Abstract
Recent advances in neuroscience have provided new insights into the understanding of heart–brain interaction and communication. Cardiac information to the brain relies on two pathways, terminating in the insular cortex (IC) and anterior cingulate cortex (ACC), along with the somatosensory cortex (S1-S2). Interoception relying on these neuroanatomical pathways has been shown to modulate social cognition. We report the case study of C.S., a patient with an ‘external heart’ (an extracorporeal left-univentricular cardiac assist device, LVAD). The patient was assessed with neural/behavioral measures of cardiac interoception complemented by neuropsychological and social cognition measures. The patient’s performance on the interoception task (heartbeat detection) seemed to be guided by signals from the artificial LVAD, which provides a somatosensory beat rather than by his endogenous heart. Cortical activity (HEP, heartbeat-evoked potential) was found decreased in comparison with normal volunteers, particularly during interoceptive states. The patient accurately performed several cognitive tasks, except for interoception-related social cognition domains (empathy, theory of mind and decision making). This evidence suggests an imbalance in the patient’s cardiac interoceptive pathways that enhances sensation driven by the artificial pump over that from the cardiac vagal-IC/ACC pathway. A patient with two hearts, one endogenous and one artificial, presents a unique opportunity to explore models of interoception and heart–brain interaction.
Keywords: interoception, insula, heart-brain, social cognition, HEP
INTRODUCTION
Interoception, the processing of bodily signals from the viscera and somatic tissues, has been recently proposed as a core aspect of motivational regulation of behavior and cognition (Craig, 2002; Bechara and Naqvi, 2004; Singer et al., 2009). A systematic method for assessing interoception is to measure performance on heartbeat detection (HBD) tasks (Cameron, 2002; Craig, 2002). HBD capability has been shown to correlate with affective and cognitive psychological states (Domschke et al., 2010) as well as cardio-dynamic parameters, such as stroke volume and momentum of the ejected blood mass (Schandry et al., 1993).
Furthermore, it has been found that HBD is also associated with neurophysiological measures. A heartbeat-evoked potential (HEP) was initially described in scalp electroencephalography (EEG) (Schandry and Weitkunat, 1990). Further evidence showed that it was generated by the insular cortex (IC) and anterior cingulate cortex (ACC) (Pollatos et al., 2005), which are considered key nodes for interoception (Craig, 2003; Critchley et al., 2004).
Meanwhile, current interoception research suggests different neural pathways mediating body awareness: (i) visceral afferents projecting to the IC and ACC; (ii) skin afferents projecting to secondary somatosensory cortex (S2) or (iii) both of them. The first pathway comprises vagal fibers endings in low-threshold mechano-receptors in the atria, ventroatrial and ventricles (Malliani et al., 1986; Longhurst, 2004), which project to the main homeostatic integration sites in the brainstem. There, they converge with the lamina-1 spinothalamocortical pathway that conveys homeostatic information from other tissues and finally to IC–ACC (Craig, 2002). Functional neuroimaging studies of normal subjects while performing HBD tasks have evidenced that interoceptive accuracy and sensitivity is related to activations of IC (specifically right anterior IC) (Critchley et al., 2004; Pollatos et al., 2007b). However, these same studies additionally reported S2 activation related to cardiac interoception (the second pathway), which suggests that skin afferent projections might also be involved in heartbeat interoception. Further functional magnetic resonance imaging (fMRI) research with pharmacologically enhanced interoception (Cameron and Minoshima, 2002) and source EEG estimation, found activation of IC, ACC and S2 (Pollatos et al., 2007a). Moreover, subjects located their perceived heartbeat sensations mostly in the lower left chest, the neck, belly and head, locations close to major arteries’ trajectories (Khalsa et al., 2009b). These results converge with the fact that individuals with lower body mass index are better HBD performers (Rouse et al., 1988), suggesting that receptors in the skin might play a role in the cardiac interoceptive awareness. Furthermore, a lesion study provided results that both pathways of cardiac interoception independently mediate the heartbeats awareness (Khalsa et al., 2009a). They assessed a patient with bilateral damage to IC–ACC and intact bilateral S2, who had preserved HBD performance. However, after applying topical anesthetic to the chest skin the patient showed impaired interoceptive awareness while performance in healthy participants was unaffected. Taken together, these two results support the existence of relatively independent pathways for cardiac interoceptive signaling.
A privileged and direct approach to the study of cardiac interoception is provided by the assessment of patients who carry external devices for cardiac assistance, such as the left ventricular assist device (LVAD) that is aimed at supporting ventricular pumping. Pulsatile external versions of the LVAD consist of a paracorporeal polyurethane membrane pump that lies over the patient’s abdominal skin and moves slightly up and down with every beat. This movement generates stimulation on the patient’s skin that can be sensed by somatosensory receptors. However, because the LVAD is not directly innervated by the brain, it constitutes a unique opportunity to separate the neural heart–insula/ACC pathway from mechanic perturbations elicited by somatosensation.
One recently proposed aspect of interoception seems to rely on its influence on other high-level cognitive domains. Particularly, interoception affects emotion recognition (Wiens, 2005), social emotion (Fukushima et al., 2011), theory of mind (Lutz et al., 2009) and decision making (Dunn et al., 2010b; Kirk et al., 2011). Nevertheless, it remains a topic of debate whether each pathway contributes to different aspects of interoception and what the relationship of each of them to emotional and social cognition is.
We report behavioral and neural measures of interoception in C.S., who is a patient with an extracorporeal LVAD and a sample of matched control participants. This study is based on one main working hypothesis:
Given that the LVAD interferes with physiological cardiac interoception rhythms, the patient should present impaired interoception. If interoception is driven by mechanical sensation, then the rhythm created by the pump should be more strongly associated with interoceptive reports. However, if interoception is mediated by direct vagal enervation, the heart would drive interoception despite somatosensory interference.
To test this hypothesis, we assessed the behavioral interoception of C.S. using a HBD task and we compared his differential performance related to both his endogenous and artificial heartbeats. In addition, the HEP was recorded and compared during states of interoception, exteroception and mind wandering in C.S. and the controls.
As a satellite hypothesis, we speculate that C.S. would exhibit a selective impaired performance on emotional and cognitive tasks (emotion recognition, empathy, theory of mind and decision making) which are suggested to be related to interoceptive feedback from the body, whereas cognitive tasks not related to interoception would remain preserved.
MATERIALS AND METHODS
Participants
Patient description
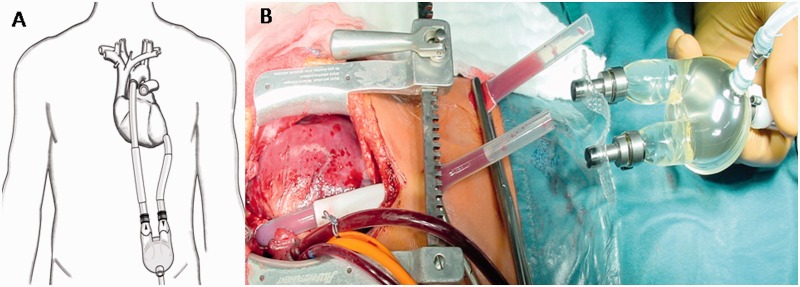
Patient C.S. is a 32-year-old male with a history of heart failure that has progressed over 4 years. He additionally suffers from dilated cardiomyopathy of an uncertain etiology. At the time of admission, he was in cardiogenic shock despite inotropic support, with severe fixed pulmonary hypertension. The patient presented with a failing native left ventricle and received the LVAD to replace pumping function, facilitate mechanical unloading and reduce pulmonary hypertension as a bridge to a heart transplant. A Berlin Heart (Excor) was implanted (Figure 1). This is a paracorporeal device with pneumatic-driven ejection and a volume displacement pump to provide effective circulatory support with pulsatile perfusion. The implant required an inflow cannulation of the left ventricle apex and an outflow cannulation of the aorta with mechanical tilting disc valves connected to a 60 ml blood chamber with a polyurethane membrane. This pump is loud due to displacement of air, and its ejection does not correlate with the native heartbeat.
Fig. 1.
Schematic and picture of the LVAD implantation procedure. (A) Schematic view of the LVAD with the pump lying over the patient’s abdominal skin and canulae connecting it to the apex and aortic artery. (B) Implantation procedure; canulae can be seen exiting from the thoracic cavity to the abdominal skin and connecting to the pump (images provided by Berlin Heart).
This pump lies on top of the patient’s abdominal skin and is connected to a stationary driving unit that allows a fixed pumping frequency to be set. The pump vibrates up and down slightly with every beat and generates somatosensory stimulation on the patient’s abdominal skin at the constant rate of 80 beats per minute. Further physiological, physical and psychological health state parameters are described in Supplementary Data (Section 1.1.1.).
Control sample
Three groups of right-handed male and female participants with no history of neurological or psychiatric conditions were evaluated. Demographic characteristics (age, gender and education level) were controlled (see the demographic and neuropsychological results below). The first group consisted of five male participants (mean age = 30.2 years, s.d. = 8.5, mean education level = 15.2, s.d. = 3.83), who were registered with EEG (HEP; descriptive statistics available in Table 1). The remaining two groups of controls were assessed for comparison in the neurocognitive and social cognition (Supplementary Data, Section 1.1.2).
Table 1.
Demographic data of C.S. and EEG controls
| Raw Score | t-value | P-value | Controls | |
|---|---|---|---|---|
| Age | 32 | 0.19 | 0.43 | mean = 30,2; s.d. = 8.5 |
| Formal education | 8 | −1.72 | 0.08 | mean = 15.2; s.d. = 3.83 |
| Gender | No difference | Male = 100% |
Experimental tasks design
Interoceptive measures
Heartbeat detection task
Electrocardiogram (EKG) signal was recorded with an ad hoc circuit composed of an amplifier AD620 and a band-pass filter (low 0.05 Hz, high 40 Hz) and then analogically fed to a laptop computer’s audio card. Three Ag/Ag-Cl adhesive electrodes were placed to the patient in lead-II positions, together with headphones for audio stimuli delivery. The signal was processed online with a PsychToolbox (Brainard, 1997) script, running on Matlab platform (Math Works). We carried out a behavioral HBD interoception test, in which the patient had to tap a computer keyboard along with his heartbeat in different conditions. External electrodes were used in the EEG setup (see below) to collect the EKG signal, which was processed in real time for peak detection and audio stimulation following the heartbeats. First, as a motor control condition, the patient was instructed to follow an audio recording of a sampled heartbeat. Next, he was told to follow his heartbeat with no external stimulation or feedback (intero-precondition). He was then given the same instructions along with simultaneous auditory feedback of his heart provided through online ECG register (feedback condition). Finally, he was once again told to follow his heartbeat without any feedback (intero-postcondition). The full instructions and also data for the heartbeat detection task’s validation and reliability are detailed in Supplementary Data (Sections 1.1–1.4). Using a measure of response synchronization, we compared the patient’s performance across the conditions to determine whether it was dependent on his own heartbeat or on the pumping frequency of the external device (see ‘Data analysis’ section below).
Heart-evoked potential measures during rest, exteroception and interoception
The HEP is a cortical evoked-related potentials (ERP) that has been widely described (Schandry et al., 1986; Gray et al., 2007) as a neural measure of cardiac pulsatile and contractile activity. It is obtained by sampling EEG epochs time-locked to the EKG-R-wave (electrocardiography) and consists of a negative deflection in the frontal-central electrodes within a 200–400 ms latency post-R-wave. We measured the HEP based on EEG data from the patient and control participants. Data acquisition was conducted under three state conditions: in the ‘mind-wandering’ condition, the participants were asked to think freely and pay attention to nothing in particular; in the exteroceptive condition, the participants were asked to count the occurrence of high-pitched sounds in a musical recording played through headphones and in the interoceptive condition, the participants were asked to pay attention to their heartbeat and their breathing. The conditions lasted 7 min each and were conducted with the participants seated in a resting state with closed eyes and the same music being played.
ERP recordings
EEG signals were sampled at 2048 Hz (and offline resampled at 512 Hz) with a Biosemi Active-two 128-channel system. The data were band-pass filtered (0.1–100 Hz) during recording and (0.3–40 Hz) off-line to remove undesired frequency components. The reference was set by default to link mastoids and re-referenced offline to the average electrodes. Two bipolar derivations monitored vertical and horizontal ocular movements. EEG data were segmented from 200 ms prior to the R-wave-EKG onset to 800 ms after its onset. All segments with eye movement contamination were removed from further analysis using a visual procedure. For all participants, the number of trials for each condition was mean = 422.4; s.d. = 53.75 for the interoception condition, mean = 409; s.d. = 62.1 for the exteroception condition and mean = 423.8; s.d. = 29.44 for the mind-wandering condition.
Neurocognitive and social cognition assessment
C.S. was evaluated in a wide variety of general neurocognitive variables reported in Supplementary Data such as intelligence (IQ), attention orientation, memory, language, visuo-spatial skills, motor programming, conflicting instructions, inhibition, executive functions (EF) and affective state. Additionally, the patient and controls were assessed with a battery of emotion and social cognition measures, including emotion recognition from dynamic facial (emotional morphing) and contextual paraverbal cues (TASIT, The Awareness of Social Inference Test), an empathy-for-pain task (EPT), theory of mind (ToM; Reading the mind in the eyes; Baron-Cohen et al., 1997) and a decision-making test (IGT, Iowa Gambling Task). For a detailed description of these tests, procedures and their corresponding results, we refer the readers to Supplementary Data (Sections 1.2 and 2, respectively).
Procedure
Before the study, all participants signed an informed consent form in accordance with the Declaration of Helsinki. The study was approved by the Institute of Cognitive Neurology’s ethics committee. Subsequently, the patient and control participants were registered with EEG during the interoception tasks. The session lasted 21 min in a sound-attenuated room with a dim light and the participants sat on a reclining chair with closed eyes in a resting state. In addition, the patient and control participants were presented with the battery of emotion recognition and social cognition tests as well as the neuropsychological and affective screening questionnaires.
Data analysis
Behavioral interoception synchronization measure
One of the main goals of this study was to assess whether the patient had an impaired interoception affected by external signals, given the hypothesis that the mechanical sensation of the LVAD could interfere with the sensing of his endogenous cardiac rhythm. If interoception is driven in a somatosensorial way, then the rhythm created by the LVAD would trigger the interoceptive awareness. However, if in C.S., interoception is mediated by a direct vagal enervation, the endogenous heart would drive interoception despite somatosensory interference. Furthermore, current research studies use mainly two kinds of tasks in order to assess interoception (Knapp-Kline and Kline, 2005): heartbeat mental tracking tasks (Schandry, 1981) and heartbeat (HB) tone detection tasks (Whitehead et al., 1977; Katkin et al., 1982; Stormer et al., 1989). However, these methods do not enable to discriminate if the examinee is following the rhythm of his heart or other somatic sensation like the ones produced by a LVAD. In heartbeat mental tracking tasks, subjects are asked to silently count their heartbeats but they do not discriminate the precise time in which the subjects count each of the heartbeats, as the output is just a single number representing the total of heartbeats counted in a specific time window. On the other hand, the tone detection paradigms (Whitehead et al., 1977) test the ability of subjects to discriminate between auditory stimuli that are either synchronized or delayed with respect to their own heartbeat. This was completely unsuitable for our patient’s LVAD condition as the artificial device would have interfered with his performance, and the task would have not given information about which sensation he was using to guide his responses.
Thereby, we needed a HBD task that would allow us to know whether C.S. was following his endogenous heart or the LVAD somatosensory stimuli. The synchronization measures analysis performed to the data gave us the possibility of doing this inference from the results. This measure quantifies the extent to which C.S. tapping was related to either the patient’s endogenous, non-functional heart or to the LVAD external pump. The measure (Z) is the square of the absolute value of the sum of terms, one for each tap, normalized to the total number of taps:
| (1) |
Each term tj entering the sum is defined as:
| (2) |
where i is the imaginary unit and  is the phase of the tap, given by:
is the phase of the tap, given by:
| (3) |
where Tj is the instantaneous period (or time between heartbeats at the tap) and ΔTj is the time difference between the tap and the last heartbeat.
The measure Z lies between 0 and 1, with 1 indicating maximum synchronization with the beat (Zcardio: ECG peak, Zexternal: pump peak) and that the taps occur at a fixed time after each beat. It is important to note that a fixed phase (or time delay) between the tap and the beat is irrespective of the measure because taking the absolute value erases all phase information. In particular, the phase was arbitrary in the case of the external pump, in which the frequency was known but the timing of the beats was not. Thus, we were only interested in measuring phase locking rather than the absolute value of the phase. In the case of the motor condition, the measure was taken with respect to the external audio beat instead of the heartbeat. In order to assess the significance of the synchronization, for each condition, we compared the computed Z-value with surrogates obtained from randomly sorting the inter-taps intervals. To decide which reference the taps were more synchronized to (cardio or external), we computed the difference in Z-values and performed a two-tailed permutation test.
HEP analysis
After preprocessing the EEG data, we performed the HEP analysis. First, off-line R-wave-EKG detection values were used to segment each of the 128 channels of EEG data. R-wave-EKG detection was achieved by the function Peakfinder implemented on Matlab, which quickly finds local peaks or valleys (local extrema) in a noisy vector using the alternating nature of the derivatives and a user-defined magnitude threshold to determine if each peak is significantly larger (or smaller) than the data around it (Yoder, 2009). We compared the experimental conditions (mind-wandering, exteroception and interoception) as well as the performance of the patient compared to that of the control group using Monte Carlo permutation tests (Manly, 1997) combined with bootstrapping. This simple method offers a straightforward solution for multiple comparison problems and does not depend on multiple comparisons correction or Gaussian assumptions about the probability distribution of the data. The data from each of the three conditions separately underwent a random partition and a t-value was then calculated. This process was repeated 5000 times to construct the t-value distribution under the null hypothesis. The null hypothesis is rejected if an obtained t-value is greater than the most extreme 1% of the distribution (e.g. P < 0.01). We compared the ERP signal with and without subtracting the cardiac signal artifact (skin-conducted EKG signal to the scalp) from the EEG signal. Finally, an analysis of the frontocentral electrodes (66–68, 74–78, Biosemi) was conducted using a region of interest approach as reported elsewhere (Schandry et al., 1986; Fukushima et al., 2011). Contrast comparisons between interoception–exteroception and interoception–mind-wandering conditions were made between the patient and the control sample.
RESULTS
Demographics
The comparison between C.S. and the EEG control sample showed no significant differences in terms of age (t = 0.19, P = 0.43, zcc = −0.21) and gender (five male), although a trend toward lower education (t = −1.72, P = 0.08, zcc = −1.88) was observed (Table 1). For demographic comparison with remaining control groups see Supplementary Data (Section 1.3).
Interoceptive and brain measures
Behavioral interoception
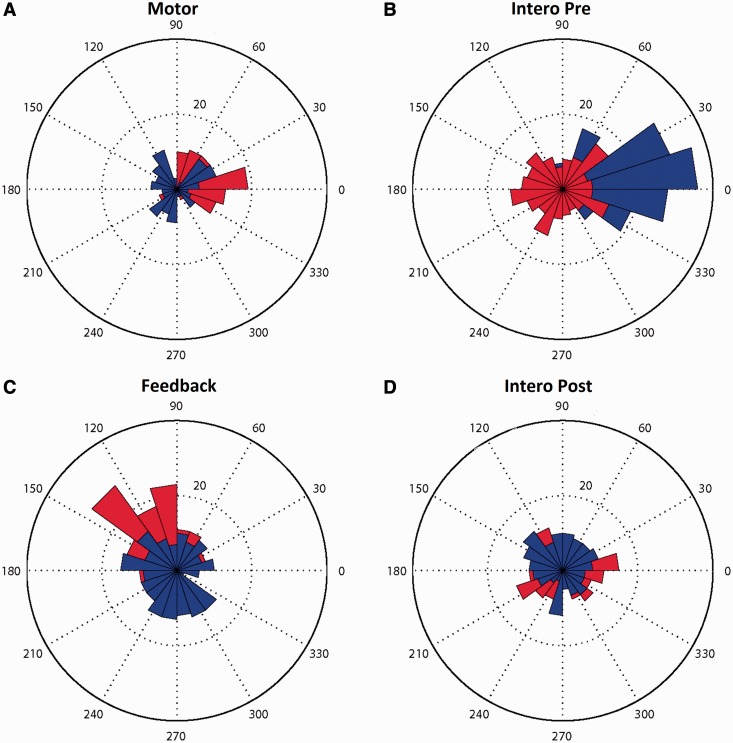
The synchronization analysis of the patient’s reaction times for both his endogenous non-functional heartbeat and the heartbeat from his LVAD pump (Table 2) showed that his tapping was synchronized to his artificial pump as opposed to his endogenous heart during the two interoception conditions (pre and post) in which no feedback was provided (results available in Table 2). In the feedback condition, C.S. tapped following his endogenous heartbeats (which were fed back to him aurally through headphones); this was similar to his performance in the motor condition, in which he also synchronized with the audio signal of a sampled heart (results available in Table 2). In Figure 2, we show these results with polar histograms of the number of taps at different delays with respect to either the endogenous heart (red in Figure 2B, C and D), or the external pump (blue, phase reference is arbitrary). In the motor condition (Figure 2A), C.S. synchronized his tapping to the sampled audio signal (red). In the first interoceptive condition (Figure 2B), the taps observed in the patient were clearly locked to the external pump. However, when presented with audio feedback he tended to follow his endogenous heartbeat (Figure 2C). Finally, after becoming attuned to his endogenous heartbeat in the feedback condition, C.S. still followed the external pump in the postinteroceptive condition. This finding indicates the patient may have attained some awareness of his actual endogenous heartbeat, presumably through an estimation of the rate (Figure 2D). Nevertheless, he was primarily following the external pump.
Table 2.
Behavioral interoception measures for each condition of the HBD
| Motor | Preinteroception | Feedback | Postinteroception | |
|---|---|---|---|---|
| Zcardio | 0.5221 | 0.0479 | 0.3780 | 0.0076 |
| Zexternal | 0.0692 | 0.6441 | 0.1031 | 0.1284 |
Zcardio, index comparing patient’s RTs with his own organic heartbeats
Zexternal, index comparing patient’s RTs with his LVAD, RT, reaction times
Fig. 2.
Behavioral performance on HBD tasks. Polar histograms of relative synchronization between the patient’s responses and his actual endogenous heartbeats (in red bars) and artificial pump-beats (blue bars). (A) motor control condition; (B) interoception pre-condition; (C) auditory feedback condition; (D) interoception post-condition. A large proportion of blue area (artificial pump-beats) can be seen in the interoception conditions (B and D), whereas a greater proportion of red area (endogenous heartbeats) can be seen in the motor control and auditory feedback conditions (A and C).
HEP on scalp EEG
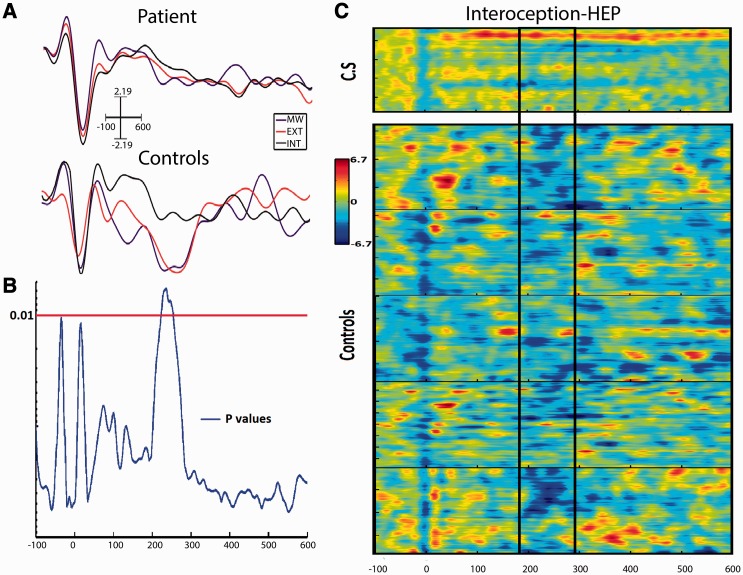
The comparisons of the HEP for each condition in the patient yielded no differences between the conditions, either before or after correcting for cardiac artifacts in the EEG signal (Figure 3A). In the controls, significant differences were observed before artifact correction between the mind-wandering and interoceptive conditions (P < 0.01) during the latency of 288–298 ms. Additionally, after cardiac artifact correction, significant differences were observed for the comparison between the mind-wandering and interoception conditions in the latencies between 193 and 233 ms and, 250 and 270 ms. A similar result was observed comparing the mind-wandering and exteroception conditions (P < 0.001) during the latency of 193–272 ms. When comparing the same conditions in C.S. vs controls, before cardiac artifact correction, we found that significant differences existed only for the interoceptive condition (P < 0.01) during the latency of 224–254 ms (Figure 3B and C). After removing cardiac artifacts from the EEG epochs, significant differences were observed for all three conditions: interoception (P < 0.001) during the latency of 221–237 ms, exteroception (P < 0.001) at a latency of 224–242 ms and mind-wandering (P < 0.001) at a latency of 214–256 ms.
Fig. 3.
HEP comparison. (A) HEP per condition in the patient and control sample during the three states (MW, mind wandering; Ext, exteroception; Int, interoception). Note the lack of modulation in the interoception condition between 130 and 300 ms in the patient. (B) Significance curve of permutations comparing the interoceptive condition between controls and the patient. (C) Raster plots of HEP single trials during the interoceptive condition in the patient and control sample. The box indicates the HEP time window.
Neurocognitive and social cognition tests
C.S. showed spared general neurocognitive functions, namely IQ, executive functions, including working memory (verbal and spatial) and speed of processing. Furthermore, he presented no affective state changes. Regarding emotions and social cognition, he performed significantly worse than controls in theory of mind, failed to recognize accidental pain and neutral conditions from the empathy for pain task and showed worse global performance in the IGT. Supplementary Figures S1 and S2 summarize the main findings on the spared domains and those in which the patient presented impairments, respectively. Supplementary Tables S2–S8 provide a detailed description of all of the results.
DISCUSSION
In this single-case report of patient C.S. with an external cardiac assist device (LVAD), we investigated the relationship between two different cardiac afferent pathways and behavioral and neural correlates of interoception. We found two pieces of relevant evidence:
during natural conditions (e.g. without auditory heartbeat amplification), the behavioral HBD responses of C.S. followed the external pump rather than his endogenous heart.
When measuring the HEP during three mental states (interoception, mind-wandering and exteroception), the controls presented HEP differences between the states but the patient did not. Further, C.S.’s HEP was reduced in the interoceptive condition compared with controls.
These behavioral and neural measures provide convergent evidence of different, and to some extent deregulated or imbalanced, somatosensory and visceral-vagal interoceptive pathways.
The first finding comes from the behavioral interoceptive assessment. In the HBD task, C.S. tapped in synchrony with his LVAD instead of his endogenous heartbeats in the interoceptive conditions, while he followed the audio stimulus when it was present in the motor and feedback conditions. These results suggest that the somatosensory input produced by the artificial pump (lying over the patient’s abdominal skin) dominates the input from his endogenous heartbeat, which supports our first hypothesis. However, we observed an interesting ‘learning’ effect after the feedback condition. After having listened to his heartbeat, when asked to follow his heart, C.S. still locked his responses primarily to the external pump, but not as strongly as before the feedback. This finding might suggest an enhancement of afferent information from the cardiac pathways over the purely somatosensitive afferent information from the pump; however, this result might also reflect a higher level effect of the patient profiting from a better estimation of his actual heart rate, that he acquired during the feedback condition.
Furthermore, in the EEG analysis of C.S., we found diminished cortical responses to afferent cardiac signals related to vagus-driven interoceptive pathway suggesting an impaired cortical processing of them that was more accentuated during the interoceptive brain macrostate. The HEP results show a significant difference in HEP amplitude in the latency range from 100 to 300 ms after the EKG-R-wave between the patient and the control sample. Moreover, when control subjects pay attention to their body signals (interoceptive macro-state), there was a modulation in amplitude of the HEP component (Figure 3A). This interoceptive modulation could be interpreted in the light of a top–down or attentional facilitation of interoceptive processing by the cortical sources of the HEP, insula and ACC. Second, our results show that this interoceptive awareness modulation is lacking in the patient’s HEP. Conversely, the bottom–up afferents should be preserved, given that the patient’s HEP was similar to controls regarding exteroceptive and mind-wandering states. For as C.S.’s brain receives the cardiac-evoked signal in a comparable way to controls, evidenced by preserved HEP amplitude in exteroception and mind-wandering conditions, it would imply that the patient’s heartbeat signal is indeed reaching the interoceptive sensory cortices, although the HEP produced is not being modulated in amplitude by awareness to body signals. In other words, the patient’s HEP was not influenced by top–down mechanisms of attention (interoceptive awareness, see fig. 3A). This diminished amplitude modulation of the HEP component in the patient interoceptive macro-state is in line with enhancement of somatosensory-driven interoception and might lead to the pattern of behavioral HBD performance observed in the patient. Thus, we think that a top–down effect of interoceptive awareness explanation seems more suitable than a bottom–up sensorial interference of the LVAD.
Finally, a secondary piece of evidence suggests that the patient’s selective domains related with interoception are also affected. Specifically, C.S. showed impairments on some empathy for pain conditions (accidental scenarios of EPT), ToM and a decision-making task (IGT). Conversely, the patient presented a normal performance on the evaluation of several cognitive domains such as emotion recognition, intelligence, attention-orientation, memory, language, visuo-spatial skills, motor programming, conflicting instructions, inhibition, EFs and even empathic judgment during intentional scenarios (where more explicit cues of personage’s intention to harm actions were provided (Baez et al., 2012), see figure S1). As proposed in models by Lamm and Singer (2010), the participation of interoception in social emotion and social behavior is related to bodily arousal (Dunn et al., 2010a). Moreover, social cognition processes (e.g. emotions, decision making, empathy and ToM) activate neural networks that involve interoceptive cortices (IC and ACC; Ochsner et al., 2008; Dunn et al., 2012; Ibanez and Manes, 2012; Couto et al., 2013a, b; Melloni et al., 2013). Hence, we speculate that lower interoceptive performance in C.S. would be related to specific impairments on social cognition. Nevertheless, these observations remain in a highly speculative grounding and need further research.
It is important to note that the present results were observed in a patient with relatively preserved cognitive and executive functions and absence of clinical depression or trait anxiety. However, we cannot rule out that the high level of state anxiety observed in the patient might be related to these results. Although C.S.’s cognitive impairments could be due to interoceptive disturbances or to anxiety and stress, all the points made above give support to the hypothesis of a specific link between this patient’s interoception imbalance and his social cognition impairments rather than an anxiety state-interoception linking.
In summary, we present a patient with external cardiac assistance whose behavioral performance and cortical responses suggest an imbalance between pathways of cardiac interoception, characterized by an enhancement of the somatosensory pathway compared to the vagal-insular pathway.
Our results raise the question of possible interference between these two pathways of cardiac interoception and suggest there may be some degree of plasticity after peripheral sensory modification. In humans and other mammals, it is well known that basic modalities exhibit plasticity, including somatosensory (Merzenich et al., 1983; Mogilner et al., 1993; Ramachandran, 1993; Manger et al., 1996), visual (Wiesel and Hubel, 1963; Cohen et al., 1997; Thaler et al., 2011) and more complex modalities such as extended-mind tools (Iriki et al., 1996). Furthermore, ‘reliable’ coupling activity between the brain and physical devices is key evidence for the extended-mind hypothesis (Clark Chalmers, 1998). This hypothesis states that either bodily actions (e.g. counting with the fingers) or usage of simple (a pencil) to sophisticated (a computer) tools, can be considered an extension of the human mind as long as they perform a constant role and are accessible when needed for any cognitive task the brain intends to accomplish. Malafouris (2010) proposes the term brain–artifact interfaces (BAIs) to describe interactions between these objects and actions. These can be physical objects or cultural inventions and may function as triggers of neural plasticity (or metaplasticity) and should be seen as continuous integral connections and extra-neural nodes of human cognition (Iriki et al., 1996; Malafouris, 2008; Malafouris and Renfrew, 2010). The construct of BAIs allows the brain to delegate part of a cognitive process to a physical artifact and through this can initiate a structural rewiring of existing neural pathways and rearrangement of the functional architecture of the engaged brain system. Consequently, we suggest that the external LVAD pump can be thought of as a BAI for the patient’s cardiac interoception and our results provide indirect support for neuroplasticity and functional changes in somatosensory cortices S1–S2 as well as interoceptive central effectors IC–ACC. In this way, the external LVAD could be considered an interoceptive-BAI for the maintenance of homeostasis in afferent cardiac modulation of cognitive processes. Thus, the somatosensory pathway may supply a vicarious function until the patient receives a new endogenous heart by transplantation. It has not been well studied whether parasympathetic re-innervation proceeds effectively after cardiac transplantation and no clinical non-invasive markers for this process have been described. However, the behavioral and cortical measures of cardiac interoception outlined here could be employed as cognitive and electrophysiological markers of evolution and follow-up in patients with an LVAD and patients with heart transplantation. This is a topic for further research and a potential new assessment of the patient described in this study.
In clinical heart transplant candidates, it is known that fixed pulmonary hypertension is a contraindication, mainly due to the potential development of severe right ventricular failure and a higher early mortality rate. A ventricular assist device effectively reverses pulmonary hypertension and offers benefits such as unloading of the failing left ventricle as well as improved functional capacity, quality of life and survival. This can allow for cardiac orthotopic transplantation (bridge to heart transplant) in patients who were not initially considered candidates (Mikus et al., 2011; Torre-Amione et al., 2010). This study has clinical relevance for these individuals, suggesting that LVAD and heart transplanted patients may suffer from a subtle and subclinical interoceptive imbalance that may impact their social cognition abilities. This may present a new feature to monitor in the post-surgical evaluation of these patients, which should also be studied in future research and within a pre-post-surgical design.
In spite of these applications, this study contains several limitations that should be accounted for in future studies. First of all, inferences made here are based on single case evidence, that might not be enough to completely clarify the nature of cardiac interoceptive pathways and future clinical vs control–sample studies should be conducted. Moreover, the small sample size of the control group and the presence of different samples are issues that could be avoided in future research. The patient’s high anxiety state could represents a confounding factor in interoceptive behavioral measures, given that a relationship between these two processes has previously been suggested (Stein et al., 2007). However, this is the first neurocognitive report in a patient with LVAD providing convergent evidence on interoceptive impairments and related deficits.
CONCLUSION
We have shown that external cardiac-assisted patients represent an extraordinary opportunity for testing different hypotheses on cardiac interoception, specifically regarding different pathways of cardiac interoception in patients presenting modified heart–brain afferent inputs. Furthermore, to some extent, this model mirrors models of IC lesion, in which the cortical afferent representation of the vagal interoceptive pathway is missing (Khalsa et al., 2009a). A new emergent mode of heart–brain communication involving artificial hearts and their cognitive effects is a topic for future interoception research.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by grants of the Comisión Nacional de Investigación Científica y Tecnológica CONICYT/FONDECYT Regular (1130920 to A.I.), Fondo para la Investigación Científica y Tecnológica (FONCyT- PICT) 2012-0412 (F.M.), FONCyT- PICT 2012-1309 (A.I.), James McDonnell Foundation (M.S.), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and INECO Foundation. There are no conflicts of interest including any financial, personal or other relationships with persons or organizations for any author related to the work described in this article.
REFERENCES
- Baez S, Rattazzi A, Gonzalez-Gadea ML, et al. Integrating intention and context: assessing social cognition in adults with Asperger syndrome. Frontiers in Human Neuroscience. 2012;6:302. doi: 10.3389/fnhum.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. Journal of Child Psychology and Psychiatry. 1997;38(7):813–22. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Naqvi N. Listening to your heart: interoceptive awareness as a gateway to feeling. Nature Neuroscience. 2004;7(2):102–3. doi: 10.1038/nn0204-102. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–6. [PubMed] [Google Scholar]
- Cameron OG. Visceral Sensory Neuroscience: Interoception. New York: Oxford University Press; 2002. [Google Scholar]
- Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosomatic Medicine. 2002;64(6):851–61. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- Clark A, Chalmers D. The extended mind. Analysis. 1998;58(1):7–19. [Google Scholar]
- Cohen LG, Celnik P, Pascual-Leone A, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–3. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Couto B, et al. Insular networks for emotional processing and social cognition: Comparison of two case reports with either cortical or subcortical involvement. Cortex. 2013a;49:1420–34. doi: 10.1016/j.cortex.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Couto B, Manes F, Montañes P, et al. Structural neuroimaging of social cognition in in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front. Hum. Neurosci. 2013b;7:467. doi: 10.3389/fnhum.2013.00467. doi: 10.3389/fnhum.2013.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, et al. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion Neurobioliogy. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clinical Psychology Review. 2010;30(1):1–11. doi: 10.1016/j.cpr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, et al. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behaviour Research and Therapy. 2010a;48(11):1133–8. doi: 10.1016/j.brat.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Evans D, et al. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychological Science. 2010b;21(12):1835–44. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Evans D, Makarova D, White J, Clark L. Gut feelings and the reaction to perceived inequity: the interplay between bodily responses, regulation, and perception shapes the rejection of unfair offers on the ultimatum game. Cognitive and Affective Behavioral Neuroscience. 2012;12(3):419–29. doi: 10.3758/s13415-012-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Terasawa Y, Umeda S. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. International Journal of Psychophysiology. 2011;79(2):259–65. doi: 10.1016/j.ijpsycho.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Gray MA, Taggart P, Sutton PM, et al. A cortical potential reflecting cardiac function. Proceedings of the National Academy of Sciences, USA. 2007;104(16):6818–23. doi: 10.1073/pnas.0609509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78:1354–62. doi: 10.1212/WNL.0b013e3182518375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7(14):2325–30. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, Ohman A. Individual differences in heartbeat discrimination. Psychophysiology. 1982;19(2):160–6. doi: 10.1111/j.1469-8986.1982.tb02538.x. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nature Neuroscience. 2009a;12(12):1494–6. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. International Journal of Psychophysiology. 2009b;72(1):34–45. doi: 10.1016/j.ijpsycho.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Frontal Neuroscience. 2011;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: slow and steady wins the race. Biological Psychology. 2005;69(3):387–96. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure and Function. 2010;214(5–6):579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Longhurst J. Cardiac and other visceral afferents. In: Roberston D, editor. Primer on the Autonomic Nervous System. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage. 2009;47(3):1038–46. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafouris L. Beads for a plastic mind: the ‘blind man’s stick’ (BMS) hypotheis and the active nature of material culture. Cambridge Archaeological Journal. 2008;18:401–14. [Google Scholar]
- Malafouris L. The brain-artefact interface (BAI): a challenge for archaeology and cultural neuroscience. Social Cognitive and Affective Neuroscience. 2010;5(2–3):264–73. doi: 10.1093/scan/nsp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafouris L, Renfrew C. The Cognitive Life of Things: Recasting the Boundaries of the Mind. Cambridge: McDonald Institute; 2010. Knapping intentions and the marks of the mental. In: pp. 13–22. [Google Scholar]
- Malliani A, Lombardi F, Pagani M. Sensory innervation of the heart. Prog Brain Res. 1986;67:39–48. doi: 10.1016/s0079-6123(08)62755-7. [DOI] [PubMed] [Google Scholar]
- Manger PR, Woods TM, Jones EG. Plasticity of the somatosensory cortical map in macaque monkeys after chronic partial amputation of a digit. Proceedings of the Royal Society B: Biological Sciences. 1996;263(1372):933–9. doi: 10.1098/rspb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. Boca Raton, FL: Chapman & Hall/CRC; 1997. [Google Scholar]
- Melloni M, Lopez V, Ibanez A. Empathy and contextual social cognition. Cogn Affect Behav Neurosci. 2013 doi: 10.3758/s13415-013-0205-3. doi: 10.3758/s13415-013-0205-3. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Mikus E, Stepanenko A, Krabatsch T, et al. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. European Journal Cardiothoracic Surgery. 2011;40(4):971–7. doi: 10.1016/j.ejcts.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Grossman JA, Ribary U, et al. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proceedings of the National Academy of Sciences, USA. 1993;90(8):3593–7. doi: 10.1073/pnas.90.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Social Cognitive and Affective Neuroscience. 2008;3(2):144–60. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Human Brain Mapping. 2005;26(1):54–64. doi: 10.1002/hbm.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007a;28(1):9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007b;1141:178–87. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proceedings of the National Academy of Sciences USA. 1993;90(22):10413–20. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse CH, Jones GE, Jones KR. The effect of body composition and gender on cardiac awareness. Psychophysiology. 1988;25:400–7. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–8. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schandry R, Weitkunat R. Enhancement of heartbeat-related brain potentials through cardiac awareness training. International Journal of Neuroscience. 1990;53(2–4):243–53. doi: 10.3109/00207459008986611. [DOI] [PubMed] [Google Scholar]
- Schandry R, Sparrer B, Weitkunat R. From the heart to the brain: a study of heartbeat contingent scalp potentials. International Journal of Neuroscience. 1986;30(4):261–75. doi: 10.3109/00207458608985677. [DOI] [PubMed] [Google Scholar]
- Schandry R, Bestler M, Montoya P. On the relation between cardiodynamics and heartbeat perception. Psychophysiology. 1993;30(5):467–74. doi: 10.1111/j.1469-8986.1993.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Science. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Stormer SW, Heilitag U, Knoll JF. Hearbeat perception and knowledge of results: a new method and some theoretical thoughts. Journal of Psychophysiology. 1989;3(4):340–50. [Google Scholar]
- Thaler L, Arnott SR, Goodale MA. Neural correlates of natural human echolocation in early and late blind echolocation experts. PLoS One. 2011;6(5):e20162. doi: 10.1371/journal.pone.0020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Amione G, Southard RE, Loebe MM, et al. Reversal of secondary pulmonary hypertension by axial and pulsatile mechanical circulatory support. Journal of Heart and Lung Transplant. 2010;29(2):195–200. doi: 10.1016/j.healun.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P, Blackwell B. Relation of heart rate control to heartbeat perception. Applied Psychophysiology and Biofeedback. 1977;2(4):371–92. [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18(4):442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yoder N. PeakFinder. 2009 in http://www.mathworks.com/matlabcentral/fileexchange/25500-peakfinder (ed), Matlab, MathWorks. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.