Abstract
Hypnosis can affect perception, motor function and memory. However, so far no study using neuroimaging has investigated whether hypnosis can influence reward processing and decision-making. Here, we assessed whether posthypnotic suggestions can diminish the attractiveness of unhealthy food and whether this is more effective than diminishing attractiveness by one’s own effort via autosuggestion. In total, 16 participants were hypnotized and 16 others were instructed to associate a color cue (blue or green) with disgust regarding specific snacks (sweet or salty). Afterwards, participants bid for snack items shown on an either blue or green background during functional magnetic resonance imaging measurement. Both hypnosis and autosuggestion successfully devalued snacks. This was indicated by participants’ decision-making, their self-report and by decreased blood oxygen level-dependent signal in the ventromedial prefrontal cortex (vmPFC), a region known to represent value. Different vmPFC subregions coded for cue and snack type. The cue had significantly stronger effects on vmPFC after hypnosis than after autosuggestion, indicating that hypnosis was more effective in genuinely reducing value. Supporting previous findings, the precuneus was involved in the hypnotic effects by encoding whether a snack was sweet or salty during hypnotic cue presentation. Our results demonstrate that posthypnotic suggestions can influence valuation and decision-making.
Keywords: hypnosis, precuneus, self-control, value-based decision-making, ventromedial prefrontal cortex
INTRODUCTION
Hypnosis is a state of altered attention and heightened suggestibility, which is typically induced by verbal instructions. During hypnosis, suggestions can be given to participants, and these may either take effect immediately or after the hypnotic state. In the latter case, they are termed ‘posthypnotic’ suggestions (Raz et al., 2002, 2005; Wheatley and Haidt, 2005; Iani et al., 2006, 2009). Hypnotic suggestions can lead to strong and genuine effects in susceptible individuals, such as paralysis (Halligan et al., 2000; Cojan et al., 2009), experiencing own movements as externally caused (Blakemore et al., 2003), seeing letters in colors (Cohen Kadosh et al., 2009), sensory pain without an affective component (Rainville et al., 1997), visual illusions (Kosslyn et al., 2000) or auditory hallucinations (Szechtman et al., 1998). Hypnotic suggestions can also affect moral judgments and moral behavior (Wheatley and Haidt, 2005; Brüne et al., 2012). So far, however, no study using neuroimaging has investigated whether hypnosis can also influence reward processing and value-based decision-making. This is a crucial question given the wide use of hypnosis to treat maladaptive decision-making such as in nicotine addiction or obesity (Kirsch et al., 1995; Allison and Faith, 1996; Carmody et al., 2008; Barnes et al., 2010).
It is well established that the subjective value people place on decision options is represented in the ventromedial prefrontal cortex (vmPFC), which includes the rostral anterior cingulate cortex (rACC) and the medial orbitofrontal cortex (mOFC; Kable and Glimcher, 2007; Hare et al., 2008; Lebreton et al., 2009; Basten et al., 2010; Peters and Büchel, 2010; Plassmann et al., 2010; Grabenhorst and Rolls, 2011; Brosch et al., 2012). For example, the greater the activity in vmPFC, the more people are willing to pay for an item (Plassmann et al., 2007; Chib et al., 2009; Janowski et al., 2013), or the more they desire to consume it (Hare et al., 2009; Litt et al., 2011). Some studies have shown that value signals in vmPFC can be modulated by self-control or attention (Hare et al., 2009; Hollmann et al., 2011) or by mindsets (Bhanji and Beer, 2012; for studies on modulating pleasantness of tasting or smelling rewards, see de Araujo et al., 2005; Grabenhorst and Rolls, 2008; Grabenhorst et al., 2008; Plassmann et al., 2008). For example, when participants focus their attention on health aspects of food items, vmPFC responds more strongly to the healthiness of the food, and participants also make healthier decisions (Hare et al., 2011). Moreover, when participants focus on the (negative) long-term rather than the (positive) short-term consequences of consuming food or cigarettes, vmPFC responds less strongly to pictures showing food or cigarettes (Kober et al., 2010). However, overall, evidence regarding experimental manipulations of vmPFC value signals during decision-making has been scarce.
Here we investigated whether posthypnotic suggestions can influence the value people place on unhealthy food during decision-making, as indicated by behavior, self-report and vmPFC activation. We further asked whether hypnosis can achieve stronger effects than self-controlled down-regulation of the attractiveness of food, termed here ‘autosuggestion’ (Baudouin, 2003; Coué, 2009). Autosuggestion refers to the process of implementing a mental change in oneself (e.g. by repeating suggestions to oneself and by engaging in goal-directed imagery). It is a novel approach to use an autosuggestion (or self-control) group as a comparison group for hypnosis. Previous studies have typically used control participants who were either instructed to ‘simulate’ hypnotic behavior (Cojan et al., 2009) or who received the same suggestions as the hypnotized group without a hypnotic induction (Iani et al., 2006). Another approach is to compare hypnotic effects between highly suggestible and less suggestible participants (Raz et al., 2002). Here, we compared hypnosis to autosuggestion, because it is clinically and practically relevant to determine if hypnosis is more effective than attempts to implement a mental change by oneself.
We further assessed whether the hypnotic manipulation of value-based decision-making involves the precuneus. Previous findings indicated that the precuneus is important for hypnotic effects. In a study by Cojan et al. (2009), participants were given hypnotic suggestions for left-hand paralysis. When they were instructed to move their left hand—which they were unable to do—there was precuneus activation; and precuneus showed enhanced functional connectivity with primary motor cortex. The authors related their findings to studies showing that the precuneus is involved in mental imagery and self-related processing (Lou et al., 2004; Cavanna and Trimble, 2006). They proposed that during hypnotic effects, behavior is guided by increased self-monitoring processes and by internal representations produced by imagery and by the hypnotic suggestions.
We hypothesized that hypnosis would be able to change decision-making about unhealthy snacks behaviorally, and that this would be reflected in diminished value signals in vmPFC during those decisions. We further predicted that hypnosis would lead to stronger effects than autosuggestion. Finally, we hypothesized that the precuneus would be functionally involved in the hypnotic effects by encoding relevant information about the content of the suggestions.
METHODS
Participants
We tested 32 participants (18 female, mean age = 24.94 years, s.d. = 3.70), 16 in a hypnosis group and 16 in an autosuggestion group. Inclusion criteria were: right-handedness, normal or corrected-to-normal vision, no history of eating disorders or other psychiatric or neurological disorders, no medication which may influence brain activation, no current diet, liking of both sweet and salty snacks, and upper medium to high hypnotic suggestibility (prescreened using the Harvard Group Scale of Hypnotic Susceptibility: Form A; HGSHS:A; required score: 7–12; Shor and Orne, 1962). Participants of the two groups were matched in terms of age, sex and hypnotic suggestibility (Supplementary Table S1). Participants received monetary compensation for participation and gave written informed consent. The study was approved by the local ethics committee.
Procedure
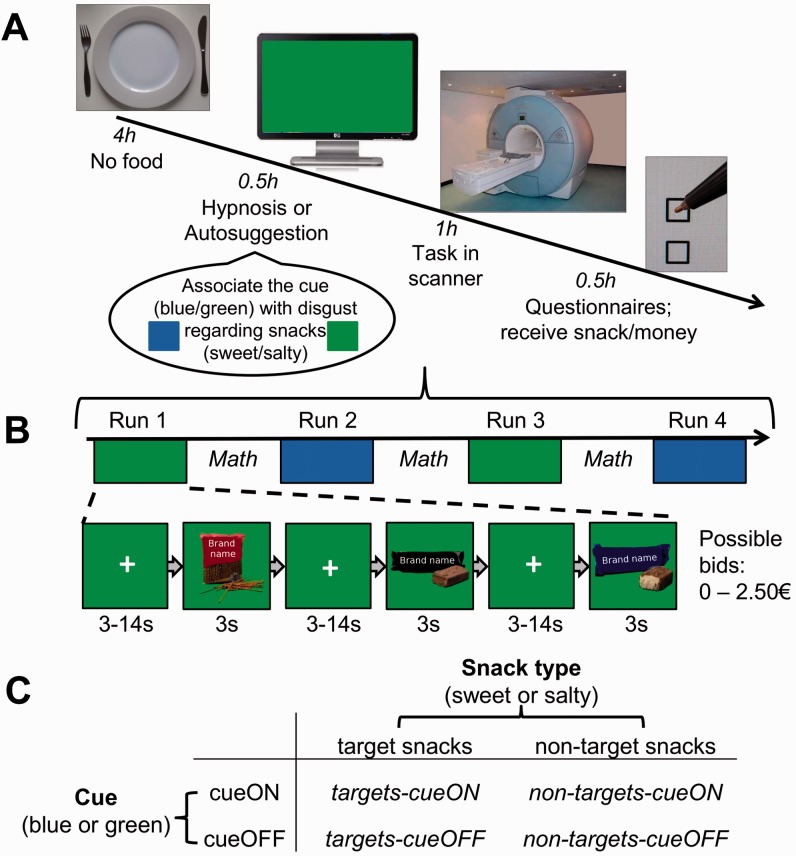
Participants arrived at the laboratory hungry, as they had been asked to not eat for 4 h before the experiment (Figure 1A). The 16 participants of the hypnosis group were then hypnotized in a one-to-one setting by a professional hypnotist (H.K.). During hypnosis, participants were suggested to open their eyes and to look at a color on a monitor (blue or green, counterbalanced across subjects). They were suggested that this color would be associated with a strong feeling of disgust regarding either sweet or salty snacks (counterbalanced across subjects). This is a posthypnotic suggestion as it is activated when the posthypnotic color cue is encountered ‘after’ hypnosis in a normal state. Note that hypnosis did not involve induction of amnesia; participants were therefore aware of what had been suggested to them. We refer to those snacks (i.e. sweet or salty) that were associated with disgust as ‘target snacks’ and to the others as ‘non-target snacks’. The 16 other participants were instructed—without hypnosis—to associate the color cue (blue/green) with disgust regarding either sweet or salty snacks. They were given as much time as the hypnotized subjects to make the association by themselves, and they were also sitting in front of the monitor with the color while engaging in the autosuggestion. Participants were free to use their own strategy to make this association, which we inquired about after the experiment (Supplementary Table S2).
Fig. 1.
Methods. (A) Procedure: hungry participants were either hypnotized or they used autosuggestion in order to feel disgust regarding either sweet or salty snacks upon seeing either a blue or a green color cue. Afterwards, they carried out an auction on sweet and salty snacks shown on an either blue or green background in the fMRI scanner. They could buy a real snack at the auction. (B) Experimental auction. (C) Overview of the trial types. Note that in this figure, example stimuli have been modified so that the brands of the snacks are not recognizable.
After the intervention, we assessed participants’ decision-making and brain activation using functional magnetic resonance imaging (fMRI) during an auction on unhealthy snacks (Figure 1B). Note that participants in the hypnosis group were in a normal state during scanning as hypnosis was finished beforehand. After scanning, participants completed a questionnaire about their experience. The procedures are described in more detail in the Supplementary Data.
Task
The auction was a variant of a Becker–DeGroot–Marshak method (Becker et al., 1964; Plassmann et al., 2007) and took ∼35 min. Participants saw pictures of sweet and salty snack items (e.g. chocolate bars, chips) and could bid between 0€ and 2.50€ for each of them (in 0.50€ steps). Snacks were shown on an either blue or green background. That is, during two out of the four runs of the auction, snacks were shown on the color cue associated with disgust (cueON-runs) and during the two other runs snacks were shown on the neutral color (cueOFF-runs). There were, hence, four trial types: targets-cueON, non-targets-cueON, targets-cueOFF, and non-targets-cueOFF (Figure 1C). Runs were separated by math problems for distraction. Our stimulus set comprised 50 high-resolution pictures of appetitive snack items (25 sweet and 25 salty) that were highly familiar in Germany. For each run, 40–43 stimuli were randomly selected for presentation out of the stimulus set. Approximately, half of the snacks in each run were target snacks and half were non-target snacks (i.e. half were sweet and half were salty), presented randomly. No stimulus was shown more than once during one run.
The auction was set up such that participants would treat each decision as a real decision and as the only one that counts (see Supplementary Data). To incentivize honest responses, participants could buy a real snack on the auction. If participants did not buy a snack, they had to stay hungry for another half hour after leaving the scanner.
Postexperimental questionnaire
The postexperimental questionnaire differed partly between groups. The following questions were overlapping (translated from German): ‘How much disgust did you experience for salty snacks on blue background?’ (the same question reoccurred three more times, with sweet on blue, salty on green, and salty on blue); ‘To what degree did you feel physical disgust?’, ‘How automatic vs. self-controlled did the disgust appear to you, in case that you felt disgust?’, ‘Please be honest: during the experiment, did you sometimes just pretend to feel disgust?’ and ‘Did you often consciously think about the meaning of the color cue during the experiment and consciously/voluntarily recalled the association with disgust?’ Answers were given on Likert-scales from 1–7. Some open response questions about participants’ experiences were also included (e.g. regarding the strategies that participants in the autosuggestion group used).
Analysis of behavior and self-report
Mixed analysis of variances (ANOVAs) were used to analyze bids, mean reaction times (RTs) for bids and postexperimental disgust ratings. ANOVAs included the within-subject factors snack type (target/non-targets) and cue (cueON/cueOFF) and the between-subject factor group (hypnosis/autosuggestion). Prior to the analysis, bids were log-transformed in order to meet the assumption of normality (see Supplementary Data for more information). To compare the two groups regarding the remaining self-report questions, we used independent t-tests where data were normally distributed and non-parametric Mann–Whitney tests where data were not normal. For ANOVAs, η2 is reported as a measure of effect size. It represents the proportion of variance in the dependent variable explained by an effect. Effect sizes r for t-tests and Mann–Whitney tests were calculated following Rosenthal (1991), interpretation of r: 0.10: small, 0.30: medium, and 0.50 large (Cohen, 1988).
fMRI data acquisition and preprocessing
Data were collected using a 3T Siemens Tim Trio MRI scanner with a 12-channel head coil. For each run, 185 functional images including 33 axial slices were acquired in descending order using a T2*-sensitive one-shot gradient-echo echo-planar imaging (EPI) sequence. The following parameters were used: repetition time = 2 s, echo time = 25 ms, field of view = 24 cm, matrix size = 64 × 64, voxel size = 3 × 3 × 3 mm and inter-slice gap = 0.75 mm. Functional images were realigned and unwarped based on fieldmaps, slice-time-corrected, spatially normalized to the standard Montreal National Institute (MNI) EPI template and smoothed using an isotropic 8 mm full-width half-maximum Gaussian kernel (see also Supplementary Data). Coordinates reported in this article are MNI-coordinates.
fMRI data analysis
We calculated general linear models on the single-subject level. In a first model, we modeled target trials, non-target trials and missed trials as box-car functions of 3 s, convolved with the hemodynamic response function. Depending on the run, the target and non-target regressors encoded ‘targets-cueON’ and ‘non-targets-cueON’ or ‘targets-cueOFF’ and ‘non-targets-cueOFF’. Motion parameters were included as regressors of no interest. High-pass temporal filtering (128 s) was applied. A second model was set up in the same way as the first model but it included the parametric modulator bid size. This served to identify regions correlating with bids. Relevant contrasts were calculated on the first level for each subject separately. On the second level, we used one-sample t-tests for determining the effects for all subjects together and independent t-tests for the group comparisons. We restricted our main analyses to voxels within our regions of interest (ROIs) in vmPFC and precuneus (Supplementary Figure S1). We corrected the results using family-wise error (FWE) correction at P < 0.05 within ROIs using small-volume correction. For completeness, we also report other relevant effects within ROIs at a liberal threshold of P < 0.001, uncorrected. Moreover, we conducted exploratory whole-brain analyses at P < 0.001, uncorrected.
Regions of interest
For the two areas of interest—vmPFC and precuneus—we created a priori ROIs (see Supplementary Data). These regions served to spatially restrict the main analyses and for small-volume alpha error adjustment. For vmPFC, we created a probabilistic ROI that takes into account the coordinates of several previous studies on valuation (Supplementary Figure S1A and Supplementary Table S5). For precuneus, we used the peak coordinate from Cojan et al. (2009) with a 15 mm sphere around it (Supplementary Figure S1B).
RESULTS
Bids
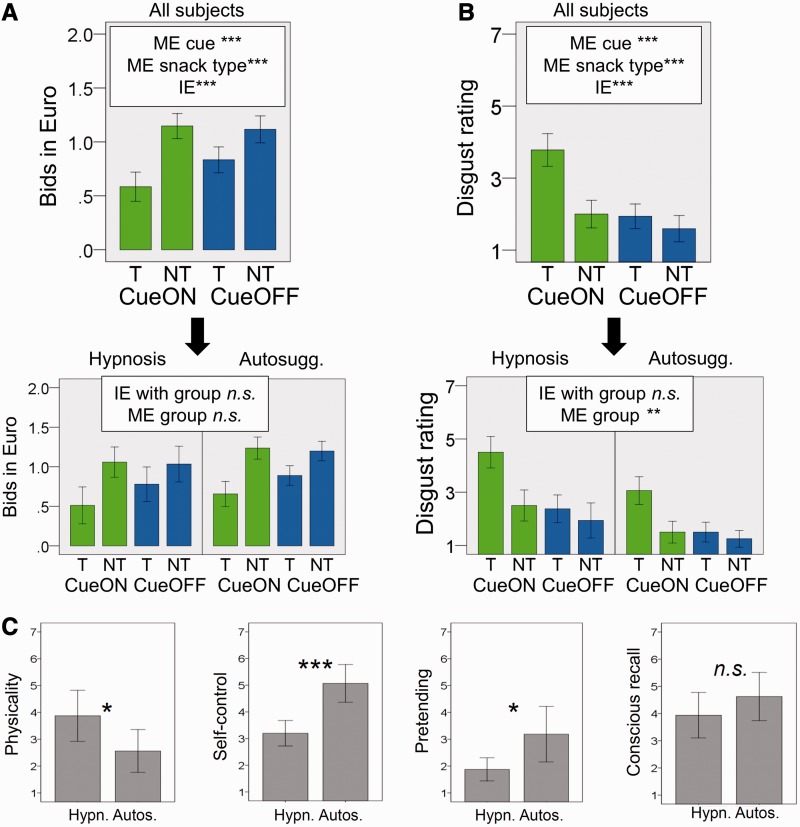
Bids per condition are shown in Figure 2A. For all subjects together, there was a main effect of snack type, F(1,30) = 16.095, P < 0.001, η2 = 0.29, as participants bid less for target snacks than for non-target snacks. There was also a main effect of cue, F(1,30) = 17.686, P < 0.001, η2 = 0.02, as participants bid less for snacks during cueON-runs than during cueOFF-runs. Finally, there was an interaction of cue × snack type, F(1,30) = 22.953, P < 0.001, η2 = 0.04, as the difference between bids for target snacks and non-target snacks was bigger during cueON-runs than during cueOFF-runs. Furthermore, there were no interactions of group × snack type, group × cue or group × snack type × cue (all P-values >0.50), and also no main effect of group (P = 0.16). Hence, the effects for bids did not differ between groups.
Fig. 2.
Behavior and self-report. (A) Bids. (B) Postexperimental disgust ratings. (C) Postexperimentally reported degree of physicality of disgust, experience of self-control (as opposed to automaticity) regarding the feeling of disgust, pretending of disgust and conscious recall of suggestion/instruction during the experiment. Ninety-five percent confidence interval (CI) of the mean are shown, adjusted for within-subject designs where appropriate Loftus and Masson, (1994). T: target snacks (sweet or salty). NT: non-target snacks. CueON: cue color shown in the background. CueOFF: neutral background color. ME: Main effect. IE: Interaction effect. ***P < 0.001, **P < 0.01, *P < 0.05; n.s., not significant.
Pairwise comparisons for all subjects together further showed that bids for targets-cueON were lower than those for each of the other three conditions (all P-values < 0.001; all P-values reported here are Bonferroni-corrected.). There was no difference between non-targets-cueON and non-targets-cueOFF (P > 0.50). Unexpectedly, bids for targets-cueOFF were also lower than bids for non-targets-cueON (P < 0.05), and tended to be lower than those for non-targets-cueOFF (P < 0.10). In sum, both hypnosis and autosuggestion successfully devalued target snacks during cue presentation, as measured by bidding behavior.
RTs for bid responses (Table 1) did not differ between groups or conditions (P-values > 0.10 for all main effects and interactions).
Table 1.
Mean RTs per condition in seconds
| Hypnosis (n = 16) | Autosuggestion (n = 16) | ||
|---|---|---|---|
| Cue | Snack type | Mean (s.d.) | Mean (s.d.) |
| CueON | Targets | 1.434 (0.247) | 1.457 (0.229) |
| Non-targets | 1.475 (0.216) | 1.440 (0.150) | |
| CueOFF | Targets | 1.476 (0.192) | 1.409 (0.198) |
| Non-targets | 1.442 (0.203) | 1.381 (0.205) |
s.d., standard deviation.
Self-report
Results concerning the postexperimental questionnaires are shown in Figure 2B and C (disgust ratings and other questions, respectively). For disgust ratings, there was a main effect of snack type, F(1,30) = 17.177, P < 0.001, η2 = 0.18, of cue, F(1,30) = 48.661, P < 0.001, η2 = 0.20 and an interaction of cue × snack type, F(1,30) = 23.236, P < 0.001, η2 = 0.08. There were no interactions of group × cue, group × snack type or group × cue × snack type (all P-values >0.15). However, there was a main effect of group, as the hypnosis group reported higher average disgust than the autosuggestion group, F(1,30) = 10.76, P = 0.003, η2 = 0.26. As predicted, t-tests for all participants together showed that reported disgust was higher for targets-cueON than for each of the other conditions (all P-values <0.001, Bonferroni-corrected). The remaining three conditions did not differ from each other (all corrected P-values >0.40).
Thus, disgust ratings per condition indicated that both interventions were equally successful in devaluing target snacks experientially. However, participants in the hypnosis group reported their disgust to be more bodily, t(30) = 2.250, P = 0.03, r = 0.38 and less self-controlled (i.e. more automatic) than participants in the autosuggestion group, U = 22.500, P < 0.001, r = −0.70 (two values were missing here). Moreover, participants in the autosuggestion group reported merely having pretended to feel disgust to a stronger degree than participants in the hypnosis group, t(20.033) = −2.499, P = 0.02, r = 0.49 (degrees of freedom adjusted due to unequal variances). In terms of recalling the suggestion or instruction during the experiment, the groups did not differ, U = 91.500, P = 0.16, r = −0.25.
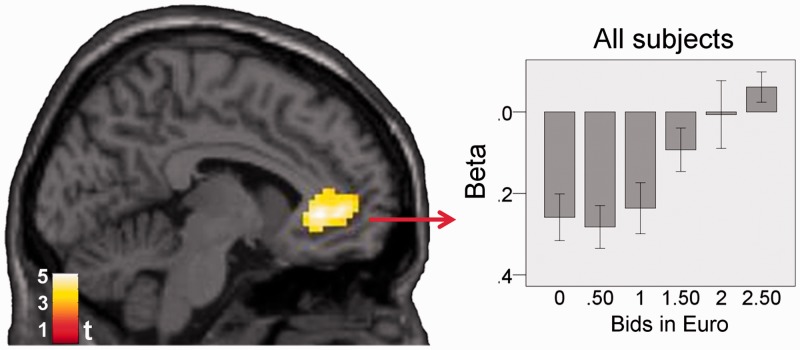
Correlation between vmPFC activation and bids independent of condition
In the fMRI analysis, we first tested whether vmPFC correlated with subjective value, as shown by previous studies. Thus, we determined whether any voxels within our vmPFC ROI (Supplementary Figure S1A) correlated with bids independent of condition in all 32 participants. As expected, rACC and parts of mOFC correlated with bids at P < 0.05, corrected (Figure 3).
Fig. 3.
Correlation with bids independent of condition for participants of both groups analyzed together (n = 32; peak: − 6, 35, − 2, t = 5.09). Results are masked by the a priori defined vmPFC ROI (Supplementary Figure S1A). For visualization purpose, activations are shown at P < 0.005, k = 10. The beta-plot visualizes the results (mean beta ± 1 s.e.). The plot was constructed using a procedure that ensures independency from the main analysis (see Supplementary Data; Litt et al., 2011).
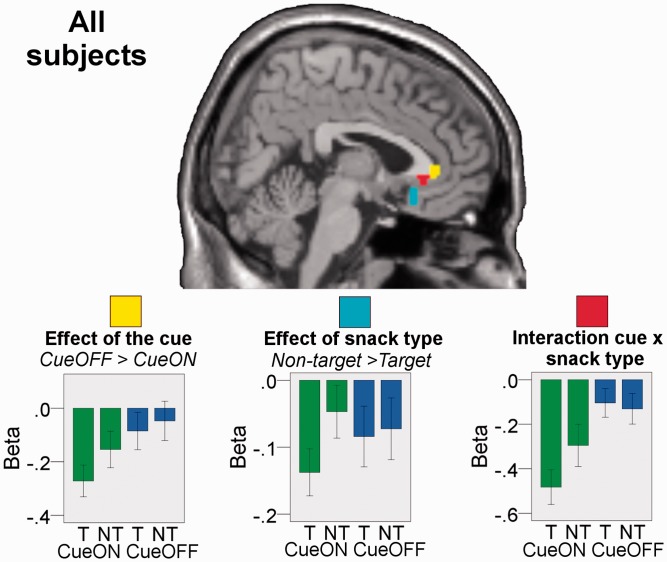
Effects on vmPFC
Our next goal was to corroborate our behavioral findings across the four conditions with corresponding activation patterns in vmPFC. We tested for the effects of cue (OFF > ON), of snack type (non-target > target) and for the interaction of cue × snack type (stronger effect for non-targets > targets during cueON-runs than during cueOFF-runs) within our vmPFC ROI (Supplementary Figure S1A). Diminished attractiveness of snacks should be reflected in diminished blood oxygen level-dependent (BOLD) signal in vmPFC. We initially analyzed the fMRI data for all subjects together and then assessed group differences.
Indeed, for all these contrasts we found BOLD signal changes in vmPFC (Figure 4). The effects of cue and snack type were both significant at P < 0.05, corrected, while the interaction of cue × snack type was significant at P < 0.001, uncorrected. Interestingly, activations for the three effects were found in distinct but partly overlapping regions of vmPFC. Note that it is not problematic that the cue × snack type interaction was non-significant after correction. This interaction might also be encoded by the pattern of activation across the two vmPFC subregions showing effects for cue and snack type. Thus, there does not necessarily need to be a subregion representing the interaction as such.
Fig. 4.
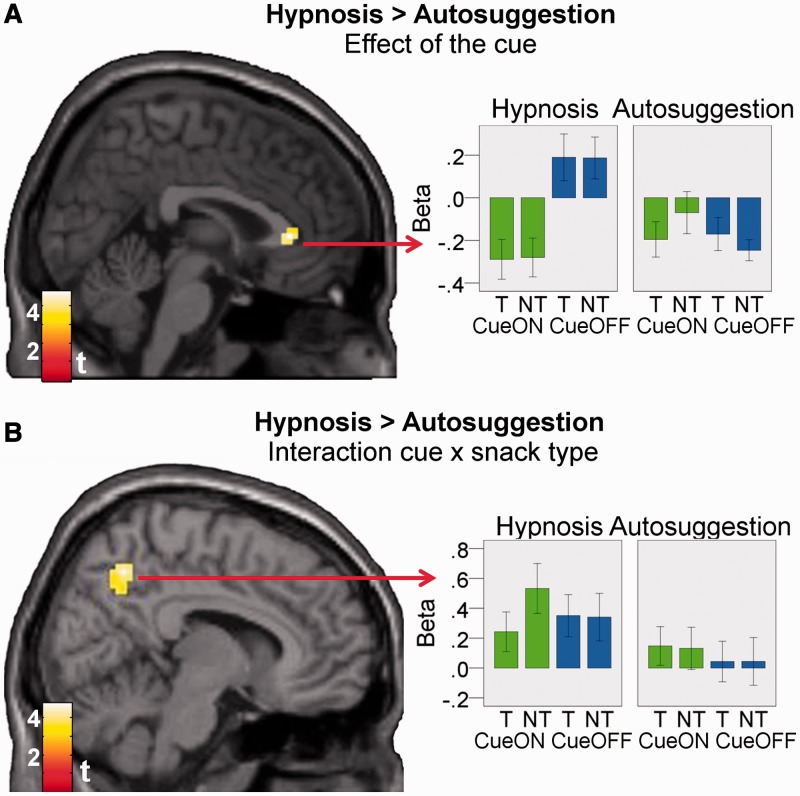
Effect of the cue (yellow), of snack type (cyan) and of the interaction of cue × snack type (red) in vmPFC for participants of both groups analyzed together (n = 32; peaks within ROI for cue: −6, 35, −2, t = 4.01; snack type: 6, 26, −17, t = 4.29; cue × snack type: 0, 32, −2, t = 3.63). Results are masked by the vmPFC ROI. For visualization, activations are shown at P < 0.005, k = 10. beta-plots visualize the results (mean beta ± 1 s.e.) and were constructed in a way that ensures independency from the main analysis (see Supplementary Data). T: target snacks (sweet or salty). NT: non-target snacks. CueON: cue color shown in the background. CueOFF: neutral background color.
The effects of cue, snack type and of their interaction (Figure 4) overlapped with the region that correlated with bids ‘within’ the four conditions (Figure 3). This indicates that hypnosis and autosuggestion indeed altered ‘valuation’ of food stimuli. Hence, when analyzing all subjects together, the results of the fMRI analysis corroborated the behavioral results as vmPFC was responsive to the experimental manipulations in the expected directions.
When comparing the two groups, we further found that the effect of the cue on rACC (a subregion of the vmPFC ROI) was stronger in the hypnosis group than in the autosuggestion group, at P < 0.05, corrected (Figure 5A). The effect of snack type and the interaction of cue × snack type, in contrast, did not differ significantly between groups within the vmPFC ROI.
Fig. 5.
Group differences. (A) There was a stronger effect of the cue on vmPFC activation in the hypnosis group (peak: 3, 32, 4, t = 4.73). (B). There was also a stronger interaction effect of cue × snack type on the precuneus in the hypnosis group (peak: 9, −58, 46, t = 4.00). Results are masked by the vmPFC ROI in panel A and by the precuneus ROI in panel B (Supplementary Figure S1 gives a depiction of the ROIs). For visualization, activations are shown at P < 0.005, k = 10. beta-plots visualize the results (mean beta ± 1 s.e.) and were constructed in a way that ensures independency from the main analysis (see Supplementary Data). T: target snacks (sweet or salty); NT: non-target snacks. CueON: cue color shown in the background. CueOFF: neutral background color.
Effects on the precuneus
If the precuneus was functionally involved in the effects of hypnosis or autosuggestion, we would expect finding an effect of cue, snack type or of their interaction in this region. As above, we first tested for these three effects in all subjects together (n = 32) in all voxels of the precuneus ROI (Supplementary Figure S1B). There was no effect of cue or snack type, but there was an interaction of cue × snack type at P < 0.05, corrected (peak: 6, −55, 52, t = 4.25).
The cue × snack type interaction in the precuneus was stronger in the hypnosis group than in the autosuggestion group, at P < 0.05, corrected. Figure 5B shows mean β-values of the interaction voxels per condition. The pattern shows that the group × cue × snack type interaction arises because only in the hypnosis group, precuneus differentiated between targets/non-targets during cueON-runs but not during cueOFF-runs. This indicates that the precuneus was indeed functionally involved in the effects of the hypnotic suggestions because it encoded relevant information concerning the hypnotic suggestions. Finally, we also found stronger activation of the precuneus for all trials (against baseline) for the hypnosis group compared with the autosuggestion group, at P < 0.05, corrected (peak: 0, −61, 34, t = 3.89).
Whole-brain analysis
Our exploratory whole-brain analyses revealed effects of snack type and of the interaction of cue × snack type on several regions outside of our ROIs when all participants were analyzed together. Among other findings, the right anterior insula was more active for target snacks than for non-target snacks. An interaction of cue × snack type was, for example, found in the fusiform gyrus, the posterior cingulate cortex and the parahippocampal gyrus (Supplementary Table S3 shows the complete results). The analyses further showed that group differences between hypnosis and autosuggestion were largely specific to vmPFC and precuneus (Supplementary Table S4).
DISCUSSION
In this study, participants were either given posthypnotic suggestions or they used autosuggestion to associate a color cue (blue or green) with the feeling of disgust regarding particular unhealthy snacks (either sweet or salty). Both hypnosis and autosuggestion successfully diminished the attractiveness of snacks in suggestible participants, as indicated by behavior, self-report and vmPFC activation. Participants who underwent hypnosis reported the effects to be more physical, automatic and genuine (not simulated) compared with participants who used autosuggestion. Moreover, while the behavioral effects of hypnosis and autosuggestion were equally strong, the color cue had significantly stronger effects on vmPFC activation (specifically: rACC) in the hypnosis group compared with the autosuggestion group.
As both groups behaved indistinguishably during the experiment, the neural differences between groups cannot be attributed to participants’ behavior (Egner et al., 2005). The finding that vmPFC correlates with subjective value or attractiveness is one of the most established findings in neuroeconomics (Plassmann et al., 2007, 2010; Kable and Glimcher, 2007; Hare et al., 2008, 2009, 2011; Chib et al., 2009; Basten et al., 2010; Peters and Büchel, 2010; Grabenhorst and Rolls, 2011; Litt et al., 2011; Janowski et al., 2013). Therefore, a possible explanation for the group difference is that hypnosis induced more genuine decreases of the perceived attractiveness of snacks. This interpretation should, however, be treated with caution, because it cannot be excluded that vmPFC activation can be reduced when the value of an object has not truly changed (e.g. when participants think of something negative while looking at pictures, or due to other unrelated processes). However, the interpretation is supported by the finding that only participants in the autosuggestion group reported sometimes having pretended to feel disgust. Moreover, we could show that BOLD signal in the exact same region which was affected more by hypnosis than autosuggestion correlated with bids independent of condition within the current study. Notably, only the effect of the color cue on vmPFC (and not of snack type or the interaction of cue × snack type) was stronger after hypnosis compared with autosuggestion. A possible explanation for this is that the color cue was especially important for the hypnotic effects. This could be due to the fact that the salient color cue can be processed quickly and automatically, whereas it takes more cognitive processing to categorize snacks as sweet or salty.
Regarding the mechanisms of hypnotic effects, we additionally found evidence for involvement of the precuneus, in line with previous findings (Cojan et al., 2009). There was higher precuneus activation for all trials against baseline after hypnosis compared with autosuggestion. More importantly, there was a three-way interaction of cue × snack type × group. This arose because only in the hypnosis group, the precuneus differentiated between sweet and salty snacks during cueON-runs. Thus, in this group the precuneus activated less for the devalued target snacks than for non-target snacks, but only when the posthypnotic cue was shown. Precuneus, hence, encoded specific information regarding the suggestions.
Two points should be kept in mind with respect to our results, particularly those concerning group differences. First, all our conclusions apply to ‘free’ autosuggestion rather than highly structured or trained autosuggestion. That is, participants in the autosuggestion group chose and used their own mental strategies. In contrast, participants in the hypnosis group received a relatively standardized, structured intervention. This was done to approximate a real-life situation in the autosuggestion group in which people attempt to reduce their craving for food completely by their own effort. Importantly, the effectiveness of autosuggestion might increase when participants receive specific instructions or training concerning the use of autosuggestion. Second, we selected participants based on their responsiveness to hypnosis. We assumed that participants who respond well to hypnosis are also good at using autosuggestion. For example, highly hypnotizable as compared to low hypnotizable participants respond better to autogenic training (involving autosuggestion; Schultz and Luthe, 1959) and to cognitive self-hypnosis in the treatment of headaches (ter Kuile et al., 1994). Moreover, suggestible participants respond very well to suggestions given outside hypnosis (Kirsch and Braffman, 2001; Raz et al., 2006; McGeown et al., 2012). Nevertheless, it cannot be excluded that autosuggestion would have had even stronger effects in participants specifically selected to be good at using autosuggestion.
The finding that not only hypnosis, but also free autosuggestion could influence decision-making is remarkable. On the postexperimental questionnaire, participants reported their strategies for autosuggestion: they typically imagined disgusting objects and told themselves repeatedly that the snacks were disgusting (Supplementary Table S2). There is little explicit empirical work on autosuggestion in the neuroimaging literature. Exceptions are studies on autogenic training (Schlamann et al., 2010; Naglatzki et al., 2012) and the placebo effect (Wager et al., 2004; Raz, 2007; Eippert et al., 2009), which involves unconscious autosuggestion or belief that a treatment will help. Our results show that conscious autosuggestion as a tool to influence cognition or behavior is a promising field for future neuroscientific research.
Interestingly, Coué (2009) proposed that suggestion ‘does not and cannot exist except on the sine qua non condition of transforming itself into autosuggestion in the subject’. Thus, also the effects in our hypnosis group might (partly) be due to autosuggestion, as participants might have used their cognitive resources to support the suggested effects. It is likely very difficult to disentangle autosuggestive from heterosuggestive components of hypnosis. One possibility for this would be comparing hetero-hypnosis with self-hypnosis, with the exact same suggestions for both. However, self-hypnosis requires training and is usually taught through hetero-hypnosis (Sacerdote, 1981). Another possibility would be to induce posthypnotic amnesia, so that participants would be unaware of what has been suggested to them (Mendelsohn et al., 2008). However, only few subjects are susceptible to posthypnotic amnesia, and it is difficult to ascertain that participants are truly amnesic.
Finally, our results may have implications beyond the area of hypnosis. This experiment can be conceived of as an experiment about valuation of multi-attribute objects. The food pictures in this study had two salient features that varied, namely type of taste (sweet or salty) and background color (blue or green). Hypnosis and autosuggestion systematically changed the value of the levels of these features. Thus, for each subject, one type of taste and one background color was associated with a negative value (e.g. salty = disgusting and green = disgusting). When participants assigned values to stimuli, they had to perceive and evaluate both stimulus features and integrate them to an overall value (together with other variables; von Winterfeldt and Fischer, 1975). We found that different vmPFC subregions tracked the value of different stimulus attributes (Figure 4). While rACC was most responsive to the (value of the) cue, more ventral parts of rACC and mOFC tracked the value of the type of taste (sweetness/saltiness). Thus, our results—gained by using hypnosis as a tool—indicate that the values of different features of objects are represented in different vmPFC subregions. This demonstrates that hypnosis and autosuggestion can be useful for studying the neural basis of decision-making (Raz and Shapiro, 2002; Oakley and Halligan, 2009; Raz, 2011).
In sum, our results show that posthypnotic suggestions and—to a lesser extent—free autosuggestion can influence decision-making and valuation on the behavioral, phenomenological and on the neural level. Thus, these methods might be useful for studying the neural basis of decision-making, and they may be useful for helping people make better decisions in real life.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Antonio Rangel for stimulating discussions on study design and data analysis. We thank Sven Reichelt for taking the pictures for the stimulus set and Hilke Plassmann for advice regarding the creation of the stimuli. We are grateful to Walter Bongartz for providing materials for the German HGSHS:A and to Dar Meshi for proof-reading and commenting on the manuscript. We thank Dominik Bach, Björn Schott, and Susanne Erk for discussions and Torsten Wüstenberg for providing the MATLAB tool for the creation of the vmPFC ROI.
This study was funded by the VW-Foundation (II/84 051 H.W.) and the German National Academic Foundation (to V.U.L.).
REFERENCES
- Allison DB, Faith MS. Hypnosis as an adjunct to cognitive-behavioral psychotherapy for obesity: a meta-analytic reappraisal. Journal of Consulting and Clinical Psychology. 1996;64(3):513–6. doi: 10.1037//0022-006x.64.3.513. [DOI] [PubMed] [Google Scholar]
- Barnes J, Dong C, McRobbie H, Walker N, Mehta M, Stead L. Hypnotherapy for smoking cessation. Cochrane Database of Systematic Reviews. 2010;(10):1–34. [Google Scholar]
- Basten U, Biele G, Heekeren HR, Fiebach CJ. How the brain integrates costs and benefits during decision making. Proceedings of the National Academy of Sciences USA. 2010;107(50):21767–72. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C. Suggestion and Autosuggestion. Whitefish, Montana: Kessinger Publishing; 2003. [Google Scholar]
- Becker G, DeGroot M, Marschak J. Measuring utility by a single-response sequential method. Behavioral Science. 1964;9:226–32. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- Bhanji JP, Beer JS. Taking a different perspective: Mindset influences neural regions that represent value and choice. Social Cognitive and Affective Neuroscience. 2012;7(7):782–93. doi: 10.1093/scan/nsr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Oakley DA, Frith CD. Delusions of alien control in the normal brain. Neuropsychologia. 2003;41(8):1058–67. doi: 10.1016/s0028-3932(02)00313-5. [DOI] [PubMed] [Google Scholar]
- Brosch T, Coppin G, Schwartz S, Sander D. The importance of actions and the worth of an object: dissociable neural systems representing core value and economic value. Social Cognitive and Affective Neuroscience. 2012;7(5):497–505. doi: 10.1093/scan/nsr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M, Tas C, Wischniewski J, Welpinghus A, Heinisch C, Newen A. Hypnotic ingroup–outgroup suggestion influences economic decision-making in an Ultimatum Game. Consciousness and Cognition. 2012;21(2):939–46. doi: 10.1016/j.concog.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Carmody TP, Duncan C, Simon JA, et al. Hypnosis for smoking cessation: a randomized trial. Nicotine & Tobacco Research. 2008;10(5):811–8. doi: 10.1080/14622200802023833. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O'Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. Journal of Neuroscience. 2009;29(39):12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. New York: Academic Press; 1988. [Google Scholar]
- Cohen KR, Henik A, Catena A, Walsh V, Fuentes LJ. Induced cross-modal synaesthetic experience without abnormal neuronal connections. Psychological Science. 2009;20(2):258–65. doi: 10.1111/j.1467-9280.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- Cojan Y, Waber L, Schwartz S, Rossier L, Forster A, Vuilleumier P. The brain under self-control: modulation of inhibitory and monitoring cortical networks during hypnotic paralysis. Neuron. 2009;62(6):862–75. doi: 10.1016/j.neuron.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Coué E. Self Mastery Through Conscious Autosuggestion. Fairford, UK: Echo Lib; 2009. [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46(4):671–9. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Egner T, Jamieson G, Gruzelier J. Hypnosis decouples cognitive control from conflict monitoring processes of the frontal lobe. NeuroImage. 2005;27(4):969–78. doi: 10.1016/j.neuroimage.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–43. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. European Journal of Neuroscience. 2008;27(3):723–9. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cerebral Cortex. 2008;18(7):1549–59. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Athwal BS, Oakley DA, Frackowiak RS. Imaging hypnotic paralysis: implications for conversion hysteria. Lancet. 2000;355(9208):986–7. doi: 10.1016/S0140-6736(00)99019-6. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. Journal of Neuroscience. 2011;31(30):11077–87. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, et al. Neural correlates of the volitional regulation of the desire for food. International Journal of Obesity. 2011;36:648–55. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Iani C, Ricci F, Baroni G, Rubichi S. Attention control and susceptibility to hypnosis. Consciousness and Cognition. 2009;18(4):856–63. doi: 10.1016/j.concog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Iani C, Ricci F, Gherri E, Rubichi S. Hypnotic suggestion modulates cognitive conflict. Psychological Science. 2006;17(8):721–7. doi: 10.1111/j.1467-9280.2006.01772.x. [DOI] [PubMed] [Google Scholar]
- Janowski V, Camerer C, Rangel A. Empathic choice involves vmPFC value signals that are modulated by social processing implemented in IPL. Social Cognitive and Affective Neuroscience. 2013;8(2):201–8. doi: 10.1093/scan/nsr086. doi: 10.1093/scan/nsr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Braffman W. Imaginative suggestibility and hypnotizability. Current Directions in Psychological Science. 2001;10(2):57–61. [Google Scholar]
- Kirsch I, Montgomery G, Sapirstein G. Hypnosis as an adjunct to cognitive-behavioral psychotherapy: a meta-analysis. Journal of Consulting and Clinical Psychology. 1995;63(2):214–20. doi: 10.1037//0022-006x.63.2.214. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences USA. 2010;107(33):14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Costantini-Ferrando MF, Alpert NM, Spiegel D. Hypnotic visual illusion alters color processing in the brain. American Journal of Psychiatry. 2000;157(8):1279–84. doi: 10.1176/appi.ajp.157.8.1279. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64(3):431–9. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cerebral Cortex. 2011;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–90. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences USA. 2004;101(17):6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeown WJ, Venneri A, Kirsch I, et al. Suggested visual hallucination without hypnosis enhances activity in visual areas of the brain. Consciousness and Cognition. 2012;21(1):100–16. doi: 10.1016/j.concog.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Mendelsohn A, Chalamish Y, Solomonovich A, Dudai Y. Mesmerizing memories: Brain substrates of episodic memory suppression in posthypnotic amnesia. Neuron. 2008;57(1):159–70. doi: 10.1016/j.neuron.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Naglatzki RP, Schlamann M, Gasser T, et al. Cerebral somatic pain modulation during autogenic training in fMRI. European Journal of Pain. 2012;16(9):1293–301. doi: 10.1002/j.1532-2149.2012.00138.x. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Halligan PW. Hypnotic suggestion and cognitive neuroscience. Trends in Cognitive Sciences. 2009;13(6):264–70. doi: 10.1016/j.tics.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213(2):135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27(37):9984–8. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences USA. 2008;105(3):1050–4. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. Journal of Neuroscience. 2010;30(32):10799–808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raz A. Hypnobo: perspectives on hypnosis and placebo. American Journal of Clinical Hypnosis. 2007;50(1):29–36. doi: 10.1080/00029157.2007.10401595. [DOI] [PubMed] [Google Scholar]
- Raz A. Hypnosis: a twilight zone of the top-down variety. Trends in Cognitive Sciences. 2011;15(12):555–7. doi: 10.1016/j.tics.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Raz A, Fan J, Posner MI. Hypnotic suggestion reduces conflict in the human brain. Proceedings of the National Academy of Sciences USA. 2005;102(28):9978–83. doi: 10.1073/pnas.0503064102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Kirsch I, Pollard J, Nitkin-Kaner Y. Suggestion reduces the Stroop effect. Psychological Science. 2006;17(2):91–5. doi: 10.1111/j.1467-9280.2006.01669.x. [DOI] [PubMed] [Google Scholar]
- Raz A, Shapiro T. Hypnosis and neuroscience: a cross talk between clinical and cognitive research. Archives of General Psychiatry. 2002;59(1):85–90. doi: 10.1001/archpsyc.59.1.85. [DOI] [PubMed] [Google Scholar]
- Raz A, Shapiro T, Fan J, Posner MI. Hypnotic suggestion and the modulation of Stroop interference. Archives of General Psychiatry. 2002;59(12):1155–61. doi: 10.1001/archpsyc.59.12.1155. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic Procedures for Social Research. revised edn. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Sacerdote P. Teaching self-hypnosis to adults. International Journal of Clinical and Experimental Hypnosis. 1981;29(3):282–99. [Google Scholar]
- Schlamann M, Naglatzki R, Greiff Ad, Forsting M, Gizewski ER. Autogenic training alters cerebral activation patterns in fMRI. International Journal of Clinical and Experimental Hypnosis. 2010;58(4):444–56. doi: 10.1080/00207144.2010.499347. [DOI] [PubMed] [Google Scholar]
- Schultz IH, Luthe W. Autogenic training: a psychophysiologic approach in psychotherapy. New York: Georg & Stratton; 1959. [Google Scholar]
- Shor RE, Orne EC. The Harvard Group Scale of Hypnotic Susceptibility, Form A. Palo Alto, CA, USA: Consulting Psychologists Press; 1962. [Google Scholar]
- Szechtman H, Woody E, Bowers KS, Nahmias C. Where the imaginal appears real: a positron emission tomography study of auditory hallucinations. Proceedings of the National Academy of Sciences USA. 1998;95(4):1956–60. doi: 10.1073/pnas.95.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Kuile MM, Spinhoven P, Linssen ACG, Zitman FG, Van Dyck R, Rooijmans HGM. Autogenic training and cognitive self-hypnosis for the treatment of recurrent headaches in three different subject groups. Pain. 1994;58(3):331–40. doi: 10.1016/0304-3959(94)90127-9. [DOI] [PubMed] [Google Scholar]
- von Winterfeldt D, Fischer GW. Multi-attribute utility theory: models and assessment procedures. In: Wendt D, Vlek CAJ, editors. Utility, Probability, and Human Decision Making. Dordrecht: the Netherlands: Reidel; 1975. pp. 47–86. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Haidt J. Hypnotic disgust makes moral judgments more severe. Psychological Science. 2005;16(10):780–4. doi: 10.1111/j.1467-9280.2005.01614.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.