Abstract
Leprosy (also known as Hansen's Disease) is a chronic infectious disease caused by Mycobacterium leprae that primarily targets the peripheral nervous system; skin, muscle, and other tissues are also affected. Other than humans, nine-banded armadillos (Dasypus novemcinctus) are the only natural hosts of M. leprae, and they are the only laboratory animals that develop extensive neurological involvement with this bacterium. Infection in the armadillo closely recapitulates many of the structural, physiological, and functional aspects of leprosy seen in humans. Armadillos can be useful models of leprosy for basic scientific investigations into the pathogenesis of leprosy neuropathy and its associated myopathies, as well as for translational research studies in piloting new diagnostic methods or therapeutic interventions. Practical and ethical constraints often limit investigation into human neuropathies, but armadillos are an abundant source of leprotic neurologic fibers. Studies with these animals may provide new insights into the mechanisms involved in leprosy that also might benefit the understanding of other demyelinating neuropathies. Although there is only a limited supply of armadillo-specific reagents, the armadillo whole genomic sequence has been completed, and gene expression studies can be employed. Clinical procedures, such as electrophysiological nerve conduction testing, provide a functional assessment of armadillo nerves. A variety of standard histopathological and immunopathological procedures including Epidermal Nerve Fiber Density (ENFD) analysis, Schwann Cell Density, and analysis for other conserved cellular markers can be used effectively with armadillos and will be briefly reviewed in this text.
Keywords: armadillo, ENFD, gene-expression, leprosy, myopathy, neuropathy, Schwann cell, translational
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. It primarily affects the peripheral nervous system (PNS) and involves skin, skeletal, muscle, and other tissues. The distinct nerve involvement during M. leprae infection is directly associated with the remarkable capacity of M. leprae to invade adult Schwann cells, the glial cells of the PNS, which enclose and support the axons of sensory and motor neurons. Schwann cell infection causes complex biological and pathological alterations including demyelination, de-differentiation, and reprogramming of the Schwann cells. Infection eventually brings an inflammatory response, which causes nerve injury. These events, orchestrated by M. leprae and the immune response to it, underlie the extreme disability and gross deformity sometimes associated with this disease, and are the central feature in the pathology of leprosy (Scollard et al. 2006). Despite the numerous studies from patients, and cellular biology studies of M. leprae infection in vitro, relatively little is known about the pathogenesis of leprosy. Large gaps in the understanding of neuropathogenesis, in particular the early preclinical events, have severely impeded progress towards developing effective early diagnostics and therapeutics for the management of nerve damage in leprosy patients.
M. leprae targets the nerves early after the onset of infection. Peripheral nerves not only serve as the principal target for M. leprae infection, but also serve as a safe haven for the bacillus. The blood-nerve barrier protects the organism from many host immune responses. M. leprae appears to take advantage of the remarkable regenerating capacity of the adult PNS when securing its preferred niche, and regeneration of damaged peripheral nerves continues post-treatment in patients with advanced leprosy (Miko et al. 1993). Nerve damage progresses gradually over the entire course of the disease.
Nerve involvement in leprosy patients can be identified in the form of sensory and/or motor neuron damage. The disease process is progressive and can lead to deformities and disabilities unless the patient is treated to reduce the bacterial load, and inflammatory insults are controlled. Although bacterial cure can be achieved by successful multidrug antimicrobial therapy, neurological injury continues to occur in patients and is exacerbated during pathological perturbations of the host immunological response to M. leprae, known as leprosy reactions. The cause and treatment of these reactions is quite problematic, and can result in significant injury to the underlying tissues.
There are no laboratory tests to aid early detection of leprosy, and the disease can only be diagnosed once clinical symptoms appear. The incubation period in humans is usually estimated to be from 3–5 years, but much longer intervals have been described. The clinical presentation is comprised mainly of skin lesions with characteristic hypoesthesia and anesthesia; illustrating nerve involvement in even the earliest clinical stages of the disease. The clinical aspects of leprosy and cell biology of early M. leprae infection have been reviewed previously (Rambukkana 2004, 2010; Scollard et al. 2006).
Leprosy is curable with combination multi-drug therapy. Early detection and treatment are the most effective means to avoid its undesirable sequelae. However, the treatment interval is protracted and requires months to years to complete. Even after completion of effective antibacterial therapy, bacillary clearance from the involved tissues is quite slow. The residual dead bacilli retained in tissues provide chronic immunological stimulation and can be problematic for disease management (Scollard et al. 2006). The World Health Organization has recorded remarkable reductions in global prevalence of leprosy over the last few decades, but nearly a quarter of a million new leprosy cases continue to be reported globally each year (these estimates are probably low). Many of these individuals present with permanent nerve damage, and there are several million people living today with advanced disabilities owing to their leprosy (World Health Organization 2012).
Susceptibility to leprosy is rare, and appears to have a genetic component (Alter et al. 2011). Infected individuals manifest their disease over a broad clinical and histopathological spectrum that is determined by the individual's immunological response to M. leprae. On one end of the spectrum (lepromatous) there may be numerous diffuse lesions, which contain large numbers of bacilli in poorly organized granulomas; showing evidence of little cell-mediated immune response to M. leprae. On the other extreme (Tuberculoid) there may be one or few clearly defined lesions with well-organized granulomas showing few, if any, visible bacilli and evidencing active cell mediated immunity. Between these two poles, borderline forms blend the extreme forms to make 2–3 additional classifications. However, the common feature across all forms of the leprosy spectrum is focal insensitivity of the lesions, and impaired sensory and/or motor function resulting from involvement of M. leprae along with damage to the underlying peripheral nerves (Scollard et al. 2006).
M. leprae likely enters the human body through respiratory routes and disseminates hematogenously to manifest a widespread, asymmetrical disease pattern. Intraneural infection by M. leprae is the pathognomonic feature of leprosy, and perineural and intraneural inflammation are its morphological hallmarks. The granulomas, which form in these nerves, are identical in structure to those in skin lesions. The bacilli proliferate in macrophages and Schwann cells of the peripheral nerves, especially in the hands, legs, and feet, or cooler regions of the body, as M. leprae prefers cooler temperatures for growth. Inflammation in the cutaneous nerves of skin lesions may extend proximally for variable distances and involve other nerve trunks and branches. There may be pronounced edema and fibrosis (Scollard 2008). Some superficial nerves can become readily palpable, and high resolution sonography has been used to quantify and detect nerve damage in some leprosy patients (Jain et al. 2009). The elevation of circulating levels of pro-inflammatory cytokines and chemokines have been related to the onset of leprosy reactions. Studies of limited biopsy material from leprosy patients with these reactions indicate that levels of TNFα in cutaneous nerves are similar to those in the skin (Khanolkar-Young et al. 1995), but little is known about gene expression in leprotic nerves. Ethical and practical constraints generally preclude biopsy of affected human nerves. Most of the clinical understanding about leprosy neuropathy has been derived from examining amputated tissues taken from very advanced stages of infection (Scollard 2008).
Studies with primary nerve tissue culture and mouse models have yielded insight into some molecular interactions of M. leprae with Schwann cells, which likely facilitate the localization of M. leprae to the peripheral nerve and benefit its proliferation in that privileged niche (Rambukkana 2010). Attachment of M. leprae to the basal lamina that surrounds Schwann cell-axon units of nerve fibers seems to be mediated through the G domain of the laminin alpha-2 chain and alpha dystroglycan receptors on the cell surface (Rambukkana et al. 1997, 1998; Rambukkana 2000). Interestingly, the bacilli also bind and activate the receptor tyrosine kinase ErbB2, which induces the Erk1/2 signal transduction pathways and results in demyelination and dedifferentiation of the terminally differentiated Schwann cell (Noon and Lloyd 2005; Rambukkana 2004, 2010; Tapinos and Rambukkana 2005; Tapinos et al. 2006;). Recent evidence also suggests that these dedifferentiated cells are easily parasitized, and the reprogramming of infected Schwann cells to stem cell-like cells provides them a convenient vehicle to promote the spread to other tissues, including skeletal muscles (Masaki et al. 2013). However, translating this knowledge into the development of new diagnostics or therapeutic applications requires recapitulation of the events in an animal model system, which can validate the findings in the setting of a natural infection.
Management of leprosy patients requires careful monitoring of sensory and motor nerve function. Assessment of tactile sensory function in leprosy is most routinely measured using Semmes-Weinstein monofilaments (MFT), and motor function is assessed by standard voluntary muscle tests (VMT). Recent studies examining the development and progression of nerve injury in large numbers of leprosy patients have shown that impairment begins early and progresses chronically over the course of the disease. Electrophysiological nerve conduction studies, used in combination with warm perception threshold testing, and other procedures, can be used effectively to detect sensory and motor nerve function impairment, as well as predict future clinical abnormalities in sensory and motor nerves (Nicholls et al. 2005; Smith et al. 2009; van Brakel et al. 2005, 2008; Wilder-Smith and van Brakel 2008;).
Investigation of the various components of the nociceptive fibers that are associated with sensory loss in leprosy skin lesions is scant and sketchy. Tactile sensitivity is mediated by thickly myelinated Aβ fibers of the dermis, while thermal sensitivity is mediated by thin myelinated Aδ fibers and unmyelinated C type fibers that end in the epidermis as free nerve endings (Ebenezer et al. 2007). There is some evidence suggesting that the C fibers in the epidermis are the earliest to undergo degeneration, this is consistent with clinical observations that the assessment of thermal sensation can be a primary component in establishing early diagnosis (van Brakel et al. 2005, 2008).
Peripheral neuropathy in leprosy patients also contributes to the impairment of skeletal muscle function. Studies on the involvement of skeletal muscle in leprosy patients have clearly demonstrated reduced motor nerve conduction velocities, decreased compound muscle action potentials, and sometimes a complete absence of potentials (Werneck et al. 1999). However, it is unknown how M. leprae infection causes the impairment of motor functions, with subsequent muscle atrophy. In leprosy patients, disseminated M. leprae are detected in skeletal muscles, which are also known to serve as M. leprae reservoirs and play a key role in leprosy pathogenesis (Kaur et al. 1981; Werneck et al. 1999). In patient studies, plantar nerves that innervate intrinsic muscles of the foot also show loss of sensation and muscle function impairment, indicating that muscle pathogenesis is a key feature in human leprosy. Thus, the involvement of skeletal muscle in human leprosy may occur as a secondary event, resulting from peripheral neuropathy-induced denervation (Pearson et al. 1970), or may be due to primary lesions in the muscle itself.
M. leprae is an obligate intracellular parasite that cannot be cultivated on artificial laboratory media. Its long doubling time (12.5 days) severely limits the utility of many cell culture techniques. The organism prefers cool temperatures, and viability of M. leprae decreases quickly at temperatures above 35°C (Truman and Krahenbuhl 2001). Ever since discovery of the organism, investigators have attempted to propagate M. leprae in a variety of different animal species, especially those with body temperatures lower than 37°C. However, the susceptible animal host range appears to be quite narrow. Most animals readily clear the bacilli (Couret 1911; Fite et al. 1964). Limited replication can be achieved after inoculation of M. leprae into the hind foot pads of conventional mice (Shepard 1960). Although the infection remains localized to the foot, the level of growth achieved in foot pads is markedly enhanced among athymic nude mice and other immune-deficient mouse strains (Colston and Hilson 1976). However, the only immunologically intact animal which reliably becomes infected with M. leprae, and closely recapitulates leprosy, is the nine-banded armadillo (Dasypus novemcinctus).
The Armadillo
Armadillos are exotic looking animals about the size of the common housecat (Figure 1A). They have short legs, with strong claws and a hard flexible carapace that armors most of their body. They are mammals of the Order Xenarthra; relatives of sloths and anteaters. The armadillo's normal body temperature ranges from 33–35°C, and it was this trait that first attracted the attention of leprosy researchers. Experimental infection of armadillos with M. leprae requires 18–24 months to manifest as a fully disseminated disease, but prolific quantities of bacilli accumulate throughout the animal's reticuloendothelial organs and up to 1012 M. leprae can be harvested from the tissues of a single animal. The remarkable quantities of M. leprae, made available through armadillos, have been a boon to leprosy research, and armadillos are the hosts-of-choice for in vivo propagation of bulk quantities of leprosy bacilli (Truman and Sanchez 1993).
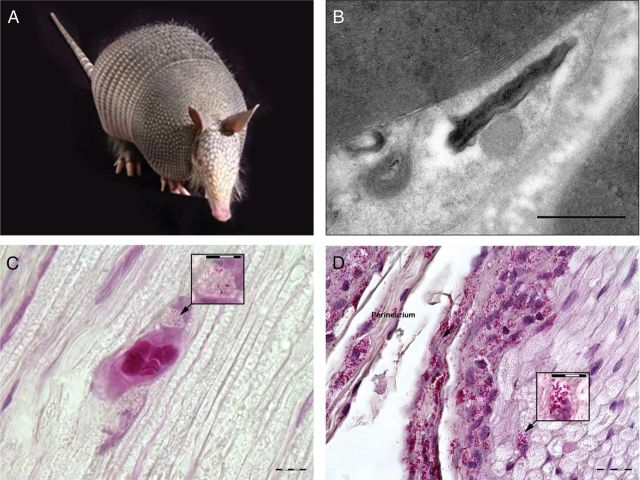
Figure 1:
Collage of armadillo, nerves and Schwann cell involvement. (A) Photo, nine-banded armadillo (Dasypus novemcinctus). (B) Electron micrograph illustrating M. leprae within armadillo Schwann cell. Bar is 500nm. (C) Fite-stained armadillo post-tibial nerve 60x magnification showing involvement of M. leprae in vascular endothelial cells, with inset at 120x magnification. (D) Fite-stained armadillo post-tibial nerve 60x magnification showing extensive perineural and endoneural involvement of M. leprae with epineural inflammation. Inset at 120x magnification illustrates endoneural involvement.
Shortly after initial discovery of the armadillo's unique susceptibility to experimental infection, a naturally occurring, systemic mycobacteriosis was found among free ranging armadillos (Walsh et al. 1975). Subsequent surveys confirmed that wild armadillos are reservoir-hosts and had harbored M. leprae for many decades prior to their ever being used in leprosy research (Truman 2005; Walsh et al. 1986). Leprosy was not present in the New World during pre-Colombian times, making it reasonable to assume that armadillos have acquired the infection from humans sometime in the last few centuries. They are recognized as the only nonhuman reservoir of M. leprae, and are part of the natural ecology of the disease in the United States. Recent reports indicate that zoonotic transmission of M. leprae from armadillos is responsible for up to 64% of all leprosy cases seen in the United States. These animals might play a role perpetuating leprosy elsewhere in the Americas. Besides humans, armadillos are the only known natural hosts of M. leprae (Truman et al. 2011).
M. leprae infection in the armadillo closely recapitulates many of the structural, physiological, and functional aspects of leprosy seen in humans. Armadillos do not exhibit many characteristic gross skin lesions. A hard, flexible carapace obscures the majority of the armadillo's body; greater involvement of the disease is seen internally. However, the feet, nose, and eyes may show asymmetrically distributed, nonspecific, focal, ulcerative dermatitis that is associated with regional insensitivity of the skin. Although the majority of armadillos appear to be susceptible to experimental infection, 15–20% readily resist the challenge. Their granulomatous response to M. leprae also appears to be histopathologically identical to that of humans; and armadillos exhibit a similar spectrum of histopathological responses to the organism. Although 70% of the animals manifest a Lepromatous-type of response to M. leprae, some animals produce full Tuberculoid or Borderline forms of the disease (Job et al. 1983; Job and Truman 2000). Beyond sharing a unique susceptibility to M. leprae, perhaps the most important similarity between humans and armadillos is that they both develop extensive neurological involvement with M. leprae. No other laboratory animal (e.g., mice, rabbits, and guinea pigs) develops neurological involvement with M. leprae. The infection path in the armadillo provides a unique opportunity to model the neuropathogenesis of leprosy.
Effective animal models can help provide pivotal new understandings about the mechanisms involved in the disease process, and the animals may serve as useful platforms for piloting new therapeutic interventions. Armadillos are well established as models for leprosy pathogenesis and they are the most abundant source of leprotic neurological fibers for basic science investigations. Among the armadillo's unique attributes are a controlled and known infection status, compressed disease duration, and a functional recapitulation of leprosy as is seen in humans. Armadillos specimens can be examined and compared in regards to rare neurological events that happen in both normal and leprotic tissues, all from time periods and in material quantities that cannot be obtained from human subjects. Studies with armadillos may help identify new targets for interventions, establish end-points that can be useful for monitoring the clinical course of infection in patients, and in evaluating the effectiveness of new therapies.
Although armadillos have been used in leprosy research for nearly 40 years, their exotic nature and lack of armadillo-adaptable research reagents has limited their use as model hosts. Recent completion of the armadillo whole genomic sequence is now facilitating the development of armadillo-specific reagents and molecular probes that are needed for modern neuropathogenesis studies. This will propel armadillos as effective models for translational research in leprosy. The development of armadillos for use in in vivo propagation of M. leprae and representative model hosts of leprosy has been reviewed before (Adams et al. 2012; Scollard and Truman 1999; Sharma et al. 2013). This paper focuses on the special advantages of the armadillo model with regards to enhancing the understanding about the pathogenesis of leprosy, highlighting some novel insights into leprosy that have been gained through work with armadillos, and emphasizing end-points and select methods that can be useful for conducting intervention studies with armadillos.
Preclinical Leprosy
A notable advantage when using the armadillo as a model is the opportunity to examine the pathogenesis of infection at preclinical stages that have never been observed in humans, which are more likely to be effectively targeted by therapeutic intervention. Following experimental infection of armadillos, M. leprae populates the peripheral nerves and reticuloendothelial tissues; then slowly disseminates systemically from these early foci. The armadillo post-tibial nerve runs for about 5 cm beneath the skin surface of the medial hind limb. This nerve has a high frequency of involvement in both armadillo and human infections. It is easily accessible in the armadillo and a useful target for studies in armadillos. Although the duration of experimental infection in armadillos (4–24 months) is highly compressed when compared to the many years involved in human infections, bacillary loads of ≥106 M. leprae/cm are common in armadillo post-tibial nerves (Figure 1).
The first essential step in leprosy neuritis is the localization of M. leprae to the peripheral nerve. Detailed histopathological studies on armadillo post-tibial nerves suggests that this localization is a multistep process, which proceeds from the outside-in as opposed to an ascending-type of infection that is sometimes described in the early literature. The bacilli first aggregate in epineural lymphatics and blood vessels, then enter the endoneural compartment through its blood supply (Scollard et al. 1999; Scollard 2000). This view gives significance to old observations of substantial involvement of endothelial cells, and suggests that the substantial perineural inflammation seen in leprosy is evidence when tracking the route of infection in the nerves themselves (Figure 1B, C, and D). In addition to implications with respect to the understanding of neuropathogenesis, these studies highlight several additional possible points of preventative, or therapeutic, intervention that might otherwise remain ignored. These points include: interrupting M. leprae binding to endothelial cells, entry into endothelium, exit from endothelial cells through the perineurium and into the endoneurium, and binding to Schwann cells. Any of these factors could be critical points to potentially negate establishment of infection in the nerve and eventual nerve injury (Scollard 2008).
Molecular Studies on Clinically Relevant Nerves
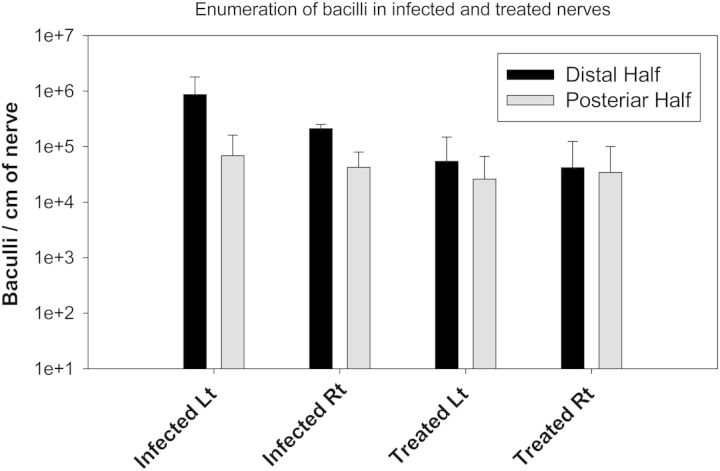
Once M. leprae populates the nerve the bacilli can grow to high numbers and spread to adjacent nerve trunks. Anti-leprosy drug therapies must have good neural penetration in order to kill bacilli sequestered in nerves. Even once effectively killed by the anti-microbial drugs, humans and armadillos show only slow clearance of bacilli from nerves and skin lesions. In one study, ten M. leprae infected armadillos were allowed to incubate their infections for twelve months, before five of them were treated with 10mg/kg rifampin once monthly per os for an additional twelve months. These and an additional five naïve control animals were later sacrificed at twenty-four months postinfection. Although each of the treated animals showed clinical improvement in skin lesions and ulcers as a result of the antimicrobial therapy, examination of their post-tibial nerves showed continued presence of M. leprae. Molecular assessment of M. leprae viability suggested the organisms had been effectively killed by rifampin therapy. However, bacterial counts averaging 104–5 bacilli per cm of post-tibial nerve were still observed even after the conclusion of a full year of treatment (Figure 2). This heavy burden of (dead) bacilli provides a rich substrate for continued immunological interaction with the host, and suggests there is insidious chronic injury to nerves involved with M. leprae.
Figure 2:
Comparison of M. leprae infiltrated to armadillo post-tibial nerves among treated and untreated animals. Mean and Standard deviation of M. leprae/cm of armadillo post-tibial nerves (five animals each group) harvested 24 months postinfection and segmented to an equal distal and proximal portion. Treated animals received 12 months anti-microbial therapy, with once monthly 10 mg/kg rifampin per os. Nerve trunks from Treated and Untreated animals were segmented to distal and proximal portions and processed for simultaneous DNA and RNA extraction. Bacteria were enumerated and normalized per segment centimeter, and values pooled to derive mean and standard deviation. Untreated with an additional 12 months of incubation showed higher overall numbers, but treated animals also retained large numbers of dead M. leprae in their nerves, even one year after therapy was initiated.Total number of leprosy bacilli in each segment were enumerated using M. leprae, specific quantitative PCR assay targeting RLEP gene and number of viable only bacilli were calculated by using quantitative RT-PCR assay targeting 16S rRNA (Truman et al. 2008). Although, no viable bacilli were detected after one year Rifampin treatment, up to 10E + 04 dead bacilli were still not cleared from the treated armadillo nerves. Bacterial counts were significantly (p < 0.05) higher in segments of distal half, compared to proximal half of PT nerve harvested from infected (p = 0.0023) animals as well as after treatment (p = 0.019).
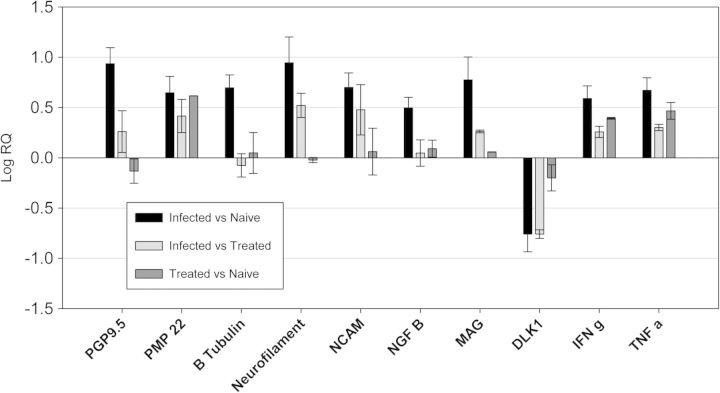
Although there are no comparable human studies, armadillo nerve segments can be used effectively for gene expression profiling and analysis of cell signaling pathways. The gene expression profiles between uninfected-normal nerves, and infected-untreated or infected-rifampin-treated armadillo post-tibial nerves can be compared with a broad array of neural specific markers. In examining the nerves described above, the gene expression profiles reflected ongoing degeneration and regeneration processes among the infected animals when compared to the naïve controls, as well as evidence of persistent inflammation with enhanced expression of both IFNg and TNFa (Figure 3). Though gene expression among treated animals was somewhat lower than those of the animals suffering active infection, the gene expression profiles of nerve segments from rifampin treated animals more closely resembled those of the untreated animals over the naïve controls (Figure 3). The slow clearance of killed bacilli can be problematic for nerve injury. Molecular markers for neurodegeneration and regeneration, along with gene expression profile of inflammatory genes, and enumeration of the bacterial load of M. leprae in the nerve are useful therapeutic end-points for laboratory studies. They highlight the importance of developing new therapies to enhance clearance of bacilli from the host in conjunction with anti-bacterial treatment in order to limit the progress of insidious neuropathy.
Figure 3:
Gene expression profiling of Naïve, M. leprae-Infected Untreated and Treated armadillo post-tibial nerves. Expression of molecular markers associated with nerve structure and function (PGP9.5 [UCHL1] PMP 22, β- tubulin, Neurofilament, NCAM, growth factors [NGF β and DLK-1,]) and inflammation (TNF-α and IFN-γ) was compared between armadillo post-tibial nerves harvested from 1) Naïve: uninfected-normal animals, 2) Infected Untreated animals, and 3) Treated, Infected animals that had received 12 months of rifampin treatment. Data was normalized using GAP3DH, and relative expression was computed by using the ΔΔCt method. Results represent mean ± SD from duplicate experiments on five animals in each group.
Electrophysiological Studies
Armadillos do not reliably respond to thermal, light, or tactile nociceptive stimulants, but measurements of nerve conduction can be used effectively to assess function of armadillo motor nerves. Nerve conduction studies are noninvasive, and are ideal for repeated, or prospective, assessments studying the onset and progress of peripheral neuropathy over time in the same subject. Normal armadillos exhibit conduction profiles similar to humans (mean NCV 62.09 ± 10.72 m/sec, mean CMAP 1.55 ± 0.33 mV). Demyelinating events result in a decreased Nerve Conduction Velocity (NCV) measured in m/sec, while axonal loss and muscular atrophy leads to a decrease in the Compound Motor Action Potential (CMAP) measured in mV (Franssen 2008). Conduction deficit is observed in the post-tibial nerves of 75% of all experimentally infected armadillos, with onset occurring as early as 90 days postinfection. Similar to observations on humans, depressed CMAP amplitude (<0.9 mV, mean – 2 SD) is the most common presentation, but abnormal nerve conduction velocity (NCV <40m/s, mean -2SD) also can be observed. Most armadillos progress from normal conduction to a total conduction block by the latest stages of their experimentally induced infections with M. leprae. Onset of conduction abnormality generally coincides with evolution of a detectable immune response to M. leprae (i.e., detectable PGL1 IgM antibodies) and is a significant predictor of other non-specific symptoms of neuropathy such as foot ulcers, and nail avulsion or hypertrophy (Sharma et al. 2013). Unfortunately, their hard carapace and thick skin limit the number of nerves that may be examined in armadillos, and sensory nerve conduction profiles have not yet been successful. However, other histopathological techniques can be substituted effectively.
Epidermal Nerve Fiber and Schwann Cell Density
The morphological and quantitative study of skin biopsy offers an alternative tool to assess thin nerve fiber structure related to the thermal sensitivity function. Immunostaining of punch skin biopsies for protein gene product 9.5 (PGP9.5), a neuronal pan axonal marker, has now been used by several investigators to visualize the intra-epidermal nerve fibers, dermal nerves, and Schwann cells in lieu of nerve conduction tests which may fail to detect small nerve fiber impairment. The small fiber innervation is length dependent and robust normative data for epidermal nerve fiber densities (ENFD) in the distal limb have been developed (McArthur et al. 1998). In small fiber sensory neuropathies associated with diabetes, HIV, and idiopathic small fiber sensory neuropathies, a decrease in epidermal density in the distal leg has been demonstrated (Goransson et al. 2006; Holland et al. 1997; Periquet et al. 1999; Polydefkis et al. 2004). Abnormalities were demonstrated in cutaneous innervation even in individuals with normal tendon reflexes at the ankles, normal sural nerve action potential amplitudes, and normal quantitative sensory tests (Gibbons et al. 2006). Although leprosy neuritis has been well described clinically and histologically, the underlying mechanisms of nerve damage remain poorly understood. Very little morphological work has been done to detect early damage to sensory nerves.
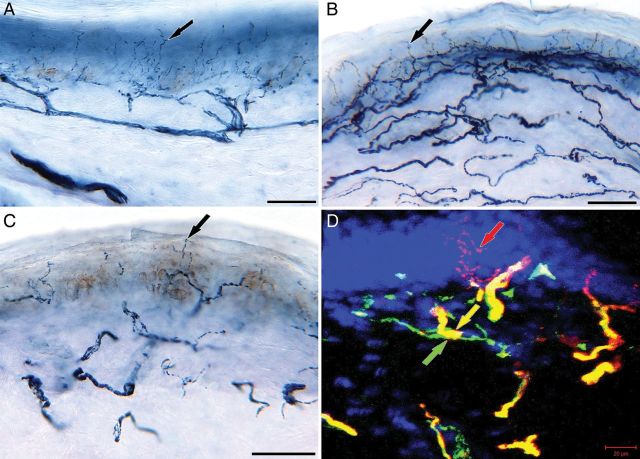
Quantitation of epidermal fibers in skin biopsies of ears, abdomen, and a distal leg of naïve armadillos has shown a length dependent innervation similar to humans (GJ Ebenezer, R Truman, D Scollard, M Polydefkis, R Lahiri, JC McArthur, unpublished data). The epidermal innervation was extremely dense in the ear and abdomen compared to the distal leg (Figure 4A, B, and C) and the lower limit of normal (defined as the 5th percentile) was 21.3 fibers/mm at the distal leg. The ENFD in armadillos was higher in comparison to the normative densities published in human healthy controls (Lauria et al. 2010). The infected animals showed a lower mean ENFD compared to naïve animals, suggesting early small fiber degeneration. Double staining of cutaneous axons and Schwann cells in naïve armadillos also mimicked the human cutaneous nerve network pattern (Figure 4D). Schwann cells of dermal cutaneous nerves in infected armadillos showed a trend towards increasing density (GJ Ebenezer, R Truman, D Scollard, M Polydefkis, R Lahiri, JC McArthur, unpublished data) and thus provided indirect evidence that during early infection Schwann cells undergo proliferation while harboring M. leprae. Though this difference was not significant statistically, it reaffirms the feasibility of studying small fibers in the armadillo using this technique, and the possibility of using it as a novel tool to test new drugs and in therapeutic interventions.
Figure 4:
Skin sections (A, B, and C) immunostained with anti-PGP 9.5, neuronal marker, and double stained confocal image (D) of axons (anti-PGP 9.5) and Schwann cells (anti nerve growth factor receptor, p75). (A), (B), and (C): Skin sections from the ear lobes (A) and abdomen (B) of naïve animals showing dense epidermal innervation (black arrows), compared to the distal leg (C). (D): Distal leg section from a naïve animal exhibiting axons entering the epidermis (black arrow) and the Schwann cells (gray arrow) are en-sheathing and co-localizing (white arrow) on the dermal axons. Scale bars: (A, B, and C) = 50um and (D) = 20um.
Impairment of Muscle Architecture and Function in Infected Armadillos
A common pathological hallmark of human leprosy, and M. leprae-infected armadillos, is the involvement of extremities. The lumbrical muscles of the foot are innervated by the medial and lateral plantar nerves, and in leprosy patients these plantar nerves are affected. Given that leprosy is a classic example of an infectious disease that can cause muscle paralysis due to neurological injury, the pathological status of lumbrical muscles from the armadillo hind limb has been examined.
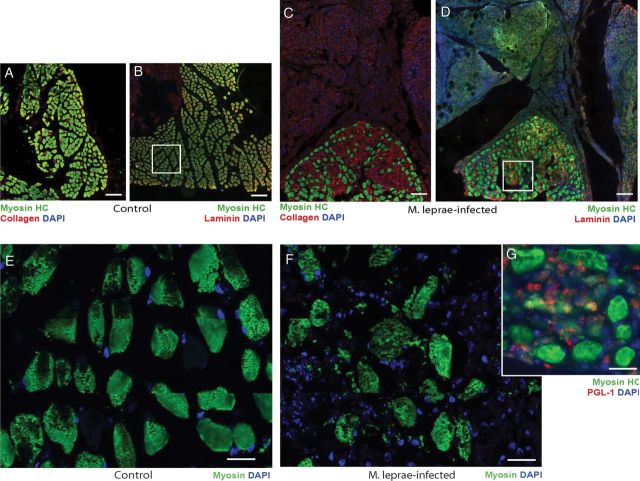
The organization of intact muscle architecture can be evaluated by labeling muscle tissues with antibodies to adult myosin, a family of ATP-dependent, actin-binding, and highly conserved muscle motor protein. Antibodies specific for myosin heavy chains (myosin HC) that react with mature myofibrils were used to detect the myosin distribution and architecture of lumbrical muscles. Labeling of transverse sections of control armadillo muscles showed a highly organized architecture of muscle fibers with clear endomysium and perimysium (Figures 5A, B, and E), similar to human and rodent skeletal muscle. In contrast lumbrical muscles from infected animals displayed a markedly disorganized pattern of muscle fibers, with disorganized endomysium and perimysium (Figures 5C, D, and F). Analysis of transverse sections of lumbrical muscles with antibodies specific for basal lamina components, laminin, and collagen showed markedly disrupted and abnormal extracellular matrix expression in infected muscles (Figures 5C and D) as compared to control animals, whose labeling was confined to the outer surface of each muscle fiber (Figures 5A and B). Co-localization of laminin and collagen with myosin HC clearly showed abnormal accumulation of matrix components within the endomysium, and additional fibrotic growth (Figures 5C and D). Detailed analysis of muscle fibers showed weak or inconsistent myosin HC expression, with a less striated pattern compared to controls (Figures 5E and F). Nuclear labeling revealed an increased accumulation of cells in the muscle, most likely mononuclear inflammatory cells or macrophages. In uninfected animals, nuclear labeling was found only adjacent to individual muscle fibers, which is similar to normal adult mouse and human muscles. Furthermore, the distribution of M. leprae within the lumbrical muscles in infected animals was studied using antibody (Ab) to M. leprae PGL-1 that specifically detects whole M. leprae (Masaki et al. 2013). These data revealed that M. leprae were predominantly localized to cells in the interstitial tissues in the perimysium, most likely within the infiltrated cells.
Figure 5:
Pathological features of skeletal muscles in infected armadillos. (A-D) Double immunolabeling of transverse sections of control (A, B) and infected (C, D) deep lumbrical muscles showing an accumulation of fibrotic tissues (C, D) and immunolabeling with antibodies to myosin heavy chain (Myosin HC; green) and laminin or collagen (red), counter stained with DAPI for nuclei (blue). Note the massive number of nucleated cells present in fibrotic tissues associated with infected muscles that were not present in the control muscles (weak myosin HC staining in green in fibrotic tissues represent non-specific background fluorescence). Also note the disorganized muscle architecture and reduced number of muscle fibers with accumulated matrix in infected muscles (C and D) as compared to controls (A and B). Higher magnification of myosin HC in the boxed areas in (B) and (D) are shown in (E), (control: myosin HC in green), (F) (infected: myosin HC in green) and (G) (infected: myosin HC in green and M. leprae labeling by PGL-1 antibody in red counter stained with DAPI for blue nuclei). Note the disorganized myosin HC labeling showing disrupted muscle fibers with infiltrated cells (blue nuclei) containing large number of M. leprae (red labeling in G) infected animals.
In agreement with these findings, functional studies also showed that the small lumbrical and flexor muscles of the armadillo foot have early involvement with M. leprae. Brand (Brand et al. 1981) showed that the PCSA (cross sectional area/mass) of muscles in the hands of leprosy patients could be used as a surrogate measure of grip strength and index muscle atrophy. Examining the PCSA of armadillo small (intrinsic) lumbrical and flexor muscles shows a qualitative reduction of muscle mass among infected armadillos, with PGL1 IgM positive animals having an average of 20% less muscle mass than naïve normal animals. Detailed histopathological studies showed that long-term infection in the armadillo also has discernible effect on the morphological and molecular composition of skeletal muscle fibers. These features of the skeletal muscles in infected armadillos resembles the muscle pathology and function impairment documented in patients with lepromatous leprosy with a high bacterial load (Werneck et al. 1999), suggesting the potential of using the armadillo model not only for neuropathies but also myopathies associated with human leprosy.
Routes and Timing of Infection
Armadillos are susceptible to experimental infection with M. leprae by a variety of routes, including intravenous, intradermal, percutaneous, and respiratory instillation (Sharma et al. 2013; Truman and Sanchez 1993). In addition, many armadillo nerve trunks are large enough to permit direct inoculation of bacilli to the peripheral nerve. Regardless of the route of administration, the type of disease that is manifest in the animals depends on that particular animal's innate or pre-existing response to M. leprae. Lepromatous-type armadillos will eventually develop a fully disseminated infection, while the level and type of involvement will be less with animals that manifest other forms of leprosy.
The duration of infection in the armadillo is somewhat idiosyncratic, but is mainly a factor of the viable challenge dose of bacilli given. The standard challenge dose is 1 × 109 highly viable M. leprae given intravenously. At this dose most animals will develop a fully disseminated infection, requiring humane euthanasia within 18-24 months. Lower challenge doses require accordingly longer periods to manifest full dissemination (Truman 2008). Studies addressing events of preclinical leprosy do not require fully disseminated infections, and can be initiated immediately following challenge.
Limitations of the Armadillo Model
Armadillos are exotic animals and are not commonly used in laboratory studies outside of leprosy research. The primary limitation in use of armadillos is the paucity of reagents, especially antibodies, to facilitate investigations. With recent completion of the armadillo whole genomic sequence, investigative reagents can be generated more easily. Highly specific molecular probes and primers are readily designed. All of these reagents require independent development and verification of their quality.
Armadillos are not available from standard commercial vendors and must be obtained from the wild for investigative purposes. Some IACUC or animal facilities may not be equipped to deal with wild animals. In addition, such wild animals are highly out-bred and may exhibit wide variations in response to challenge. Armadillos do not breed reliably in captivity, but female armadillos routinely give birth to monozygotic quadruplets, and gravid females captured from the wild will litter in captivity, making it possible to conduct studies on matched sets of identical twins (Truman 2008).
Conclusions
Leprosy is a complex infectious disease that can cause disabling damage to peripheral nerves. Although the infection can be cured bacteriologically, there are no effective therapies for reversing nerve damage or preventing additional complications after therapy. Ethical and practical constraints in studying the neurological aspects of human leprosy have left large gaps in the common knowledge of the mechanisms involved in inducing neuropathy and few strategies for the development of new diagnostic approaches or therapeutic regimen. Studies with animal models could markedly benefit the human understanding of the mechanisms of nerve injury in leprosy, and might provide a useful vehicle for evaluating new therapies.
Other than humans, the nine-banded armadillo is the only animal that develops extensive neurological involvement with M. leprae. Comparative pathological studies show that armadillos closely recapitulate many of the functional, physiological, and structural aspects of human leprosy; and a variety of routine clinical, diagnostic, and immuno-pathological techniques can be employed effectively to monitor and stage M. leprae infections in these animals. In addition, armadillos yield an abundant supply of rare leprotic tissues that can facilitate molecular and histopathological studies that would not be possible to consider with other human or animal resources.
Developing techniques to effectively detect and monitor the onset and progress of leprosy neuropathy could have significant benefit to leprosy patients and may provide additional insights for developing new intervention strategies. The compressed duration of infection in armadillos and general characteristics of the infection in these animals make them good candidates for evaluating adjunctive immunological and drug therapy combinations, new anti-neuropathic medications, and safety analysis of new drugs or vaccines before they are deployed in human populations. In addition, experimental leprosy can be viewed as an inducible neuropathy that results in demyelination of Schwann cells and chronic inflammation of peripheral nerves. The understandings gained from studies with armadillos also may be of benefit to research efforts on other demyelinating neuropathies, and will better integrate leprosy into the larger community of important neuropathic diseases.
Acknowledgments
The authors are grateful for the technical assistance of Kyle Andrews, Angelina Deming, Vilma Marks, Greg McCormick, Roena Stevenson, and Heidi Zhang at the NHDP. We also express our appreciation to Karen Burr and Sang Soo Seo at the University of Edinburgh for studies on muscle preparation and microscopy. We thank Peter Hauer at the Johns Hopkins School of Medicine for his assistance in this analysis. These studies were supported in part by funds from the Miss A. M. Urquhart Trust, UK and the University of Edinburgh, Scotland, UK. As well as the United States Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, National Hansen's Disease Program and the National Institutes of Allergy and Infectious Disease (IAA-2646).
References
- Adams LB, Pena MT, Sharma R, Hagge DA, Schurr E, Truman RW. Insights from animal models on the immunogenetics of leprosy: A review. Mem Inst Oswaldo Cruz. 2012;107:197–208. doi: 10.1590/s0074-02762012000900028. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Alter A, Grant A, Abel L, Alcaïs A, Schurr E. Leprosy as a genetic disease. Mamm Genome. 2011;22:19–31. doi: 10.1007/s00335-010-9287-1. [DOI] [PubMed] [Google Scholar]
- Brand PW, Beach BB, Thompson DE. Relative tension and potential excursion of muscles in the forearm and hand. J Hand Surg Am. 1981;3:209–219. doi: 10.1016/s0363-5023(81)80072-x. [DOI] [PubMed] [Google Scholar]
- Colston MJ, Hilson GR. Growth of Mycobacterium leprae and. M. marinum in congenitally athymic (nude) mice, Nature. 1976;5567:399–401. doi: 10.1038/262399a0. [DOI] [PubMed] [Google Scholar]
- Couret M. The behavior of bacillus leprae in cold-blooded animals. J Exp Med. 1911;5:576–589. doi: 10.1084/jem.13.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: A new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007:1059–1073. doi: 10.1097/nen.0b013e31815c8989. (66)12. [DOI] [PubMed] [Google Scholar]
- Fite GL, Wrinkle CK, Sanchez R. Inoculations of M. Leprae in Reptiles. Int J Lepr. 1964;32:272–278. [PubMed] [Google Scholar]
- Franssen H. Electrophysiology in demyelinating polyneuropathies. Expert Rev Neurother. 2008;8(3):417–431. doi: 10.1586/14737175.8.3.417. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66(2):256–258. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- Goransson LG, Brun GJ, Harboe E, Mellgren SI, Omdal R. Intraepidermal nerve fiber densities in chronic inflammatory autoimmune diseases. Arch Neurol. 2006;63(10):1410–1413. doi: 10.1001/archneur.63.10.1410. [DOI] [PubMed] [Google Scholar]
- Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48(3):708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- Jain S, Visser LH, Praveen TL, Rao PN, Surekha T, Ellanti R, Abhishek TL, Nath I. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3(8) doi: 10.1371/journal.pntd.0000498. e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job CK, Kirchheimer WF, Sanchez RM. Variable lepromin response to Mycobacterium leprae in resistant armadillos. Int J Lepr Other Mycobact Dis. 1983;51(3):347–353. [PubMed] [Google Scholar]
- Job CK, Truman RW. Comparative study of Mitsuda reaction to nude mouse and armadillo lepromin preparations using nine-banded armadillos. Int J Lepr Other Mycobact Dis. 2000;68(1):18–22. [PubMed] [Google Scholar]
- Kaur S, Malik AK, Kumar B. Pathologic changes in striated muscles in leprosy. Lepr India. 1981;53(1):52–56. [PubMed] [Google Scholar]
- Khanolkar-Young S, Rayment N, Brickell PM, Katz DR, Vinayakumar S, Colston MJ, Lockwood DN. Tumour necrosis factor-alpha (TNF-alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin Exp Immunol. 1995;99(2):196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152(1-2):51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffen JW. Epidermal nerve fiber density: Normative reference range and diagnostic efficiency. Arch Neurol. 1998;55(12):1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- Miko TL, Le Maitre C, Kinfu Y. Damage and regeneration of peripheral nerves in advanced treated leprosy. Lancet. 1993;342(8870):521–525. doi: 10.1016/0140-6736(93)91647-5. [DOI] [PubMed] [Google Scholar]
- Nicholls PG, Bakirtzief Z, Van Brakel WH, Das-Pattanaya RK, Raju MS, Norman G, Mutatkar RK. Risk factors for participation restriction in leprosy and development of a screening tool to identify individuals at risk. Lepr Rev. 2005;76(4):305–315. [PubMed] [Google Scholar]
- Noon LA, Lloyd AC. Hijacking the ERK signaling pathway: Mycobacterium leprae shuns MEK to drive the proliferation of infected Schwann cells. Sci STKE. 2005;2005(309) doi: 10.1126/stke.3092005pe52. e52. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Rees RJ, Weddell AG. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr Rev. 1970;41(3):155–166. doi: 10.5935/0305-7518.19700023. [DOI] [PubMed] [Google Scholar]
- Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy: Prospective evaluation using skin biopsy. Neurology. 1999;53(8):1641–1647. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: Studies in normal controls and in people with diabetes, with and without neuropathy. Brain 127(Pt 7) 2004:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Rambukkana A. Molecular basis of the interaction of Mycobacterium leprae with peripheral nerve: Implications for therapeutic strategies. Lepr Rev 71 Suppl. 2000:S168–169. [PubMed] [Google Scholar]
- Rambukkana A. Mycobacterium leprae-induced demyelination: A model for early nerve degeneration. Curr Opin Immunol. 2004;16(4):511–518. doi: 10.1016/j.coi.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Rambukkana A. Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog Neurobiol. 2010;91(2):102–107. doi: 10.1016/j.pneurobio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell. 1997;88(6):811–821. doi: 10.1016/s0092-8674(00)81927-3. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Yamada H, Zanazzi G, Mathus T, Salzer JL, Yurchenco PD, Campbell KP, Fischetti VA. Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 1998;282(5396):2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- Scollard DM. Endothelial cells and the pathogenesis of lepromatous neuritis: Insights from the armadillo model. Microbes Infect. 2000;2(15):1835–1843. doi: 10.1016/s1286-4579(00)01335-6. [DOI] [PubMed] [Google Scholar]
- Scollard DM. The biology of nerve injury in leprosy. Lepr Rev. 2008;79(3):242–253. [PubMed] [Google Scholar]
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard DM, McCormick G, Allen JL. Localization of Mycobacterium leprae to endothelial cells of epineurial and perineurial blood vessels and lymphatics. Am J Pathol. 1999;154(5):1611–1620. doi: 10.1016/S0002-9440(10)65414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard DS, Truman RW. Armadillos as animal models for lepromatous neuropathy. In: Deason, editor. Animal Models for Biomedical Research. New York: Academic Press; 1999. pp. 330–336. [Google Scholar]
- Sharma R, Lahiri R, Scollard DM, Pena M, Williams DL, Adams LB, Figarola J, Truman RW. The armadillo: A model for the neuropathy of leprosy and potentially other neurodegenerative diseases. Dis Model Mech. 2013;6(1):19–24. doi: 10.1242/dmm.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. Journal of Experimental Medicine. 1960;112:445–454. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Nicholls PG, Das L, Barkataki P, Suneetha S, Suneetha L, Jadhav R, Sundar Rao PS, Wilder-Smith EP, Lockwood DN, van Brakel WH. Predicting Neuropathy and Reactions in Leprosy at Diagnosis and Before Incident Events-Results from the INFIR Cohort Study. PLoS Negl Trop Dis. 2009;3(8) doi: 10.1371/journal.pntd.0000500. e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12(8):961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- Tapinos N, Rambukkana A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and. Proc Natl Acad Sci U S A. 2005;102(26):9188–9193. doi: 10.1073/pnas.0501196102. 56Lckdependent pathway from leprosy bacilli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman RW. Leprosy in wild armadillos. Leprosy Review. 2005;76:198–208. [PubMed] [Google Scholar]
- Truman RW. Leprosy. In: Vizcaino SF, Loughry WJ, editors. The Biology of the Xenarthra. Gainesville: University Press of Florida; 2008. [Google Scholar]
- Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL, Gillis TP. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl Trop Dis. 2008;2(11) doi: 10.1371/journal.pntd.0000328. e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman RW, Krahenbuhl JL. Viable. M. leprae as a research reagent, Int J Lepr Other Mycobact Dis. 2001;69(1):1–12. [PubMed] [Google Scholar]
- Truman RW, Sanchez RM. Armadillos: Models for leprosy. Lab Animal. 1993;22((1): 8-32) [Google Scholar]
- Truman RW, Singh P, Sharma R, Busso P, Rougemont J, Paniz-Mondolfi A, Kapopoulou A, Brisse S, Scollard DM, Gillis TP, Cole ST. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364(17):1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brakel WH, Nicholls PG, Das L, Barkataki P, Maddali P, Lockwood DN, Wilder-Smith E. The INFIR Cohort Study: Assessment of sensory and motor neuropathy in leprosy at baseline. Lepr Rev. 2005;76(4):277–295. [PubMed] [Google Scholar]
- van Brakel WH, Nicholls PG, Das L, Barkataki P, Maddali P, Lockwood DN, Wilder-Smith E. The INFIR Cohort Study: Investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in north India Lepr Rev. 2005;76(1):14–34. [PubMed] [Google Scholar]
- van Brakel WH, Nicholls PG, Wilder-Smith EP, Das L, Barkataki P, Lockwood DN INFIR Study Group. Early diagnosis of neuropathy in ceprosy–comparing diagnostic tests in a large prospective study (the INFIR Cohort Study) PLoS NTD. 2008;2(4) doi: 10.1371/journal.pntd.0000212. e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh GP, Meyers WM, Binford CH. Naturally acquired leprosy in the nine-banded armadillo: A decade of experience 1975-1985. J Leukoc Biol. 1986;40(5):645–656. doi: 10.1002/jlb.40.5.645. [DOI] [PubMed] [Google Scholar]
- Walsh GP, Storrs EE, Burchfield HP, Cotrell EH, Vidrine MF, Binford CH. Leprosy-like disease occurring naturally in armadillos. J Reticuloendothelial Soc. 1975;18(6):347–351. [PubMed] [Google Scholar]
- Werneck LC, Teive HA, Scola RH. Muscle involvement in leprosy. Study of the anterior tibial muscle in 40 patients Arq Neuropsiquiatr. 1999;57(3B):723–734. doi: 10.1590/s0004-282x1999000500001. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith EP, Van Brakel WH. Nerve damage in leprosy and its management. Nat Clin Pract Neurol. 2008;4(12):656–663. doi: 10.1038/ncpneuro0941. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global leprosy situation. Wkly Epidemiol Rec. 2012;34:317–328. [Google Scholar]