Abstract
Despite the progress in our understanding of pathogeneses and the identification of etiologies of peripheral neuropathy, idiopathic neuropathy remains common. Typically, attention to peripheral neuropathies resulting from exposure to environmental agents is limited relative to more commonly diagnosed causes of peripheral neuropathy (diabetes and chemotherapeutic agents). Given that there are more than 80,000 chemicals in commerce registered with the Environmental Protection Agency and that at least 1000 chemicals are known to have neurotoxic potential, very few chemicals have been established to affect the peripheral nervous system (mainly after occupational exposures). A wide spectrum of exposures, including pesticides, metals, solvents, nutritional sources, and pharmaceutical agents, has been related, both historically and recently, to environmental toxicant-induced peripheral neuropathy. A review of the literature shows that the toxicity and pathogeneses of chemicals adversely affecting the peripheral nervous system have been studied using animal models. This article includes an overview of five prototypical environmental agents known to cause peripheral neuropathy—namely, organophosphates, carbon disulfide, pyridoxine (Vitamin B6), acrylamide, and hexacarbons (mainly n-hexane, 2,5-hexanedione, methyl n-butyl ketone). Also included is a brief introduction to the structural components of the peripheral nervous system and pointers on common methodologies for histopathologic evaluation of the peripheral nerves.
Keywords: acrylamide, carbon disulfide, environmental toxicant, hexacarbon, organophosphate, peripheral neuropathy, pyridoxine, vitamin B6
Introduction
Worldwide, the incidence of peripheral neuropathy in humans due to toxicants ranges across a broad spectrum that includes acute and accidental exposures, as well as chronic low-dose exposures in industrial or occupational settings, exposures in the ambient environment as a result of increased commercial use of chemicals, and less commonly, intentional exposures associated with recreational abuse, suicide, chemical warfare, and terrorist activities. Classes of toxic agents include pesticides, chemical and organic solvents, metals, chemical contaminants, nutritional ingredients, and pharmaceutical drugs (Spencer and Schaumburg 2000). Examples of specific toxicants affecting the peripheral nervous system (PNS) include, but are not limited to, organic solvents (n-hexane, trichloroethylene, methyl-n-butyl ketone, toluene, and styrene), industrial chemicals (carbon disulfide, dimethylaminoproprionitrile, and acrylamide), metals (arsenic, organic mercury, thallium, and lead), and pharmaceutical sources (vitamin B6 or pyridoxine, immunosuppressants, and cancer chemotherapeutics). Additionally, organophosphate compounds are not only present as active ingredients in pesticides but have also been identified as contaminants in cooking oil and in illicit alcohol production (during the 1920s Prohibition era in the United States). More recently, emerging toxic neuropathies include an incidence of exposure to aerosolized porcine neural tissue that resulted in polyradiculoneuropathy in swine abattoir workers (Holzbauer et al. 2010; Lachance et al. 2010). A list of well-known toxic agents inducing peripheral neuropathy in humans is summarized in Table 1.
Table 1.
Examples of human exposures resulting in peripheral neuropathy
| Substance | Location | Comments | Reference |
|---|---|---|---|
| Industrial chemical | |||
| Acrylamide | Polyacrylamide production | (LoPachin et al. 2003) | |
| Canada | Grout worker | (Auld and Bedwell 1967; Kjuus et al. 2004) | |
| Solvents | |||
| n-Hexane | Iran | Shoemakers | (Neghab et al. 2012) |
| United States | Furniture makers | (Herskowitz et al. 1971) | |
| India | Screen printers | (Puri et al. 2007) | |
| Methyl n-butyl ketone | United States | Fabric plant workers | (Allen et al. 1975; Mendell et al. 1974) |
| United States | Spray painters | (Mallov 1976) | |
| South Africa | Polymer factory workers | (Myers and Macun 1991) | |
| Carbon disulfide | China Italy |
Viscose rayon production workers | (Chu et al. 1995; Corsi et al. 1983) |
| 2-t-Butylazo-2-hydroxy-5-methylhexane | United States | Workers in reinforced plastic bathtub manufacture | (Horan et al. 1985) |
| Organic solvent/mixtures (n-hexane, methyl ethyl ketone, toluene) | Germany United States |
Glue sniffers and Huffer's neuropathy | (Altenkirch et al. 1977; Prockop et al. 1974; Towfighi et al. 1976) |
| Trichloroethylene | United States | Contaminated well water | (White et al. 1997) |
| Pesticides | |||
| Organophosphates | Italy | Agricultural workers | (Lotti and Moretto 2005) |
| Turkey | Suicides | (Ergun et al. 2009) | |
| Iran | Chemical warfare | (Holisaz et al. 2007) | |
| Japan | Terrorist activity (Sarin) | (Yokoyama et al. 1998) | |
| Morocco | Cooking oil (triorthocresylphosphate) | (Smith and Spalding 1959) | |
| South Africa | Cooking oil (triorthocresylphosphate) | (Susser and Stein 1957) | |
| Sri Lanka | Cooking oil (triorthocresylphosphate) | (Senanayake and Jeyaratnam 1981) | |
| United States | Beverage production (alcohol during Prohibition era) (triorthocresylphosphate) | (Aring 1942; Morgan and Penovich 1978; Woolf 1995) | |
| Metals | |||
| Mercury | Japan | Minamata disease | (Miyakawa et al. 1976; Ninomiya et al. 2005) |
| Iraq | Pesticide-coated grain | (Bakir et al. 1973) | |
| Arsenic | United States | Arsenic smelter workers | (Feldman et al. 1979) |
| Thallium | United Kingdom | Intentional ingestion of rodenticides | (Cavanagh et al. 1974) |
| Lead | India | Battery factory workers | (Shobha et al. 2009) |
| Pharmaceutical agents | |||
| Thiocyanate | Nigeria | Cassava | (Osuntokun et al. 1970) |
| Vitamin B6 (pyridoxine) | United States | Nutritional supplement | (Scott et al. 2008) |
| Clioquinol | India | Antifungal and antiprotozoal drug | (Wadia 1984) |
| Cancer chemotherapeutics | Australia | Platinum compounds, taxanes, vinca alkaloids, 5-fluorouracil | (Schloss et al. 2013; Zheng et al. 2012) |
| Cholesterol-lowering statins | 3-hydroxy-3-methylglutaryl-Coenzyme A reductase inhibitors (HMG-CoA) (lovastatin, pravastatin, simavastin) | (Chong et al. 2004) | |
| Other | |||
| Aerosolized porcine neural tissue | United States | Swine abattoirs | (Holzbauer et al. 2010; Lachance et al. 2010) |
| Toxic oil syndrome | Spain | Rapeseed oil | (Altenkirch et al. 1988) |
The proportional incidence of peripheral neuropathy specifically due to environmental or industrial toxicants compared with other etiologies is unclear. Despite advances in the identification of etiologies of peripheral neuropathy, it is evident that in clinical settings idiopathic polyneuropathy remains common in humans (Weimer and Sachdev 2009). Given the vast range of neurotoxicants resulting in peripheral neuropathy, compounded with the inherent anatomic and physiologic complexity of the organization of the PNS, it is imperative that a thorough examination of the PNS be conducted during clinical evaluation in humans, in preclinical safety evaluation studies of potential pharmaceutical agents, and in the hazard identification of animal toxicity studies for screening of environmental chemicals. What follows is a brief introduction of the structural components of the PNS relevant to toxic neuropathies, a short review of five prototypical PNS toxicants, and a brief overview of current approaches to histopathologic examination of peripheral nerves in animal studies.
Overview of the PNS
The challenge with assessing the effects of environmental toxicants on the PNS lies in that, both anatomically and functionally, the organization of the PNS is remarkably extensive. Moreover, environmental toxicants can affect any component or multiple components of the PNS. In general, the PNS includes the innervation of somatic (skeletal muscle) and visceral (autonomic) components of the nervous system by afferent (sensory) and efferent (motor) fibers. Somatic afferent fibers carry information from special organs for vision, hearing, and balance; from sensory receptors in skin for pain, touch, and temperature; and from receptors controlling proprioception in muscles and joints. Visceral afferents convey impulses (pain or reflex sensations) from the internal organs, glands, and blood vessels. Somatic afferents innervate striated muscle, whereas visceral efferents supply smooth muscles of blood vessels, glands, and the gut. Peripheral neurotoxicants affecting somatic sensory-motor fibers can impact sensory loss (changes in sensitivity to vibration, touch, and proprioception) and motor weakness in distal extremities (damage to nerves or neuromuscular junctions leading to muscle atrophy). Peripheral neurotoxicants affecting autonomic fibers can result in abnormal sweating, cardiovascular changes, or disturbances in the gastrointestinal and genito-urinary systems.
Histologically, although the major components of the PNS are axons, myelin sheaths, and Schwann cells, ganglia contain neuronal cell bodies and satellite cells. Because the cell bodies of motor neurons lie within the spinal cord and brainstem and some peripheral sensory neurons have processes extending in spinal and brainstem tracts, a true distinction between the central nervous system and the PNS is superficial.
Environmental Agents Toxic to the PNS
Typically, environmental toxicants can cause neuronopathies (primarily impacting the neuronal cell bodies), axonopathies (affecting the axon), and myelinopathies (involvement of the myelin sheath or the Schwann cell). Neuronopathies have been reported after exposure to pyridoxine, arsenic, and thallium. Axonopathies are seen with organophosphates and carbon disulfide exposures, whereas myelinopathies can be induced by trichloroethylene. Knowledge and understanding of the pattern by which neural cells are affected has been gained from experimental and morphologic studies. In fact, in most of the human intoxications of the nervous system, knowledge of the structural changes is based almost exclusively on laboratory animal studies. A few select peripheral neurotoxicants taken from the list in Table 1 are reviewed in the following sections.
Organophosphorus Ester-Induced Delayed Neurotoxicity
Organophosphorus ester-induced delayed neurotoxicity (OPIDN) is an entity that has been recognized in man for more than 100 years (Ehrich and Jortner 2002, 2010). It is distinct from acute cholinergic organophosphate poisoning, although some, but not all, OPIDN-inducing agents also elicit acute toxicity. OPIDN is manifested by degeneration of distal regions of large, long axons and thus involves myelinated fibers in the peripheral (and central) nervous system. For the classical form of OPIDN (described here), neurotoxicants must have their phosphorus in a pentavalent state and include direct acting agents, such as mipafox, diisopropyl phosphofluoridate, and phenyl saligenin phosphate, and protoxicants, such as leptophos and tri-ortho-tolyl (cresyl) phosphate (Ehrich and Jortner 2010). As part of the name (i.e., “delayed”) implies, there is a latent period between exposure to a toxic dose of the organophosphate and clinical recognition of the disease. Clinical signs in man include bilateral weakness progressing to flaccidity, tingling, loss of sensation, locomotor difficulties, and abnormal reflexes of the limbs, especially distally (Ehrich and Jortner 2002). In the domestic chicken, the standard animal model, similar signs of weakness and incoordination, initially affecting the legs, are also noted (Cavanagh 1954). Peripheral nerve lesions in experimental studies in chickens include axonal swelling with associated granular degeneration of the cytoskeleton or aggregation of dark-stained membranous masses, altered mitochondria, and dense bodies (Figure 1). The altered axons are unable to maintain their myelin sheaths, and the altered fibers undergo phagocytosis and degradation within Schwann cells or macrophages (Figure 1), resembling changes seen in Wallerian degeneration. This evolves to formation of columns of Schwann cells (the so-called bands of Büngner), which provide sites for axonal regeneration (Jortner et al. 1989). As the clinical signs suggest, lesions are more severe distally but may progress proximally along the nerve trunk. There is associated similar but more slowly evolving bilateral myelinated fiber degeneration in long tracts of the central nervous system, but as expected, regeneration does not occur in these regions.
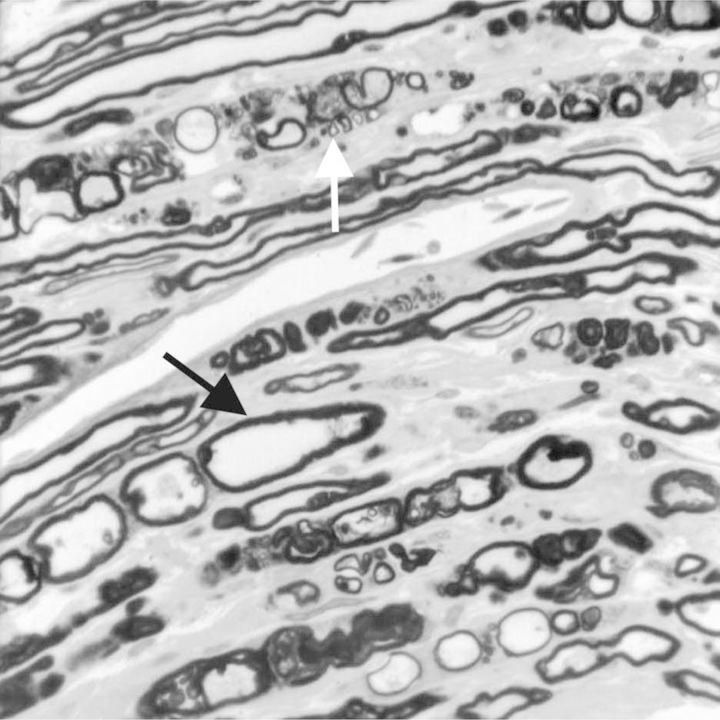
Figure 1.
Tangential section of perfusion-fixed sciatic nerve embedded in epoxy resin of a chicken exposed to a neurotoxic dose (2.5 mg/kg) of phenyl saligenin phosphate (intramuscular) 15 days before it was killed. Several stages of myelinated fiber degeneration are seen, including a swollen pale stained axon (black arrow) and a fiber undergoing degradation (Wallerian-type) within a phagocyte (white arrow). Toluidine blue and safranin stain.
The precise mechanisms of neurotoxicity are not known, but there is a well-described association of inhibition and aging, a related intramolecular rearrangement, of the nervous system enzyme neurotoxic esterase (neuropathy target esterase, NTE) induced by such agents, and the subsequent development of OPIDN. The physiologic functions of NTE are unknown, as is its role in OPIDN-induced nerve fiber degeneration (Ehrich and Jortner 2002). There are temporal and dose level–related aspects to the association of toxicant exposure, NTE inhibition, and subsequent OPIDN. As an example, using a single neurotoxic dose in the chicken leading to inhibition of at least 70% of the brain NTE by 48 hours after exposure is likely to be followed by OPIDN in a 7 to 10 day period, by which time NTE has largely regenerated. In addition to work with NTE, studies have suggested altered threshold activity of peripheral nerve, disturbed axonal transport, increased protease activity, and abnormal protein phosphorylation as mechanistic factors in the axonal degeneration of OPIDN (Abou-Donia 1995; Ehrich and Jortner 2002; El-Fawal et al. 1990; Pope et al. 1995; Richardson 1995). There appears to be an age-related issue in OPIDN, as mature chickens are more susceptible than chicks.
Carbon Disulfide
Carbon disulfide is used in the viscose production of cellophane and rayon, with approximately 76 million pounds released into the atmosphere annually in the United States during production. Exposure to carbon disulfide in humans results in tingling and numbness of the extremities and weakness of limbs. In rats exposed to carbon disulfide, neuromuscular deficits are more pronounced in the hindlimbs where there is decreased grip strength and gait alterations (Moser et al. 1998). In both human occupational settings and experimental animal models, exposure to carbon disulfide can result in prominent central and peripheral neuropathies with a predilection for long, large-diameter myelinated axons (Graham et al. 1995). The axonopathy is first observed in the long distal axons and is characterized by axonal swelling (Figure 2) with the accumulation of neurofilament proteins in distal and sensory and motor nerve tracts and is most often detected proximal to the nodes of Ranvier (Gottfried et al. 1985; Sills et al. 1998). The accumulation of neurofilaments in the axoplasm of swollen myelinated axons displaces mitochondria and neurotubules to the periphery of the axon. The swollen axons may have thin myelin sheaths (Figure 2) that have a normal configuration but are composed of a reduced number of myelin lamellae. After continuous exposure to carbon disulfide, degeneration (Figure 3) and regeneration of axons in the PNS may occur. A common mechanism has been proposed for the axonal swelling and degeneration observed after exposure to chemicals that cause axonopathies such as carbon disulfide and 2,5-hexanedione. Carbon disulfide covalently modifies neurofilament proteins. Specifically, carbon disulfide has been shown to cause Lys-Lys thiourea cross-linking of neurofilament proteins that results in the accumulation of neurofilaments in the axon, which causes the characteristic lesion of axonal swelling (Valentine et al. 1997).
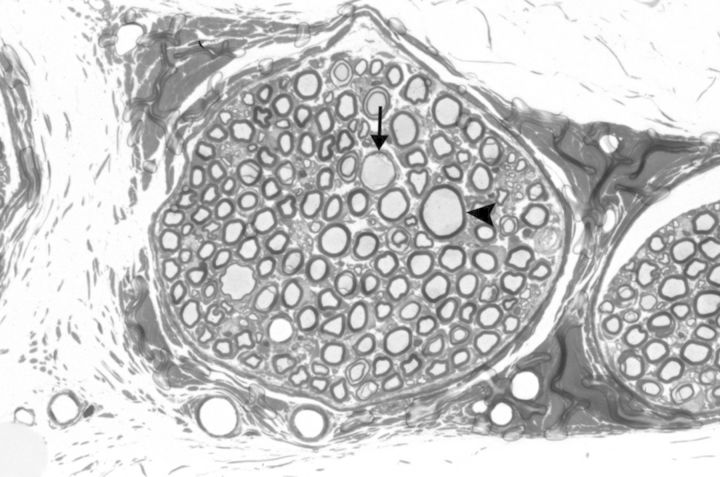
Figure 2.
Muscular branch of the posterior tibial nerve (perfusion fixed and embedded in epoxy resin) with multiple swollen axons (arrow head and arrow) within a nerve fascicle from a rat exposed to 800 ppm of carbon disulfide (by inhalation) for 13 weeks (6 hours/day for 5 days/week). Note the thinning of the myelin sheath in a swollen axon (arrow). Toluidine blue stain.
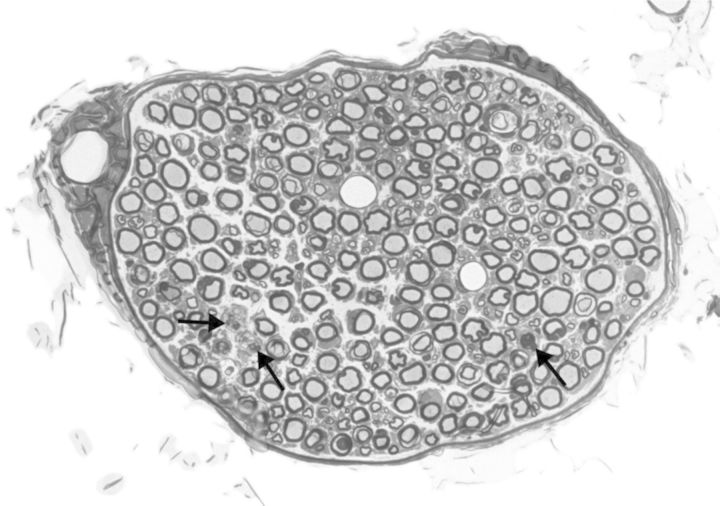
Figure 3.
Muscular branch of the posterior tibial nerve (perfusion fixed and embedded in epoxy resin) illustrating axonal degeneration (arrows) within a nerve fascicle from a rat exposed to 800 ppm of carbon disulfide (by inhalation) for 13 weeks (6 hours/day for 5 days/week). Toluidine blue stain.
Hexacarbons (n-Hexane, Methyl n-Butyl Ketone, 2,5-Hexanedione)
2,5-Hexanedione is the active γ-diketone metabolite of n-hexane and methyl n-butyl ketone. The parent hexacarbons (n-hexane and methyl n-butyl ketone) have been implicated in a multitude of occupational and intentional inhalational abuse exposure settings (Spencer and Schaumburg 1980). In humans, teased myelinated nerve fiber preparations from nerve biopsies after occupational exposure to n-hexane illustrated paranodal giant axonal swellings accompanied by myelin retraction, and electron microscopy showed neurofilament accumulation in axonal swelling (Rizzuto et al. 1977). As with carbon disulfide, experimental studies in animals have shown axonal swellings containing neurofilament masses as the morphologic hallmarks of hexacarbon toxicity (Graham et al. 1982; Spencer and Schaumburg 1980). Specifically, increased numbers of malaligned neurofilaments (Gottfried et al. 1985; Spencer and Schaumburg 1977) within areas of axonal swelling are seen in both hexanedione and carbon disulfide exposure. There are also decreased numbers of microtubules, sequestration of axoplasmic organelles, and expansion of Schwann cell cytoplasm (Gottfried et al. 1985; Spencer and Schaumburg 1977). As noted above, there may be a common molecular mechanism of toxicity between 2,5-hexanedione and carbon disulfide. In addition to axonal swelling, experimental animal studies have shown that axonal atrophy is an important pathologic change observed with various dosing paradigms (Lehning et al. 1995; LoPachin et al. 2000). Although pigeons, chickens, mice, guinea pigs, cats, dogs, and monkeys have been used in experimental studies to delineate mechanisms of peripheral neuropathy, hexacarbon neuropathy has been most thoroughly investigated in the rat (Spencer and Schaumburg 1980).
Pyridoxine (Vitamin B6) Neuropathy
Although not a classical environmental toxicant, pyridoxine (vitamin B6) is included here because it illustrates neuronopathy, an important form of toxic neuropathy. Pyridoxine is a dietary requirement and is a coenzyme in many biologic reactions, but megadoses of this vitamin are neurotoxic, eliciting bilateral sensory neuropathy in humans and experimental animals. Examples of such excessive dosages are daily oral exposures of 7 to 10 grams for 2 months in humans (Schaumburg 2000) or 100 to 300 mg/kg for 12 weeks or 600 to 1200 mg/kg intraperitoneally for 6 to 10 days in rats (Xu et al. 1989). Such toxic exposures lead to neuronopathy, a condition in which the primary insult is in the neuronal cell body (Krinke and Fitzgerald 1988). For pyridoxine, such injury is manifested by injury to the soma of larger neurons of primary sensory ganglia, such as dorsal root ganglia. This process may lead to progressive injury to the neuron, resulting in necrosis (Figure 4). A consequence is the inability of the injured neuronal cell body to maintain its long axon. Thus, there is axonal degeneration progressing to myelinated fiber degeneration, which is more severe distally (Jortner 2000; Krinke and Fitzgerald 1988). In severe or prolonged intoxication, proximal fiber levels are affected, such as dorsal spinal roots (Figure 4). Central axonal projections from affected ganglionic neurons, such as in the gracile fasciculus, are also affected. If injury is sublethal, cessation of pyridoxine exposure may result in clinical improvement, likely an expression of axonal repair or regeneration, with the latter being restricted to peripheral nerves (Schaumburg 2000; Windebank et al. 1985). If neurons are severely injured or necrotic, then such axonal restoration is not possible. The mechanism of pyridoxine neuronopathy is not fully understood, but it is thought that high circulating levels of pyridoxine might have a direct toxic effect on neurons of the peripheral sensory ganglia (Jortner 2000), which may relate to the presence of a lower (“leaky”) blood–nerve barrier in such regions (Olsson 1984). The more complete blood–brain barrier in the central nervous system, along with a saturable pyridoxine transport system, protects neurons in those regions from high blood levels of pyridoxine (Schaumburg 2000).
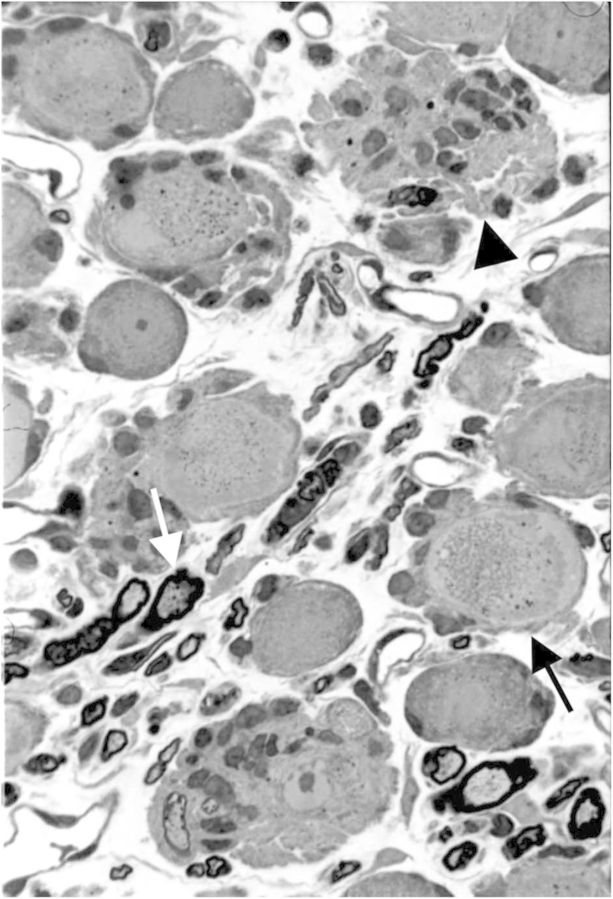
Figure 4.
Dorsal root ganglion of a rat (perfusion fixed and embedded in epoxy resin) exposed to a toxic dose (600 mg/kg) of pyridoxine administered intraperitoneally twice a day for 4 days and killed 2 days after the last exposure. Degradative change is seen in the soma of a neuron (black arrow), which progresses to necrosis manifest by satellite cell phagocytosis of an affected neuron cell body (neuronophagia) (arrowhead). There also is axonal degeneration of a myelinated dorsal root fiber within the ganglion (white arrow). Toluidine blue and safranin stain.
Acrylamide Neuropathy
Acrylamide is a water-soluble vinyl monomer used to produce polyacrylamide (a polymeric form) that is used extensively in chemical industries, in molecular research laboratories, and as a by-product in certain food preparations (Spencer and Schaumburg 1974; Tareke et al. 2000). It is important to note that only the monomeric form is neurotoxic. With the initial recognition of skeletal muscle ataxia and weakness in humans exposed to acrylamide (Prineas 1969), the elucidation of PNS toxicity preceded the demonstration of central nervous system target sites in animal studies. Today, the hallmark morphologic effects of acrylamide are considered to include the progressive loss of cerebellar Purkinje cells (Cavanagh and Nolan 1982; Lehning et al. 2002) and, peripherally, the degeneration of sensory and somatomotor nerve terminals (LoPachin et al. 2003). Although animal studies demonstrate axonal degeneration resulting from chronic and low-dose exposures (Crofton et al. 1996; Prineas 1969), it is interesting to note that even with high doses, the earliest neuropathologic features of acrylamide neurotoxicity include the PNS—specifically, degeneration at the nerve terminals (Lehning et al. 1998). It is now understood that the effects at the motor nerve terminals of hindlimb neuromuscular junctions exhibit degenerative alterations before changes in preterminal axons (DeGrandchamp and Lowndes 1990). Changes in the preterminal fibers subsequently progress to the intramuscular nerves and, finally, the main nerve tracts, suggesting a sequential “dying-back” process (Cavanagh 1964). The progressive changes in the distal nerve are seen only as a function of continued acrylamide exposure. Similar patterns of dying-back degeneration have been produced in rats, dogs, cats, and primates (Spencer and Schaumburg 1974). Elegant morphometric and ultrastructural studies of neuromuscular junctions after acrylamide exposure have shown swellings containing tubulovesicular profiles, neurofilaments, and degenerating mitochondria (DeGrandchamp and Lowndes 1990; DeGrandchamp et al. 1990). The molecular mechanism of toxicity remains to be determined. Relevant hypotheses include impaired membrane fusion processes mediating exocytosis and vesicle trafficking in the nerve terminals and Purkinje cells (centeral nervous system) as well as axoplasmic changes in ionic balance (Lehning et al. 1998).
Current Approaches to Histopathologic Evaluation of Peripheral Nerves in Animal Studies
There are several excellent references that address sampling, fixation, and tissue processing for peripheral nerves (Fix and Garman 2000; Jortner 2011; Kasukurthi et al. 2009; King 1999; Krinke et al. 2000; Lauria et al. 2005; Lauria et al. 2009). Ideally, sampling of tissues should include both proximal and distal levels for peripheral nerves, as well as peripheral ganglia such as the trigeminal ganglion or dorsal root ganglia (Jortner 2011), or autonomic ganglia in visceral organs. Although perfusion fixation is the preferred method, immersion fixation has also been used (Kasukurthi et al. 2009). Specific details on types of fixative and concentrations for immunohistochemical endpoints and/or electron microscopy have been published (Fix and Garman 2000). Although epoxy resin embedding is the preferred approach, paraffin embedding is still useful for light microscopic examination. Special procedures for histopathologic evaluation include morphometric assessments of individual teased nerve fiber preparations (Krinke et al. 2000), as well as routine cross-sections of nerves (King 1999). Recent advances include a diagnostic assessment of cutaneous nerve fibers, such as in the rat footpad, to evaluate peripheral neuropathy (Lauria et al. 2005; Lauria et al. 2009). Typically, in routine toxicity screening studies, practice recommendations for the peripheral nerve include longitudinal and transverse sections of the sciatic and/or tibial nerve trunk. Although bilateral examinations are encouraged, unilateral examinations are acceptable. In addition, bilateral examination of the eyes examined through the middle of the globe and nerve are routinely included (Bolon et al. 2013).
Conclusions
As noted in Table 1, there is a broad spectrum of chemicals that can cause peripheral neuropathy. Given that there are at least 80,000 chemicals in the United States registered for use with the US Environmental Protection Agency (US Environmental Protection Agency, Office of Pollution, Prevention, and Toxics 2010), it is important to note that at least 1000 chemicals are known to be neurotoxic in laboratory animal studies, of which 201 are known to be neurotoxic in humans (Grandjean and Landrigan 2006). At this time, it is unclear how many are PNS neurotoxicants, central nervous system neurotoxicants, or both. In conclusion, evaluation of the peripheral nerve during clinical examination in humans and routine screening in toxicity/safety animal studies is vital for complete evaluation of the nervous system. Therefore, contemporary and future directions in this field will benefit from including peripheral nerve evaluation during routine clinical examination in humans, preclinical safety evaluation studies of therapeutics in animals, and routine toxicity screening of environmental agents with unknown potential for toxicity in animal studies.
Acknowledgments
We thank the reviewers, Drs. David E. Malarkey and Arun R. Pandiri, for their input toward the improvement of this article. We are grateful to Emily Singletary for her technical expertise with the image preparation. This research was supported in part by the Division of the National Toxicology Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS). This article may be the work product of an employee or group of employees of the NIEHS, NIH; however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of the NIEHS, NIH, or US government.
References
- Abou-Donia MB. Organophosphorus Pesticides. In: Chang W, Dyer RS, editors. Handbook of Neurotoxicology. New York: Marcel Dekker. p: Marcel Dekker, Inc; 1995. pp. 419–473. [Google Scholar]
- Allen N, Mendell JR, Billmaier DJ, Fontaine RE, O'Neill J. Toxic polyneuropathy due to methyl n-butyl ketone. An industrial outbreak. Arch Neurol. 1975;32:209–218. doi: 10.1001/archneur.1975.00490460025001. [DOI] [PubMed] [Google Scholar]
- Altenkirch H, Mager J, Stoltenburg G, Helmbrecht J. Toxic polyneuropathies after sniffing a glue thinner. J Neurol. 1977;214:137–152. doi: 10.1007/BF02430351. [DOI] [PubMed] [Google Scholar]
- Altenkirch H, Stoltenburg-Didinger G, Koeppel C. The neurotoxicological aspects of the toxic oil syndrome (TOS) in Spain. Toxicology. 1988;49:25–34. doi: 10.1016/0300-483x(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Aring CD. The systemic nervous affinity of troorthocresyl phosphate (Jamaican ginger palsy). Brain. 1942;65:34–47. [Google Scholar]
- Auld RB, Bedwell SF. Peripheral neuropathy with sympathetic overactivity from industrial contact with acrylamide. Canad Med Ass J. 1967;96:652–654. [PMC free article] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Bolon B, Garman RH, Pardo ID, Jensen K, Sills RC, Roulois A, Radovsky A, Bradley A, Andrew-Jones L, Butt M, Gumprecht L. STP position paper: recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during non-clinical general toxicity studies. Toxicol Pathol. 2013;41:1028–1048. doi: 10.1177/0192623312474865. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. The toxic effects of triortho-cresyl phosphate on the nervous system; an experimental study in hens. J Neurol Neurosurg Psychiatry. 1954;17:163–172. doi: 10.1136/jnnp.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JB. The significance of the "dying back" process in experimental and human neurological disease. Int Rev Exp Pathol. 1964;3:219–267. [PubMed] [Google Scholar]
- Cavanagh JB, Fuller NH, Johnson HR, Rudge P. The effects of thallium salts, with particular reference to the nervous system changes. A report of three cases. Q J Med. 1974;43:293–319. [PubMed] [Google Scholar]
- Cavanagh JB, Nolan CC. Selective loss of Purkinje cells from the rat cerebellum caused by acrylamide and the responses of beta-glucuronidase and beta-galactosidase. Acta Neuropathol. 1982;58:210–214. doi: 10.1007/BF00690803. [DOI] [PubMed] [Google Scholar]
- Chong PH, Boskovich A, Stevkovic N, Bartt RE. Statin-associated peripheral neuropathy: Review of the literature. Pharmacotherapy. 2004;24:1194–203. doi: 10.1592/phco.24.13.1194.38084. [DOI] [PubMed] [Google Scholar]
- Chu CC, Huang CC, Chen RS, Shih TS. Polyneuropathy induced by carbon disulphide in viscose rayon workers. Occup Environ Med. 1995;52:404–407. doi: 10.1136/oem.52.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi G, Maestrelli P, Picotti G, Manzoni S, Negrin P. Chronic peripheral neuropathy in workers with previous exposure to carbon disulphide. Br J Ind Med. 1983;40:209–211. doi: 10.1136/oem.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Padilla S, Tilson HA, Anthony DC, Raymer JH, MacPhail RC. The impact of dose rate on the neurotoxicity of acrylamide: The interaction of administered dose, target tissue concentrations, tissue damage, and functional effects. Toxicol Appl Pharmacol. 1996;139:163–176. doi: 10.1006/taap.1996.0155. [DOI] [PubMed] [Google Scholar]
- DeGrandchamp RL, Lowndes HE. Early degeneration and sprouting at the rat neuromuscular junction following acrylamide administration. Neuropathol Appl Neurobiol. 1990;16:239–254. doi: 10.1111/j.1365-2990.1990.tb01160.x. [DOI] [PubMed] [Google Scholar]
- DeGrandchamp RL, Reuhl KR, Lowndes HE. Synaptic terminal degeneration and remodeling at the rat neuromuscular junction resulting from a single exposure to acrylamide. Toxicol Appl Pharmacol. 1990;105:422–433. doi: 10.1016/0041-008x(90)90146-l. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Jortner BS. Organophosphate-induced delayed neuropathy. In: Massaro EJ, editor. Handbook of Neurotoxicology. Vol. 1. Totowa, NJ: Humana Press. p; 2002. pp. 17–27. [Google Scholar]
- Ehrich M, Jortner BS. Organophosphate-induced delayed neuropathy. In: Krieger R, editor. Hayes' Handbook of Pesticide Toxicology. Vol. 2. San Diego: Elsevier. p; 2010. pp. 1479–1504. [Google Scholar]
- El-Fawal HA, Correll L, Gay L, Ehrich M. Protease activity in brain, nerve, and muscle of hens given neuropathy-inducing organophosphates and a calcium channel blocker. Toxicol Appl Pharmacol. 1990;103:133–142. doi: 10.1016/0041-008x(90)90269-z. [DOI] [PubMed] [Google Scholar]
- Ergun SS, Ozturk K, Su O, Gursoy EB, Ugurad I, Yuksel G. Delayed neuropathy due to organophosphate insecticide injection in an attempt to commit suicide. Hand (N Y) 2009;4:84–87. doi: 10.1007/s11552-008-9126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RG, Niles CA, Kelly-Hayes M, Sax DS, Dixon WJ, Thompson DJ, Landau E. Peripheral neuropathy in arsenic smelter workers. Neurology. 1979;29:939–944. doi: 10.1212/wnl.29.7.939. [DOI] [PubMed] [Google Scholar]
- Fix AS, Garman RH. Practical aspects of neuropathology: a technical guide for working with the nervous system. Toxicol Pathol. 2000;28:122–131. doi: 10.1177/019262330002800115. [DOI] [PubMed] [Google Scholar]
- Gottfried MR, Graham DG, Morgan M, Casey HW, Bus JS. The morphology of carbon disulfide neurotoxicity. Neurotoxicology. 1985;6:89–96. [PubMed] [Google Scholar]
- Graham DG, Amarnath V, Valentine WM, Pyle SJ, Anthony DC. Pathogenetic studies of hexane and carbon disulfide neurotoxicity. Crit Rev Toxicol. 1995;25:91–112. doi: 10.3109/10408449509021609. [DOI] [PubMed] [Google Scholar]
- Graham DG, Anthony DC, Boekelheide K, Maschmann NA, Richards RG, Wolfram JW, Shaw BR. Studies of the molecular pathogenesis of hexane neuropathy. II. Evidence that pyrrole derivatization of lysyl residues leads to protein crosslinking. Toxicol Appl Pharmacol. 1982;64:415–422. doi: 10.1016/0041-008x(82)90237-x. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Herskowitz A, Ishii N, Schaumburg H. N-hexane neuropathy. A syndrome occurring as a result of industrial exposure. N Engl J Med. 1971;285:82–85. doi: 10.1056/NEJM197107082850204. [DOI] [PubMed] [Google Scholar]
- Holisaz MT, Rayegani SM, Hafezy R, Khedmat H, Motamedi MH. Screening for peripheral neuropathy in chemical warfare victims. Int J Rehabil Res. 2007;30:71–74. doi: 10.1097/MRR.0b013e3280143c49. [DOI] [PubMed] [Google Scholar]
- Holzbauer SM, DeVries AS, Sejvar JJ, Lees CH, Adjemian J, McQuiston JH, Medus C, Lexau CA, Harris JR, Recuenco SE, Belay ED, Howell JF, Buss BF, Hornig M, Gibbins JD, Brueck SE, Smith KE, Danila RN, Lipkin WI, Lachance DH, Dyck PJ, Lynfield R. Epidemiologic investigation of immune-mediated polyradiculoneuropathy among abattoir workers exposed to porcine brain. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009782. e9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan JM, Kurt TL, Landrigan PJ, Melius JM, Singal M. Neurologic dysfunction from exposure to 2-t-butylazo-2-hydroxy-5-methylhexane (BHMH): A new occupational neuropathy. Am J Public Health. 1985;75:513–517. doi: 10.2105/ajph.75.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortner BS. Mechanisms of toxic injury in the peripheral nervous system: neuropathologic considerations. Toxicol Pathol. 2000;28:54–69. doi: 10.1177/019262330002800108. [DOI] [PubMed] [Google Scholar]
- Jortner BS. Preparation and analysis of the peripheral nervous system. Toxicol Pathol. 2011;39:66–72. doi: 10.1177/0192623310387618. [DOI] [PubMed] [Google Scholar]
- Jortner BS, Shell L, el-Fawal H, Ehrich M. Myelinated nerve fiber regeneration following organophosphorus ester-induced delayed neuropathy. Neurotoxicology. 1989;10:717–726. [PubMed] [Google Scholar]
- Kasukurthi R, Brenner MJ, Moore AM, Moradzadeh A, Ray WZ, Santosa KB, Mackinnon SE, Hunter DA. Transcardial perfusion versus immersion fixation for assessment of peripheral nerve regeneration. J Neurosci Methods. 2009;184:303–309. doi: 10.1016/j.jneumeth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- King RHM. London: Arnold Publishers; 1999. Atlas of Peripheral Nerve Pathology. [Google Scholar]
- Kjuus H, Goffeng LO, Heier MS, Sjoholm H, Ovrebo S, Skaug V, Paulsson B, Tornqvist M, Brudal S. Effects on the peripheral nervous system of tunnel workers exposed to acrylamide and N-methylolacrylamide. Scand J Work Environ Health. 2004;30:21–29. doi: 10.5271/sjweh.761. [DOI] [PubMed] [Google Scholar]
- Krinke GJ, Fitzgerald RE. The pattern of pyridoxine-induced lesion: Difference between the high and the low toxic level. Toxicology. 1988;49:171–178. doi: 10.1016/0300-483x(88)90190-4. [DOI] [PubMed] [Google Scholar]
- Krinke GJ, Vidotto N, Weber E. Teased-fiber technique for peripheral myelinated nerves: methodology and interpretation. Toxicol Pathol. 2000;28:113–121. doi: 10.1177/019262330002800114. [DOI] [PubMed] [Google Scholar]
- Lachance DH, Lennon VA, Pittock SJ, Tracy JA, Krecke KN, Amrami KK, Poeschla EM, Orenstein R, Scheithauer BW, Sejvar JJ, Holzbauer S, Devries AS, Dyck PJ. An outbreak of neurological autoimmunity with polyradiculoneuropathy in workers exposed to aerosolised porcine neural tissue: A descriptive study. Lancet Neurol. 2010;9:55–66. doi: 10.1016/S1474-4422(09)70296-0. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: Neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54:273–285. doi: 10.1111/j.1365-2559.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- Lehning EJ, Balaban CD, Ross JF, Reid MA, LoPachin RM. Acrylamide neuropathy: I. Spatiotemporal characteristics of nerve cell damage in rat cerebellum. Neurotoxicology. 2002;23:397–414. doi: 10.1016/s0161-813x(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Lehning EJ, Dyer KS, Jortner BS, LoPachin RM. Axonal atrophy is a specific component of 2,5-hexanedione peripheral neuropathy. Toxicol Appl Pharmacol. 1995;135:58–66. doi: 10.1006/taap.1995.1208. [DOI] [PubMed] [Google Scholar]
- Lehning EJ, Persaud A, Dyer KR, Jortner BS, LoPachin RM. Biochemical and morphologic characterization of acrylamide peripheral neuropathy. Toxicol Appl Pharmacol. 1998;151:211–221. doi: 10.1006/taap.1998.8464. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Balaban CD, Ross JF. Acrylamide axonopathy revisited. Toxicol Appl Pharmacol. 2003;188:135–153. doi: 10.1016/s0041-008x(02)00072-8. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Lehning EJ, Opanashuk LA, Jortner BS. Rate of neurotoxicant exposure determines morphologic manifestations of distal axonopathy. Toxicol Appl Pharmacol. 2000;167:75–86. doi: 10.1006/taap.2000.8984. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. Organophosphare-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- Mallov JS. MBK meuropathy among spray painters. JAMA. 1976;235:1455–1457. [PubMed] [Google Scholar]
- Mendell JR, Saida K, Ganansia MF, Jackson DB, Weiss H, Gardier RW, Chrisman C, Allen N, Couri D, O'Neill J, Marks B, Hetland L. Toxic polyneuropathy produced by methyl N-butyl ketone. Science. 1974;185:787–789. doi: 10.1126/science.185.4153.787. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Murayama E, Sumiyoshi S, Deshimaru M, Fujimoto T. Late changes in human sural nerves in Minamata disease and in nerves of rats with experimental organic mercury poisoning. Acta Neuropathol. 1976;35:131–138. doi: 10.1007/BF00690559. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Penovich P. Jamaica ginger paralysis. Forty-seven-year follow-up. Arch Neurol. 1978;35:530–532. doi: 10.1001/archneur.1978.00500320050011. [DOI] [PubMed] [Google Scholar]
- Moser VC, Phillips PM, Morgan DL, Sills RC. Carbon disulfide neurotoxicity in rats: VII. Behavioral evaluations using a functional observational battery. Neurotoxicology. 1998;19:147–157. [PubMed] [Google Scholar]
- Myers JE, Macun I. Acrylamide neuropathy in a South African factory: An epidemiologic investigation. Am J Ind Med. 1991;19:487–493. doi: 10.1002/ajim.4700190406. [DOI] [PubMed] [Google Scholar]
- Neghab M, Soleimani E, Khamoushian K. Electrophysiological studies of shoemakers exposed to sub-TLV levels of n-hexane. J Occup Health. 2012;54:376–382. doi: 10.1539/joh.12-0029-fs. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Imamura K, Kuwahata M, Kindaichi M, Susa M, Ekino S. Reappraisal of somatosensory disorders in methylmercury poisoning. Neurotoxicol Teratol. 2005;27:643–653. doi: 10.1016/j.ntt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Vascular permeability in the peripheral nervous system. In: Dyck PJ, Thomas PK, Lampert EH, Bunge R, editors. Peripheral Neuropathy. Philadelphia: WB Saunders. p; 1984. pp. 579–597. [Google Scholar]
- Osuntokun BO, Aladetoyinbo A, Adeuja AO. Free-cyanide levels in tropical ataxic neuropathy. Lancet. 1970;2:372–373. doi: 10.1016/s0140-6736(70)92913-2. [DOI] [PubMed] [Google Scholar]
- Pope C, diLorenzo K, Ehrich M. Possible involvement of a neurotrophic factor during the early stages of organophosphate-induced delayed neurotoxicity. Toxicol Lett. 1995;75:111–117. doi: 10.1016/0378-4274(94)03167-6. [DOI] [PubMed] [Google Scholar]
- Prineas J. The pathogenesis of dying-back polyneuropathies. II. An ultrastructural study of experimental acrylamide intoxication in the cat. J Neuropathol Exp Neurol. 1969;28:598–621. doi: 10.1097/00005072-196910000-00004. [DOI] [PubMed] [Google Scholar]
- Prockop LD, Alt M, Tison J. "Huffer's" neuropathy. JAMA. 1974;229:1083–1084. [PubMed] [Google Scholar]
- Puri V, Chaudhry N, Tatke M. N-hexane neuropathy in screen printers. Electromyogr Clin Neurophysiol. 2007;47:145–152. [PubMed] [Google Scholar]
- Richardson RJ. Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: A critical review of the literature. J Toxicol Environ Health. 1995;44:135–165. doi: 10.1080/15287399509531952. [DOI] [PubMed] [Google Scholar]
- Rizzuto N, Terzian H, Galiazzo-Rizzuto S. Toxic polyneuropathies in Italy due to leather cement poisoning in shoe industries. A light- and electron-microscopic study. J Neurol Sci. 1977;31:343–354. doi: 10.1016/0022-510x(77)90213-1. [DOI] [PubMed] [Google Scholar]
- Schaumburg HH. Pyridoxine, Experimental and Clinical Neurotoxicology. In: Spencer PS, Schaumburg HH, Ludolph AC, editors. New York: Oxford University Press; 2000. [Google Scholar]
- Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): A systematic review. Clin Nutr. 2013;32:888–893. doi: 10.1016/j.clnu.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Scott K, Zeris S, Kothari MJ. Elevated B6 levels and peripheral neuropathies. Electromyogr Clin Neurophysiol. 2008;48:219–223. [PubMed] [Google Scholar]
- Senanayake N, Jeyaratnam J. Toxic polyneuropathy due to gingili oil contaminated with tri-cresyl phosphate affecting adolescent girls in Sri Lanka. Lancet. 1981;1:88–89. doi: 10.1016/s0140-6736(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Shobha N, Taly AB, Sinha S, Venkatesh T. Radial neuropathy due to occupational lead exposure: Phenotypic and electrophysiological characteristics of five patients. Ann Indian Acad Neurol. 2009;12:111–115. doi: 10.4103/0972-2327.53080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills RC, Harry GJ, Morgan DL, Valentine WM, Graham DG. Carbon disulfide neurotoxicity in rats: V. Morphology of axonal swelling in the muscular branch of the posterior tibial nerve and spinal cord. Neurotoxicology. 1998;19:117–127. [PubMed] [Google Scholar]
- Smith HV, Spalding JM. Outbreak of paralysis in Morocco due to ortho-cresyl phosphate poisoning. Lancet. 1959;2:1019–1021. doi: 10.1016/s0140-6736(59)91486-2. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. A review of acrylamide neurotoxicity. Part II. Experimental animal neurotoxicity and pathologic mechanisms. Can J Neurol Sci. 1974;1:152–169. doi: 10.1017/s0317167100119201. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J Neuropathol Exp Neurol. 1977;36:276–299. doi: 10.1097/00005072-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Classification of neurotoxic disease: A morphological approach. In: Spencer PS, Schaumberg HH, editors. Experimental and Clinical Neurotoxicology Baltimore: Williams and Wilkins. p; 1980. pp. 92–99. [Google Scholar]
- Spencer PS, Schaumburg HH. New York: Oxford: University Press.; 2000. Experimental and Clinical Neurotoxicology. [Google Scholar]
- Susser M, Stein Z. An outbreak of tri-ortho-cresyl phosphate (T.O.C.P.) poisoning in Durban. Br J Ind Med. 1957;14:111–120. doi: 10.1136/oem.14.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Acrylamide: A cooking carcinogen? Chem Res Toxicol. 2000;13:517–522. doi: 10.1021/tx9901938. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Gonatas NK, Pleasure D, Cooper HS, McCree L. Glue sniffer's neuropathy. Neurology. 1976;26:238–243. doi: 10.1212/wnl.26.3.238. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency, Office of Pollution, Prevention, and Toxics. Chemical testing and data evaluation. 2010 Available online (www.epa.gov/opptintr/chemtest/ ), accessed on July 10, 2013. [Google Scholar]
- Valentine WM, Amarnath V, Graham DG, Morgan DL, Sills RC. CS2-mediated cross-linking of erythrocyte spectrin and neurofilament protein: Dose response and temporal relationship to the formation of axonal swellings. Toxicol Appl Pharmacol. 1997;142:95–105. doi: 10.1006/taap.1996.8028. [DOI] [PubMed] [Google Scholar]
- Wadia NH. SMON as seen from Bombay. Acta Neurol Scand Suppl. 1984;100:159–164. [PubMed] [Google Scholar]
- Weimer LH, Sachdev N. Update on medication-induced peripheral neuropathy. Curr Neurol Neurosci Rep. 2009;9:69–75. doi: 10.1007/s11910-009-0011-z. [DOI] [PubMed] [Google Scholar]
- White RF, Feldman RG, Eviator II, Jabre JF, Niles CA. Hazardous waste and neurobehavioral effects: A developmental perspective. Environ Res. 1997;73:113–124. doi: 10.1006/enrs.1997.3699. [DOI] [PubMed] [Google Scholar]
- Windebank AJ, Low PA, Blexrud MD, Schmelzer JD, Schaumburg HH. Pyridoxine neuropathy in rats: Specific degeneration of sensory axons. Neurology. 1985;35:1617–1622. doi: 10.1212/wnl.35.11.1617. [DOI] [PubMed] [Google Scholar]
- Woolf AD. Ginger Jake and the blues: A tragic song of poisoning. Vet Hum Toxicol. 1995;37:252–254. [PubMed] [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Araki S, Murata K, Nishikitani M, Okumura T, Ishimatsu S, Takasu N. Chronic neurobehavioral and central and autonomic nervous system effects of Tokyo subway sarin poisoning. J Physiol Paris. 1998;92:317–323. doi: 10.1016/s0928-4257(98)80040-5. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol. 2012;238:225–234. doi: 10.1016/j.expneurol.2012.08.023. [DOI] [PubMed] [Google Scholar]