Abstract
Anthropomorphism is the attribution of human characteristics or behaviour to animals, non-living things or natural phenomena. It is pervasive among humans, yet nonetheless exhibits a high degree of inter-individual variability. We hypothesized that brain areas associated with anthropomorphic thinking might be similar to those engaged in the attribution of mental states to other humans, the so-called ‘theory of mind’ or mentalizing network. To test this hypothesis, we related brain structure measured using magnetic resonance imaging in a sample of 83 healthy young adults to a simple, self-report questionnaire that measured the extent to which our participants made anthropomorphic attributions about non-human animals and non-animal stimuli. We found that individual differences in anthropomorphism for non-human animals correlated with the grey matter volume of the left temporoparietal junction, a brain area involved in mentalizing. Our data support previous work indicating a link between areas of the brain involved in attributing mental states to other humans and those involved in anthropomorphism.
Keywords: anthropomorphism, temporoparietal junction, mentalizing and VBM
INTRODUCTION
It is not uncommon to overhear someone trying to coax their car into starting on a cold morning or chastize their computer for crashing at a crucial moment. As humans, we seem to have a natural tendency to attribute social meaning to the world in which we live; many perceive anger in a grey thundercloud or deceit in an elusive set of keys. Such anthropomorphism is the attribution of human characteristics or behaviour to animals or non-living stimuli. Anthropomorphism displays a large degree of inter-individual variability. Children are, in general, more likely to anthropomorphize than adults (Carey, 1985) attributing mental states to virtually everything that surrounds them from a happy sunshine to cuddly animals (Piaget, 1929). Anthropomorphism is culturally dependant: certain cultures appear more likely to anthropomorphize than others (Asquith, 1986). Anthropomorphism also depends on the nature of the thing being anthropomorphized: objects moving at speeds similar to humans are more readily anthropomorphized (Morewedge et al., 2007), the number of facial features and dimensions of a robot head influences the perception of humanness (DiSalvo et al., 2002) and unpredictability and the motivation for predictability increase the tendency to anthropomorphize (Epley et al., 2008).
Individual differences in the extent to which people anthropomorphize are stable over time (Waytz et al., 2010b), suggesting that they reflect an enduring behavioural trait. However, very little is known about the neural correlates of anthropomorphism. One possibility is that tasks relating to anthropomorphism should involve brain regions associated with social cognition; however, the specific areas identified by such social cognition studies vary (Castelli et al., 2000, 2002; Waytz et al.. 2010a). Anthropomorphizing involves generalizing from human to non-human agents. Identification of those areas of the brain important in anthropomorphism could therefore sensibly begin by examining regions of the brain that are known to be involved in thinking about other humans.
Several studies have identified areas of the brain that are activated when people attribute mental states to others. The tendency to explain one’s own and others’ actions in terms of beliefs, desires and goals has been called ‘theory of mind (ToM)’, or mentalizing. Brain activity associated with mentalizing is seen in three principal regions: an anterior region of the medial prefrontal cortex/anterior cingulate cortex, an area in the anterior temporal lobes close to the amygdala and the temporoparietal junction (TPJ) encompassing posterior superior temporal sulcus and the angular gyrus (Frith and Frith, 2003).
Here, we adopted an individual differences approach to examine the neural basis of anthropomorphism. Much recent work has illustrated that individual variability in a range of cognitive functions can be predicted from the local structure of grey and white matter, as assessed by voxel-based morphometry (VBM) (Kanai and Rees, 2011). We hypothesized that individual variability in anthropomorphism would be associated with differences in brain structure in areas known to be activated during mentalizing tasks. To test this hypothesis we collected anthropomorphism scores and magnetic resonance imaging (MRI) brain scans in 83 healthy young adults. Anthropomorphism scores were obtained using a simple, validated self-report questionnaire (Waytz et al., 2010b). We tested whether the degree to which our participants made anthropomorphic judgements about non-human animals and non-animal stimuli predicted brain structure using optimized VBM (Ashburner, 2007). We focused our analysis on those areas of the brain known to be involved in mentalizing activities, but also conducted whole-brain analyses for completeness.
METHODS
Participants
In total, 83 healthy adult volunteers (mean ± s.d. age 24 ± 3.84 years, 50 female) were recruited from the University College London (UCL) participant pool. Written informed consent was obtained from each participant. The study was approved by the UCL ethics committee.
Individual Differences in Anthropomorphism Questionnaire
We used a questionnaire devised by Waytz et al. (2010b) that provides a measure of stable individual differences in anthropomorphism to obtain an anthropomorphism ‘score’ for each of our participants. All participants were asked to complete the Individual Differences in Anthropomorphism Questionnaire (IDAQ) online that was used to assess anthropomorphism. Factor-analysis undertaken by Waytz et al. (2010b) yielded a two factor solution of the questionnaire data as optimal: anthropomorphism of the living (i.e. animals) and non-animal (technology and nature) stimuli. We repeated the principal component analysis with our data and confirmed this result. We, therefore, used anthropomorphism of non-human animals and anthropomorphism of non-animal stimuli as independent regressors in our VBM data analysis.
MRI data acquisition
MR images were acquired on a 1.5-T Siemens Sonata MRI scanner (Siemens Medical). High-resolution anatomical images were acquired using a T1-weighted 3D modified driven equilibrium Fourier transform sequence (repetition time =12.24 ms; echo time = 3.56 ms; field of view = 256 × 256 mm; voxel size = 1 × 1 × 1 mm).
VBM pre-processing and analysis
T1-weighted MR images were first segmented for grey matter and white matter using the segmentation tools in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Subsequently, we performed diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) in SPM8 for intersubject registration of the grey matter images (Ashburner, 2007). To ensure that the total amount of grey matter was conserved after spatial transformation, we modulated the transformed images by the Jacobian determinants of the deformation field. The registered images were then smoothed with a Gaussian kernel of 8 mm full-width half-maximum and transformed to Montreal Neurological Institute (MNI) stereotactic space using affine and non-linear spatial normalization implemented in SPM8.
Region of interest analyses
A multiple regression analysis was performed on the smoothed grey matter images in SPM8 to establish if there were regions of grey matter density that showed a correlation with anthropomorphism of non-human animals or non-animal stimuli.
The total grey matter volume of individual brains was included in the design matrix to regress out any general brain size differences across the participants and we further regressed out the potential confounding variables of age and gender. Anthropomorphism of non-human animals and anthropomorphism of non-animal stimuli (nature and technology) were included as separate regressors in the design matrix, the principal component analysis (see ‘Results’ section; Waytz et al., 2010b) having shown these elements of the questionnaire to constitute the two principal factors.
To test our regionally specific hypotheses, we constructed a small volume ‘mentalizing mask’. This approach was similar to previous work that employed an anatomical mask consisting of regions of interest derived from the average stereotactic coordinates reported in previous mentalizing and social cognition studies (Dumontheil, 2010). The mask comprised six spheres (diameter = 12 mm), centred on the medial prefrontal cortex (±10, 51, 34) the temporal poles (±43, 8, −34) and the posterior superior temporal sulcus/TPJ (±52, −56, 23), MNI co-ordinates. This distributed region of interest (ROI) was used to constrain the search space of our analyses and for a small volume correction across the distributed ROI examined. Robustness of the results to different anatomical mask definitions was confirmed with the use of a second, independently defined, anatomical small-volume mask taken from a meta-analysis of mentalizing studies (Van Overwalle and Baetens, 2009). This comprised four spheres, each of radius 15 mm, centred on the medial prefrontal cortex (0, 50, 20), the precuneus (0, −60, 40) and the left and right temporoparietal junctions (50, −55, 25) and (−50, −55, 25) all coordinates in MNI space. Furthermore, we used anatomical definitions of the AAL (anatomical automatic labelling; Tzourio-Mazoyer et al., 2002) atlas to construct an anatomical mask that consisted of bilateral superior medial frontal cortex, bilateral temporal pole (middle) and bilateral angular gyrus. As our VBM analysis revealed qualitatively identical results regardless of the anatomical mask used, we report the results from the analysis with the mask constructed based on Dumontheil et al.’s study (2010).
We used a threshold of P < 0.05, corrected for multiple comparisons across the mentalizing mask volume using the family-wise error rate (FWE). In addition to these analyses, we also conducted exploratory whole-brain analyses with correction for multiple comparisons across the whole brain volume.
RESULTS
VBM analysis revealed that inter-individual variability in the grey matter volume of the left TPJ positively correlated with anthropomorphism of non-human animals as indexed by the IDAQ animal score (Figure 1), t(77) = 4.80, P = 0.004, MNI co-ordinate x = −45, y = −54, z = 27, cluster size 24 voxels (81 mm3) at FWE-corrected threshold of P < 0.05 using the small volume correction defined by the mentalizing mask from Dumontheil (2010). This result was also seen when using a different mask for small volume correction taken from the meta-analysis of Van Overwalle and Baetens (2009), t(77) = 4.80, P = 0.005, cluster size 23 voxels (78 mm3) MNI co-ordinates x = −45, y = −54, z = 27. Cluster sizes for both masks for P < 0.001 uncorrected are reported in Table 1.
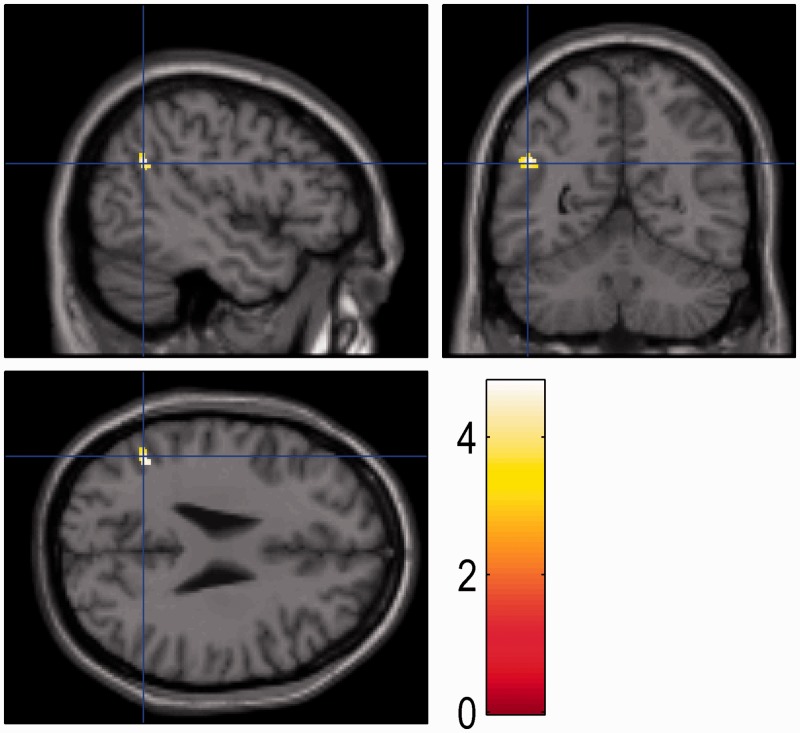
Fig. 1.
The region where grey matter volume showed a correlation with anthropomorphism of non-human animals is shown overlaid on a T1-weighted MRI anatomical image in the stereotactic space of the MNI template. Cross-sectional cuts are: top-left sagittal, top-right coronal and bottom left axial, respectively. The cross hair identifies the cluster at the left temporoparietal junction (−45,−54, 27, MNI co-ordinates) showing a statistically significant (P < 0.05 FWE-corrected for volume examined) positive correlation with anthropomorphism of non-human animals as measured by the animal IDAQ. The threshold is set to P < 0.001 uncorrected, extent threshold = 10, for illustrative purposes, cluster size = 230 mm3 (81 mm3 at P < 0.05 FWE-corrected for volume examined). The colour scale indicates the t-value for the data.
Table 1.
Cluster size for both masks (P < 0.001, uncorrected)
| Mask | t-value | Cluster size | MNI co-ordinates |
|---|---|---|---|
| (Dumontheil, 2010) | 4.8 | 68 (230 mm3) | −45, −54, 27 |
| (Van Overwalle and Baetens, 2009) | 4.8 | 68 (230 mm3) | −45, −54, 27 |
Regions reported in the table are significant after small volume correction, P < 0.05. Cluster size is the number of contiguous voxels in the cluster at P < 0.001, uncorrected.
No other brain region reached statistical significance for a positive correlation between anthropomorphism of non-human animals and grey matter volume (P < 0.05, FWE corrected for either whole-brain or mentalizing mask volume). No regions showed a negative correlation with anthropomorphism of non-human animals that survived our threshold for statistical significance. Specifically, no correlation was observed between degree of anthropomorphism and grey matter volume in the right TPJ.
No regions were found that displayed a positive correlation with anthropomorphism of non-animal stimuli and survived correction for multiple comparisons across the ROI. Outside the ROIs, selected on the basis of our hypothesis, we undertook a whole-brain analysis to search for additional brain regions that correlated with anthropomorphism of non-human animals or non-animal stimuli: however, no regions were found that displayed a positive or negative correlation with either factor and survived correction for multiple comparisons across the whole brain.
DISCUSSION
We investigated whether inter-individual variability in tendency to anthropomorphize was reflected in human brain structure. We hypothesized that the degree to which individuals engaged in anthropomorphic thinking would be reflected in the structure of brain regions implicated in thinking about others’ mental states. In support of this hypothesis, our VBM results established that the degree to which individuals anthropomorphize non-human animals was correlated with variability in regional grey matter density of the left TPJ—a brain area involved in mentalizing.
Previous functional neuroimaging work on anthropomorphism has approached this question from two different perspectives. Some studies have tried to identify the network of brain areas involved in ToM, that is, attributing beliefs and mental states to others (Castelli et al., 2000; Waytz et al. 2010a, 2010b) in anthropomorphization; others have used anthropomorphism to ask how action observation is used by the so-called ‘mirror neuron systems’ in the primate brain to interpret goals and intentions (Chaminade et al., 2007; Gazzola et al., 2007).
Gallagher and colleagues looked at brain activations when participants were engaged in a ToM task implemented by stories that were written in text or displayed by a single frame cartoon. The idea was that modality-independent brain activity related to mental state attribution should be common to both formats of story. Indeed, the conjunction analysis showed that strongest activations common to both modalities were found in the left and right TPJ (Gallagher et al., 2000). Castelli et al. (2000) used positron emission tomography to probe neural activity during mental state attribution. The stimulus they employed was a silent computer animation based on a film (Heider and Simmel, 1944), which depicts moving geometric shapes. Observers describe the film as reflecting the interactions of human-like characters. The work by Castelli et al. (2000) indicated that areas involved in ToM were more active when participants observed animated shapes and characters engaged in social or intentional motion as compared to non-social, random or mechanical motion. Increased activation in association with mental state attribution is seen in four main regions bilaterally: medial prefrontal cortex, temporoparietal junction, basal temporal regions and extrastriate cortex. All of these areas have been implicated in prior studies of mentalizing.
Waytz et al. (2010a) also examined neural correlates of anthropomorphism, exploring the hypothesis that anthropomorphism occurs in part to satisfy ‘effectance motivation’ (the motivation to acquire mastery of one’s environment). They showed that participants are more likely to attribute a mind to gadgets described as unpredictable, than those described as predictable. Their results indicate that evaluating the mental capacity of unpredictable gadgets is associated with relative increases in fMRI activity in an area centred in the ventral medial prefrontal cortex (vMPFC) and anterior cingulate cortex, a region shown to be involved in socio-cognitive processes including mentalizing about similar others.
Mirror neurons were first found in the monkey premotor cortex (Gallese et al., 1996; Rizzolatti et al., 1996; Rizzolatti and Craighere, 2004); these neurons fire both during the execution of a specific goal directed action and during the observation of equivalent actions that fulfil the same goal. The existence of homologous mirror neurons in the human brain has been the subject of much controversy (Dinstein et al., 2000) but cross-modal fMRI adaptation from action observation to execution and vice versa (Kilner et al. 2009) has now offered evidence for the existence of these neurons in the human brain. The discovery of mirror neurons has led to the ‘motor theory of social cognition’ (Gallese and Goldman, 1998; Blakemore and Decety, 2001; Gallese, 2003; Metzinger and Gallese, 2003; Wolpert et al., 2003), which proposes that understanding the mental states of other agents is accomplished by mapping their observed actions onto the observer’s own motor repertoire without undertaking the action. In this vein, studying the mirror neuron ‘systems’ in the human brain has proved a controversial, yet fruitful approach to understanding the neural substrates of understanding goals (Hamilton and Grafton, 2006) and possibly intentions (Brass et al., 2007) via action observation without necessarily having to resort to ToM, which has proved difficult to demonstrate in non-human primates (Frith and Frith, 2003).
With this approach in mind, Chaminade and colleagues have asked how ‘human-like’ should the appearance of an acting agent be for the mirror-neuron systems in the human brain to respond to the agent’s actions, thereby investigating the neuronal basis of what might be termed ‘motor anthropomorphization’. They presented participants with animations of walking avatars whose appearance progressively departed from the standard human figure, spanning the range of human through imaginary alien and robot to abstract, point-light-walker (Chaminade et al., 2007). Irrespective of the avatar’s appearance, its motion data was either captured from human actors (‘biological motion’) or from synthetic trajectories devised by an animator (‘synthetic motion’) and the participant’s task was to decide if the motion was biological or synthetic. Activity in response to these animations in the human left TPJ and anterior cingulate cortex was critically modulated by the individual differences between participants in their bias for distinguishing biological from synthetic motion. Observers who restricted biological motion exclusively to human form (i.e. weak motor anthropomorphizers) show much less left TPJ activity, whereas those for whom the aliens, robots and point-light-walkers appear human-like (i.e. strong motor anthropomorphizers) show a stronger TPJ response to motion. Chaminade et al. (2007) and our current study have both investigated individual differences in anthropomorphization, but employed two very different behavioural methods (bias in biological motion perception and IDAQ) and two different forms of neuroimaging (functional and structural MRI). Given these differences, it is striking that both studies converge in identifying the same neuronal substrate: the left TPJ. It is also reassuring that both studies found a positive correlation between their respective neural and behavioural measures.
ToM tasks are more frequently associated with right (Saxe and Wexler, 2005) rather left TPJ (Frith and Frith, 2003) activation, raising the question why we did not find any evidence for a relationship between individual differences in anthropomorphization and the structure of the right TPJ. One simple possibility is that there is no necessary relationship between individual differences in brain structure and evoked activity associated with a particular mental process (Kanai and Rees, 2011). However, it is also possible that the asymmetrical anatomical relationship we observed reflects differences in function. In a meta-analysis of more than 70 studies of ToM, Decety and Lamm (2007) argued that right TPJ involvement in ToM strongly overlaps with the activations of the same area found in the more domain-general process of reorienting attention to salient stimuli. This is also consistent with the suggestion and fMRI findings (Waytz et al., 2010a) that anthropomorphization may be a cognitive strategy to cope with unpredictability of non-human agents’ (e.g. animals, gadgets, etc.) actions. This suggestion can be neatly subsumed under the same domain-general function of the right TPJ. However, the questionnaire method used here did not assess anthropomorphization in any way that could incorporate unpredictability factors or variations in bottom–up salience, which perhaps explains why no relationship was found between right TPJ and variations in self-reports of tendency for anthropomorphization. Moreover, a recent study (Zink et al., 2011) demonstrated that only left (but not right) TPJ is activated by social unfamiliarity leading one to conjecture that anthropomorphization may be a cognitive process involving social interpretation of entities that are not socially familiar per se (such as animals and gadgets).
But how do anthropomorphism, mentalizing, action perception and the left TPJ relate? Recent work in non-human primates suggests that the TPJ is likely to be the ‘connecting hub’ between the mirror neuron system for action understanding and the affective mirror system for empathizing, bringing together understanding of others’ affective mental states and others’ goals and intentions in non-human primates (Iriki, 2006). A recent study (Mars et al., 2012) that traced the structural and functional connectivity patterns of the human (right but not left) TPJ suggests that this area consists of at least three components differentially connected to brain areas implicated in social cognition and attention. The connectivity-based parcellation of the right TPJ revealed that the posterior TPJ is functionally connected with a network of brain regions associated with social cognition (e.g. posterior cingulate/precuneus and anterior medial prefrontal cortex), whereas the anterior TPJ shows functional connectivity with attention-related regions (see also Decety and Lamm, 2007).
With the caveat in mind that these previous parcellation and meta-analysis studies both focused on the right TPJ, we asked if the left TPJ cluster identified in our study overlapped with the anterior or the posterior subregion of the left TPJ. We flipped the masks delineating the right TPJ parcellations (Mars et al., 2012) to the left hemisphere and found a close spatial correspondence between our cluster and the posterior TPJ subregion. This is consistent with our hypothesis that individual differences in the tendency to anthropomorphize reflect structural variation in brain regions linked with social cognition ability. However, one should be cautious in drawing conclusions as little is known about connectivity patterns of the left TPJ and whether application of a procedure similar to Mars et al. (2012) will result in symmetrically identical parcellation of the left TPJ or not. Further studies are required to test whether similar functional distinction along the posterior–anterior axis applies to left TPJ. Notwithstanding these cautions, the correlation we report here between left TPJ structure and anthropomorphism is consistent with the role of the TPJ as the point of convergence of neural processes subserving mentalizing (Castelli et al., 2000) and action understanding (Chaminade et al., 2007).
In line with this admittedly speculative conjecture, a very recent study (Suzuki, 2012) showed that when human agents interact with each other, separable neural correlates are found for agent’s estimated prediction error of the other’s reward (in vMPFC) as well as for the other’s ‘action prediction errors’ (in bilateral TPJ). A clear prediction following from the above synthesis is that the ‘connectivity’ between the network representing the other’s reward and action prediction errors should be mediated via the TPJ. Future studies can directly address this prediction.
Further evidence for locating anthropomorphic thinking within the mentalizing network is found in autistic individuals. Autistic individuals have difficulties attributing mental states to other humans and show reduced activation of areas of the brain known to be involved in theory of mind activities (Castelli et al., 2002). If we hypothesize that these are the areas involved in anthropomorphic thinking, then we might logically predict these subjects to exhibit an impaired ability to anthropomorphize; this is indeed the case. Studies using the short film by Heider and Simmel (1944) or similar films have shown that the tendency to describe it in anthropomorphic terms is attenuated in autism (Abell et al., 2000; Bowler and Thommen, 2000; Castelli et al., 2000; Klin, 2000).
There are a number of possible caveats to our work. The question of how best to measure the extent to which individuals anthropomorphize is difficult, yet clearly central to the study. Different approaches have been adopted including rating scales and analysis of narratives. Any method adopted will suffer from limitations, confounding anthropomorphism with animacy or attachment being two obvious examples. Our study used a self-report questionnaire that asked participants to rate their response to a selection of questions probing anthropomorphism on a scale of 1–5. The questionnaire was devised by Waytz et al. (2010b) and constitutes a relatively robust tool for measurement of anthropomorphism in as much as it has been shown to provide a measure of stable individual differences. Perhaps, a useful next step would be to combine the self-report questionnaire with psychophysical measurements similar to those employed by Chaminade et al. (2007) to characterize the individual’s explicit and implicit tendency for anthropomorphization more cohesively.
Our study recruited participants from a relatively narrow cross section of society, a relatively homogenous population of young people aged between 19 and 34 years. We are therefore unable to make generalizations about the wider population as a whole. VBM findings have indicated that brain structure can be modulated by experience and training (Dragansk et al., 2004; Driemeyer et al., 2008). The cross-sectional nature of our study prevents us from commenting on the likely temporal evolution of structure that might occur with changing anthropomorphic thinking. Indeed, the question of how anthropomorphic thinking might evolve with time, or if it displays any temporal plasticity remains, as far as we are aware, unaddressed.
We have shown an association between the structure of the left TPJ and anthropomorphic thinking and like all correlational analyses, this finding does not imply causation. A natural progression of this study would be an intervention study using brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS); these would shed light on the question of a causal link between size of the left TPJ and anthropomorphic thinking.
CONCLUSION
In conclusion, here we hypothesized that the degree to which individuals engaged in anthropomorphic thinking would be reflected in the structure of brain regions implicated in thinking about others’ mental states. Supporting this hypothesis, we observed a correlation between tendency to anthropomorphize and grey matter volume in the left TPJ, a brain area previously implicated both in mentalizing and action understanding. These results are corroborated by a previous functional MRI finding (Chaminade et al., 2007) relating the functional brain activity in the left TPJ with a very different behavioural measure to quantify individual differences in anthropomorphism. Together, these findings are consistent with the left TPJ as a point of convergence of neural processes subserving mentalizing and action understanding. Further research should examine the relationship between the structure and function of this region and medial prefrontal areas involved in making anthropomorphic judgments, and the relationship between anthropomorphizing and mentalizing judgments more generally.
Conflict of Interest
None declared.
Acknowledgments
This work was funded by the Wellcome Trust (GR), a British Academy Postdoctoral Fellowship and a Starting Grant from the European Research Council (ERC, project number 309865 to B.B.); the Japan Society for the Promotion of Science and Japan Science and Technology Agency (to R.K.).
We express our gratitude to Rogier Mars for generously providing the right TPJ parcellations.
REFERENCES
- Abell F, Happe F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. J Cogn Dev 2000. 2000;15:1–20. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Asquith PJ. Anthropomorphism and the Japaneses and Western Tradition in Primatology. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Blakemore S, Decety J. From the perception of action to the understanding of intention. Nature Neuroscience. 2001;2:561–71. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Bowler D, Thommen E. Attribution of mechanical and social causality to animated displays by children with autism. Autism. 2000;4:147–71. [Google Scholar]
- Brass M, Schmitt R, Spengler S, Gergely G. Investigation action understanding: Inferential processes versus action simulation. Current Biology. 2007:2117–21. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Carey S. Conceptual Change in Childhood. Cambridge: MA: MIT Press; 1985. [Google Scholar]
- Castelli F, Frith C, Happe F, Frith C. Autism and Brain Mechanisms for the Attribution of Mental States to Animated Shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Hodgins J, Kawato J. Anthropomorphism influences perception of computer-animated characters’ actions. Social Cognitive and Affective Neuroscience. 2007;2(3):206–16. doi: 10.1093/scan/nsm017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Gardner J, Jazayeri M, Heeger D. Executed and observed movements have different distributed representations in human aIPS. Journal of Neuroscience. 2000;28:11231–9. doi: 10.1523/JNEUROSCI.3585-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–93. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- DiSalvo C, Gemperle F, Forlizzi J, Kiesler S. All robots are not created equal: the design and perception of humanoid robot heads. DIS Conference Proceedings. 2002 ACM Press, London, 321–6. [Google Scholar]
- Dragansk B, Gase C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning-revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil L. Taking perspective into account in a communicative task. Neuroimage. 2010;52:1574–83. doi: 10.1016/j.neuroimage.2010.05.056. [DOI] [PubMed] [Google Scholar]
- Epley N, Akalis S, Waytz A, Cacioppo J. Creating social connection through inferential reproduction: loneliness and perceived agency in gadgets, Gods and greyhounds. Psychological Science. 2008;19:114–20. doi: 10.1111/j.1467-9280.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizign. Philosophy Transcripts Royal Society London Biology of Science. 2003:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H, Happe F, Brunswick N, Fletcher P, Frith U, Frith C. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind' in verbal adn nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallese V. The manifold nature of interpersonal relations: the quest for a common mechanism. In: Wolpert C, Frith D, editors. The Neuroscience of Social Interaction. Oxford: Oxford University Press; 2003. pp. 159–82. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mindreading. Trends in Cognitive Science. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35(4):1674–84. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulculs. Journal of Neuroscience. 2006;26(4):1133–7. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psycology. 1944;57:243–59. [Google Scholar]
- Iriki A. The neural origins and implications of imitation, mirror neurons and tool use. Current Opinion in Neurobiology. 2006;16:660–7. doi: 10.1016/j.conb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differnces in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12:231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neuroscience. 2009;29:10153–9. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and asperger syndrome: the social attribution task. Journal of Child Psychology and Psychiatry. 2000;41(7):831–46. [PubMed] [Google Scholar]
- Mars R, Sallet J, Urs S, Jbabdi S, Ivan T, Rushworth M. Connectivity-based subdivision of the human right “temporoparietal junction area” evidence for different areas participating in different cortical networks. Cerebral Cortex. 2012;22(8):1894–903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Metzinger T, Gallese V. The emergence of a shared ontology: building blocks for a theory. Conscious Cognition. 2003;12:549–71. doi: 10.1016/s1053-8100(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Morewedge C, Preston J, Wegner D. Timescale bias in the attribution of mind. Journal of Personality and Social Psychology. 2007;93(1):1–11. doi: 10.1037/0022-3514.93.1.1. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Child's Conception of the World. New York: Harcourt, Brace Jovanovich; 1929. [Google Scholar]
- Rizzolatti G, Craighere L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3(2):131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind:the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Harasawa N, Ueno K, et al. Learning to simulate others' decisions. Neuron. 2012;74(6):1125–37. doi: 10.1016/j.neuron.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Waytz A, Morewedge C, Epley N, Monteleone G, Gao J, Cacioppo J. Making sense by making sentient: effectance motivation increases anthropomorphism. Journal of Personality and Social Psycology. 2010a;99(3):410–35. doi: 10.1037/a0020240. [DOI] [PubMed] [Google Scholar]
- Waytz A, Cacioppo J, Epley N. Who sees human? The stability and importance of individual differences in anthropomophism. Perspectives on Psycological Science. 2010b;5:219–32. doi: 10.1177/1745691610369336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. In: Wolpert D, Frith C, editors. The Neuroscience of Social Interaction. Oxford: University Press; 2003. pp. 305–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Kempf L, Hakimi S, Rainey CA, Stein JL, Meyer-Lindenberg A. Vasopressin modulates social recognitin-related activity in the left temporoparietal junction in humans. Translational Psychiatry. 2011 doi: 10.1038/tp.2011.2. , 1, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]