Abstract
It is presumed that resolution of hepatitis C, as evidenced by normalization of liver function tests and disappearance of hepatitis C virus (HCV) RNA from serum, as determined by conventional laboratory assays, reflects virus eradication. In this study, we examined the expression of the HCV genome in the sera, peripheral blood mononuclear cells (PBMC), and, on some occasions, monocyte-derived dendritic cells (DC) long after resolution of hepatitis C by using a highly sensitive reverse transcription (RT)-PCR-nucleic acid hybridization (RT-PCR-NAH) assay. The samples obtained from 16 randomly selected patients (5 with spontaneous and 11 with treatment-induced resolution), monitored for up to 5 years, were studied by qualitative and semiquantitative RT-PCR-NAH and by real-time RT-PCR to detect the HCV RNA positive strand. The replicative HCV RNA negative strand was examined in PBMC after culture with a T-cell proliferation stimulating mitogen. The findings show that HCV RNA was carried in the convalescent-phase sera and/or PBMC in all 16 individuals investigated. Also, DC from six of seven patients were reactive for the HCV genome. Importantly, traces of the HCV RNA negative strand, suggesting progressing virus replication, were detected in the majority of mitogen-stimulated PBMC, including four samples collected 5 years after recovery. Sequencing of the HCV 5′ untranslated region fragment revealed genotype 1b in four of nine individuals examined and genotypes 1a and 2a in three and two patients, respectively. These results imply that HCV RNA can persist at very low levels in the serum and peripheral lymphoid cells and that an intermediate replicative form of the HCV genome can persist in PBMC for many years after apparently complete spontaneous or antiviral therapy-induced resolution of chronic hepatitis C.
Hepatitis C virus (HCV) is a small positive-strand RNA virus of approximately 9,400 nucleotides that chronically infects an estimated 170 to 350 million people worldwide. Of those acutely afflicted, only 15% recover, while the remaining 85% succumb to chronic hepatitis (4). Furthermore, up to one-fifth of the individuals with chronic hepatitis C progress to cirrhosis, and these patients are at a greater risk of developing hepatocellular carcinoma (9).
It is generally accepted that HCV replicates by making a cRNA strand known as the negative or replicative strand. Although the liver is the main site of virus replication, there is an increasing body of evidence for virus propagation in extrahepatic locations, including cells of the lymphatic and the central nervous systems (17, 28). In regard to infection of lymphoid cells, HCV positive and negative strands were detected in the peripheral blood mononuclear cells (PBMC) and the bone marrow from chronically infected patients (26, 29, 37). It was also shown that HCV can propagate in lymphoid cell cultures and that the virus derived is infectious (33, 34). The notion of natural HCV tropism for lymphoid cells is supported by a significant overrepresentation of certain lymphoproliferative disorders in the HCV-infected population. For instance, type II mixed cryoglobulinemia occurs 11 times more frequently in patients with HCV than in those without (6). Also, non-Hodgkin lymphoma appears to be, albeit less strongly, associated with HCV infection (24).
The current RT-PCR-based assay approved for clinical diagnostics, i.e., the Amplicor HCV v2.0 assay (Roche Molecular Diagnostics, Pleasanton, Calif.), detects HCV RNA with a sensitivity of 1,000 virus genomic equivalents (vge) per ml (or 500 IU/ml). Other assays can identify HCV RNA at 52 vge/ml (or 10 IU/ml) (i.e., the Versant HCV RNA qualitative assay; Bayer Corp., Tarrytown, N.J.). This implies that small quantities of HCV occurring either in serum or within cells may escape detection. Therefore, considering the natural history of HCV infection, there exists a possibility that the virus may not be completely eradicated at the time of clinical and serological resolution of hepatitis. This situation may occur following spontaneous recovery or antiviral therapy.
Lymphotropism is a characteristic of many DNA and RNA viruses capable of inducing persistent infection (8, 27). A number of studies, including those with highly hepatotropic hepatitis B virus (19, 30) and woodchuck hepatitis virus (3, 21, 22), have demonstrated that pathogenic virions can persist at low levels in cells of the lymphatic system years after resolution of liver disease, if not for life. In the present study, considering the fact that a symptomatic long-term hepatitis C is a frequent consequence of HCV infection, we asked whether individuals convalescent from hepatitis C may also persistently carry the virus. In addition, although HCV infection of PBMC has been documented in patients with chronic hepatitis C (17, 26, 28, 31, 37), a similar investigation after resolution of hepatitis C has not been conducted. This line of research is important, since silent persistence of small quantities of HCV might have potentially epidemiologic implications and could be responsible for reactivation of the disease after termination of antiviral therapy or in immunocompromised patients.
To assess whether HCV may persist after resolution of hepatitis C, we examined sera and PBMC from individuals with either spontaneous or therapeutically induced resolution of chronic hepatitis C, as evidenced by sustained normalization of biochemical indicators of liver function tests and clearance of HCV RNA from serum, as determined by conventional laboratory assays. In this study, the samples were analyzed using reverse transcription (RT) and nested PCRs followed by Southern blot hybridization of the amplified products to virus-specific probes (RT-PCR-NAH assay; sensitivity, ≤10 vge/ml). Applying this enhanced detection method, we found HCV genomes in sera and/or PBMC in all recovered individuals investigated and observed that the genome can persist for at least 60 months after apparently complete sustained clearance of HCV RNA, as assessed by the current RT-PCR assays. In addition, traces of the virus RNA negative strand were identified in mitogen-stimulated PBMC collected at the end of the observation period, suggesting that virus replication persisted in lymphoid cells long after clinical resolution of hepatitis C.
MATERIALS AND METHODS
Patients and samples.
Parallel serum and PBMC samples from 16 randomly selected patients (12 men and 4 women; ages 32 to 57), who had recovered from HCV infection based on clinical, biochemical, and virological criteria, were investigated in this study (Table 1). Five persons (patients 1 to 5) had evidence of past HCV infection; i.e., they were seropositive for anti-HCV (EIA; Abbott Diagnostics, Maidenhead, United Kingdom) and had undetectable levels of HCV RNA in serum (Roche Amplicor HCV v2.0) and normal levels of alanine aminotransferase in serum. Four of these five individuals had risk factors for exposure to HCV, i.e., multiple transfusions of factor VIII since childhood because of hemophilia (n = 1) or long-term intravenous drug use (IVDU) (n = 3) (Table 1). Prior to inclusion in this study, these five subjects had been monitored regularly for a period of between 12 and 36 months. The blood results repeatedly showed normal liver function and undetectable HCV RNA in the serum on three or more occasions, as determined by the Roche Amplicor HCV v2.0 assay using total RNA extracted with guanidine thiocyanate, as recommended by the manufacturer (sensitivity, 1,000 copies/ml).
TABLE 1.
Clinical characteristics and detection of HCV RNA in sera and lymphoid cells from patients with serological resolution of hepatitis Ca
| Recovery type and patient | Age/ sex | Route of infection | Estimated duration of infection (yr) | Antiviral treatment type and duration (wk) | Follow-up after SVR to treatment (mo)b | HCV RNA
|
HCV geno- typee | Over all positivityf | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum: positive strandc | PBMC
|
DC: positive strandc | |||||||||
| Positive strandc | Negative strandd | ||||||||||
| Spontaneous | |||||||||||
| 1 | 44/M | Blood tx | 41 | None | NA | + | + | NA | NA | 1b | + |
| 2 | 42/M | IVDU | 22 | None | NA | + | + | − | NA | NT | + |
| 3 | 32/F | IVDU | 12 | None | NA | + | + | + | NA | 1a | + |
| 4 | 34/F | IVDU | 10 | None | NA | − | + | + | NA | 2a | + |
| 5 | 55/M | Sporadic | ND | None | NA | + | − | NT | NA | NT | + |
| Therapeutically inducedg | |||||||||||
| 6 | 46/M | IVDU | 20 | IFN/R (24) | 60 | + | + | − | + | 1a | + |
| 7 | 43/M | IVDU | 15 | IFN/R (48) | 60 | + | + | + | NA | 2a | + |
| 8 | 37/M | IVDU | 12 | IFN/R (48) | 60 | + | + | + | + | NT | + |
| 9 | 52/F | Blood tx | 9 | IFN/R (48) | 60 | + | + | + | + | 1a | + |
| 10 | 43/F | IVDU | ND | IFN/R (48) | 60 | + | + | + | − | NT | + |
| 11 | 50/M | Sporadic | ND | IFN/R (48) | 36 | + | + | + | + | NT | + |
| 12a | 49/M | IVDU | 20 | IFN/R (48) | 30 | + | − | NT | + | 1b | + |
| 13 | 48/M | IVDU | 25 | IFN (48) | 24 | + | + | − | NA | NT | + |
| 14 | 44/M | IVDU | 25 | IFN/R (48) | 24 | + | NA | NA | NA | NT | + |
| 15 | 34/M | IVDU | 17 | IFN/R (24) | 17 | + | − | NT | NA | 1b | + |
| 12b | 48/M | IVDU | 20 | IFN/R (48) | 15 | − | + | + | NA | 1b | + |
| 16 | 57/M | IVDU | 35 | IFN/R (24) | 12 | + | + | + | + | 1b | + |
| HCV RNA positive/ total tested (%) | 15/17 (88) | 13/16 (81) | 9/12 (75) | 6/7 (86) | 17/17 (100) | ||||||
M, male; F, female; Blood tx, blood transfusion; NA, not available or not applicable; ND, not determined; NT, not tested; IFN, alpha interferon; R, ribavirin; 12a and 12b, samples from the same patient collected at 30 (a) and 15 (b) months after clinical recovery.
HCV RNA measured by the Roche Amplicor HCV v2.0 assay (sensitivity, 1,000 vge/ml).
HCV RNA measured by nested RT-PCR-NAH with UTR and E2 region-specific primers (sensitivity, ≤10 vge/ml).
HCV RNA measured by nested RT-PCR-NAH with UTR-specific primers (sensitivity, ∼102 vge/ml).
HCV genotype determined in recovered serum and/or PBMC samples as described in Materials and Methods.
Overall positivity defined as HCV RNA reactivity in either serum, PBMC, or a combination thereof.
Patients with SVR after a 24- to 48-week therapy with IFN or combination treatment with IFN/R.
Eleven other patients were known to have chronic hepatitis C, as proven by biopsy and serology; i.e., they were seropositive for anti-HCV and HCV RNA and had received antiviral treatment for 24 or 48 weeks (Table 1). Ten of these eleven patients received combination treatment with alpha interferon 2b (Intron-A) at 3 million U three times per week and ribavirin at 1,000 or 1,200 mg daily (Schering-Plough, Kenilworth, N.J.), while one patient received alpha interferon monotherapy with Roferon-A (Roche, Welwyn Garden City, United Kingdom) at 4.5 million U three times a week for 48 weeks. Although some of these patients acquired HCV infection due to IVDU in the past (Table 1), none were injecting drugs during the treatment or the follow-up. All 11 patients showed sustained virological response (SVR) to antiviral treatment; i.e., HCV RNA was undetectable in the serum 6 months after the end of treatment, and every 6 to 12 months liver function tests and HCV RNA were checked. During the follow-up, HCV RNA was not detectable in these individuals by the Roche Amplicor HCV v2.0 assay (patients 6 to 16; Table 1). In addition, serum samples from the five patients with the 5-year follow-up (patients 6 to 10; Table 1) were also screened for the presence of HCV RNA by the NGI assay (National Genetics Institute, Los Angeles, Calif.) (lower limit of detection, 100 copies/ml). The liver function tests were repeatedly normal in all 11 cases during follow-up. The samples (serum and PBMC) used in this study were obtained between 12 and 60 months after these 11 patients showed SVR to treatment. Serum and PBMC from patient 12 were collected on two occasions, at 15 and 30 months after clearance of HCV RNA as tested by the conventional assay. A serum sample from a serologically evident phase from patient 16 prior to antiviral treatment was also available for analysis. The study was approved by the Human Investigation Committee and samples were collected after an informed consent had been obtained.
Isolation and mitogen stimulation of lymphoid cells.
PBMC were isolated from whole blood by Ficoll-Hypaque (Pharmacia Biotech, Quebec, Canada) gradient centrifugation (18). After washing, cells were resuspended in RPMI 1640 medium (Invitrogen Life Technologies, Burlington, Ontario, Canada) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen), 2 mM glutamine, 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 0.1 mM nonessential amino acids and were counted. Approximately 1 × 107 cells were plated in a 25-cm2 flask. Nonadherent cells were removed for subsequent mitogen stimulation, whereas adherent cells were allowed to differentiate to dendritic cells (DC) for 2 weeks in the presence of 10 ml of the same medium as above, supplemented with 500 U of granulocyte-monocyte colony-stimulating factor (GM-CSF)/ml (BD Biosciences Pharmingen, San Diego, Calif.) and 0.2 ng of interleukin-4 (IL-4)/ml (Roche Diagnostics, Quebec, Canada), as described by others (32). After 5 to 7 days, the cells were resuspended in fresh medium containing 200 ng of lipopolysaccharide (LPS)/ml (Sigma, Mississauga, Ontario, Canada) to aid in DC maturation (16). Finally, cells were spun down at 370 × g for 10 min, and the resulting supernatant was ultracentrifuged at 40,000 × g for 22 h in a Beckman SW50.1 rotor (Beckman Instruments, Inc., Palo Alto, Calif.). RNA was extracted as described below. We have previously observed, by investigating occult hepadnavirus infection in the woodchuck model of hepatitis B (3, 20) and by following earlier findings by others in the same experimental system (13), that stimulation of lymphoid cells with nonspecific mitogens can augment virus replication and expression of its genome, which otherwise were undetectable by PCR methods. In a series of preliminary experiments, we tested whether this approach would enhance identification of HCV in lymphoid cells. The experiments showed that PBMC from patients with either chronic or resolved hepatitis C treated with IL-2 and phytohemagglutinin (PHA) (see below) consistently showed greater expression of both HCV positive and replicative RNA strands relative to that of untreated cells (T. N. Q. Pham and T. I. Michalak, unpublished observations). Therefore, this method was adopted as a routine step in this study. In brief, ∼1 × 107 nonadherent cells were cultured with IL-2 (20 U/ml; Roche Diagnostics Inc., Indianapolis, Ind.) for 48 h and then supplemented with PHA (5 μg/ml; ICN Biomedicals Inc., Aurora, Ohio) and cultured for an additional 72 h. Finally, the cells were spun down, and their pellet and supernatant were preserved for analysis. Where available, samples from mitogen-untreated cells were prepared as controls.
RNA extraction and RT-PCR-NAH assay.
Total RNA was extracted at ambient temperature from ∼1 × 107 cells or 250 μl of serum by using Trizol or Trizol LS (Invitrogen), respectively, for 20 min. After an overnight precipitation in a 0.5× Trizol volume of isopropanol and a wash in 75% RNase-free ethanol, the recovered RNA pellet was resuspended in TE buffer (10 mM Tris with 1 mM EDTA, pH 6.8). In some experiments, the lymphoid cell culture supernatant was ultracentrifuged (as above), and the pellet was treated with Trizol LS to recover RNA. With each RNA extraction of test samples, a mock sample (contamination control), serum, or PBMC from a healthy donor (negative control) and serum or lymphoid cells from a patient with serologically evident chronic HCV infection (positive control) were included. RNA from cells (2 to 4 μg) and all RNA extracted from 250 μl of serum were transcribed with Moloney murine leukemia virus reverse transcriptase (Invitrogen) (20). For PCR amplification of the HCV 5′-untranslated region (UTR), the following primer pairs were used: 5′-CTGTGAGGAACTACTGTCTTC (sense; UTR1) and 5′-GCGGTTGGTGTTACGTTT (antisense; RTU1) for the direct round, and 5′-GCAGAAAGCGTCTAGCCATGGC (sense; UTR2) and 5′-CTGCAAGCACCCTATGAGGCAGT (antisense; RTU2) for the nested round. Reaction mixtures were amplified for 35 cycles in a PTC-200 thermocycler (MJR Research, Watertown, Mass.) by using the following conditions: denaturation at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min. In selected samples (depending upon availability of test material), the presence of the HCV genome was also examined by amplification with the virus E2 region-specific primers. For this purpose, primers 5′-GCGTTGGTAGTAGCTCAACTGCTCC (sense; E2-1) and 5′-GCTTCCGTTAGCGTGACTGATGG (antisense; 2E-1) were used for the direct run and primers 5′-GCGTTGGTAGTAGCTCAACTGCTCC (sense; E2-2) and antisense 2E-1 for the seminested reaction. Both PCR amplifications were carried out for 40 cycles with each cycle at 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min. Samples containing water instead of test cDNA were included as contamination controls. A mock sample and cDNA derived from the serum or PBMC of a healthy donor, prepared as outlined above, were included in each PCR run. As positive controls, cDNA from serum or PBMC from a patient with serologically confirmed chronic HCV infection with a virus load of 3 × 107 vge/ml quantified by real-time PCR (see below) and serial dilutions of the cloned HCV UTR-E2 fragment (see below) were used. The final products were subjected to electrophoresis and NAH with a 32P-labeled HCV UTR-E2 fragment as a probe. This probe was generated by transcribing RNA isolated from a patient chronically infected with HCV genotype 1a. Thus, the cDNA was amplified with primers 5′-GCAGAAAGCGTCTAGCCATGGCGT (sense; UTR3) and 5′-CCACCACMACRGGGCTGGGRGT (antisense; 2E-2) for 40 cycles under the same conditions as those used for E2 fragment amplification. The final product of 1,821 bp was gel purified and cloned into the dual promoter PCRII plasmid vector using the TOPO-TA cloning system (Invitrogen). The identity of several randomly selected clones was ascertained by sequencing. Semiquantitative estimation of HCV RNA load by RT-PCR-NAH was done by amplification of 10-fold serial dilutions of the cloned fragment in parallel with test samples. In a preliminary experiment, it was established that amplification of the predicted amounts of the cloned UTR-E2 sequence gave density signals comparable to those generated by amplification in equivalent amounts of HCV vge obtained from the serum of a patient chronically infected with HCV in which the HCV RNA load was quantified by real-time PCR, as mentioned below. Density of autoradiographic images of signals obtained after Southern blot hybridization were analyzed using a Chemi Genius-2 bio-imaging system (Syngene, Cambridge, United Kingdom). The sensitivity of the nested RT-PCR-NAH assay with either UTR or E2 region-specific primers was ≤10 vge/ml.
HCV RNA real-time RT-PCR.
In selected cases, cDNA was analyzed using LightCycler fast start master hybridization probes (Roche) in the presence of 4 mM MgCl2. cDNA from serum (equivalent of 25 μl) was amplified for 50 cycles (95°C for 10 s, 55°C for 10 s, and 72°C for 5 s) using 5 pM (each) primer: 5′-GCAGAAAGCGTCTAGCCAT (sense; UTR4) and 5′-CTCGCAAGCACCCTATCAG (antisense; RTU3). For the purpose of signal detection through fluorescence resonance energy transfer, probe 1 (5′-CCGCAAGACTGCTAGCCGAGTAGTG) was labeled at the 5′ end with LC Red 640, while probe 2 (5′-ATGCCTGGAGATTTGGGCGTGC) was labeled at the 3′ end with fluorescein isothiocyanate. Melting of the products was done at 95°C by 0.2°C/s increments. Specific HCV amplicons melted at 90.2°C. Tenfold serial dilutions of the recombinant UTR-E2 fragment were used for enumeration of viral load in test samples. The specificity of amplifications and the validity of controls (the same as those used in the RT-PCR-NAH assay) was confirmed by NAH of the products with the HCV UTR-E2 fragment.
Detection of HCV RNA negative strand.
The HCV negative strand was detected by conducting cDNA synthesis using rTth DNA polymerase (Promega Corp., Madison, Wis.) and an approach described by others (15). The sensitivity and specificity of the detection was established using synthetic HCV RNA positive and negative strand fragments, which were generated from the HCV UTR-E2 fragment clones, as templates. For this purpose, randomly chosen clones were sequenced using a fmol DNA cycle sequencing system (Promega) to select those carrying a positive or a negative strand. After plasmid linearization, the strands were transcribed with T7 RNA polymerase from a Megascript T7 high-yield transcription kit (Ambion, Austin, Tex.). For negative-strand detection, 5 μg of test RNA in 10 μl of RNase-free TE buffer was denatured for 1 min at 95°C, and then the temperature was lowered to 70°C. All subsequent steps were carried out at this temperature. The RNA sample was supplemented with 10 μl of preheated 2× RT buffer (20 mM Tris-HCl buffer, pH 8.3, with 180 mM KCl, 2 mM MnCl2, 1.6 mM deoxynucleoside triphosphate mix, 15 pM UTR1 primer, and 5 U of rTth DNA polymerase) and cycled for 2 min at 60°C. cDNA extension was continued for an additional 15 min. Then, 80 μl of preheated PCR mixture containing a chelating buffer (10 mM Tris-HCl buffer, pH 8.3, with 100 mM KCl, 750 μM EGTA, 0.05% Tween 20, and 5% glycerol) with 2.5 mM MgCl2 and 15 pM antisense RTU1 primer was added, and amplification was carried out for 45 cycles. Finally, 10 to 20 μl of the direct PCR product was used as the template for the second round of amplification, which was done under the same conditions as those described for standard RT-PCR. The resulting PCR products were analyzed by agarose gel electrophoresis and detected by NAH, as described above. The strand-specific PCR combined with hybridization analysis was capable of detecting ∼102 vge of the correct (negative) strand, while unspecifically detecting the incorrect (positive) strand at ≥106 molecules. As controls, amplifications of a synthetic HCV RNA positive strand, a synthetic HCV RNA negative strand, a mock sample, cDNA from PBMC of a healthy donor, and water were performed with each assay run to ensure specific detection.
Sequencing.
PCR amplicons of the 5′-UTR 244-bp fragment derived from selected test serum and PBMC samples (subject to availability of material) were directly sequenced by the Sanger dideoxy chain termination method with the use of a fmol DNA cycle sequencing system (Promega Corp.) and 32P-labeled UTR2 primer. By using this method, on average 68% ± 20% (standard deviation) of the UTR product sequence was determined. Analysis of the sequencing data and their comparison with the published consensus sequences of HCV genotypes were done with the aid of Sequencher software (Gene Codes Corporation, Ann Arbor, Mich.).
RESULTS
HCV RNA in sera after resolution of hepatitis C.
Sera were collected from individuals monitored for 12 to 60 months after spontaneous or antiviral treatment-induced recovery from chronic hepatitis C. These persons were repeatedly nonreactive for HCV RNA when tested under standard clinical laboratory RNA isolation and RT-PCR amplification conditions. However, the same sera subjected to RNA extraction with Trizol and analyzed by nested RT-PCR-NAH with UTR and/or E2 region-specific primers (sensitivity, ≤10 vge/ml) demonstrated persistent presence of HCV genomes (Table 1). Thus, four of the five individuals with apparently complete virological recovery from self-limiting hepatitis C continued to carry circulating HCV RNA when tested by the in-house assay. Among the persons who resolved hepatitis C due to antiviral therapy, the HCV genome was identified in sera of all of the 11 patients examined. However, two serum samples collected from one individual (patient 12) at 15 and 30 months after recovery gave opposing results. Thus, while the former sample was HCV RNA nonreactive, the latter was evidently positive when tested by nested RT-PCR-NAH. Overall, HCV RNA was detected in 15 (88%) out of 17 serum samples analyzed. The quantities of HCV RNA carried in the late-convalescent-phase sera usually varied between 10 and 102 vge per ml, as estimated by densitometric analysis of the RT-PCR-NAH signals. The rigorous precautions undertaken, which included contamination and negative controls at each step of RNA and cDNA preparation and PCR amplification as well as validation of the positive signals and controls by NAH, unequivocally ensured the authenticity of HCV RNA detection.
Quantification of HCV RNA by real-time RT-PCR.
Serum samples from 10 patients, 4 with self-limiting and 6 with antiviral treatment-induced resolution of hepatitis C, which were HCV positive by the nested RT-PCR-NAH, were examined for viral RNA load by our in-house-developed real-time RT-PCR (sensitivity, ≥102 vge/ml). Of the 10 samples, 4 (cases 1, 3, 6, and 15) showed quantifiable amounts of HCV genome by a Light Cycler (Roche), while the remaining 6 were entirely nonreactive (data not shown). The HCV RNA loads measured in the positive samples were around 5 × 102 vge/ml. The results indicated that, while HCV RNA could be detected and quantified in some convalescent-phase sera by real-time RT-PCR, overall this technique provides only a moderate advantage over standard laboratory RT-PCR assays and was not superior to the RT-PCR-NAH protocol normally used for detection of the silent presence of HCV sequences.
Upregulation of HCV RNA expression in mitogen-stimulated PBMC.
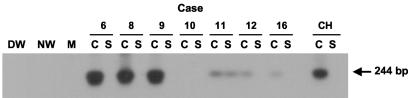
Taking advantage of previous observations that mitogen stimulation of lymphoid cells may enhance replication of the residing virus and allow a more ready detection of its genome (3, 13), we included cultures of PBMC from the recovered individuals with IL-2 and PHA as a routine step prior to RNA extraction. As illustrated in Fig. 1, this measure allowed for detection of HCV RNA in apparently virus-nonreactive PBMC or augmented HCV RNA expression in cells which, prior to the treatment, displayed weak HCV RNA signals. Using this approach, the HCV RNA positive strand was detected in 13 (81%) out of the 16 cases investigated (Table 1). In contrast, when unstimulated cells were examined, three of the nine tested were found reactive (data not shown).
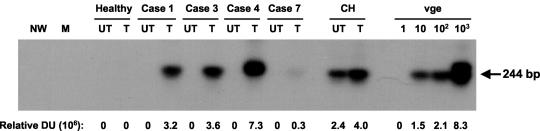
FIG. 1.
Effect of mitogen stimulation on the detection of HCV RNA in PBMC from individuals with resolved chronic hepatitis C. Peripheral lymphoid cells isolated from individuals with spontaneous (cases 1, 3, and 4) and therapeutically induced (case 7) resolution of chronic hepatitis C were treated (T) by culture with IL-2 and PHA for 72 h or were untreated (UT). Total RNA (3 μg) was transcribed to cDNA and amplified by nested PCR with HCV 5′-UTR primers, and the amplicons were detected by Southern blot hybridization. For contamination controls water was added instead of cDNA, amplified by direct and nested reactions (NW), and mock (M) treated as test RNA. Negative controls included untreated and treated PBMC from a healthy donor. The positive controls included RNA extracted from PBMC of a patient with clinically evident chronic hepatitis C (CH) and serial dilutions of a recombinant HCV UTR-E2 fragment as quantitative standards (vge). Positive signals showed the expected 244-bp oligonucleotide fragments. Numbers under the panel represent relative densitometric units (DU) given by hybridization signals.
Detection of HCV RNA in parallel sera and PBMC.
Examination of paired sera and IL-2- and PHA-treated PBMC using the RT-PCR-NAH assay with UTR and E2 region-specific primers revealed that in fact all individuals investigated remained HCV RNA reactive (Table 1). Figure 2a illustrates the detection of HCV RNA in three cases after spontaneous recovery from chronic hepatitis C. Of the five individuals examined in this group, the viral genome was identified in three (60%) in both serum and PBMC. The other two (40%) were positive in either serum or PBMC (Table 1). Figure 2b depicts the identification of HCV RNA in parallel serum and PBMC samples collected from three individuals 5 years after apparently complete SVR due to antiviral treatment. In all five recovered individuals who were tested after the 5-year observation period, HCV RNA signals were detected in both serum and PBMC.
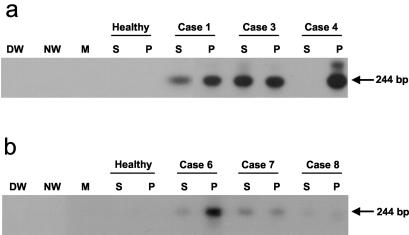
FIG. 2.
Detection of HCV RNA in parallel serum and PBMC samples from individuals convalescent from chronic hepatitis C. Sera (S) and IL-2- and PHA-stimulated PBMC (P) from individuals who (a) resolved hepatitis spontaneously and were monitored for at least 12 months thereafter or (b) resolved hepatitis due to antiviral therapy and were monitored for 60 months after the apparently complete virological recovery were tested for HCV RNA by nested RT-PCR-NAH assay. Serum and PBMC from a healthy individual (Healthy) and water (DW and NW) and cDNA-free mock (M) samples were used as negative controls. Positive samples showed the expected 244-bp band.
In selected cases, sera and parallel PBMC samples (as availability of material permitted) were evaluated for the presence of HCV RNA by RT-PCR-NAH not only using UTR primers but also E2 region-specific primers. Figure 3 illustrates the identification of these genomic sequences in cases 2 and 13. Thus, the serum and PBMC of case 2 were reactive for both the UTR and E2 regions. Interestingly, in case 13, although HCV RNA was negative in serum by amplification with the UTR primers, the same sample was reactive with the E2 primers. PBMC from this patient showed HCV reactivity detectable with both genomic regions. The discrepancy observed was likely related to minor differences in the efficacy of PCR amplification and/or in RNA or cDNA loading. This situation was observed in other studies where trace quantities of virus genomes occurring at the lower limit of the sensitivity of the nested PCR-NAH were tested (3, 20, 22).
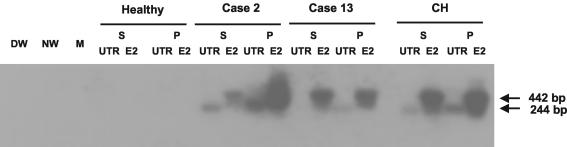
FIG. 3.
Identification of HCV RNA in parallel samples of serum and PBMC by RT-PCR-NAH using both UTR and E2 region-specific primers. Total RNA was extracted from sera (S) and PBMC (P) from case 2, with self-limited acute infection, and from case 13, with therapeutically induced recovery from chronic hepatitis. Serum and PBMC from a healthy individual and a patient with clinically evident chronic hepatitis C (CH) served as negative and positive controls, respectively. Water (DW and NW) and cDNA-free mock (M) samples were included as contamination controls. Positive samples showed the expected 244-bp band for UTR and the 442-bp fragment for the E2 region by Southern blot hybridization analysis.
Overall, as summarized in Table 1, of the 11 cases with therapeutically induced resolution of hepatitis C, 10 (91%) were found positive for HCV RNA in serum, and 9 (90%) out of 10 whose IL-2- and PHA-treated PBMC were tested also carried viral sequences. Although, one should be cautioned to note that detection of the HCV RNA positive-strand sequences by RT-PCR does not necessarily reflect the existence of the entire virus genome.
HCV RNA sequence analysis.
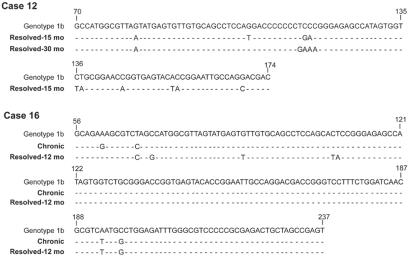
Direct sequencing of the nested 5′-UTR amplicons derived from sera and, on some occasions, from in vitro stimulated PBMC allowed for determination of the HCV genotype in 2 of 5 individuals with spontaneous recovery and in 6 of 11 patients with SVR due to antiviral therapy (Table 1). Genotype 1b was identified in four cases, and genotype 1a was identified in three individuals, whereas genotype 2a was prominent in two patients. In two instances (cases 12 and 16), samples obtained at two different time points of follow-up were sequenced. Thus, in patient 12, the sequences from the 15- and 30-month points after resolution of hepatitis were compared. In case 16, the first sample was obtained during symptomatic chronic hepatitis and the second 12-month sample after clinical recovery. As documented in Fig. 4, the sequences detected were identical in both samples from each of the patients, and they were of genotype 1b (10), albeit there were minor differences between these sequences and the prototype sequence of HCV genotype 1b.
FIG. 4.
Nucleotide sequence alignment of the 5′-UTR fragments of HCV RNA amplified from serum or PBMC of individuals with apparently complete serological resolution of chronic hepatitis C following antiviral therapy. Samples from case 12 were obtained 15 (PBMC) or 30 months (serum) after sustained response to treatment. In case 16, the first sample was taken during symptomatic chronic hepatitis (Chronic) and the second 12 months after evidence of sustained clinical resolution of hepatitis. The sequences obtained were aligned with the prototype HCV genotype 1b (10). Nucleotides in the sequences from the patients' samples identical to those in the HCV genotype reference (top line) are shown as dashes, and differences are identified by letters.
HCV RNA in DC.
DC derived from the monocyte population of PBMC were available for examination from seven convalescent cases (Table 1). When comparable amounts of total RNA extracted from these DC were transcribed and cDNA was analyzed by the nested RT-PCR-NAH, six cell preparations were found to be HCV RNA reactive (Fig. 5). Interestingly, DC from one individual (case 12, sample collected 30 months after SVR) showed HCV RNA despite the fact that the total PBMC population was HCV nonreactive by RT-PCR-NAH (Table 1). DC culture supernatants concentrated by ultracentrifugation were HCV RNA negative, except in a single specimen (case 11). This indicated that a potential contribution of extracellular HCV RNA to positive signals detected in DC was unlikely.
FIG. 5.
Expression of the HCV RNA positive strand in monocyte-derived DC from individuals with long-term recovery from chronic hepatitis C. Cultured DC (C) and concentrates of their culture supernatant (S) were tested for HCV RNA by nested RT-PCR-NAH. Three micrograms of DC RNA and all RNA extracted from the pellet obtained after ultracentrifugation of 10 ml of DC culture supernatant were used for analysis. Water (DW and NW) instead of cDNA and mock (M) sample treated as a test RNA sample served as negative controls, while DC and their culture supernatant derived from a patient with chronic hepatitis C (CH) were used as positive controls. Hybridization signals showed 244-bp bands.
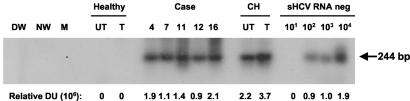
HCV RNA negative strand in PBMC.
As indicated, 81% of the PBMC samples examined after culture in the presence of IL-2 and PHA displayed the HCV RNA positive strand (Table 1). To determine whether this expression was accompanied by the presence of the replicating virus genome, the HCV RNA negative strand, which is considered to be a reliable indicator of actually progressing replication, was quantified. Since this replication intermediate normally occurs at frequencies lower than those of the positive strand, the replicative strand was examined only in PBMC which were found reactive for the positive strand. Figure 6 illustrates the detection of the HCV RNA negative strand in IL-2- and PHA-treated PBMC isolated from patient 4 with a 12-month follow-up after spontaneous recovery and from four individuals with follow-up between 12 and 60 months after apparently complete SVR due to antiviral treatment (Table 1). Overall, this form of RNA was detected in 9 (75%) out of 12 PBMC samples investigated. The expression of the replicative strand varied between 30 and 3 × 103 vge/μg of total cellular RNA, as estimated by quantification of hybridization signals. It is of note that culture of PBMC with IL-2 and PHA enhanced expression of the HCV RNA negative strand by approximately twofold (Fig. 6).
FIG. 6.
Detection of the HCV RNA negative strand in PBMC from individuals with clinical and apparently complete virological resolution of hepatitis C. Total RNA was extracted from IL-2- and PHA-stimulated PBMC isolated from individuals with self-limited (case 4) or therapeutically induced (cases 7, 11, 12, and 16) recovery from hepatitis and from control untreated (UT) and IL-2- and PHA-treated (T) PBMC obtained from a healthy donor and a patient with actively progressing chronic hepatitis C (CH). RNA was reverse transcribed and cDNA was amplified by PCR with HCV RNA negative strand-specific 5′-UTR primers. Serial dilutions of a synthetic HCV RNA negative strand (sHCV RNA neg) amplified in parallel were used as semiquantitative standards. Water (DW and NW) and mock (M) samples were used as negative and contamination controls. The positive signals (244-bp fragments) were visualized by hybridization to the recombinant HCV UTR-E2 probe. Values under the panel represent relative densitometric units (DU) given by positive hybridization signals.
DISCUSSION
In the present study, by applying a highly sensitive HCV RNA detection assay, we show, for the first time, evidence of the long-term persistence of HCV genomes in sera and circulating lymphoid cells in individuals considered to be clinically and serologically cleared of HCV infection. Serum samples of these persons have been repeatedly HCV RNA nonreactive by a standard laboratory assay during follow-up between 12 and 60 months after normalization of liver function tests and apparently complete spontaneous or antiviral treatment-induced sustained virus clearance. However, as the enhanced sensitivity of our assay showed, all of the patients in fact continued to carry low levels of HCV RNA in serum and/or PBMC. The RT-PCR-NAH applied in this study was at least 10-fold more sensitive than the current laboratory tests. The enhanced level of HCV RNA detection was achieved, among other factors, by using a greater amount of RNA for testing, following a classical RNA extraction method, the employment of two rounds of PCR amplification, and the use of hybridization analysis to further increase the sensitivity and specificity of virus identification. The nucleotide sequence analysis of the HCV 5′-UTR confirmed the specificity of the HCV genome detection and revealed that different virus genotypes were associated with the occult long-term carriage of HCV genomes.
Analysis of PBMC, which were collected in parallel with test sera and routinely treated with IL-2 and PHA prior to RNA extraction, gave a significant diagnostic advantage by allowing the identification of HCV genomes in the sera of recovered persons who were HCV RNA nonreactive (i.e., cases 4 and 12; Table 1) and by confirming positive results obtained by serum testing. However, most importantly, the availability of these cells permitted examination of the negative strand of virus RNA, providing an insight into the replication capability of the HCV genome carried by lymphoid cells in the recovered individuals. This analysis showed that trace virus replication persisted in the lymphatic system in the majority (75%) of persons from whom sufficient numbers of PBMC were available for investigation. This finding is not surprising, considering that several laboratories have shown the presence of the HCV RNA replicative strand in peripheral and organ lymphoid cells of patients with chronic hepatitis C (7, 17, 23, 37), as we also found in this study (Fig. 6). The present data clearly indicate that carriage of HCV in peripheral lymphoid cells is not terminated at the time of clinical resolution of chronic hepatitis C, but rather it subsides to a level that is not readily detectable by the currently used laboratory assays, and the virus genome persists at these low levels for a long time after apparently complete recovery.
In the present study, culture of lymphoid cells with IL-2 and PHA markedly enhanced detection of both the HCV RNA positive and replicative strands. PHA is a potent nonspecific inducer of T-cell proliferation, whereas IL-2 is a cytokine important for T-cell growth and in vitro survival (12). In this context, it has been shown that T-cell (11, 25, 33) as well as B-cell (23) lines can be infected in culture by HCV, although the infection was usually inefficient and transient. Furthermore, the most recent and unambiguous study documented that HCV infects B cells and that these cells are capable of producing infectious HCV virions (35). Although the molecular mechanism of mitogen-induced upregulation of HCV replication and genome expression in lymphoid cells is unknown and will require separate studies, this finding is not unique. Similar observations have been made in infections with other viruses which display a tropism for lymphoid cells, including serologically occult infection caused by woodchuck hepatitis virus that invariably involves the host's lymphatic system (3, 20). The strategy of enhancing HCV genome detection in PBMC established in this study provides a valuable aid to the diagnosis of serologically silent HCV persistence. Subsequent work should include a definition of culture conditions under which an increased HCV genome expression can be achieved in both T and B cells. This might further enhance the identification of HCV genomes in situations where low virus loads are expected and where sera and resting lymphoid cells remain seemingly virus nonreactive.
In light of recent findings demonstrating that monocyte-derived DC from patients with chronic hepatitis C might be a reservoir of replicating HCV (2, 7), we assessed whether DC obtained from the recovered individuals by a similar procedure also carry the virus. Here, we provide molecular evidence for the presence of HCV RNA in DC from six of the seven cases examined (Table 1 and Fig. 3). Although the amount of the genomic material was not sufficient to determine the presence of the HCV replicative strand, the absence of a viral RNA signal in the concentrated DC culture supernatant, except for that of a single specimen (case 11), strongly suggests that the HCV RNA detected originated from the cells but not from a culture aliquot. The allostimulatory function and maturation of DC in individuals with resolved hepatitis C do not seem to be noticeably affected (1, 2). However, the persistence of trace amounts of the virus in DC, which are essential for T-cell activation, may play an important role by providing a sustained stimulus to HCV-specific T cells. Consistent with this interpretation is the existence of strong HCV-specific cytotoxic T- and Th-lymphocyte responses for many years after resolution of hepatitis C (5, 14, 36).
The present study shows that HCV RNA can persist, albeit at very low levels, in the serum and circulating lymphoid cells for years after apparently complete clinical and virological resolution of chronic hepatitis C. If the present findings reflect the existence of the biologically competent, infectious virus, this silent persistence may have important epidemiological and pathogenic implications. Among other factors, these trace amounts of the virus may lead to reactivation of hepatitis C after termination of antiviral therapy or due to severe immunosuppression and may support perpetuation on the subclinical level of liver disease which becomes symptomatic years after exposure to the virus. They may also constitute a source from which HCV may spread through blood and organ donations to susceptible individuals.
Acknowledgments
This study was supported by grant EOP-41538 provided to T. I. Michalak from the Canadian Institutes of Health Research and the Health Canada Hepatitis C Initiative. P. M. Mulrooney is a recipient of a Doctoral Fellowship Award from the Canadian Blood Services. T. I. Michalak is the Canada Research Chair (Tier 1) in Viral Hepatitis/Immunology sponsored by the Canada Research Chair Program and funds from the Canadian Institutes of Health Research and the Canada Foundation for Innovation.
REFERENCES
- 1.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 2.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 3.Coffin, C. S., and T. I. Michalak. 1999. Persistence of infectious hepadnavirus in offspring of woodchuck mothers recovered from viral hepatitis. J. Clin. Investig. 104:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J. 1999. The scientific challenge of hepatitis C virus. Science 285:26-30. [DOI] [PubMed] [Google Scholar]
- 5.Cramp, M. E., P. Carucci, S. Rossol, S. Chokshi, G. Maertens, R. Williams, and N. V. Naoumov. 1999. Hepatitis C virus (HCV) specific immune responses in anti-HCV positive patients without hepatitis C viraemia. Gut 44:424-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag, H. B., H. Hampel, C. Yeh, and L. Rabeneck. 2002. Extrahepatic manifestation of hepatitis C among United States male veterans. Hepatology 36:1439-1445. [DOI] [PubMed] [Google Scholar]
- 7.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 187:1951-1958. [DOI] [PubMed] [Google Scholar]
- 8.Grosjean, I., C. Caux, C. Bella, I. Berger, I. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory property for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoofnagle, H. J. 2002. Course and outcome of hepatitis C. Hepatology 36:S21-S29. [DOI] [PubMed] [Google Scholar]
- 10.Kato, N., M. Hijikata, Y. Ootsuyama, M. Nakagawa, S. Ohkoshi, T. Sugimura, and K. Shimotohno. 1990. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc. Natl. Acad. Sci. USA 87:9524-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato, N., T. Nakazawa, T. Mizutani, and K. Shimotohno. 1995. Susceptibility of human T lymphotropic virus type 1 infected cell line MT-2 to hepatitis C virus infection. Biochem. Biophys. Res. Commun. 206:863-869. [DOI] [PubMed] [Google Scholar]
- 12.Kay, J. E. 1991. Mechanisms of T lymphocyte activation. Immunol. Lett. 29:51-54. [DOI] [PubMed] [Google Scholar]
- 13.Korba, B. E., P. J. Cote, and J. L. Gerin. 1988. Mitogen-induced replication of woodchuck hepatitis virus in cultured peripheral blood lymphocytes. Science 241:1213-1216. [DOI] [PubMed] [Google Scholar]
- 14.Koziel, M. J., D. K. Wong, D. Dudley, M. Houghton, and B. D. Walker. 1997. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell response in seronegative persons. J. Infect. Dis. 176:859-866. [DOI] [PubMed] [Google Scholar]
- 15.Lanford, R. E., and D. Chavez. 1998. Strand-specific rTth RT-PCR for the analysis of HCV replication, p. 471-481. In J. Y.-N. Lau (ed.), Hepatitis C protocols. Humana Press, Totowa, N. J. [DOI] [PubMed]
- 16.Larsson, M., D. Messmer, S. Somersan, J. F. Fonteneau, S. M. Donahoe, M. Lee, P. R. Dunbar, V. Cerundolo, I. Julkunen, D. F. Nixon, and N. Bhardwaj. 2000. Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells. J. Immunol. 165:1182-1190. [DOI] [PubMed] [Google Scholar]
- 17.Lerat, H., F. Berby, M. A. Traubaud, O. Vidalin, M. Major, C. Trepo, and G. Inchauspe. 1996. Specific detection of hepatitis C virus strand RNA in hematopoietic cells. J. Clin. Investig. 97:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lew, Y.-Y., and T. I. Michalak. 2001. In vitro and in vivo infectivity and pathogenicity of the lymphoid cell-derived woodchuck hepatitis virus. J. Virol. 75:1770-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalak, T. I., C. Pasquinelli, S. Guilhot, and F. V. Chisari. 1994. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Investig. 93:230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michalak, T. I., I. U. Pardoe, C. S. Coffin, N. D. Churchill, D. S. Freake, P. Smith, and C. L. Trelegan. 1999. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 29:928-938. [DOI] [PubMed] [Google Scholar]
- 21.Michalak, T. I. 2000. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol. Rev. 174:98-111. [DOI] [PubMed] [Google Scholar]
- 22.Michalak, T. I., P. M. Mulrooney, and C. S. Coffin. 2004. Low doses of hepadnavirus induce infection of the lymphatic system that does not engage the liver. J. Virol. 78:1730-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morsica, G., G. Tambussi, G. Sitia, R. Novati, A. Lazzarin, L. Lopalco, and S. Mukenge. 1999. Replication of hepatitis C virus in B lymphocytes (CD19+). Blood 94:1138-1139. [PubMed] [Google Scholar]
- 24.Musto, P. 2002. Hepatitis C virus infection and B cell non-Hodgkin's lymphomas: more than a simple association. Clin. Lymphoma 3:150-160. [DOI] [PubMed] [Google Scholar]
- 25.Nissen, E., M. Hohne, and H. Schreier. 1994. In vitro replication of hepatitis C virus in a human lymphoid cell line (H9). J. Hepatol. 20:437-442. [DOI] [PubMed] [Google Scholar]
- 26.Okuda, M., K. Hino, M. Korenaga, Y. Yamaguchi, Y. Katoh, and K. Okita. 1999. Differences in hypervariable region 1 quasispecies of hepatitis C virus in human serum, peripheral blood mononuclear cells, and liver. Hepatology 29:217-222. [DOI] [PubMed] [Google Scholar]
- 27.Oldstone, M. B. 1996. Virus-lymphoid cell interactions. Proc. Natl. Acad. Sci. USA 93:12756-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radkowski, M., J. Wilkinson, M. Nowicki, D. Adair, H. Varga, C. Ingui, J. Rakela, and T. Laskus. 2002. Search for Hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J. Virol. 76:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radowski, M., J. Kubicka, E. Kisiel, A. Cianciara, M. Nowicki, J. Rakela, and T. Laskus. 2000. Detection of active hepatitis C virus and hepatitis G virus/GB virus C replication in bone marrow in human subjects. Blood 95:3986-3990. [PubMed] [Google Scholar]
- 30.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of cytotoxic T-lymphocyte response. Nat. Med. 2:1104-1108. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Inigo, E., M. Casqueiro, S. Navas, J. Bartolome, M. Pardo, and V. Carreno. 2000. Fluorescent “in situ” hybridization of hepatitis C virus RNA in peripheral blood mononuclear cells from patients with chronic hepatitis C. J. Med. Virol. 60:269-274. [DOI] [PubMed] [Google Scholar]
- 32.Romani, N., S. Grunner, D. Brang, E. Kampen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu, Y. K., A. Iwamoto, M. Hijikata, R. H. Purcell, and H. Yoshikura. 1992. Evidence for in vitro replication of hepatitis C virus genome in a human T cell line. Proc. Natl. Acad. Sci. USA 89:5477-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu, Y. K., H. Igarashi, T. Kiyohara, M. Shapiro, D. C. Wong, R. H. Purcell, and H. Yoshikura. 1998. Infection of a chimpanzee with hepatitis C virus grown in cell culture. J. Gen. Virol. 79:1383-1386. [DOI] [PubMed] [Google Scholar]
- 35.Sung, V. M. H., S. Shimodaira, A. L. Doughty, G. R. Picchio, H. Can, B. T. S. Yen, K. L. Lindsay, A. M. Levine, and M. M. C. Lai. 2003. Establishment of B cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J. Virol. 77:2134-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 37.Willems, M., K. Peerlinck, H. Moshage, I. Deleu, C. Van den Eynde, J. Vermylen, and S. H. Yap. 1994. Hepatitis C virus RNAs in plasma and in peripheral blood mononuclear cells of hemophiliacs with chronic hepatitis C: evidence for viral replication in peripheral blood mononuclear cells. J. Med. Virol. 42:272-278. [DOI] [PubMed] [Google Scholar]