Abstract
Perceived threat from outgroups is a consistent social-environmental antecedent of intergroup bias (i.e. prejudice, ingroup favoritism). The serotonin transporter gene polymorphism (5-HTTLPR) has been associated with individual variations in sensitivity to context, particularly stressful and threatening situations. Here, we examined how 5-HTTLPR and environmental factors signaling potential outgroup threat dynamically interact to shape intergroup bias. Across two studies, we provide novel evidence for a gene–environment interaction on the acquisition of intergroup bias and prejudice. Greater exposure to signals of outgroup threat, such as negative prior contact with outgroups and perceived danger from the social environment, were more predictive of intergroup bias among participants possessing at least one short allele (vs two long alleles) of 5-HTTLPR. Furthermore, this gene x environment interaction was observed for biases directed at diverse ethnic and arbitrarily-defined outgroups across measures reflecting intergroup biases in evaluation and discriminatory behavior. These findings reveal a candidate genetic mechanism for the acquisition of intergroup bias, and suggest that intergroup bias is dually inherited and transmitted through the interplay of social (i.e. contextual cues of outgroup threat) and biological mechanisms (i.e. genetic sensitivity toward threatening contexts) that regulate perceived intergroup threats.
Keywords: intergroup bias, prejudice, 5-HTTLPR, gene × environment interactions, behavioral genetics
INTRODUCTION
Intergroup bias, which manifests as prejudice toward outgroups and/or favoritism for one’s ingroup, produces pernicious social ills, such as the systematic deprivation of rights and opportunities, victimization and violence. While intergroup bias may be widely transmitted and inherited through cultural mechanisms, through stereotypes, socialization and the media (Allport, 1954; Dixon, 2008; Weisbuch et al., 2009) wide inter-individual variation in intergroup bias have also been observed (Livingston and Drwecki, 2007; Sibely and Duckitt, 2008).
Individual differences associated with intergroup bias may be regulated by individual variations in biological mechanisms, such as hormones (De Dreu et al., 2010, 2011) and fertility cycles (Navarrete et al., 2009). Notably, like many individual differences, propensity to report attitudes reflecting intergroup bias has been associated with significant levels of genetic heritability, with heritability indices ranging from 0.34 to 0.55, based on twin study research (Tesser, 1993; Olson et al., 2001; Lewis and Bates, 2010). These findings suggest that (i) intergroup bias may have a genetically-regulated component and (ii) non-genetic environmental influences may also contribute significantly to intergroup bias.
Despite the value of prior evidence from twin studies on the heritability of intergroup attitudes, the specific genes and associated endophenotypes that may regulate intergroup bias remains unknown. Identifying specific candidate genetic and environmental mechanisms that contribute to the acquisition and transmission of intergroup bias provides novel promise for developing comprehensive gene-by-environmental models for understanding how intergroup bias is inherited, transmitted, perpetuated and could potentially be reduced (Chiao et al., 2012, 2013).
Genes that regulate sensitivity and reactivity to threatening contexts may serve as promising candidate genes influencing the acquisition of intergroup bias. The experience of threat and its associated psychological states, such as anxiety, fear, uncertainty, vigilance and risk, have been implicated as consistent antecedents of intergroup bias for both dispositional and contextual/environmental models of intergroup bias. For instance, social cues and experiences that heighten perceptions of outgroups as a source of threat have been reported as contextual antecedents of prejudice and ingroup favoritism (Stephan and Stephan, 2000; Riek et al., 2006; Neuberg et al., 2011). This relationship between perceived outgroup threat and intergroup bias has been observed across diverse domains and manifestations of potential outgroup threat that converge upon subjective experiences of anxiety, fear or uncertainty. Such outgroup threats include competition for resources and opportunities (LeVine and Campbell, 1972; Esses et al., 2001), danger or harm (Schaller et al., 2003), symbolic or value threats (Sears, 1988; Greenberg and Kosloff, 2008) or pathogenic infection threats (Schaller and Park, 2011). Likewise, individual differences and personality dispositions predictive of intergroup bias typically reflect inter-individual variations in sensitivity or tolerance of threat and uncertainty. Compared to those exhibiting more tolerant personalities, individuals with more ‘prejudiced personalities’ (see Allport, 1954) typically exhibit greater levels of sensitivity to fear conditioning (Livingston and Drwecki, 2007), right-wing authoritarianism, which is associated with personal insecurity (Altemeyer, 1988; Van Hiel et al., 2004), heightened susceptibility to infection (Navarrete et al., 2007), and intolerance of ambiguity, uncertainty and unpredictability (Altemeyer, 1988; Kruglanski et al., 2006; Sibely and Duckitt, 2008).
Given the central role of the subjective experience of threat in intergroup bias, we propose that a functional polymorphism, 5-HTTLPR, of the serotonin transporter gene (SLC6A4) may act as a genetic mechanism facilitating the acquisition of intergroup bias in the presence of outgroup threat. The polymorphism is represented by two allele variants, short (S) or long (L), reflecting the length of the promoter region of SLC6A4, which influences the regulation of serotonergic neurotransmission. The less transcriptionally efficient S-allele of the polymorphism is associated with higher concentrations of serotonin remaining in the synaptic cleft compared to the more efficient L-allele, and this difference in the reuptake of serotonin has been associated with variations in affective processing based on genotype (Canli and Lesch, 2007). Behavioral genetics has implicated this polymorphism in a number of anxiety and threat-relevant outcomes. Specifically, those possessing at least one copy of the S-allele (S/S and S/L) tend to exhibit a psychological phenotypic profile of heightened sensitivity and reactivity to threatening contexts and stimuli, evidenced by greater propensities for anxiety, vigilance, fear conditioning, risk aversion and collectivistic values (Canli and Lesch, 2007; Crisan et al., 2009; Kuhnen and Chiao, 2009; Lonsdorf et al., 2009; Chiao and Blizinsky, 2010; Caspi et al., 2010). People who carry the S-allele also exhibit greater reactivity to fear-relevant stimuli in the amygdala (Hariri et al., 2002; Munafo et al., 2008), a brain structure previously associated with negative implicit racial biases (Phelps et al., 2000; Cunningham et al., 2004).
Importantly, 5-HTTLPR has been implicated in gene × environment interactions for anxiety and stress-related outcomes. Most notably, those carrying the S-allele of 5-HTTLPR, who may be disposed to greater contextual sensitivity, tend to exhibit greater symptomatology of anxiety and depressive disorders with heightened exposure to threatening and stressful events (see Capsi et al., 2010; Karg et al., 2011). Yet, recent evidence suggests that possessing the S-allele of 5-HTTLPR may not only confer greater levels of anxiety in response to stressful or adverse environments in a unidirectional manner, but may also promote more positive affective and behavioral outcomes in the absence of adverse experiences or in the presence of relatively positive environmental influences (van Ijzendoorn et al., 2012). For instance, magnitude of stressful life events (SLE) has been positively associated with greater levels of neuroticism among S-allele carriers, such that S-allele carriers exhibit greater levels of neuroticism compared with L/L-allele carriers when experiencing high SLEs, but also exhibit lower levels of neuroticism compared with L/L-allele carriers when experiencing low SLEs (Pluess et al., 2010; Kuepper et al., 2012). Yet, in these studies, there was an absence of a relationship between SLEs and neuroticism among L/L-allele carriers. Together, these findings suggest that 5-HTTLPR may reflect a differential susceptibility or plasticity to the presence (or absence) of environmental threats and stressors, such that S-allele carriers, compared to those with two L-alleles, may exhibit greater or lower levels of threat-related psychological outcomes in response to negative or positive environments, respectively.
Similarly, we predicted a gene × environment interaction for intergroup bias and prejudice, such that greater exposure to signals of outgroup threat would be especially predictive of intergroup bias among individuals carrying one or two copies of the S-allele. One well-studied environmental moderator of intergroup bias is the quality of prior contact one has experienced with outgroup members. Greater quality of prior contact has been associated with greater levels of intergroup tolerance (see Pettigrew and Tropp, 2006), though negative quality of intergroup contact may be a particularly strong determinant of negative intergroup attitudes and prejudice (Barlow et al., 2012). Thus, we predicted that among people possessing the S-allele, exposure to negative or threatening intergroup contact with a group will be especially predictive of bias against that group.
Another critical moderator associated with the broader environment is one’s subjective perception and expectation of threat and danger from the social environment. Though particular outgroups are associated with danger, such groups may be evaluated especially negatively among individuals who maintain internal working models or active expectations about the possibility of being victimized or exploited by others. Indeed, individuals who believe the social world is full of danger typically exhibit greater biases toward threat-cuing groups (Schaller et al., 2003; Miller et al., 2010). For instance, Miller et al. (2010) revealed that belief in a dangerous world interacted with heightened situational fear to shape White participants’ tendency of rating racially ambiguous targets that cue threat (i.e. having angry faces) as outgroup members (i.e. Black rather than White). Similarly, we predicted that perceptions of a dangerous world will be predictive of biases against groups specifically associated with threat to a greater extent among people carrying the S-allele compared with those carrying two L-alleles.
Overall, support for these hypotheses would reveal: (i) for the first time, to our knowledge, specific genetic–environment mechanisms for the acquisition and transmission of intergroup bias and prejudice1 and (ii) that intergroup bias is transmitted and inherited through the convergence of social influences (i.e. negative intergroup contact) and genetic mechanisms that may regulate and heighten subjective perceptions of threat.
Rather than 5-HTTLPR and environmental factors independently influencing intergroup bias, genotype may alternatively predispose individuals to experience more negative intergroup contact or dangerous perceptions of the environment, which may ultimately shape intergroup bias. Such gene–environment correlations (see Scarr and McCartney, 1983) may be an alternative mechanism in which genetic and environmental influences shape intergroup bias, and will be tested by examining whether 5-HTTLPR genotype is associated with variations in prior quality of intergroup contact and beliefs in a dangerous world.
STUDY 1
Participants and procedure
We recruited 116 Caucasian-American participants (73 females; Age M = 22.42, s.d. = 9.66) to complete measures of intergroup bias toward a variety of outgroups, quality of prior intergroup contact and belief in a dangerous world.2 As a measure of evaluative biases, participants completed feeling thermometer ratings (0–100) reflecting global evaluations of target groups. A range of target groups were included to reflect the ingroup (i.e. White Americans), ethnic groups associated with danger and threat (i.e. Blacks, Arabs), an ethnic group typically unassociated with danger (i.e. Asian-Americans), a non-ethnic group associated with danger (i.e. people with schizophrenia) and ethnic groups for whom prominent stereotypes may not be available in American culture (i.e. Bangladeshis, Singaporeans). Feeling thermometer ratings for each outgroup was subtracted from the ingroup (White Americans) to produce an index of evaluative intergroup bias toward each outgroup. To verify the extent to which target groups were associated with threat, participants completed semantic differential ratings of threat-relevant traits (i.e. dangerous-safe, immoral-moral, dishonest-honest, etc.) associated with each target group. Participants rated their level of global quality of prior contact with each of these groups on a seven-point scale (‘very positive’ to ‘very negative’). Similar one-item measures of global quality of intergroup contact have been predictive of intergroup bias in prior research (see Pettigrew and Tropp, 2006; Barlow et al., 2012). Participants also completed the Belief in a Dangerous World Scale (BDW; Altemeyer, 1988), and the Attitude Towards Blacks Scale (ATB; Brigham, 1993), as an additional measure of evaluative and discriminatory biases toward Blacks.
Saliva samples were collected using Oragene Saliva DNA Self-Collection Kits (DNA Genotek). DNA extraction and 5-HTTLPR genotyping were conducted by ACGT, Inc. (Wheeling, IL). All saliva DNA samples were genotyped in one batch after the completion of data collection for Studies 1 and 2. DNA was extracted from each kit using the Oragene DNA purification reagent as per manufacturer’s instructions. DNA concentrations were evaluated using spectroscopy (NanoDrop Technologies, USA). Each DNA sample was PCR amplified for the 5HTT repeat region target with the forward primer (5′-GCCAGCACCTAACCCCTAAT-3′) labeled with 6-FAM (6-carboxyfluorescein) and a reverse primer (5′-GAGGGACTGAGCTGGACAACCAC-3′). A PCR of 20 μl consisting of 1.5 μl of genomic DNA from the test sample, PCR buffer, 1 mM each of forward and reverse primers, 10 mM deoxyribonucleotides, KapaTaq polymerase and 50 mM MgCl2 was performed using a 7900 thermocycler (Applied Biosystems Inc.). Water was included in each assay as a negative control. Cycling conditions included an initial 15 min denaturation at 95°C, and 35 cycles of 94°C (30 s), 60°C (60 s), 72°C (60 s), and a final extension of 72°C for 10 min. PCRs were genotyped with an ABI 3730xl Genetic Analyzer (Applied Biosystems Inc.) and normalized with GeneScan 600 LIZ (Applied Biosystems, Inc.) size standards run on each sample. The genotype data was analyzed using GeneMapper ID (Applied Biosystems, Inc.).
Results
The distribution of genotypes for the 5-HTTLPR polymorphism consisted of 71 participants in the S-group (S/S and S/L genotypes) and 45 participants in the L/L-group (Table 1). Genotype distributions were in Hardy–Weinberg Equilibrium (χ2 = 2.86, n.s.).
Table 1.
Distribution of participants based on genotype and gender
| Full sample | Males | Females | |
|---|---|---|---|
| Study 1 | |||
| L/L-group | 45 | 16 | 29 |
| S-group | 71 | 27 | 44 |
| Study 2 | |||
| L/L-group | 24 | 9 | 15 |
| S-group | 39 | 14 | 25 |
There were no significant main effects of 5-HTTLPR genotype on measures of intergroup bias or prior quality of contact with any group, or BDW scores (P’s > 0.10).
Gene–environment interactions between 5-HTTLPR and prior quality of contact on intergroup bias were tested using multiple regression. Genotype (dummy coded: 0 = L/L, 1 = S/S and S/L), standardized quality of contact with a specific outgroup and their interaction term were used to predict intergroup bias toward the respective outgroup. Separate models were tested for each target outgroup. As hypothesized, a pattern of gene–environment interactions on intergroup bias was observed. The 5-HTTLPR genotype and quality of prior contact with a group significantly interacted to influence intergroup biases toward Blacks and Arabs, and also toward Singaporeans and Bangladeshis, despite the lack of prominent stereotypes toward these two groups (Table 2). There were no significant interactions between genotype and quality of contact on biases toward Asian-Americans or people with schizophrenia.
Table 2.
Correlation between measures of intergroup bias and prior quality of contact with target outgroups as a function of 5-HTTLPR genotype, and statistics for tests of genotype-contact interactions. Greater values for contact reflect more negative quality of prior contact, and greater values for intergroup bias measures reflect greater levels of intergroup bias toward the target group. *P < 0.05, **P < 0.005
| Measure of intergroup bias | S-group | L/L-group | Model F and P | Interaction β and P |
|---|---|---|---|---|
| ATB | r = 0.52** | r = −0.11 | F = 7.67, P < 0.001 | β = 0.55, P = 0.003 |
| Black bias | r = 0.51** | r = 0.33* | F = 10.89, P < 0.001 | β = 5.34, P = 0.06 |
| Asian-American bias | r = 0.44** | r = 0.44** | F = 9.31, P < 0.001 | β = −2.17, n.s. |
| Arab bias | r = 0.50** | r = 0.04 | F = 6.53, P < 0.001 | β = 9.67, P = 0.06 |
| Singaporean bias | r = 0.42** | r = 0.01 | F = 5.11, P = 0.002 | β = 7.57, P = 0.02 |
| Bangladeshi bias | r = 0.47** | r = 0.12 | F = 6.56, P < 0.001 | β = 6.14, P = 0.08 |
| Schizophrenic bias | r = 0.26* | r = 0.48** | F = 5.37, P = 0.002 | β = −7.31, n.s. |
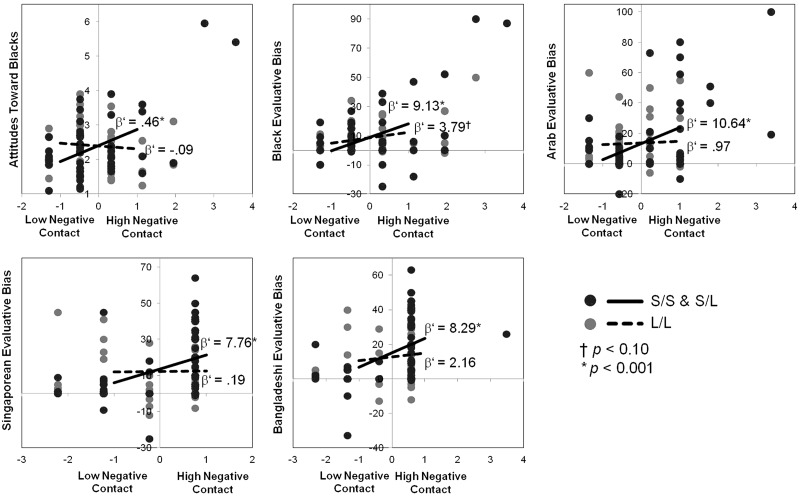
For each of the measures reflecting a significant gene–environment interaction, simple slopes analysis (Aiken and West, 1991) was used to test whether the relationship between outgroup quality of contact and intergroup bias against the outgroup was significantly stronger among the S-group compared with the L/L-group for each of the target outgroups for whom a significant or marginally significant 5-HTTLPR-contact interaction was observed (Figure 1).3 Simple slopes (β’) analyses revealed that for the S-group, negative prior contact with a specific outgroup was significantly associated with intergroup biases against the respective outgroup (ATB: β’ = 0.46, P < 0.001; Black evaluative bias: β’ = 9.13, P < 0.001; Arab evaluative bias: β’ = 10.64, P < 0.001; Singaporean evaluative bias: β’ = 7.76, P < 0.001; Bangladeshi evaluative bias: β’ = 8.29, P < 0.001). Yet, for the L/L-group, the analyses revealed that negative contact with a specific outgroup were more weakly related to intergroup bias, or not significantly related (ATB: β’ = −0.09, n.s.; Black evaluative bias: β’ = 3.79, P = 0.097; Arab evaluative bias: β’ = 0.97, n.s.; Singaporean evaluative bias: β’ = 0.19, n.s.; Bangladeshi evaluative bias: β’ = 2.16, n.s.). These analyses also revealed a pattern suggesting that while the presence of negative quality of contact was related to more intergroup biases among the S-group (compared with the L/L-group), low levels of negative contact (or higher of positive contact) was related to less intergroup biases among the S-group (compared with the L/L-group).
Fig. 1.
Interactions of 5-HTTLPR genotype and quality of contact with outgroups on intergroup bias. For each of the comparisons below, a genotype x quality of contact interaction was observed, such that greater negative quality of prior contact with an outgroup was more predictive of intergroup bias among the S-group compared with the L/L-group. Negative contact (x-axis) is represented in Z-score values. Dots reflect participant scatter plot data. Lines reflect simple slopes for the interactions at low (−1 s.d.) and high (+1 s.d.) levels of negative contact.
To determine whether the relationship between quality of contact and intergroup bias is stronger among the S-allele group broadly across all outgroups rather than for individual outgroups, correlations between contact and measures of bias for each outgroup were aggregated into a summary correlation coefficient. Each correlation coefficient of contact and bias toward an outgroup was transformed into its respective Fisher’s exact Z value, and was weighted according to the variance of each Z value. These values were averaged among the S- and L/L-group before being converted into the respective Pearson correlation coefficient that reflects a single summary correlation coefficient of the relationship between quality of contact and intergroup bias across all outgroups for the S- and L/L-groups. Among the L/L-group, the summary estimate of the correlation between negative prior contact and intergroup bias toward all groups was significant, r = 0.21, P = 0.0004, 95% confidence interval (CI) [0.10–0.33]. Among the S-group, the summary estimate of the correlation was greater, r = 0.44, P < 0.0001, 95% CI [0.37–0.52]. Consistent with meta-analyses summarizing the relationship between prior contact and intergroup bias (see Pettigrew and Tropp, 2006; Barlow et al., 2012), our findings suggest an overall significant relationship between negative quality of contact and intergroup bias regardless of 5-HTTLPR genotype. Yet, this general relationship between prior contact and intergroup bias is stronger among the S-group, supporting the notion that S-allele carriers may exhibit greater plasticity in intergroup bias in response to the presence or absence of environmental threats.
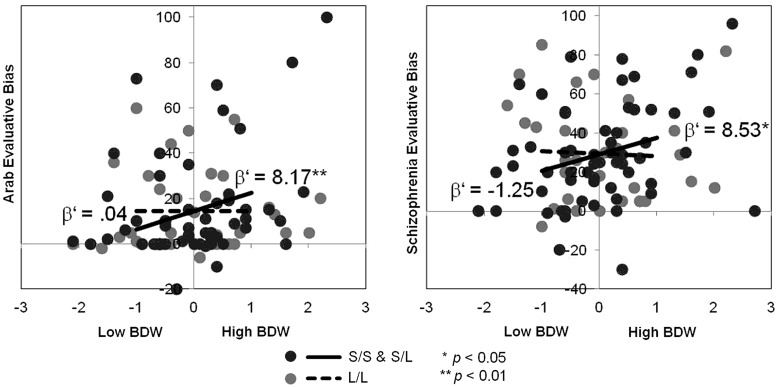
Interactions between 5-HTTLPR and BDW on intergroup bias toward threatening groups were similarly tested. An analysis of variance revealed that the groups varied in semantic differential ratings, F(3, 267) = 56.40, P < 0.001, such that the ingroup (White-Americans) was rated less threatening than all other groups, P < 0.01, except Asian-Americans. Arabs and people with schizophrenia were associated with the most threat-relevant attributes compared with any other group, P’s < 0.05. Thus, interactions between 5-HTTLPR and BDW were tested for biases against Arabs and people with schizophrenia by regressing measures of intergroup bias toward these groups on dummy-coded genotype, standardized BDW and their interaction term. Interactions of 5-HTTLPR and BDW were observed for intergroup biases toward Arabs, F(3, 87) = 2.44, P = 0.07, β = 8.13, P = 0.09, and people with schizophrenia, F(3, 90) = 2.27, P = 0.09, β = 9.78, P = 0.07 (Figure 2). Simple slopes analyses revealed that for the S-group, higher BDW was associated with greater biases against Arabs, β’ = 8.17, P = 0.008, and greater biases against people with schizophrenia, β’ = 8.53, P = 0.01. Yet, among the L/L-group, there was no significant relationship between BDW and intergroup biases against Arabs, β’ = 0.04, n.s., or people with schizophrenia, β’ = -1.25, n.s. There were no significant genotype × BDW interactions on bias toward any other group, n.s.
Fig. 2.
Interactions of 5-HTTLPR genotype and BDW on intergroup bias. Higher BDW was more predictive of intergroup bias among the S-group compared with the L/L-group. This gene x environment interaction was selectively observed only for attitudes toward the two groups rated to be the most threatening (Arabs and people with schizophrenia). BDW (x-axis) is represented in Z-score values. Dots reflect participant scatter plot data. Lines reflect simple slopes for the interactions at low (−1 s.d.) and high (+1 s.d.) levels of BDW.
Study 1 discussion
Support for our hypotheses provides novel evidence for the following conclusions: (i) 5-HTTLPR may represent a candidate genetic mechanism influencing the acquisition of intergroup bias. (ii) Intergroup bias may be regulated by gene × environment interactions, in which those with heightened genetically modulated susceptibility or plasticity to threat may exhibit heightened intergroup bias when exposed to social cues and experiences signaling outgroup threat, yet also exhibit lower levels of intergroup bias when exposed to relatively more positive signals from outgroups.
Though these results provide initial support of our hypotheses, there are some limitations. First, while these findings demonstrate a novel association between the serotonin transporter gene and evaluative intergroup bias, they do not necessarily generalize to a genetic contribution to intergroup bias in behavior, such as discrimination. Second, Study 1 measured the role of recollected past intergroup contact on intergroup bias, without situating participants within actual intergroup contexts. Finally, given the relatively limited sample size, a conceptual replication would further confirm the validity of the proposed gene × environment interaction on intergroup bias.
STUDY 2
To remedy these concerns, a second study was conducted that employed a minimal group paradigm (MGP; Tajfel et al., 1971) to expose participants to members of novel, arbitrarily defined outgroups. Because minimal groups are arbitrary in nature, there are no preexisting stereotypes, biases or histories of contact with these groups. Thus, participants’ acquisition of biases toward these groups can be examined by introducing opportunities for threatening interactions with the groups without contaminating influences from preexisting experiences and stereotypes associated with the groups. To examine whether 5-HTTLPR may modulate the acquisition of intergroup bias against threatening outgroups, participants in Study 2 were exposed to interactions with members of two minimal groups (threatening and non-threatening), before discriminatory behaviors toward these groups were measured. Participants from Study 1 were re-recruited for Study 2, since their genotypes were already known and measures of moderating variables (i.e. BDW, prior outgroup contact) had already been collected.
We predicted an interaction between 5-HTTLPR and BDW on discriminatory behavior, such that BDW will be predictive of discrimination selectively toward a minimal group associated with threatening characteristics to a greater extent among the S-group relative to the L/L-group. This finding would conceptually replicate the genotype × BDW interactions on selective biases against real outgroups that are associated with threat (i.e. Arabs and schizophrenics) observed in Study 1, but using a novel group constructed during the experiment to elicit associations of threat. Additionally, we also sought to test a replication of the interaction between 5-HTTLPR and prior contact with outgroups measured in Study 1 (i.e. ethnic outgroups and people with schizophrenia) on new measures of intergroup bias assessing social distance toward these groups administered in Study 2.
Participants and procedure
Sixty-three participants from Study 1 returned to complete Study 2 (40 females; Age M = 19.53, s.d. = 1.29). There were no significant differences in 5-HTTLPR allele frequencies or on any measures of intergroup bias measured in Study 1 between those who did and did not return for Study 2, P’s > 0.10.
Participants were ostensibly assigned to one of three minimal groups based on their preferences for randomly generated inkblot patterns, and provided instructions for the Trust Game (Berg et al., 1995). Participants were informed they would be playing the game with ostensible members of the two minimal outgroups via a computer network. Participants were assigned to play as the investor (first-mover) for every round of the Trust Game and that points earned across the rounds by players would be used as raffle entries for gift certificates. Importantly, participants were also informed that those assigned to be investors and receivers (the second mover position) would be drawing from separate raffles, to minimize potential competitive mindsets among the participants toward the receivers or beliefs that being generous may undermine the participants’ own probability of winning raffles.
The Trust Game was modified in this experiment to serve as a manipulation of outgroup threat. Participants played eight rounds with members from each of the two minimal outgroups in a randomized order, interacting with a new player every round. In each round, participants were shown a label identifying the group the other player belongs to, as well as an individual player number. To reduce potential confusion about players from the two outgroups, the player numbers for the two outgroups did not overlap. In each round, participants decided how to split 10 points with receiver. The points ‘invested’ in the receiver were tripled, and the ostensible receiver then decided how much of the tripled amount to return to the participant. Exposure to signals of potential outgroup threat was simulated by manipulating how members of each outgroup played the Trust Game. While members of the non-threatening outgroup generally returned around 40–55% of the participants’ tripled investment and never defected with the participants’ investments, members of the threatening outgroup played in a less predictable and more untrustworthy manner, defecting with all of the participants’ investments during four of the eight rounds to elicit feelings of threat and betrayal. On the non-defecting rounds, behaviors of the threatening outgroup resembled the non-threatening group.
Following all 16 rounds of the game, participants completed two separate resource allocation matrices, in which they decided how to split 10 bonus points between a member of their ingroup and a novel member of the threatening group, and between another member of their ingroup and a novel member of the non-threatening group. Neither of the outgroup members involved in the decisions to split bonus points had been encountered during the trust game. Participants also completed semantic differential scales to measure associations of threat (e.g. risky-safe, untrustworthy-trustworthy, dishonest-honest, etc.) with the threatening and non-threatening outgroup. Finally, two surveys of desired social distance from ethnic outgroups (Wolsko et al., 2006) and people with schizophrenia (Link et al., 1999) were included to further replicate the relationship between quality of contact and intergroup bias observed in Study 1, using new measures of intergroup bias reflecting avoidance and social distance.
Results
The sample composition based on genotype is outlined in Table 1. Genotype distributions were in Hardy–Weinberg Equilibrium (X2 = 2.21, n.s.).
As expected, the threatening group was perceived as being more threatening than both the ingroup, t(59) = 6.33, P < 0.001, and the non-threatening group, t(59) = 4.89, P < 0.001, on semantic differential ratings.
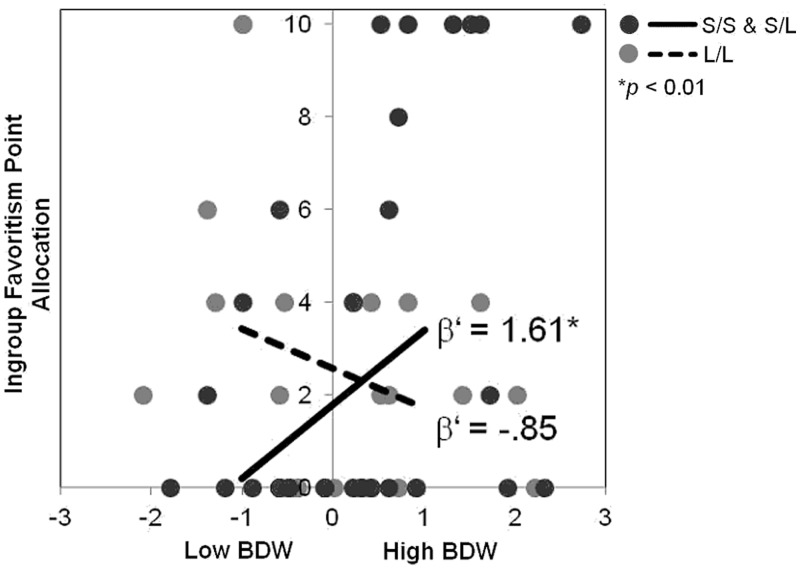
To test the interaction of genotype and BDW on discriminatory behaviors toward the threatening outgroup, allocation decisions toward the threatening outgroup were regressed on 5-HTTLPR genotype (dummy coded), standardized BDW scores from Study 1 and their interaction term. Consistent with the findings of Study 1, there was a significant interaction of BDW and 5-HTTLPR on intergroup bias, F(3, 58) = 3.05, P = 0.04, R2 = 0.14, β = 2.46, P = 0.009 (Figure 3). Simple slopes analyses revealed that for the S-group, greater BDW was associated with allocating fewer points to a novel member of the threatening group relative to an ingroup member, β’ = 1.61, P = 0.008. Conversely, for those with the L/L genotype, BDW was not associated with allocations decisions, β’ = −0.85, n.s. Testing an identical model for allocation decisions for a member of the non-threatening group did not yield a significant interaction of genotype and BDW, F(3, 58) = 1.09, n.s., R2 = 0.05, β = 0.13, n.s.
Fig. 3.
Interaction of 5-HTTLPR and BDW on intergroup bias in Study 2. BDW was associated with greater resource allocations favoring a member of the ingroup relative to a novel member of the threatening group for only the S-group, but not the L/L-group. Allocation decision (y-axis) reflects surplus points distributed to the ingroup member over the outgroup member (zero reflects an equal distribution). BDW (x-axis) is represented in Z-score values. Dots reflect participant scatter plot data. Lines reflect simple slopes for the interactions at low (−1 s.d.) and high (+1 s.d.) levels of BDW.
Since the ethnic social distance scale (Wolsko et al., 2006) measures responses toward ‘ethnic groups’ rather than a specific group, participants’ ratings of prior negative contact with ethnic outgroups from Study 1 (i.e. Blacks, Arabs, Asian-Americans, Singaporeans, Bangladeshis) were averaged to form a composite of negative quality of prior ethnic intergroup contact. Social distance scores were regressed on this composite, genotype and their interaction term. Similarly, social distance from schizophrenia scores were regressed on Study 1 schizophrenia contact scores, genotype and their interaction term. Though the interaction terms for these models did not reach significance, an overall pattern consistent with Study 1 was observed, such that among the S-group, more negative prior contact was predictive of greater desire for social distance from ethnic outgroups, r(37) = 0.35, P = 0.03, and people with schizophrenia, r(37) = 0.48, P = 0.002., but among the L/L-group, prior contact was not associated with social distance from ethnic outgroups, r(21) = 0.11, n.s., or people with schizophrenia, r(21) = 0.22, n.s.
Discussion
Overall, the results of Study 2 supports and extends the findings of Study 1 by demonstrating that 5-HTTLPR interacts with perceived or expected threat from one’s social environment to facilitate the acquisition of intergroup bias among S-allele carriers. Importantly, this gene × environment interaction was observed even within a minimal group context in which participants of both genotype groups encountered identical cues of outgroup threat and no prior expectations about the outgroups existed. Analogous to Study 1, 5-HTTLPR and perceptions of a threatening social environment moderated bias directed specifically toward a novel individual from a minimal group that participants associated with threat. Importantly, these biases were expressed through discriminatory behaviors that would have negative consequences on the outcomes and opportunities of their targets, though our resource allocation measure did not allow us to determine whether discrimination was motivated by ingroup favoritism or outgroup derogation. Further supporting and extending Study 1’s findings, the relationship between negative prior intergroup contact with real groups and heightened intergroup bias among the S-group was again observed, but on measures of outgroup avoidance. One reason that the 5-HTTLPR × genotype interaction for these measures of social distance may not have reached statistical significance, despite exhibiting a pattern of results similar to Study 1, may be due to the smaller sample size of the participants who returned to complete Study 2.
The design of Study 2 also offers some potential insights into the role of BDW in its interaction with 5-HTTLPR genotype on discriminatory bias. Though the threatening outgroup consisted of a mix of trustworthy and untrustworthy players on the Trust Game, discrimination was measured specifically toward a single new member of this outgroup who was not encountered in the Trust Game. Thus, whether this target should be considered and treated as untrustworthy, or treated comparably with an ingroup member may have been ambiguous to participants. Miller et al. (2010) identified that those with greater levels of BDW who experienced heightened fear were more likely categorize ambiguous targets that cue threat as outgroup members. Similarly, in our study, internal expectations about exploitation or harm from others (BDW) and possessing the S-allele may interact to contribute to whether a novel member of the threatening group is disambiguated as threatening, and ultimately subjected to discrimination. Though the current studies demonstrate that 5-HTTLPR genotype and perceptions of threat from others may predict intergroup bias, investigating whether this interaction also affects more fundamental processes involved in group categorization would be a promising topic for future inquiry.
GENERAL DISCUSSION
Together, our findings provide direct and novel evidence that intergroup bias in evaluation, desire for avoidance and discriminatory behavior is a result of the interaction of both genetic and environmental mechanisms. While perceived threat, anxiety and uncertainty associated with outgroups are often sources of intergroup bias, individuals genetically predisposed to heightened sensitivity and reactivity to these experiences may develop intergroup bias more readily when exposed to intergroup threat cues.
One potential alternative mechanism for these findings is that the S-group may be more likely to encounter situations signaling outgroup threat, or predisposed to interpreting non-threatening or ambiguous intergroup interactions as threatening. These gene–environment correlations were not supported by our findings since we observed no difference in quality of prior contact or perceptions of a dangerous world as a function of genotype. Moreover, Study 2 exposed participants to identical signals of outgroup threat in the minimal group context, yet produced different patterns of intergroup bias as a function of genotype. Our findings suggest that the S-group may appraise or react to cues of outgroup threat differently when they are encountered, rather than experiencing a greater likelihood of encountering or provoking such cues from the social environment. Yet given the important role that personality and individual differences play on shaping how individuals navigate their social environment, and how others may respond to such traits (see Scarr and McCartney, 1983; Funder, 1991), future studies should not dismiss the potential role of gene–environment correlations on group processes.
Despite the relationship between 5-HTTLPR and intergroup bias in our studies, the serotonin transporter gene should not be conceptualized as a gene for intergroup bias or the ‘prejudice gene’, given the likely contribution of multiple genetic mechanisms to intergroup bias, and the diverse phenotypes and outcomes associated with 5-HTTLPR. Furthermore, the relationship between 5-HTTLPR and intergroup bias emerges only after sufficient exposure to experiences that signal outgroup members as a potential source of threat. Moreover, S-allele carriers may exhibit the lowest levels of intergroup bias in the absence of clear signals of outgroup threat. Our findings suggest that heightened intergroup bias is not a direct or fixed psychological phenotype of 5-HTTLPR genotype. Rather, 5-HTTLPR may have an indirect effect on intergroup bias by influencing more basic processes unrelated to intergroup evaluation. Such processes may include the modulation of physiological and neurobiological mechanisms underlying affective conditioning and emotion-based learning (Lonsdorf et al., 2009; Klucken et al., 2012a,b) or attention to contextual emotional cues, which has downstream psychological consequences on how cues of outgroup threat are processed, as well as their motivational salience. Our findings are consistent with prior work suggesting that the S-allele is not always deterministic of negative psychological outcomes, and that the supposed ‘deficits’ associated with the S-allele may facilitate adaptive responses to contextual demands (see Homberg and Lesch, 2011; van Ijzendoorn et al., 2012). Finally, the label of being prejudiced is a highly stigmatizing mark in multicultural societies. Framing the S-allele as a genetic marker for prejudice poses the risk of portraying and stigmatizing S-allele carriers as incorrigible and essentialized bigots.
The present findings lend support to the general notion that 5-HTTLPR genotype may be associated with differential susceptibility to environmental influences (van Ijzendoorn et al., 2012), but critically extends these findings to understanding plasticity of intergroup bias. For instance, in Study 2, S-allele carriers who expect low levels of harm and exploitation from others (i.e. low BDW) were most likely to provide an equal split of resources between an ingroup member and novel member of the threatening group. Those with genetically predisposed sensitivity to environmental influences who chronically perceive the world as relatively stable, secure and full of trustworthy people may be more likely to give ‘the benefit of the doubt’ and fair treatment to a novel individual, even if he or she shares group membership with others who are deemed untrustworthy or threatening. One promising avenue of future research examining the relationship between 5-HTTLPR and intergroup bias may be examining whether interventions or methodologies to reduce prejudice and intergroup bias, such as exposure to positive outgroup exemplars (see Plant et al., 2009), may be asymmetrically more efficacious among S-allele carriers. Another important question to address may be determining what types of interventions and social experiences may be especially effective for eliciting changes in intergroup biases among those possessing two L-alleles, who may exhibit lower levels of plasticity of intergroup bias in response to social cues and experiences.
Though intergroup attitudes may exhibit moderate to high levels of heritability through genes (Tesser, 1993; Olson et al., 2001; Lewis and Bates, 2010), the present findings reveal that the biological inheritance of the propensity for intergroup bias may result from the transmission of genetic mechanisms that regulate the processing of threat. Likewise, intergroup bias may be transmitted culturally through shared social environments and cultural messages that portray or cue outgroups as a source of threat. Consequently, inter-individual and cross-situational variations in intergroup bias may be determined by the joint contributions of these dual mechanisms for the cultural and genetic inheritance/transmission of intergroup threat perceptions.
ACKNOWLEDGEMENTS
This work was funded by the Kellogg School of Management, APA Dissertation Research Award, SPSSI Grant in Aid Award, and NSF/NRF EAPSI fellowship. The preparation of this article was partially supported by Academic Research Fund Tier 2 grant (MOE2012-T2-1-051) from the Singapore Ministry of Education awarded to Y.Y.H. and J.Y.C. We would like to thank G. Bodenhausen, J. Richeson, V. Mathur and M. Schurgin for their helpful comments and support.
Footnotes
1 One exception is that Forbes et al. (2011) have shown genetic influences on gender biases among brain lesion samples.
2 Due to logistical constraints, 24 participants did not complete measures pertaining to Arab targets (feel thermometer, semantic differential, quality of contact), and 21 of these same participants did not complete the ATB Scale and BDW Scale. One additional participant did not complete the BDW Scale.
3 Some of the genotype × quality of contact interactions included data points simultaneously reflecting extremely high negative prior contact and high intergroup bias (i.e. for ATB and Arab Evaluative Biases) (Figure 1). When these data points are excluded from analysis, the interaction on the respective measure of intergroup bias is no longer significant. But these data points were included in the analysis since: (i) there is no indication they represent erroneous responses; (ii) they conform to the broader trend of the data within the graph (positive association between negative contact and intergroup bias), as well as the same trends observed in the accompanying graphs; (iii) they are consistent with findings from prior research on intergroup contact (i.e. those who experienced extremely negative contact with an outgroup are expected to harbor more extreme biases against that group); and (iv) given the sample size within the S-group and L/L-group, the removal of any data points is likely to attenuate sensitivity for detecting effects.
REFERENCES
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Allport GW. The Nature of Prejudice. Reading, MA: Addison-Wesley; 1954. [Google Scholar]
- Altemeyer B. Enemies of Freedom: Understanding Right-Wing Authoritarianism. San Francisco: Jossey-Bass; 1988. [Google Scholar]
- Barlow FK, Paolini S, Pedersen A, et al. The contact caveat: negative contact predicts increased prejudice more than positive contact predicts reduced prejudice. Personality and Social Psychology Bulletin. 2012;38(12):1629–43. doi: 10.1177/0146167212457953. [DOI] [PubMed] [Google Scholar]
- Berg J, Dickhaut J, McCabe K. Trust, reciprocity, and social history. Games and Economic Behavior. 1995;10:122–42. [Google Scholar]
- Brigham JC. College students’ racial attitudes. Journal of Applied Psychology. 1993;23:1933–67. [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10(9):1103–9. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167(5):509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene (5-HTTLPR) Proceedings of the Royal Society B: Biological Sciences. 2010;277(1681):529–37. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Cheon BK, Bebko GM, Livingston RW, Hong YY. Gene x environment interaction in social cognition. In: Fiske ST, Macrae CN, editors. Sage Handbook of Social Cognition. Thousand Oaks: Sage Publications; 2012. [Google Scholar]
- Chiao JY, Cheon BK, Pornpattanangkul N, Mrazek AJ, Blizinsky KD. Cultural neuroscience: progress and promise. Psychological Inquiry. 2013;24:1–19. doi: 10.1080/1047840X.2013.752715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan LG, Pana S, Vulturar R, et al. Genetic contributions of the serotonin transporter to social learning of fear and economic decision making. Social Cognitive and Affective Neuroscience. 2009;4:399–408. doi: 10.1093/scan/nsp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby JC, Gore JC, Banaji MR. Separable neural components in the processing of Black and White faces. Psychological Science. 2004;15(12):806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon TL. Network news and racial beliefs: exploring the connection between national television news exposure and stereotypical perceptions of African Americans. Journal of Communication. 2008;58:321–37. [Google Scholar]
- Esses VM, Dovidio JF, Jackson LM, Armstrong TL. The immigrant dilemma: the role of perceived competition, ethnic prejudice, and national identity. Journal of Social Issues. 2001;57(3):389–412. [Google Scholar]
- Forbes CE, Poore JC, Barbey AK, et al. BDNF polymorphism-dependent OFC and DLPFC plasticity differentially moderates implicit and explicit bias. Cerebral Cortex. 2011;22(11):2602–9. doi: 10.1093/cercor/bhr337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder DC. Global traits - a neo-allportian approach to personality. Psychological Science. 1991;2(1):31–39. [Google Scholar]
- Greenberg J, Kosloff S. Terror management theory: implications for understanding prejudice, stereotyping, intergroup conflict, and political attitudes. Social and Personality Psychology Compass. 2008;2(5):1881–94. [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. Journal of Personality and Social Psychology. 1998;74(6):1464–80. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lsch K-P. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–9. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Alexander N, Schweckendiek J, et al. Individual differences in neural correlates of fear conditioning as a function of 5-HTTLPR and stressful life events. Social Cognitive and Affective Neuroscience. 2012a;8(3):318–25. doi: 10.1093/scan/nss005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, et al. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Human Brain Mapping. 2012b doi: 10.1002/hbm.22085. doi: 10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglanski AW, Pierro A, Mannetti L, De Grada E. Groups as epistemic providers: need for closure and the unfolding of group-centrism. Psychological Review. 2006;113:84–100. doi: 10.1037/0033-295X.113.1.84. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, Wielpuetz C, Alexander N, Mueller E, Grant P, Hennig J. 5-HTTLPR S-allele: a genetic plasticity factor regarding the effects of life events on personality? Genes, Brain and Behavior. 2012;11:643–50. doi: 10.1111/j.1601-183X.2012.00783.x. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Chiao JY. Genetic determinants of financial risk taking. PLoS one. 2009;4:e4362. doi: 10.1371/journal.pone.0004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine RA, Campbell DT. Ethnocentrism: Theories of Conflict, Ethnic Attitudes, and Group Behavior. New York: Wiley; 1972. [Google Scholar]
- Lewis GJ, Bates TC. Genetic evidence for multiple biological mechanisms underlying in-group favoritism. Psychological Science. 2010;21:1623–28. doi: 10.1177/0956797610387439. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan JC, Bresnahan M, Stueve A, Pescosolido BA. Public conceptions of mental illness: labels, causes, dangerousness, and social distance. American Journal of Public Health. 1999;89(9):1328–33. doi: 10.2105/ajph.89.9.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston RW, Drwecki BB. Why are some individuals not racially biased? Susceptibility to affective conditioning predicts nonprejudice toward blacks. Psychological Science. 2007;18(9):816–23. doi: 10.1111/j.1467-9280.2007.01985.x. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Öhman A. Genetic gating of human fear learning and extinction. Psychological Science. 2009;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Miller SL, Maner JK, Becker DV. Self-protective biases in group categorization: threat cues shape the psychological boundary between ‘us’ and ‘them’. Journal of Personality and Social Psychology. 2010;99:62–77. doi: 10.1037/a0018086. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63(9):852–57. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete CD, Fessler DMT, Eng SJ. Elevated ethnocentrism in the first trimester of pregnancy. Evolution and Human Behavior. 2007;28(1):60–65. [Google Scholar]
- Navarrete CD, Fessler DMT, Fleischman DS, Geyer J. Race bias tracks conception risk across the menstrual cycle. Psychological Science. 2009;20(6):661–5. doi: 10.1111/j.1467-9280.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- Neuberg SL, Kenrick DT, Schaller M. Human threat management systems: self-protection and disease avoidance. Neuroscience & Biobehavioral Reviews. 2011;35:1042–51. doi: 10.1016/j.neubiorev.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant EA, Devine PG, Cox WTL, et al. The Obama effect: decreasing implicit prejudice and stereotyping. Journal of Experimental Social Psychology. 2009;45(4):961–4. [Google Scholar]
- Olson JM, Vernon PA, Harris JA, Jang KL. The heritability of attitudes: a study of twins. Journal of Personality and Social Psychology. 2001;80(6):845–60. [PubMed] [Google Scholar]
- Pettigrew TF, Tropp LR. A meta-analytic test of intergroup contact theory. Journal of Personality and Social Psychology. 2006;90(5):751–83. doi: 10.1037/0022-3514.90.5.751. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12(5):729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Way BM, Taylor SE. 5-HTTLPR moderates effects of current life events on neuroticism: differential susceptibility to environmental influences. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(6):1070–4. doi: 10.1016/j.pnpbp.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek BM, Mania EW, Gaertner SL. Intergroup threat and outgroup attitudes: a meta-analytic review. Personality and Social Psychology Review. 2006;10(4):336–53. doi: 10.1207/s15327957pspr1004_4. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype? environment effects. Child development. 1983;54:424–35. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schaller M, Park JH. The behavioral immune system (and why it matters) Current Directions in Psychological Science. 2011;20:99–103. [Google Scholar]
- Schaller M, Park JH, Mueller A. Fear of the dark: interactive effects of beliefs about danger and ambient darkness on ethnic stereotypes. Personality and Social Psychology Bulletin. 2003;29(5):637–49. doi: 10.1177/0146167203029005008. [DOI] [PubMed] [Google Scholar]
- Sears DO. Symbolic racism. In: Katz PA, Taylor DA, editors. Eliminating Racism. New York: Plenum; 1988. pp. 53–84. [Google Scholar]
- Sibley CG, Duckitt J. Personality and prejudice: a meta-analysis and theoretical review. Personality and Social Psychology Review. 2008;12(3):248–79. doi: 10.1177/1088868308319226. [DOI] [PubMed] [Google Scholar]
- Stephan WF, Stephan CW. An integrated threat theory of prejudice. In: Oskamp S, editor. Claremont Symposium on Applied Social Psychology. Hillsdale, NJ: Erlbaum; 2000. pp. 23–46. [Google Scholar]
- Tajfel H, Billig MG, Bundy RP, Flament C. Social categorization and intergroup behavior. European Journal of Social Psychology. 1971;1(2):149–77. [Google Scholar]
- Tesser A. The importance of heritability in psychological research: the case of attitudes. Psychological Review. 1993;100(1):129–42. doi: 10.1037/0033-295x.100.1.129. [DOI] [PubMed] [Google Scholar]
- Van Hiel A, Pandelaere M, Duriez B. The impact of need for closure on conservative beliefs and racism: differential mediation by authoritarian submission and authoritarian dominance. Personality and Social Psychology Bulletin. 2004;30:824–37. doi: 10.1177/0146167204264333. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5-HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;7(2):e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbuch M, Pauker K, Ambady N. The subtle transmission of race bias via televised nonverbal behavior. Science. 2009;326:1711–4. doi: 10.1126/science.1178358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsko C, Park B, Judd CM. Considering the Tower of Babel: correlates of assimilation and multiculturalism among ethnic minority and majority groups in the United States. Social Justice Research. 2006;19:277–306. [Google Scholar]