drug targets. In particular, OPN was markedly upregulated and there were less pronounced increases in biglycan and TGFβ. Another paper from this group emphasized that genes involved in cell signaling, particularly survival cascades, were differentially expressed in DMD (Haslett et al. 2003), thus bringing attention to an important, but somewhat overlooked, role of the dystrophin glycoprotein complex.
A recent meta-analysis of DMD mRNA array data largely confirmed conclusions reached through earlier published studies (Baron et al. 2011). In particular, differentially expressed genes fell largely into two clusters, namely overexpressed genes coding for proteins involved in extracellular cell-matrix adhesion and inflammatory-immune response and underexpressed genes coding for mitochondrion proteins. The meta-analysis validated published observations that differentially expressed genes largely reflect infiltrating inflammatory cells and proliferating connective tissue and that the patterns of gene expression correspond to major pathological features of DMD.
Protein studies in GRMD dogs have identified abnormalities in energy metabolism (Guevel et al. 2011) and signaling (Feron et al. 2009) consistent with those defined by gene expression microarrays in DMD. Extending those studies, we have assessed mRNA microarrays in tandem with real time quantitative reverse transcription PCR (qRT-PCR) and protein expression (proteomic profiling, Western blotting, and immunohistochemistry) to define the molecular basis for phenotypic variation and, in particular, CS hypertrophy in GRMD dogs (Nghiem et al. 2013). Notably, we found that CS size was inversely correlated with myostatin expression in 6-month-old GRMD dogs. Utrophin mRNA was upregulated in the CS, but levels did not correlate with size. Using a quantitative trait analysis, we queried mRNAs that strongly correlated with CS hypertrophy at 6 months. A total of 250 transcripts were entered into Ingenuity Pathway Analysis (Redwood City, CA) software to determine molecular networks associated with CS hypertrophy. The top-ranked pathway included genes coding for two dystrophin-associated mRNAs, namely dystrophin-associated glycoprotein 1 (DAG1) and LARGE enzyme responsible for O-linked glycosylation of DAG1. DAG1 qRT-PCR expression values directly correlated with CS circumference at 6 months, validating the microarray data and suggesting that DAG1 may support CS hypertrophy. Spectrin was identified as an additional potential dystrophin surrogate via proteomic profiling. The growth factor myotrophin was also increased in the CS, implying that it might play a role in muscle hypertrophy. Taken together, these data indicated that multiple factors, including myostatin downregulation, upregulation of potential dystrophin surrogates DAG1 and spectrin, and increased expression of the growth factor myotrophin are involved in CS hypertrophy.
In another collaboration with colleagues at Vanderbilt University, we have studied mRNA arrays from skeletal and cardiac muscle at three different ages (6 and 12 months and 4–8 years) of separate groups of GRMD dogs. Preliminary data demonstrate a substantial downregulation of many skeletal muscle genes associated with metabolism in the younger dogs but not the other two groups. Thus, GRMD dogs seem to undergo a “metabolic crisis” akin to that of DMD patients and then recover to some extent (CL Galindo, CL Brinkmeyer-Langford, LW Markham, JH Soslow, JN Kornegay, DB Sawyer, unpublished data).
GWAS
GWAS are used to identify genetic markers (typically single nucleotide polymorphisms [SNPs]) that may be associated with complex traits (Edwards et al. 2013; Hou and Zhao, 2013). GWAS offer a powerful tool to identify common genetic variants that mark regions of the genome to correlate with phenotypic variation in DMD. Genes within these regions may play a role in the manifestation of the phenotype, making them potential targets for drug therapy. The GRMD model is well suited to complement DMD studies (Brinkmeyer-Langford and Kornegay 2013). Completion of a draft sequence of the canine genome has provided data for the design of SNP chips to support canine GWAS to define the genetic bases for phenotypic features in dogs, including, as an example, the wide variation in canine skull shapes (Schoenebeck and Ostrander 2013). We have utilized GWAS to identify SNPs associated with individual variation for phenotypic markers in age-matched normal and GRMD dogs. For sake of these studies, the focus has been on the association between TTJ flexion and extension torque values and genetic profiles of the involved cranial tibialis and medial head of the gastrocnemius muscles (Figure 7) (Brinkmeyer-Langford and Kornegay 2013). Several candidate genes marked by SNPs associated with phenotypic diversity are currently being validated.
Figure 7.

Data from GWAS and gene expression microarrays help to identify trends that provide important clues for drug development. Panels A and B illustrate that the same gene (“Gene A”) may have distinct effects on a particular biomarker (here, force) in different muscles (“Muscles A and B”). (A) Expression of Gene A positively correlates with force values in GRMD and normal (unaffected) dogs. (B) Expression of Gene A only minimally correlates with force values (if at all), though higher values are still clearly found in unaffected dogs. Therefore, Gene A expression levels are distinct in the two different muscles from which RNA was extracted. This suggests that Gene A could be considered as a therapeutic target in Muscle A but not Muscle B.
Gene expression profiling using microarrays (or RNA sequencing) works in tandem with GWAS to elucidate the genetic background underlying phenotypic variation in DMD and its animal models. The types of information generated by these two distinct, yet complementary, methods should be used concurrently to provide a multifocal, integrated pathway toward identifying drug targets. GWAS data show the extent to which a particular region of the genome (and the genes therein) are statistically associated with some phenotype of interest, thus providing context but not insight into the mechanism affected; gene expression data cannot directly give context to the differentially expressed genes but can show pathways that are variably affected and, thereby, provide insight into possible mechanistic effects. Together, both GWAS and gene expression microarray paint a holistic picture of the genetic factors influencing phenotypic variation.
The template offered by Plenge et al. (2013) in their recent review of drug target identification should be carefully considered when using GWAS and other genetic studies to identify potential drug targets. The review cited a number of human genes for which alleles (often a SNP) associated with disease phenotype. These were identified using various biochemical, genetic linkage, and GWAS methods. Plenge et al. (2013) stressed that certain criteria must ideally be fulfilled in identifying gene-drug pairs in drug discovery (Figure 8). Although not cited in the review, the protein OPN offers a contemporary example relevant to DMD. As discussed above under Muscle Inflammation, an OPN promoter polymorphism is associated with a more severe phenotype in DMD (Bello et al. 2012; Pegoraro et al. 2011) and enhanced muscle mass in normal human females (Hoffman et al. 2013). OPN was identified as a candidate gene by correlating DMD functional and gene expression data. Confidence in OPN's relevancy was provided by (1) an established high rate of spontaneous human polymorphisms (Giacopelli et al. 2004) and their association with disease (Chiocchetti et al. 2004), (2) the influence that the same polymorphism had on normal human muscle mass, and (3) the association of increased OPN expression with disease in the mdx mouse. Therapeutic strategies to target OPN, both nonspecifically as with NF-κB inhibition and, potentially, with humanized antibodies, could have benefit in DMD.
Figure 8.
Criteria proposed by Plenge et al. (2013) to apply genetic findings to target validation in human disease. These criteria generally fit with the role that OPN plays in DMD disease pathogenesis. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Drug Discovery. Validating therapeutic targets through human genetics, 12:581–594, © 2013.
Epigenetics
The numerous SNPs identified through GWAS account for only a small proportion of phenotypic variation in complex diseases (Butcher and Beck 2008). Nongenetic factors encompassed by the field of epigenetics play an additional major role in disease pathogenesis. Epigenetics has traditionally been defined as the study of “heritable changes in gene expression that are not due to changes in DNA sequence” (Eccleston et al. 2007). Bird offered a more contemporary definition of “the structural adaptations of chromosomal regions so as to register, signal or perpetuate altered activity states” (Bird 2007). The epigenome consists of chemical agents that modify, or mark, the genome, but are separate from the DNA itself. Effects of epigenetics are principally achieved by DNA and histone modifications that enhance or suppress gene expression (Barros and Offenbacher 2009). Epigenome-wide association studies are done to determine whether differences in histone and/or DNA modifications are associated with a trait (Michels et al. 2013). Any agent that interferes with the normal programming of the epigenome can interfere with activities of the affected gene(s) and their downstream targets. In some cases, drugs targeting the epigenome can be used to restore normal function, for example, by inhibiting DNA methyltransferase and histone deacetylase to enable the transcription of affected genes (Baer-Dubowska et al. 2011). Relevant to this review, epigenetic cues likely are important in driving differentiation of stem cells during muscle regeneration, potentially dictating whether pleuripotency is maintained or cell death and fibrosis occur (Giordani and Puri 2013). Puri and his colleagues have shown that histone deacetylase inhibitors promote muscle regeneration and reduce fibrosis in mdx mice (Consalvi et al. 2013) and have suggested that this effect is mediated through inhibition of fibroadipogenic progenitors while promoting differentiation of satellite cells (Mozzetta et al. 2013).
Conclusions
Pharmacologic approaches have been used to increase DMD gene expression and enhance production of dystrophin surrogates. Drugs that target genes downstream from the primary biochemical defect are also critically needed to lessen DMD disease morbidity and mortality. Unlike many genetic diseases for which animal models are lacking, the mdx mouse and GRMD dog provide homologous models in which disease pathogenesis and therapeutic efficacy can be tested. However, just as with other disease states, data derived from these models do not uniformly translate to DMD patients. This failure of translation likely occurs due to both physiologic differences between animals and humans and the lack of sufficient rigor in preclinical studies. With regard to preclinical studies, more care should be taken in experimental design to include random assignment of animals to treatment groups, blinding of data assessment, greater attention to power analysis of outcome parameters used to establish benefit, and increased stringency in data handling. Consideration should also be given to applying criteria of the “Animal Rule” to therapy development, especially if treatments carry substantial risk. Pharmacologic approaches have typically targeted characteristic pathologic changes, such as necrosis, inflammation, and fibrosis. But, although the mdx and GRMD models are genetically homologous to DMD, they are not analogous. Differences in histologic changes and disease progression undoubtedly reflect disparate pathogenetic mechanisms among species. New assays provide a means to better identify genes/drug targets more closely tied to disease pathogenesis, especially if genetic changes correlate with objective phenotypic outcome parameters. Genes, such as OPN, in which polymorphisms have been shown to affect the DMD phenotype are particularly attractive targets for therapy.
Acknowledgments
The authors acknowledge the efforts of the many individuals who contributed to collection of data reviewed here and those involved in animal care. We hope that knowledge gained through these studies will improve not just the lives of humans but also animals with analogous diseases.
Funding was provided by NINDS/NIAMS 1U24NS059696-01A1 (J.N.K.); NICHD/Child Health Research Career Development Award K12HD001399-04 (C.F.S.); NIAMS F32 NRSA grant 1F32AR060703-01 (P.P.N.); NICHD Wellstone Muscular Dystrophy Center 1U54HD053177-01A1 (E.P.H.); NICHD National Center for Medical Rehabilitation Research 5R24HD050846 (E.P.H.); the Muscular Dystrophy Association (J.N.K., C.J.S., E.P.H., and K.N.); Department of Defense US Army Medical Research Acquisition Activity W81XWH-05-1-0616 (K.N. and E.P.H.); W81XWH-05-1-0659 (K.N.); W81XWH-11-1-0782 (K.N.); NIH K26OD011171 (K.N.); NIAMS R01-AR050478 (K.N.); and Parent Project Muscular Dystrophy (K.N.).
References
- Abdel-Salam E, Abdel-Meguid I, Korraa SS. Markers of degeneration and regeneration in Duchenne muscular dystrophy. Acta Myol. 2009;28:94–100. [PMC free article] [PubMed] [Google Scholar]
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88:83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- Ambrósio CE, Valadares MC, Zucconi E, Cabral R, Pearson PL, Gaiad TP, Canovas M, Vainzof M, Miglino MA, Zatz M. Ringo, a Golden Retriever Muscular Dystrophy (GRMD) dog with absent dystrophin but normal strength. Neuromuscul Disord. 2008;18:892–893. doi: 10.1016/j.nmd.2008.06.385. [DOI] [PubMed] [Google Scholar]
- Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108:762–767. doi: 10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Kakulas BA, Jacobsen PF, Johnsen RD, Kornegay JN, Grounds MD. Comparison of basic fibroblast growth factor in X-linked dystrophin-deficient myopathies of human, dog and mouse. Growth Factors. 1993;9:107–121. [PubMed] [Google Scholar]
- Angus LM, Chakkalakal JV, Méjat A, Eibl JK, Bélanger G, Megeney LA, Chin ER, Schaeffer L, Michel RN, Jasmin BJ. Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1α, drives utrophin gene expression at the neuromuscular junction. Am J Physiol Cell Physiol. 2005;289:C908–C917. doi: 10.1152/ajpcell.00196.2005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Yokota T, Nagata T, Nakamura A, Tanihata J, Saito T, Duguez SM, Nagaraju K, Hoffman EP, Partridge T, Takeda S. Bodywide skipping of exons 45-55 in dystrophic mdx52 mice by systemic antisense delivery. Proc Natl Acad Sci U S A. 2012;109:13763–13768. doi: 10.1073/pnas.1204638109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E, Nakamura K, Nakao K, Kameya S, Kobayashi O, Nonaka I, Kobayashi T, Katsuki M. Targeted disruption of exon 52 in the mouse dystrophin gene induced muscle degeneration similar to that observed in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1997;238:492–497. doi: 10.1006/bbrc.1997.7328. [DOI] [PubMed] [Google Scholar]
- Arechavala-Gomeza V, Graham IR, Popplewell LJ, Adams AM, Aartsma-Rus A, Kinali M, Morgan JE, van Deutekom JC, Wilton SD, Dickson G, Muntoni F. Comparative analysis of antisense oligonucleotide sequences for targeted skipping of exon 51 during dystrophin pre-mRNA splicing in human muscle. Hum Gene Ther. 2007;18:798–810. doi: 10.1089/hum.2006.061. [DOI] [PubMed] [Google Scholar]
- Arechavala-Gomeza V, Kinali M, Feng L, Guglieri M, Edge G, Main M, Hunt D, Lehovsky J, Straub V, Bushby K, Sewry CA, Morgan JE, Muntoni F. Revertant fibres and dystrophin traces in Duchenne muscular dystrophy: Implication for clinical trials. Neuromuscul Disord. 2010;20:295–301. doi: 10.1016/j.nmd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Argyle DJ, Nasir L. Telomerase: A potential diagnostic and therapeutic tool in canine oncology. Vet Pathol. 2003;40:1–7. doi: 10.1354/vp.40-1-1. [DOI] [PubMed] [Google Scholar]
- Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care. 2008;11:408–416. doi: 10.1097/MCO.0b013e328302f3fe. [DOI] [PubMed] [Google Scholar]
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Bäckman E, Nylander E, Johansson I, Henriksson KG, Tagesson C. Selenium and vitamin E treatment of Duchenne muscular dystrophy: No effect on muscle function. Acta Neurol Scand. 1988;78:429–435. doi: 10.1111/j.1600-0404.1988.tb03681.x. [DOI] [PubMed] [Google Scholar]
- Badalamente MA, Stracher A. Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve. 2000;23:106–111. doi: 10.1002/(sici)1097-4598(200001)23:1<106::aid-mus14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Baer-Dubowska W, Majchrzak-Celińska A, Cichocki M. Pharmocoepigenetics: A new approach to predicting individual drug responses and targeting new drugs. Pharmacol Rep. 2011;63:293–304. doi: 10.1016/s1734-1140(11)70498-4. [DOI] [PubMed] [Google Scholar]
- Baron D, Dubois E, Bihouée A, Teusan R, Steenman M, Jourdon P, Magot A, Péréon Y, Veitia R, Savagner F, Ramstein G, Houlgatte R. Meta-analysis of muscle transcriptome data using the MADMuscle database reveals biologically relevant gene patterns. BMC Genomics. 2011;12:113. doi: 10.1186/1471-2164-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrie ES, Smith RM, Sanford JC, Sadee W. mRNA transcript diversity creates new opportunities for pharmacological intervention. Mol Pharmacol. 2012;81:620–630. doi: 10.1124/mol.111.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros SP, Offenbacher S. Epigenetics: Connecting environment and genotype to phenotype and disease. J Dent Res. 2009;88:400–408. doi: 10.1177/0022034509335868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy I, Barrey E, Aguilar P, Uriarte A, Le Chevoir M, Thibaud JL, Voit T, Blot S, Hogrel JY. Longitudinal ambulatory measurements of gait abnormality in dystrophin-deficient dogs. BMC Musculoskelet Disord. 2011;12:75. doi: 10.1186/1471-2474-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy I, Uriarte A, Drougard C, Unterfinger Y, Thibaud JL, Blot S. Effects of an immunosuppressive treatment in the GRMD dog model of Duchenne muscular dystrophy. PLoS One. 2012;7:e48478. doi: 10.1371/journal.pone.0048478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett RJ, Stockinger S, Denis MM, Bartlett WT, Inverardi L, Le TT, Man NT, Morris GE, Bogan DJ, Metcalf-Bogan J, Kornegay JN. In vivo targeted repair of a point mutation in the canine dystrophin gene by a chimeric RNA/DNA oligonucleotide. Nat Biotech. 2000;18:615–622. doi: 10.1038/76448. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L, Piva L, Barp A, Taglia A, Picillo E, Vasco G, Pane M, Previtali SC, Torrente Y, Gazzerro E, Motta MC, Grieco GS, Napolitano S, Magri F, D'Amico A, Astrea G, Messina S, Sframeli M, Vita GL, Boffi P, Mongini T, Ferlini A, Gualandi F, Soraru’ G, Ermani M, Vita G, Battini R, Bertini E, Comi GP, Berardinelli A, Minetti C, Bruno C, Mercuri E, Politano L, Angelini C, Hoffman EP, Pegoraro E. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology. 2012;79:159–162. doi: 10.1212/WNL.0b013e31825f04ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatar M. Lost in translation: Treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Hoshiya H, Tedesco FS. Repair or replace? Exploiting novel gene and cell therapy strategies for muscular dystrophies. FEBS J. 2013;280:4263–4280. doi: 10.1111/febs.12178. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine . J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni C, Lau C, Rando TA. Restoration of dystrophin expression in mdx muscle cells by chimeraplast-mediated exon skipping. Hum Mol Genet. 2003;12:1087–1099. doi: 10.1093/hmg/ddg133. [DOI] [PubMed] [Google Scholar]
- Bessou C, Giugia JB, Franks CJ, Holden-Dye L, Ségalat L. Mutations in the Caenorhabditis elegans dystrophin-like gene dys-1 lead to hyperactivity and suggest a link with cholinergic transmission. Neurogenetics. 1998;2:61–72. doi: 10.1007/s100480050053. [DOI] [PubMed] [Google Scholar]
- Betts CA, Wood MJ. Cell penetrating peptide delivery of splice directing oligonucleotides as a treatment for Duchenne muscular dystrophy. Curr Pharm Des. 2013;19:2948–2962. doi: 10.2174/1381612811319160009. [DOI] [PubMed] [Google Scholar]
- Binder HJ, Herting DC, Hurst V, Finch SC, Spiro HM. Tocopherol deficiency in man. N Engl J Med. 1965;273:1289–1297. doi: 10.1056/NEJM196512092732401. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Bish LT, Sleeper MM, Forbes SC, Morine KJ, Reynolds C, Singletary GE, Trafny D, Pham J, Bogan J, Kornegay JN, Vandenborne K, Walter GA, Sweeney HL. Long-term systemic myostatin inhibition via liver-targeted gene transfer in golden retriever muscular dystrophy. Hum Gene Ther. 2011;22:1499–1509. doi: 10.1089/hum.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Sleeper MM, Forbes SC, Wang B, Reynolds C, Singletary GE, Trafny D, Morine KJ, Sanmiguel J, Cecchini S, Virag T, Vulin A, Beley C, Bogan J, Wilson JM, Vandenborne K, Kornegay JN, Walter GA, Kotin RM, Garcia L, Sweeney HL. Long-term restoration of cardiac dystrophin expression in golden retriever muscular dystrophy following rAAV6-mediated exon skipping. Mol Ther. 2012;20:580–589. doi: 10.1038/mt.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Yarchoan M, Sleeper MM, Gazzara JA, Morine KJ, Acosta P, Barton ER, Sweeney HL. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One. 2011;6:e20856. doi: 10.1371/journal.pone.0020856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet E, Annicotte JS, Pradelli LA, Hugon G, Matecki S, Mornet D, Rivier F, Fajas L. E2F transcription factor-1 deficiency reduces pathophysiology in the mouse model of Duchenne muscular dystrophy through increased muscle oxidative metabolism. Hum Mol Genet. 2012;21:3910–3917. doi: 10.1093/hmg/dds219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy KN, Roche BM, Schwartz DS, Nakayama T, Hamlin RL. Evaluation of the six-minute walk test in dogs. Am J Vet Res. 2004;65:311–313. doi: 10.2460/ajvr.2004.65.311. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978;28:439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle Nerve. 2013;48:336–342. doi: 10.1002/mus.23793. [DOI] [PubMed] [Google Scholar]
- Bojesen SE. Telomeres and human health. J Intern Med. 2013;274:399–413. doi: 10.1111/joim.12083. [DOI] [PubMed] [Google Scholar]
- Bonuccelli G, Sotgia F, Capozza F, Gazzerro E, Minetti C, Lisanti MP. Localized treatment with a novel FDA-approved proteasome inhibitor blocks the degradation of dystrophin and dystrophin-associated proteins in mdx mice. Cell Cycle. 2007;6:1242–1248. doi: 10.4161/cc.6.10.4182. [DOI] [PubMed] [Google Scholar]
- Briggs D, Morgan JE. Recent progress in satellite cell/myoblast engraftment: Relevance for therapy. FEBS J. 2013;280:4281–4293. doi: 10.1111/febs.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeyer-Langford C, Kornegay JN. Comparative genomics of X-linked muscular dystrophies: The golden retriever model. Curr Genomics. 2013;14:330–342. doi: 10.2174/13892029113149990004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, Province MA. Clinical investigation in Duchenne dystrophy: 2. Determination of the "power" of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PAL, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi R, Rolland JF, Fraysse B, Litvinova K, Cozzoli A, Giannuzzi V, Liantonio A, Camerino GM, Sblendorio V, Capogrosso RF, Palmieri B, Andreetta F, Confalonieri P, De Benedictis L, Montagnani M, De Luca A. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J Appl Physiol. 2009;106:1311–1324. doi: 10.1152/japplphysiol.90985.2008. [DOI] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C; DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- Bushby KM, Gardner-Medwin D, Nicholson LV, Johnson MA, Haggerty ID, Cleghorn NJ, Harris JB, Bhattacharya SS. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. II. Correlation of phenotype with genetic and protein abnormalities. J Neurol. 1993;240:105–112. doi: 10.1007/BF00858726. [DOI] [PubMed] [Google Scholar]
- Bushby KM, Hill A, Steele JG. Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet. 1999;353:557–558. doi: 10.1016/s0140-6736(98)05279-9. [DOI] [PubMed] [Google Scholar]
- Butcher LM, Beck S. Future impact of integrated high-throughput methylome analyses on human health and disease. J Genet Genomics. 2008;35:391–401. doi: 10.1016/S1673-8527(08)60057-0. [DOI] [PubMed] [Google Scholar]
- Buyse GM, Van der Mieren G, Erb M, D'hooge J, Herijgers P, Verbeken E, Jara A, Van Den Bergh A, Mertens L, Courdier-Fruh I, Barzaghi P, Meier T. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: Cardiac protection and improved exercise performance. Eur Heart J. 2009;30:116–124. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyse GM, Goemans N, van den Hauwe M, Thijs D, de Groot IJ, Schara U, Ceulemans B, Meier T, Mertens L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord. 2011;21:396–405. doi: 10.1016/j.nmd.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Carlson CG, Samadi A, Siegel A. Chronic treatment with agents that stabilize cytosolic IkappaB-alpha enhances survival and improves resting membrane potential in MDX muscle fibers subjected to chronic passive stretch. Neurobiol Dis. 2005;20:719–730. doi: 10.1016/j.nbd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Carnwath JW, Shotton DM. Muscular dystrophy in the mdx mouse: Histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci. 1987;80:39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- Carpenter JL, Hoffman EP, Romanul FC, Kunkel LM, Rosales RK, Ma NS, Dasbach JJ, Rae JF, Moore FM, McAfee MB, Pearce LK. Feline muscular dystrophy with dystrophin deficiency. Am J Pathol. 1989;135:909–919. [PMC free article] [PubMed] [Google Scholar]
- Carre-Pierrat M, Lafoux A, Tanniou G, Chambonnier L, Divet A, Fougerousse F, Huchet-Cadiou C, Ségalat L. Pre-clinical study of 21 approved drugs in the mdx mouse. Neuromuscul Disord. 2011;21:313–327. doi: 10.1016/j.nmd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, Davies KE, Cornelio F, Pozza O, Karpati G, Gilbert R, Mora M. Dystrophic phenotype of canine X-linked muscular dystrophy is mitigated by adenovirus-mediated utrophin gene transfer. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Phelps SF, Cox GA, Maichele AJ, Greenwood A. PCR analysis of muscular dystrophy in mdx mice. Mol Cell Biol Hum Dis Ser. 1993;3:167–189. doi: 10.1007/978-94-011-1528-5_7. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci U S A. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Cheng SC. Functional roles of protein splicing factors. Biosci Rep. 2012;32:345–359. doi: 10.1042/BSR20120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- Chen Y-W, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: Identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers MK, Bogan JR, Bogan DJ, Greiner H, Holder M, Grange RW, Kornegay JN. Chronic administration of a leupeptin-derived calpain inhibitor fails to ameliorate severe muscle pathology in a canine model of duchenne muscular dystrophy. Front Pharmacol. 2012;2:89. doi: 10.3389/fphar.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti A, Indelicato M, Bensi T, Mesturini R, Giordano M, Sametti S, Castelli L, Bottarel F, Mazzarino MC, Garbarini L, Giacopelli F, Valesini G, Santoro C, Dianzani I, Ramenghi U, Dianzani U. High levels of osteopontin associated with polymorphisms in its gene are a risk factor for development of autoimmunity/lymphoproliferation. Blood. 2004;103:1376–1382. doi: 10.1182/blood-2003-05-1748. [DOI] [PubMed] [Google Scholar]
- Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Cirak S, Feng L, Anthony K, Arechavala-Gomeza V, Torelli S, Sewry C, Morgan JE, Muntoni F. Restoration of the dystrophin-associated glycoprotein complex after exon skipping therapy in Duchenne muscular dystrophy. Mol Ther. 2012;20:462–467. doi: 10.1038/mt.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IA. Proposed treatment of Duchenne muscular dystrophy with desferrioxamine. Med Hypotheses. 1984;13:153–160. doi: 10.1016/0306-9877(84)90026-4. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M,, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12:917–925. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- Consalvi S, Mozzetta C, Bettica P, Germani M, Fiorentini F, Del Bene F, Rocchetti M, Leoni F, Monzani V, Mascagni P, Puri PL, Saccone V. Preclinical studies in the mdx mouse model of duchenne muscular dystrophy with the histone deacetylase inhibitor givinostat. Mol Med. 2013;19:79–87. doi: 10.2119/molmed.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BJ, Winand WN, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott M-O, Fischbeck KH, Kornegay JN, Avery RJ, Williams JR, Schmickel RD, Sylvester JE. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- Cowan J, Macdessi J, Start A, Morgan G. Incidence of Duchenne muscular dystrophy in New South Wales and the Australian Capital Territory. J Med Genet. 1980;17:245–249. doi: 10.1136/jmg.17.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli A, Capogrosso RF, Sblendorio VT, Dinardo MM, Jagerschmidt C, Namour F, Camerino GM, De Luca A. GLPG0492, novel selective androgen receptor modulator, improves muscle performance in the exercised-mdx mouse model of muscular dystrophy. Pharmacol Res. 2013;72:9–24. doi: 10.1016/j.phrs.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Cozzoli A, Nico B, Sblendorio VT, Capogrosso RF, Dinardo MM, Longo V, Gagliardi S, Montagnani M, De Luca A. Enalapril treatment discloses an early role of angiotensin II in inflammation- and oxidative stress-related muscle damage in dystrophic mdx mice. Pharmacol Res. 2011;64:482–492. doi: 10.1016/j.phrs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros D, Harnden P, Pellissier JF, Serratrice G. Muscle hypertrophy in Duchenne muscular dystrophy. A pathological and morphometric study. J Neurol. 1989;236:43–47. doi: 10.1007/BF00314217. [DOI] [PubMed] [Google Scholar]
- Cross SH, Allshire RC, McKay SJ, McGill NI, Cooke HJ. Cloning of human telomeres by complementation in yeast. Nature. 1989;338:771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: Success rates for investigational drugs. Clin Pharmacol Ther. 2010;87:272–277. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- Dai J, Li B, Shi J, Peng L, Zhang D, Qian W, Hou S, Zhao L, Gao J, Cao Z, Zhao J, Wang H, Guo Y. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59:355–366. doi: 10.1007/s00262-009-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- Delfín DA, Xu Y, Peterson JM, Guttridge DC, Rafael-Fortney JA, Janssen PM. Improvement of cardiac contractile function by peptide-based inhibition of NF-κB in the utrophin/dystrophin-deficient murine model of muscular dystrophy. J Transl Med. 2011;9:68. doi: 10.1186/1479-5876-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A. Pre-clinical drug tests in the mdx mouse as a model of dystrophinopathies: An overview. Acta Myol. 2012;31:40–47. [PMC free article] [PubMed] [Google Scholar]
- Dent KM, Dunn DM, von Niederhausern AC, Aoyagi AT, Kerr L, Bromberg MB, Hart KJ, Tuohy T, White S, den Dunnen JT, Weiss RB, Flanigan KM. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A. 2005;134:295–298. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- De Paepe B, De Bleecker JL. Cytokines and chemokines as regulators of skeletal muscle inflammation: Presenting the case of Duchenne muscular dystrophy. Mediators Inflamm. 2013;2013:540370. doi: 10.1155/2013/540370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012;3:e418. doi: 10.1038/cddis.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: A marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009;68:762–773. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- de Semir D, Aran JM. Targeted gene repair: The ups and downs of a promising gene therapy approach. Curr Gene Ther. 2006;6:481–504. doi: 10.2174/156652306777934847. [DOI] [PubMed] [Google Scholar]
- DeVanna JC, Kornegay JN, Bogan DJ, Bogan JR, Dow JL, Hawkins EC. Respiratory dysfunction in unsedated dogs with golden retriever muscular dystrophy. Neuromuscul Disord. 2014;24:63–73. doi: 10.1016/j.nmd.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB. Muscle-specific BCL2 expression ameliorates muscle disease in lamininα2-deficient, but not in dystrophin-deficient, mice. Hum Mol Genet. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum Mol Genet 15 Spec No. 2006;2:R253–R61. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham WJ, Arbogast S, Gerken E, Li YP, Reid MB. Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34:298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, Straub V, Bushby K. Managing Duchenne muscular dystrophy--the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17:470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Eccleston A, DeWitt N, Gunter C, Marte B, Nath D. Epigenetics. Nature. 2007;447:395. [Google Scholar]
- Edwards RHT, Jones DA, Newham DJ, Chapman SJ. Role of mechanical damage in the pathogenesis of proximal myopathy in man. Lancet. 1984;323:548–552. doi: 10.1016/s0140-6736(84)90941-3. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: Illuminating the dark road from association to function. Am J Hum Genet. 2013;93:779–797. doi: 10.1016/j.ajhg.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Arahata K. Mononuclear cells in myopathies: Quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986;17:704–721. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Levin LA. Solving the lost in translation problem: Improving the effectiveness of translational research. Curr Opin Pharmacol. 2013;13:108–214. doi: 10.1016/j.coph.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NP, Call JA, Bassaganya-Riera J, Robertson JL, Grange RW. Green tea extract decreases muscle pathology and NF-kappaB immunostaining in regenerating muscle fibers of mdx mice. Clin Nutr. 2010;29:391–398. doi: 10.1016/j.clnu.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW. Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R. 2009;1:755–768. doi: 10.1016/j.pmrj.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough RJ, Bareja A, Davies KE. Progress in therapy for Duchenne muscular dystrophy. Exp Physiol. 2011;96:1101–1113. doi: 10.1113/expphysiol.2010.053025. [DOI] [PubMed] [Google Scholar]
- Fan K, Dai J, Wang H, Wei H, Cao Z, Hou S, Qian W, Wang H, Li B, Zhao J, Xu H, Yang C, Guo Y. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum. 2008;58:2041–2052. doi: 10.1002/art.23490. [DOI] [PubMed] [Google Scholar]
- Fan K, Zhang B, Yang H, Wang H, Tan M, Hou S, Qian W, Li B, Wang H, Dai J, Guo Y. A humanized anti-osteopontin antibody protects from Concanavalin A induced-liver injury in mice. Eur J Pharmacol. 2011;657:144–151. doi: 10.1016/j.ejphar.2011.01.041. [DOI] [PubMed] [Google Scholar]
- Feron M, Guevel L, Rouger K, Dubreil L, Arnaud MC, Ledevin M, Megeney LA, Cherel Y, Sakanyan V. PTEN contributes to profound PI3 K/Akt signaling pathway deregulation in dystrophin-deficient dog muscle. Am J Pathol. 2009;174:1459–1470. doi: 10.2353/ajpath.2009.080460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DM, Shin JH, Yue Y, Volkmann D, Leach SB, Smith BF, McIntosh M, Duan D. Age-matched comparison reveals early electrocardiography and echocardiography changes in dystrophin-deficient dogs. Neuromuscul Disord. 2011;21:453–461. doi: 10.1016/j.nmd.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- Flanigan KM, Campbell K, Viollet L, Wang W, Gomez AM, Walker CM, Mendell JR. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: Prevalence and a glucocorticoid treatment effect. Hum Gene Ther. 2013;24:797–806. doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Sharkey M, Mealey K, Ostrander EA, Martinez M. Pharmacogenetic and metabolic differences between dog breeds: Their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008;10:110–119. doi: 10.1208/s12248-008-9011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H, Popplewell L, Dickson G. Genetic therapeutic approaches for Duchenne muscular dystrophy. Hum Gene Ther. 2012;23:676–687. doi: 10.1089/hum.2012.099. [DOI] [PubMed] [Google Scholar]
- Gamstorp I, Gustavson KH, Hellström O, Nordgren B. A trial of selenium and vitamin E in boys with muscular dystrophy. J Child Neurol. 1986;1:211–214. doi: 10.1177/088307388600100306. Erratum in: New Eng J Med 2011; 365:1361. [DOI] [PubMed] [Google Scholar]
- Gaschen F, Gaschen L, Seiler G, Welle M, Jaunin VB, Jmaa DG, Neiger–Aeschbacher G, Adé–Damilano M. Lethal peracute rhabdomyolysis associated with stress and general anesthesia in three dystrophin–deficient cats. Vet Pathol. 1998;35:117–123. doi: 10.1177/030098589803500205. [DOI] [PubMed] [Google Scholar]
- Gaschen FP, Hoffman EP, Gorospe JR, Uhl EW, Senior DF, Cardinet GH, 3rd, Pearce LK. Dystrophin deficiency causes lethal muscle hypertrophy in cats. J Neurol Sci. 1992;110:149–159. doi: 10.1016/0022-510x(92)90022-d. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Assereto S, Bonetto A, Sotgia F, Scarfì S, Pistorio A, Bonuccelli G, Cilli M, Bruno C, Zara F, Lisanti MP, Minetti C. Therapeutic potential of proteasome inhibition in Duchenne and Becker muscular dystrophies. Am J Pathol. 2010;176:1863–1877. doi: 10.2353/ajpath.2010.090468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig SM, van der Poel C, Hoeflich A, Naim T, Lynch GS, Metzger F. Therapeutic potential of PEGylated insulin–like growth factor I for skeletal muscle disease evaluated in two murine models of muscular dystrophy. Growth Horm IGF Res. 2012;22:69–75. doi: 10.1016/j.ghir.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Giacopelli F, Marciano R, Pistorio A, Catarsi P, Canini S, Karsenty G, Ravazzolo R. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol Genomics. 2004;20:87–96. doi: 10.1152/physiolgenomics.00138.2004. [DOI] [PubMed] [Google Scholar]
- Gibbs EM, Horstick EJ, Dowling JJ. Swimming into prominence: The zebrafish as a valuable tool for studying human myopathies and muscular dystrophies. FEBS J. 2013;280:4187–4197. doi: 10.1111/febs.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JM. Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse. J Muscle Res Cell Motil. 1999;20:605–625. doi: 10.1023/a:1005545325254. [DOI] [PubMed] [Google Scholar]
- Giordani L, Puri PL. Epigenetic control of skeletal muscle regeneration: Integrating genetic determinants and environmental changes. FEBS J. 2013;280:4014–4025. doi: 10.1111/febs.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta. 2010;1797:1113–1118. doi: 10.1016/j.bbabio.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin R, Daussin F, Matecki S, Li T, Petrof BJ, Burelle Y. Peroxisome proliferator–activated receptor γ coactivator1– gene α transfer restores mitochondrial biomass and improves mitochondrial calcium handling in post–necrotic mdx mouse skeletal muscle. J Physiol. 2012;590:5487–5502. doi: 10.1113/jphysiol.2012.240390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N, Holling T, Janson AA, Platenburg GJ, Sipkens JA, Sitsen JM, Aartsma-Rus A, van Ommen GJ, Buyse G, Darin N, Verschuuren JJ, Campion GV, de Kimpe SJ, van Deutekom JC. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. Erratum in: N Engl J Med 365:1361. [DOI] [PubMed] [Google Scholar]
- Gorospe JR, Tharp MD, Hinckley J, Kornegay JN, Hoffman EP. A role for mast cells in the progression of Duchenne muscular dystrophy? Correlations in dystrophin-deficient humans, dogs, and mice. J Neurol Sci. 1994;122:44–56. doi: 10.1016/0022-510x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE. Pentoxifylline fails to attenuate fibrosis in dystrophic (mdx) diaphragm muscle. Muscle Nerve. 2006;33:820–823. doi: 10.1002/mus.20523. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve. 2004;30:645–653. doi: 10.1002/mus.20150. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Williams JE, Personius K, Farkas GA. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: Impact of pirfenidone. Muscle Nerve. 2007;35:208–216. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- Graham IR, Dickson G. Gene repair and mutagenesis mediated by chimeric RNA-DNA oligonucleotides: Chimeraplasty for gene therapy and conversion of single nucleotide polymorphisms (SNPs) Biochim Biophys Acta. 2002;1587:1–6. doi: 10.1016/s0925-4439(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Graham KM, Singh R, Millman G, Malnassy G, Gatti F, Bruemmer K, Stefanski C, Curtis H, Sesti J, Carlson CG. Excessive collagen accumulation in dystrophic (mdx) respiratory musculature is independent of enhanced activation of the NF-kappaB pathway. J Neurol Sci. 2010;294:43–50. doi: 10.1016/j.jns.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granchelli JA, Pollina C, Hudecki MS. Pre-clinical screening of drugs using the mdx mouse. Neuromuscul Disord. 2000;10:235–239. doi: 10.1016/s0960-8966(99)00126-1. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Moxley RT, 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, Miller JP, Cwik VA, Pandya S, Robison J, King W, Signore L, Schierbecker J, Florence J, Mateson-Burden N, Wilson B. Duchenne dystrophy: Randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Rennie MJ. Muscle wasting in muscular dystrophy: Decreased protein synthesis or increased degradation? Ann Neurol. 1983;13:125–132. doi: 10.1002/ana.410130204. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell Mol Life Sci. 2008;65:1621–1625. doi: 10.1007/s00018-008-7574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerron AD, Rawat R, Sali A, Spurney CF, Pistilli E, Cha HJ, Pandey GS, Gernapudi R, Francia D, Farajian V, Escolar DM, Bossi L, Becker M, Zerr P, de la Porte S, Gordish-Dressman H, Partridge T, Hoffman EP, Nagaraju K. Functional and molecular effects of arginine butyrate and prednisone on muscle and heart in the mdx mouse model of Duchenne Muscular Dystrophy. PLoS One. 2010;5:e11220. doi: 10.1371/journal.pone.0011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevel L, Lavoie JR, Perez-Iratxeta C, Rouger K, Dubreil L, Feron M, Talon S, Brand M, Megeney LA. Quantitative proteomic analysis of dystrophic dog muscle. J Proteome Res. 2011;10:2465–2478. doi: 10.1021/pr2001385. [DOI] [PubMed] [Google Scholar]
- Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg. 2009;109:1043–1048. doi: 10.1213/ane.0b013e3181aa5cf6. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci U S A. 2002:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Han M, Bennett RR, Kohane IS, Beggs AH, Kunkel LM. Gene expression profiling of Duchenne muscular dystrophy skeletal muscle. Neurogenetics. 2003:163–171. doi: 10.1007/s10048-003-0148-x. [DOI] [PubMed] [Google Scholar]
- Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5:1569–1585. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG. Threats to validity in the design and conduct of preclinical efficacy studies: A systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10:e1001489. doi: 10.1371/journal.pmed.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques-Pons A, Yu Q, Rayavarapu S, Cohen TV, Ampong B, Cha HJ, Jahnke V, Van der Meulen J, Wang D, Jiang W, Kandimalla ER, Agrawal S, Spurney CF, Nagaraju K. Role of toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle. 2014 doi: 10.1093/hmg/ddt656. Hum Mol Genet Jan 14. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, Hwang S, Leonard EM, Almstead NG, Ju W, Peltz SW, Miller LL. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47:430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- Hnia K, Gayraud J, Hugon G, Ramonatxo M, De La Porte S, Matecki S, Mornet D. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am J Pathol. 2008;172:1509–1519. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown KJ, Eccleston E. New molecular research technologies in the study of muscle disease. Curr Opin Rheumatol. 2003;15:698–707. doi: 10.1097/00002281-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Connor EM. Orphan drug development in muscular dystrophy: Update on two large clinical trials of dystrophin rescue therapies. Discov Med. 2013;16:233–239. [PubMed] [Google Scholar]
- Hoffman EP, Gordish-Dressman H, McLane VD, Devaney JM, Thompson PD, Visich P, Gordon PM, Pescatello LS, Zoeller RF, Moyna NM, Angelopoulos TJ, Pegoraro E, Cox GA, Clarkson PM. Alterations in osteopontin modify muscle size in females in both humans and mice. Med Sci Sports Exerc. 2013;45:1060–1068. doi: 10.1249/MSS.0b013e31828093c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Gorospe RM. The animal models of Duchenne muscular dystrophy: Windows on the pathophysiological consequences of dystrophin deficiency. Curr Top Membranes. 1991;38:113–155. [Google Scholar]
- Hollinger K, Yang CX, Montz RE, Nonneman D, Ross JW, Selsby JT. Dystrophin insufficiency causes selective muscle histopathology and loss of dystrophin-glycoprotein complex assembly in pig skeletal muscle. 2013a doi: 10.1096/fj.13-241141. FASEB J 2013 Dec 17 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger K, Gardan-Salmon D, Santana C, Rice D, Snella E, Selsby JT. Rescue of dystrophic skeletal muscle by PGC-1α involves restored expression of dystrophin-associated protein complex components and satellite cell signaling. Am J Physiol Regul Integr Comp Physiol. 2013b;305:R13–R23. doi: 10.1152/ajpregu.00221.2012. [DOI] [PubMed] [Google Scholar]
- Holysz H, Lipinska N, Paszel-Jaworska A, Rubis B. Telomerase as a useful target in cancer fighting-the breast cancer case. Tumour Biol. 2013;34:1371–1380. doi: 10.1007/s13277-013-0757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhao H. A review of post-GWAS prioritization approaches. Front Genet. 2013;4:280. doi: 10.3389/fgene.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Cheng G, Lu H, Aronica M, Ransohoff RM, Zhou L. Impaired respiratory function in mdx and mdx/utrn(+/-) mice. Muscle Nerve. 2011;43:263–267. doi: 10.1002/mus.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner C, Lehr HA, Bodlaj R, Finckh B, Oexle K, Marklund SL, Freudenberg K, Kontush A, Speer A, Terwolbeck K, Voit T, Kohlschütter A. Wheat kernel ingestion protects from progression of muscle weakness in mdx mice, an animal model of Duchenne muscular dystrophy. Pediatr Res. 1996;40:444–449. doi: 10.1203/00006450-199609000-00013. [DOI] [PubMed] [Google Scholar]
- Hunter AJ. Have animal models of disease helped or hindered the drug discovery process? Ann N Y Acad Sci. 2011;1245:1–2. doi: 10.1111/j.1749-6632.2011.06375.x. [DOI] [PubMed] [Google Scholar]
- Huynh T, Uaesoontrachoon K, Quinn JL, Tatem KS, Heier CR, Van Der Meulen JH, Yu Q, Harris M, Nolan CJ, Haegeman G, Grounds MD, Nagaraju K. Selective modulation through the glucocorticoid receptor ameliorates muscle pathology in mdx mice. J Pathol. 2013;231:223–235. doi: 10.1002/path.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannitti T, Capone S, Feder D, Palmieri B. Clinical use of immunosuppressants in Duchenne muscular dystrophy. J Clin Neuromuscul Dis. 2010;12:1–21. doi: 10.1097/CND.0b013e3181d4a4f9. [DOI] [PubMed] [Google Scholar]
- Inkley SR, Oldenburg FC, Vignos PJ., Jr Pulmonary function in Duchenne muscular dystrophy related to stage of disease. Am J Med. 1974;56:297–306. doi: 10.1016/0002-9343(74)90611-1. [DOI] [PubMed] [Google Scholar]
- Jahnke VE, Van Der Meulen JH, Johnston HK, Ghimbovschi S, Partridge T, Hoffman EP, Nagaraju K. Metabolic remodeling agents show beneficial effects in the dystrophin-deficient mdx mouse model. Skelet Muscle. 2012;2:16. doi: 10.1186/2044-5040-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Chakraborty G, Bulbule A, Kaur R, Kundu GC. Osteopontin: An emerging therapeutic target for anticancer therapy. Expert Opin Ther Targets. 2007;11:81–90. doi: 10.1517/14728222.11.1.81. [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Kuzon WM., Jr. Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- Jones DA, Round JM, Edwards RHT, Grindwood SR, Tofts PS. Size and composition of the calf and quadriceps muscles in Duchenne muscular dystrophy. A tomographic and histochemical study. J Neurol Sci. 1983;60:307–322. doi: 10.1016/0022-510x(83)90071-0. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. Experimental muscle diseases. Methods Achiev Exp Pathol. 1975;7:109–131. [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Morris GE, Davies KE, Guerin C, Holland P. Localization and quantitation of the chromosome 6-encoded dystrophin-related protein in normal and pathological human muscle. J Neuropathol Exp Neurol. 1993;52:119–128. doi: 10.1097/00005072-199303000-00004. [DOI] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Prescott S. Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle Nerve. 1988;11:795–803. doi: 10.1002/mus.880110802. [DOI] [PubMed] [Google Scholar]
- Kharraz Y, Guerra J, Mann CJ, Serrano AL, Muñoz-Cánoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana TS, Watkins SC, Chafey P, Chelly J, Tomé FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1:185–189. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Kissel JT, Lynn DJ, Rammohan KW, Klein JP, Griggs RC, Moxley RT, 3rd, Cwik VA, Brooke MH, Mendell JR. Mononuclear cell analysis of muscle biopsies in prednisone- and azathioprine-treated Duchenne muscular dystrophy. Neurology. 1993;43:532–536. doi: 10.1212/wnl.43.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012;31:184–195. [PMC free article] [PubMed] [Google Scholar]
- Klymiuk N, Blutke A, Graf A, Krause S, Burkhardt K, Wuensch A, Krebs S, Kessler B, Zakhartchenko V, Kurome M, Kemter E, Nagashima H, Schoser B, Herbach N, Blum H, Wanke R, Aartsma-Rus A, Thirion C, Lochmüller H, Walter MC, Wolf E. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum Mol Genet. 2013;22:4368–4382. doi: 10.1093/hmg/ddt287. [DOI] [PubMed] [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Konieczny P, Swiderski K, Chamberlain JS. Gene and cell-mediated therapies for muscular dystrophy. Muscle Nerve. 2013;47:649–663. doi: 10.1002/mus.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay JN, Bogan DJ, Bogan JR, Childers MK, Cundiff DD, Petroski GF, Schueler RO. Contraction force generated by tibiotarsal joint flexion and extension in dogs with golden retriever muscular dystrophy. J Neurol Sci. 1999;166:115–121. doi: 10.1016/s0022-510x(99)00118-5. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Grange RW. Golden retriever muscular dystrophy (GRMD): Developing and maintaining a colony and physiological functional measurements. In: Duan D, editor. Muscle Gene Therapy: Methods and Protocols. Methods in Molecular Biology. 709th vol. New York: Humana Press; 2011. pp. 105–123. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Li J, Nghiem P, Detwiler DA, Larsen CA, Grange RW, Bhavaraju-Sanka RK, Tou S, Keene BP, Howard JF, Jr, Wang J, Fan Z, Schatzberg SJ, Styner MA, Flanigan KM, Xiao X, Hoffman EP. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome. 2012a;23:85–108. doi: 10.1007/s00335-011-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay JN, Childers MK, Bogan DJ, Bogan JR, Nghiem P, Wang J, Fan Z, Howard JF, Jr, Schatzberg SJ, Dow JL, Grange RW, Styner MA, Hoffman EP, Wagner KR. The paradox of muscle hypertrophy in muscular dystrophy. In: Carter GT (ed). Recent Advancements in Neuromuscular Medicine. Phys Med Rehabil Clin N Am. 2012b;23:149–172. doi: 10.1016/j.pmr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay JN, Cundiff DD, Bogan DJ, Bogan JR, Okamura CS. The cranial sartorius muscle undergoes true hypertrophy in dogs with golden retriever muscular dystrophy. Neuromuscul Disord. 2003;13:493–500. doi: 10.1016/s0960-8966(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, Wang B, Qiao C, Howard JF, Jr, Xiao X. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay JN, Tuler SM, Miller DM, Levesque DC. Muscular dystrophy in a litter of golden retriever dogs. Muscle Nerve. 1988;11:1056–1064. doi: 10.1002/mus.880111008. [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994;107:1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- Lawler JM. Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J Physiol. 2011;589:2161–2170. doi: 10.1113/jphysiol.2011.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-J. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Pastoret C, Sebille A. Phenotype of dystrophinopathy in old mdx mice. Anat Rec. 1995;242:70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- Lesko LJ, Rowland M, Peck CC, Blaschke TF, Breimer D, de Jong HJ, Grahnen A, Kuhlmann JJ, Stewart B. Optimizing the science of drug development: Opportunities for better candidate selection and accelerated evaluation in humans. Eur J Pharm Sci. 2000;10:iv–xiv. doi: 10.1016/s0928-0987(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Lin JH. Species similarities and differences in pharmacokinetics. Drug Metab Dispos. 1995;23:1008–1021. [PubMed] [Google Scholar]
- Lis Y, Roberts MH, Kamble S, J Guo J, Raisch DW. Comparisons of Food and Drug Administration and European Medicines Agency risk management implementation for recent pharmaceutical approvals: Report of the International Society for Pharmacoeconomics and outcomes research risk benefit management working group. Value Health. 2012;15:1108–1118. doi: 10.1016/j.jval.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Liu JMK, Okamura CS, Bogan DJ, Bogan JR, Childers MK, Kornegay JN. Effects of prednisone in canine muscular dystrophy. Muscle Nerve. 2004;30:767–773. doi: 10.1002/mus.20154. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hart K, Klamut H, Thomas N, Bodrug S, Burghes A, Bobrow M, Harper P, Thompson M, Ray P, Worton R. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988;242:755–759. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- Malik V, Rodino-Klapac LR, Mendell JR. Emerging drugs for Duchenne muscular dystrophy. Expert Opin Emerg Drugs. 2012;17:261–277. doi: 10.1517/14728214.2012.691965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, Morgan JE, Partridge TA, Wilton SD. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci U S A. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- Martin PT, Xu R, Rodino-Klapac LR, Oglesbay E, Camboni M, Montgomery CL, Shontz K, Chicoine LG, Clark KR, Sahenk Z, Mendell JR, Janssen PM. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol. 2009;296:C476–488. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo T, Nakatani S, Jin Y, Uemura K, Sugimachi M, Ueda-Ishibashi H, Kitakaze M, Ohe T, Sunagawa K, Miyatake K. Evaluation of transmural distribution of viable muscle by myocardial strain profile and dobutamine stress echocardiography. Am J Physiol Heart Circ Physiol. 2007;292:H921–H927. doi: 10.1152/ajpheart.00019.2006. [DOI] [PubMed] [Google Scholar]
- Mazzone ES, Pane M, Sormani MP, Scalise R, Berardinelli A, Messina S, Torrente Y, D'Amico A, Doglio L, Viggiano E, D'Ambrosio P, Cavallaro F, Frosini S, Bello L, Bonfiglio S, De Sanctis R, Rolle E, Bianco F, Magri F, Rossi F, Vasco G, Vita G, Motta MC, Donati MA, Sacchini M, Mongini T, Pini A, Battini R, Pegoraro E, Previtali S, Napolitano S, Bruno C, Politano L, Comi GP, Bertini E, Mercuri E. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS One. 2013;8:e52512. doi: 10.1371/journal.pone.0052512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Preswick G, Garner S. The sick motoneurone hypothesis of muscular dystrophy. Prog Neurobiol. 1988;30:309–331. doi: 10.1016/0301-0082(88)90026-3. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Sica REP, Currie S. Muscular dystrophy. Evidence for a neural factor . Nature. 1970;226:1263–1264. doi: 10.1038/2261263a0. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases. Duchenne muscular dystrophy . Am J Phys Med Rehabil. 1995;74:S70–S92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Henricson EK, Abresch RT, Florence JM, Eagle M, Gappmaier E, Glanzman AM. Spiegel R, Barth J, Elfring G, Reha A, Peltz S, editors. PTC124-GD-007-DMD Study Group. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: Longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013a;48:343–356. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, Gappmaier E, Glanzman AM. Spiegel R, Barth J, Elfring G, Reha A, Peltz SW, editors. PTC124-GD-007-DMD Study Group. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013b;48:357–368. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDouall RM, Dunn MJ, Dubowitz Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J Neurol Sci. 1990;99:199–217. doi: 10.1016/0022-510x(90)90156-h. [DOI] [PubMed] [Google Scholar]
- McEntee K, Amory H, Pypendop B, Balligand M, Clercx, Michaux C, Robert JF, Gerard P, Pochet T, Henroteaux M. Effects of dobutamine on isovolumic and ejection phase indices of cardiac contractility in conscious healthy dogs. Res Vet Sci. 1998;64:45–80. doi: 10.1016/s0034-5288(98)90114-x. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Engel WK, Derrer EC. Duchenne muscular dystrophy: Functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- Meregalli M, Farini A, Belicchi M, Parolini D, Cassinelli L, Razini P, Sitzia C, Torrente Y. Perspectives of stem cell therapy in Duchenne muscular dystrophy. FEBS J. 2013;280:4251–4262. doi: 10.1111/febs.12083. [DOI] [PubMed] [Google Scholar]
- Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006b;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina S, Bitto A, Aguennouz M, Mazzeo A, Migliorato A, Polito F, Irrera N, Altavilla D, Vita GL, Russo M, Naro A, De Pasquale MG, Rizzuto E, Musarò A, Squadrito F, Vita G. Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: A comparison study with methylprednisolone. Exp Neurol. 2009;220:349–358. doi: 10.1016/j.expneurol.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, Squadrito F, Vita G. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol. 2006a;198:234–241. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Messina S, Bitto A, Aguennouz M, Vita GL, Polito F, Irrera N, Altavilla D, Marini H, Migliorato A, Squadrito F, Vita G. The soy isoflavone genistein blunts nuclear factor kappa-B, MAPKs and TNF-α activation and ameliorates muscle function and morphology in mdx mice. Neuromuscul Disord. 2011;21:579–589. doi: 10.1016/j.nmd.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Michels KB, Binder AM, Dedeurwaerder S, Epstein CB, Greally JM, Gut I, Houseman EA, Izzi B, Kelsey KT, Meissner A, Milosavljevic A, Siegmund KD, Bock C, Irizarry RA. Recommendations for the design and analysis of epigenome-wide association studies. Nat Methods. 2013;10:949–955. doi: 10.1038/nmeth.2632. [DOI] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J, Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB, Girgenrath M. The role of apoptosis in neuromuscular diseases and prospects for anti-apoptosis therapy. Trends Mol Med. 2006;12:279–286. doi: 10.1016/j.molmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Miura P, Jasmin BJ. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: How close are we? Trends Mol Med. 2006;12:122–129. doi: 10.1016/j.molmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Nonaka I, Hirai S, Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993;119:43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- Mok E, Constantin B, Favreau F, Neveux N, Magaud C, Delwail A, Hankard R. l-Glutamine administration reduces oxidized glutathione and MAP kinase signaling in dystrophic muscle of mdx mice. Pediatr Res. 2008;63:268–273. doi: 10.1203/PDR.0b013e318163a259. [DOI] [PubMed] [Google Scholar]
- Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- Moorwood C, Soni N, Patel G, Wilton SD, Khurana TS. A cell-based high-throughput screening assay for posttranscriptional utrophin upregulation. J Biomol Screen. 2013;18:400–406. doi: 10.1177/1087057112465648. [DOI] [PubMed] [Google Scholar]
- Morales MG, Vazquez Y, Acuña MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, Cabello-Verrugio C. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol. 2012;44:1993–2002. doi: 10.1016/j.biocel.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 2007. 2007;3:779–786. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley RT, 3rd, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25:1116–1129. doi: 10.1177/0883073810371004. [DOI] [PubMed] [Google Scholar]
- Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, Sacco A, Puri PL. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5:626–639. doi: 10.1002/emmm.201202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Willmann R TREAT-NMD Network and the Wellstone Muscular Dystrophy Cooperative Research Network. Developing standard procedures for murine and canine efficacy studies of DMD therapeutics: Report of two expert workshops on ‘‘Pre-clinical testing for Duchenne dystrophy’’: Washington DC, October 27th-28th 2007 and Zürich, June 30th–July 1st 2008. Neuromuscul Disord. 2009;19:502–506. doi: 10.1016/j.nmd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae Y, Dorchies OM, Stoward PJ, Zimmermann BF, Ritter C, Ruegg UT. Quantitative evaluation of the beneficial effects in the mdx mouse of epigallocatechin gallate, an antioxidant polyphenol from green tea. Histochem Cell Biol. 2012;137:811–827. doi: 10.1007/s00418-012-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Models for Biomedical Research. A New Perspective. Washington DC: The National Academies Press; 1985. [Google Scholar]
- National Research Council. Biomedical Models and Resources: Current Needs and Future Opportunities. Washington DC: The National Academies Press; 1998. [PubMed] [Google Scholar]
- Nelson CA, Hunter RB, Quigley LA, Girgenrath S, Weber WD, McCullough JA, Dinardo CJ, Keefe KA, Ceci L, Clayton NP, McVie-Wylie A, Cheng SH, Leonard JP, Wentworth BM. Inhibiting TGF-β activity improves respiratory function in mdx mice. Am J Pathol. 2011;178:2611–2621. doi: 10.1016/j.ajpath.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Metzger JM, Claflin DR, Faulkner JA. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol. 2008;295:C146–150. doi: 10.1152/ajpcell.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem PP, Hoffman EP, Mittal P, Brown KJ, Schatzberg SJ, Ghimbovschi S, Wang Z, Kornegay JN. Sparing of the dystrophin-deficient cranial sartorius muscle is associated with classical and novel hypertrophy pathways in GRMD dogs. Am J Pathol. 2013;183:1411–1424. doi: 10.1016/j.ajpath.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TM, Ellis JM, Love DR, Davies KE, Gatter KC, Dickson G, Morris GE. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: Presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991;115:1695–1700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LV. The "rescue" of dystrophin synthesis in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 1993a;3:525–531. doi: 10.1016/0960-8966(93)90109-w. [DOI] [PubMed] [Google Scholar]
- Nicholson LV, Johnson MA, Bushby KM, Gardner-Medwin D. Functional significance of dystrophin positive fibres in Duchenne muscular dystrophy. Arch Dis Child. 1993b;68:632–636. doi: 10.1136/adc.68.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LV, Johnson MA, Bushby KM, Gardner-Medwin D, Curtis A, Ginjaar IB, den Dunnen JT, Welch JL, Butler TJ, Bakker E, van Ommen G-JB, Harris JB. Integrated study of 100 patients with Xp21 linked muscular dystrophy using clinical, genetic, immunochemical, and histopathological data. Part 1. Trends across the clinical groups . J Med Genet. 1993c;30:728–736. doi: 10.1136/jmg.30.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonneman DJ, Brown-Brandl T, Jones SA, Wiedmann RT, Rohrer GA. A defect in dystrophin causes a novel porcine stress syndrome. BMC Genomics. 2012;13:233. doi: 10.1186/1471-2164-13-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, Chen F, Schacht J, Pilch DS, Ben-Yosef T, Baasov T. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 2009;52:2836–2845. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chen C, Shen Y, Zhu CH, Wang G, Wang XC, Chen HQ, Zhu MS. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol Cells. 2008;25:531–537. [PubMed] [Google Scholar]
- Partridge T. The potential of exon skipping for treatment for Duchenne muscular dystrophy. J Child Neurol. 2010;25:1165–1170. doi: 10.1177/0883073810371130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 2013;280:4177–4186. doi: 10.1111/febs.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaquin AC, Metzinger L, Léger JJ, Warter JM, Poindron P. Prednisolone enhances myogenesis and dystrophin-related protein in skeletal muscle cell cultures from mdx mouse. J Neurosci Res. 1993;35:363–372. doi: 10.1002/jnr.490350403. [DOI] [PubMed] [Google Scholar]
- Passerini L, Bernasconi P, Baggi F, Confalonieri P, Cozzi F, Cornelio F, Mantegazza R. Fibrogenic cytokines and extent of fibrosis in muscle of dogs with X-linked golden retriever muscular dystrophy. Neuromuscul Disord. 2002;12:828–835. doi: 10.1016/s0960-8966(02)00071-8. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Pearce GW, Walton JN. Progressive muscular dystrophy: The histopathological changes in skeletal muscle obtained by biopsy. J Pathol Bacteriol. 1962;83:535–550. doi: 10.1002/path.1700830228. [DOI] [PubMed] [Google Scholar]
- Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM. Cooperative International Neuromuscular Research Group. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011;76:219–226. doi: 10.1212/WNL.0b013e318207afeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz SW, Morsy M, Welch EM, Jacobson A. Ataluren as an agent for therapeutic nonsense suppression. Annu Rev Med. 2013;64:407–425. doi: 10.1146/annurev-med-120611-144851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012;228:77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter AK, Marshall JL, Crosbie RH. Sarcospan reduces dystrophic pathology: Stabilization of the utrophin-glycoprotein complex. J Cell Biol. 2008;183:419–427. doi: 10.1083/jcb.200808027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JM, Kline W, Canan BD, Ricca DJ, Kaspar B, Delfín DA, DiRienzo K, Clemens PR, Robbins PD, Baldwin AS, Flood P, Kaumaya P, Freitas M, Kornegay JN, Mendell JR, Rafael-Fortney JA, Guttridge DC, Janssen PM. Peptide-based inhibition of NF-κB rescues diaphragm muscle contractile dysfunction in a murine model of Duchenne muscular dystrophy. 2011 . Mol Med. 2011;17:508–515. doi: 10.2119/molmed.2010.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MF, Quinlivan R. Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD004571.pub2. CD004571 (updated in 2011, Issue 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigozzo SR, Da Re L, Romualdi C, Mazzara PG, Galletta E, Fletcher S, Wilton SD, Vitiello L. Revertant fibers in the mdx murine model of Duchenne muscular dystrophy: An age- and muscle-related reappraisal. PLoS One. 2013;8:e72147. doi: 10.1371/journal.pone.0072147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- Politano L, Nigro G, Nigro V, Piluso G, Papparella S, Paciello O, Comi LI. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22:15–21. [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Leahy P, Gong B, Feuerman J, Cheng G, Khanna S. Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy. Hum Mol Genet. 2004;13:257–269. doi: 10.1093/hmg/ddh033. [DOI] [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Leahy P, Gong B, Khanna S. Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) mice. Hum Mol Genet. 2003;12:1813–1821. doi: 10.1093/hmg/ddg197. [DOI] [PubMed] [Google Scholar]
- Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Rüegg UT. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett. 1998;439:357–362. doi: 10.1016/s0014-5793(98)01399-4. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Zheng H, Bogan J, Li J, Yuan Z, Zhang C, Bogan D, Kornegay J, Xiao X. Hydrodynamic limb vein injection of adeno-associated virus serotype 8 vector carrying canine myostatin propeptide gene into normal dogs enhances muscle growth. Hum Gene Ther. 2009;20:1–10. doi: 10.1089/hum.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LF, Wang WC, Fang H, Mao XZ, Huang GL, Chen Y, Zhou HD, Shen Y, Qin LH, Peng D. Expression of NF-κB and osteopontin of synovial fluid of patients with knee osteoarthritis. Asian Pac J Trop Med. 2013;6:379–382. doi: 10.1016/S1995-7645(13)60042-5. [DOI] [PubMed] [Google Scholar]
- Quinn JL, Huynh T, Uaesoontrachoon K, Tatem K, Phadke A, Van der Meulen JH, Yu Q, Nagaraju K. Effects of dantrolene therapy on disease phenotype in dystrophin deficient mdx mice. PLoS Curr. 2013 doi: 10.1371/currents.md.e246cf493a7edb1669f42fb735936b46. 5pii: ecurrents.md.e246cf 493a7edb1669 f42fb 735936b46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;2006:387–397. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation. 2011;124:582–588. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- Reay DP, Yang M, Watchko JF, Daood M, O'Day TL, Rehman KK, Guttridge DC, Robbins PD, Clemens PR. Systemic delivery of NEMO binding domain/IKKγ inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology. Neurobiol Dis. 2011;43:598–608. doi: 10.1016/j.nbd.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus EW, Lyle AN, Weiss D, Landàzuri N, Weber M, Searles C, Taylor WR. miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells. Atherosclerosis. 2013;228:168–174. doi: 10.1016/j.atherosclerosis.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Millward DJ, Wolman SL, Halliday D, Matthews DE. Effects of Duchenne muscular dystrophy on muscle protein synthesis. Nature. 1982;296:165–167. doi: 10.1038/296165a0. [DOI] [PubMed] [Google Scholar]
- Rifai Z, Welle S, Moxley RT, 3rd, Lorenson M, Griggs RC. Effect of prednisone on protein metabolism in Duchenne dystrophy. Am J Physiol. 1995;268:E67–74. doi: 10.1152/ajpendo.1995.268.1.E67. [DOI] [PubMed] [Google Scholar]
- Roberts R, McCune SK. Animal studies in the development of medical countermeasures. Clin Pharmacol Ther. 2008;83:918–920. doi: 10.1038/clpt.2008.23. [DOI] [PubMed] [Google Scholar]
- Rouger K, Larcher T, Dubreil L, Deschamps JY, Le Guiner C, Jouvion G, Delorme B, Lieubeau B, Carlus M, Fornasari B, Theret M, Orlando P, Ledevin M, Zuber C, Leroux I, Deleau S, Guigand L, Testault I, Le Rumeur E, Fiszman M, Chérel Y. Systemic delivery of allogenic muscle stem (MuStem) cells induces long-term muscle repair and clinical efficacy in Duchenne muscular dystrophy dogs. Am J Pathol. 2011;179:2501–2518. doi: 10.1016/j.ajpath.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP. Pathogenesis of muscular dystrophies. Arch Neurol. 1976;33:315–321. doi: 10.1001/archneur.1976.00500050001001. [DOI] [PubMed] [Google Scholar]
- Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Curr Opin Neurol. 2013;26:577–584. doi: 10.1097/WCO.0b013e328364fbaf. [DOI] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Guerron AD, Gordish-Dressman H, Spurney CF, Iantorno M, Hoffman EP, Nagaraju K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One. 2012;7:e34204. doi: 10.1371/journal.pone.0034204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimena MC, Lagrota-Candido J, Quírico-Santos T. Gender dimorphism influences extracellular matrix expression and regeneration of muscular tissue in mdx dystrophic mice. Histochem Cell Biol. 2004;122:535–544. doi: 10.1007/s00418-004-0707-8. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordingnon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Sandri M, Coletto L, Grumati P, Bonaldo P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci. 2013;126:5325–5333. doi: 10.1242/jcs.114041. [DOI] [PubMed] [Google Scholar]
- Schatzberg SJ, Anderson LV, Wilton SD, Kornegay JN, Mann CJ, Solomon GG, Sharp NJ. Alternative dystrophin gene transcripts in golden retriever muscular dystrophy. Muscle Nerve. 1998;21:991–998. doi: 10.1002/(sici)1097-4598(199808)21:8<991::aid-mus2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schatzberg SJ, Olby NJ, Breen M, Anderson LVB, Langford CF, Dickens HF, Wilton SD, Zeiss CJ, Binns MM, Kornegay JN, Morris GE, Sharp NJH. Molecular analysis of a spontaneous dystrophin “knockout” dog. Neuromuscul Disord. 1999;9:289–295. doi: 10.1016/s0960-8966(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Schoenebeck JL, Ostrander EA. The genetics of canine skull shape variation. Genetics. 2013;193:317–325. doi: 10.1534/genetics.112.145284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsby J, Pendrak K, Zadel M, Tian Z, Pham J, Carver T, Acosta P, Barton E, Sweeney HL. Leupeptin-based inhibitors do not improve the mdx phenotype. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1192–1201. doi: 10.1152/ajpregu.00586.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433–439. doi: 10.1016/j.tins.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Serafin WE, Dement SH, Brandon S, Hill EJ, Park CR, Park JH. Interactions of vitamin E and penicillamine in the treatment of hereditary avian muscular dystrophy. Muscle Nerve. 1987;10:685–697. doi: 10.1002/mus.880100804. [DOI] [PubMed] [Google Scholar]
- Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S, Hung WY, Constantinou CD, Dykstra MJ, Roses AD, Bartlett RJ. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Chai J, Maley K, Cozens G, Grounds G, Winn N, Rosenthal N, Grounds MD. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J Cell Sci. 2010;123:960–971. doi: 10.1242/jcs.061119. [DOI] [PubMed] [Google Scholar]
- Shcherbata HR, Yatsenko AS, Patterson L, Sood VD, Nudel U, Yaffe D, Baker D, Ruohola-Baker H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007;26:481–493. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, Tomohiro M, Kornegay JN, Nonaka I, Takeda S. Canine X-linked muscular dystrophy in Japan (CXMDJ) Exp Anim. 2003;52:93–97. doi: 10.1538/expanim.52.93. [DOI] [PubMed] [Google Scholar]
- Shin J, Tajrishi MM, Ogura Y, Kumar A. Wasting mechanisms in muscular dystrophy. Int J Biochem Cell Biol. 2013;45:2266–2279. doi: 10.1016/j.biocel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Siegel AL, Bledsoe C, Lavin J, Gatti F, Berge J, Millman G, Turin E, Winders WT, Rutter J, Palmeiri B, Carlson CG. Treatment with inhibitors of the NF-kappaB pathway improves whole body tension development in the mdx mouse. Neuromuscul Disord. 2009;19:131–139. doi: 10.1016/j.nmd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Siegel AS, Henley S, Zimmerman A, Miles M, Plummer R, Kurz J, Balch F, Rhodes JA, Shinn GL, Carlson CG. The influence of passive stretch and NF-κB inhibitors on the morphology of dystrophic muscle fibers. Anat Rec (Hoboken) 2011;294:132–144. doi: 10.1002/ar.21294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BF, Yue Y, Woods PR, Kornegay JN, Shin J-H, Williams RR, Duan D. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab Invest. 2011;91:216–231. doi: 10.1038/labinvest.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet. 2002;11:2645–2655. doi: 10.1093/hmg/11.21.2645. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4[+]) and cytotoxic (CD8[+]) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Spurney C, Yu Q, Nagaraju K. Speckle tracking analysis of the left ventricular anterior wall shows significantly decreased relative radial strain patterns in dystrophin deficient mice after 9 months of age. Version 2. PLoS Curr. 2011a;3:RRN1273. doi: 10.1371/currents.RRN1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurney CF. Cardiomyopathy of Duchenne muscular dystrophy: Current understanding and future directions. Muscle Nerve. 2011d;44:8–19. doi: 10.1002/mus.22097. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, Van Der Meulen JH, Cha HJ, Pistilli EE, Partridge TA, Hoffman EP, Nagaraju K. Preclinical drug trials in the mdx mouse: Assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurney CF, Guerron AD, Yu Q, Sali A, van der Meulen JH, Hoffman EP, Nagaraju K. Membrane sealant Poloxamer P188 protects against isoproterenol induced cardiomyopathy in dystrophin deficient mice. BMC Cardiovasc Disord. 2011c;11:20. doi: 10.1186/1471-2261-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, Rayavarapu S, van der Meulen J, Hoffman EP, Nagaraju K. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011b;16:87–95. doi: 10.1177/1074248410381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol. 2012;233:597–605. doi: 10.1016/j.expneurol.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Su JB, Cazorla O, Blot S, Blanchard-Gutton N, Ait Mou Y, Barthélémy I, Sambin L, Sampedrano CC, Gouni V, Unterfinger Y, Aguilar P, Thibaud JL, Bizé A, Pouchelon JL, Dabiré H, Ghaleh B, Berdeaux A, Chetboul V, Lacampagne A, Hittinger L. Bradykinin restores left ventricular function, sarcomeric protein phosphorylation, and e/nNOS levels in dogs with Duchenne muscular dystrophy cardiomyopathy. Cardiovasc Res. 2012;95:86–96. doi: 10.1093/cvr/cvs161. [DOI] [PubMed] [Google Scholar]
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Swimmer RA, Rozanski EA. Evaluation of the 6-minute walk test in pet dogs. J Vet Intern Med. 2011;25:405–406. doi: 10.1111/j.1939-1676.2011.0689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Reay DP, Salay MN, Mi MY, Clemens PR, Guttridge DC, Robbins PD, Huard J, Wang B. Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice. Gene Ther. 2010;17:1476–1483. doi: 10.1038/gt.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-β1 blocker. Muscle Nerve. 2011;43:82–87. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitts J. Issues related to the use of canines in toxicologic pathology–issues with pharmacokinetics and metabolism. Toxicol Pathol. 2003;31:17–24. doi: 10.1080/01926230390174896. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Albrecht DE, Lokensgard BE, Spencer MJ. Apoptosis precedes necrosis of dystrophin-deficient muscle. J Cell Sci. 1995;108:2197–2204. doi: 10.1242/jcs.108.6.2197. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, Squire SE, Powell DS, Cozzoli A, Capogrosso RF, Lambert A, Wilson FX, Wren SP, De Luca A, Davies KE. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One 6. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- Townsend D, Turner I, Yasuda s, Martindale J, Davis J, Shillingford M, Kornegay JN, Metzger JM. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest. 2010;120:1140–1150. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BS, Zhao P, Pattison JS, Gordon SE, Granchelli JA, Madsen RW, Folk LC, Hoffman EP, Booth FW. Regenerated mdx mouse skeletal muscle shows differential mRNA expression. J Appl Physiol. 2002;93:537–545. doi: 10.1152/japplphysiol.00202.2002. [DOI] [PubMed] [Google Scholar]
- Uaesoontrachoon K, Wasgewatte Wijesinghe DK, Mackie EJ, Pagel CN. Osteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Dis Model Mech. 2013;6:197–205. doi: 10.1242/dmm.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, Fiel MI, Nieto N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3 K/pAkt/NFκB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine BA, Cooper BJ, Cummings JF, de Lahunta A. Canine X-linked muscular dystrophy: morphologic lesions. J Neurol Sci. 1990;97:1–23. doi: 10.1016/0022-510x(90)90095-5. [DOI] [PubMed] [Google Scholar]
- Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: Clinical studies . J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Valentine BA, Cooper BJ, Gallagher EA. Intracellular calcium in canine muscle biopsies. J Comp Pathol. 1989:223–230. doi: 10.1016/0021-9975(89)90099-6. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H, Shansky J, Benesch-Lee F, Skelly K, Spinazzola JM, Saponjian Y, Tseng BS. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J. 2009;23:3325–3334. doi: 10.1096/fj.09-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119:1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignos PJ, Jr, Spencer GE, Jr, Archibald JC. Management of muscular dystrophy of childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- Vulin A, Barthélémy I, Goyenvalle A, Thibaud JL, Beley C, Griffith G, Benchaouir R, le Hir M, Unterfinger Y, Lorain S, Dreyfus P, Voit T, Carlier P, Blot S, Garcia L. Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther. 2012;20:2120–2133. doi: 10.1038/mt.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadosky KM, Li L, Rodríguez JE, Min J, Bogan D, Gonzalez J, Kornegay JN, Willis M. Regulation of the calpain and ubiquitin proteasome systems in a canine model of Duchenne muscular dystrophy. Muscle Nerve. 2011;44:553–562. doi: 10.1002/mus.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Hamed S, Hadley DW, Gropman AL, Burstein AH, Escolar DM, Hoffman EP, Fischbeck KH. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci U S A. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- Walker DK. The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. Br J Clin Pharmacol. 2004;58:601–608. doi: 10.1111/j.1365-2125.2004.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley GL, Arechavala-Gomeza V, Fernandez-Fuente M, Burke MM, Nagel N, Holder A, Stanley R, Chandler K, Marks SL, Muntoni F, Shelton GD, Piercy RJ. A Duchenne muscular dystrophy gene hot spot mutation in dystrophin-deficient Cavalier King Charles Spaniels is amenable to exon 51 skipping. PloS One. 2010;5:e8647. doi: 10.1371/journal.pone.0008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, Chamberlain JS, Kuhr CS. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: Implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988;52:503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Wehling-Henricks M, Lee JJ, Tidball JG. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- Weller B, Massa R, Karpati G, Carpenter S. Glucocorticoids and immunosuppressants do not change the prevalence of necrosis and regeneration in mdx skeletal muscles. Muscle Nerve. 1991;14:771–774. doi: 10.1002/mus.880140812. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R, De Luca A, Benatar M, Grounds M, Dubach J, Raymackers JM, Nagaraju K TREAT-NMD Neuromuscular Network. Enhancing translation: Guidelines for standard pre-clinical experiments in mdx mice. Neuromuscul Disord. 2012;22:43–49. doi: 10.1016/j.nmd.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord. 2009;19:241–249. doi: 10.1016/j.nmd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Wilson LA, Cooper BJ, Dux L, Dubowitz V, Sewry CA. Expression of utrophin (dystrophin-related protein) during regeneration and maturation of skeletal muscle in canine X-linked muscular dystrophy. Neuropathol Appl Neurobiol. 1994;20:359–367. doi: 10.1111/j.1365-2990.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Yang Q, Tang Y, Imbrogno K, Lu A, Proto JD, Chen A, Guo F, Fu FH, Huard J, Wang B. AAV-based shRNA silencing of NF-κB ameliorates muscle pathologies in mdx mice. Gene Ther. 2102;19:1196–1204. doi: 10.1038/gt.2011.207. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Nakamura A, Nagata T, Saito T, Kobayashi M, Aoki Y, Echigoya Y, Partridge T, Hoffman EP, Takeda S. Extensive and prolonged restoration of dystrophin expression with vivo-morpholino-mediated multiple exon skipping in dystrophic dogs. Nucleic Acid Ther. 2012;22:306–315. doi: 10.1089/nat.2012.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zheng L, Li Y, Li C, Ma C, Li Y, Li X, Hao P. A cross-species analysis method to analyze animal models’ similarity to human's disease state. BMC Syst Biol. 2012;6:S18. doi: 10.1186/1752-0509-6-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K, Miyagoe Y, Yamamoto K, Nabeshima Y, Dickson G, Takeda S. Effective restoration of dystrophin-associated proteins in vivo by adenovirus-mediated transfer of truncated dystrophin cDNAs. FEBS Lett. 1998;425:329–336. doi: 10.1016/s0014-5793(98)00251-8. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, Hijikata T, Miyagoe-Suzuki Y, Takeda S. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Yugeta N, Urasawa N, Fujii Y, Yoshimura M, Yuasa K, Wada MR, Nakura M, Shimatsu Y, Tomohiro M, Takahashi A, Machida N, Wakao Y, Nakaura A, Takeda S. Cardiac involvement in Beagle-based canine x-linked muscular dystrophy in Japan (CXMDJ): Electrocardiographic, echocardiographic, and morphologic studies. BMC Cardiovasc Disord. 2006;6:47–59. doi: 10.1186/1471-2261-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu Y, Pan L, Chen T, Chen Z, Zhao R. Effect of simvastatin on collagen I deposition in non-infarcted myocardium: Role of NF-κB and osteopontin. Can J Physiol Pharmacol. 2010;88:1026–1034. doi: 10.1139/y10-075. [DOI] [PubMed] [Google Scholar]
- Ziter FA, Allsop KG, Tyler FH. Assessment of muscle strength in Duchenne muscular dystrophy. Neurology. 1977;27:981–984. doi: 10.1212/wnl.27.10.981. [DOI] [PubMed] [Google Scholar]
- Zucconi E, Valadares MC, Vieira NM, Bueno CR, Jr, Secco M, Jazedje T, da Silva HC, Vainzof M, Zatz M. Ringo: Discordance between the molecular and clinical manifestation in a golden retriever muscular dystrophy dog. Neuromuscul Disord. 2010;20:64–70. doi: 10.1016/j.nmd.2009.10.011. [DOI] [PubMed] [Google Scholar]