Abstract
Human beings are constantly engaged in thought. Sometimes thoughts occur repetitively and can become distressing. Up to now the neural bases of these intrusive or unwanted thoughts is largely unexplored. To study the neural correlates of unwanted thoughts, we acquired resting-state fMRI data of 41 female healthy subjects and assessed the self-reported amount of unwanted thoughts during measurement. We analyzed local connectivity by means of regional homogeneity (ReHo) and functional connectivity of a seed region. More unwanted thoughts (state) were associated with lower ReHo in right dorsolateral prefrontal cortex (DLPFC) and higher ReHo in left striatum (putamen). Additional seed-based analysis revealed higher functional connectivity of the left striatum with left inferior frontal gyrus (IFG) in participants reporting more unwanted thoughts. The state-dependent higher connectivty in left striatum was positively correlated with rumination assessed with a dedicated questionnaire focussing on trait aspects. Unwanted thoughts are associated with activity in the fronto-striatal brain circuitry. The reduction of local connectivity in DLPFC could reflect deficiencies in thought suppression processes, whereas the hightened activity in left striatum could imply an imbalance of gating mechanisms housed in basal ganglia. Its functional connectivity to left IFG is discussed as the result of thought-related speech processes.
Keywords: rumination, intrusive thoughts, resting state, striatum, dorsolateral prefrontal cortex
INTRODUCTION
Humans spend a good deal of their waking time with mental activity. But the flow of human thought does not always follow purposeful or intended paths. We all have experienced that certain thoughts that come to mind are not welcome and that unwanted cognitive activity interferes with our day-to-day affairs. Sometimes unwanted thoughts occur recurrently and can become difficult to control. In the literature, these phenomena have been explored under various terms such as intrusive thoughts, repetitive thoughts, rumination and worries. In the following, we will refer to unwanted thoughts as thoughts that are (i) unwanted, (ii) recurrent in nature and (iii) self-related.
There are a multitude of studies on extreme forms of unwanted thoughts in psychiatric disorders such as obsessive-compulsive disorder (OCD, Julien et al., 2007), depression (Koster et al., 2011), anxiety (Yook et al., 2010) or post-traumatic stress disorder (Speckens et al., 2007). However, some studies revealed a certain degree of universality of unwanted thoughts and assessed their frequency in non-clinical populations (O’Neill et al., 2009; for an overview: Julien et al., 2007).
To our knowledge, the neural correlates of unwanted thoughts in healthy populations are largely unexplored, but might be an important measure for psychopathological vulnerabilities. The existing neuroimaging studies that focussed on unwanted thoughts in the broadest sense have mostly investigated the trait aspects of rumination and have presented task-related fMRI data in which patients with mental disorders were compared with healthy controls (Ray et al., 2005; Berman et al., 2011; Cooney et al., 2010; Hamilton et al., 2011). The most basic question of how unwanted thoughts manifest neurally in a healthy population in ecologically valid situations—namely during free thought—has never been addressed. An electrophysiological study has associated alpha asymmetries with multiple longitudinal self-report measures of rumination over a period of 7 days. However, predictive brain activity of rumination was only found in patients with major depression in bilateral prefrontal cortex (Putnam and McSweeney, 2008). In a similar vein, we have conducted a study in which we associated deactivations during a speeded forced-choice task with experience-based sampling of unwanted thoughts over a period of 100 days (Kühn et al., 2013). We observed a correlation between the frequency of self-reported unwanted thoughts and stronger reductions of BOLD signal during task performance in prefrontal brain regions. Unfortunately, it is difficult to interpret deactivations during task performance due to its dependency on the kind of task that the so-called baseline or fixation periods are compared to (Gusnard and Raichle, 2001; Morcom and Fletcher, 2007). This problem is avoided in resting-state fMRI measurements. Resting state offers the possibility to study brain activity under natural conditions because of its stimulus-independence, which might be especially fruitful to explore the neural basis of unwanted thought. In a previous study on resting-state data, we have shown a negative association between the habitual tendency to ruminate and resting-state activity in the right inferior frontal gyrus (IFG) (Kühn et al., 2012).
In the present study, we instructed participants to keep their eyes closed and relaxed during the measurement and asked three questions about the presence of unwanted thoughts during the measurement immediately afterwards. The focus of the present study is therefore on state aspects of unwanted thoughts, not on personality aspects of trait rumination. Our analysis is focussed on regional homogeneity (ReHo) measures that capture the synchrony of resting-state brain activity in neighbouring voxels, or so-called local connectivity. While functional connectivity reveals the synchronization of remote brain regions, ReHo measures the local synchronization of spontaneous fMRI signals by calculating similarity of dynamic fluctuations of voxels within a given cluster (Zang et al., 2004; Long et al., 2008; Zou et al., 2009). Zou et al. (2009) investigated the static and dynamic characteristics of cerebral blood flow (CBF) in resting-state using an arterial spin labeling (ASL) perfusion imaging technique. Consistent with previous positron emission tomography results, static CBF measured by ASL was significantly higher in the posterior cingulate cortex, thalamus, insula/superior temporal gyrus and medial prefrontal cortex than the average CBF of the brain. These brain regions also had high temporal synchrony, as measured by ReHo. Long et al. (2008) employed three methods to analyze resting-state fMRI data: ReHo, linear correlation and independent component analysis (ICA). All three methods revealed the existence of the default mode network (e.g. Buckner et al., 2008).
The aim of the present study was to relate the self-reported presence of unwanted thoughts during rest to the neural activity recorded concurrently and we expected a negative relationship between unwanted thoughts and resting-state prefrontal activity based on our previous study in which we found a negative correlation between habitual tendency to ruminate and gray matter volume in prefrontal cortex (Kühn et al., 2012).
METHOD
Participants
A total of 41 female healthy volunteers recruited from a database of healthy participants at Ghent University took part on the basis of informed written consent, with ethical committee approval of the University hospital Ghent and according to the Declaration of Helsinki. No subject had a history of neurological, major medical or psychiatric disorders—according to personal interviews (Mini-International Neuropsychiatric Interview, Lecurbier et al., 1997) carried out by a psychologist. The participants were all female, had a mean age of 21.4 years (ranging from 18 to 31 years) and were all right-handed as assessed by means of a handedness questionnaire (Oldfield, 1971).
Scanning procedure
Images were collected with a 3 T Magnetom Trio MRI scanner system (Siemens Medical Systems, Erlangen, Germany) using an eight-channel radiofrequency head coil. First, high-resolution anatomical images were acquired using a T1-weighted 3D MPRAGE sequence (TR = 1550 ms, TE = 2.39 ms, TI = 900 ms, acquisition matrix = 256 × 256 × 176, sagittal FOV = 220 mm, flip angle = 9°, voxel size = 0.9 × 0.9 × 0.9 mm3). Whole brain functional images were collected using a T2*-weighted EPI sequence sensitive to BOLD contrast (TR = 2000 ms, TE = 35 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 17%, voxel size 3.5 × 3.5 × 3 mm3, 30 axial slices). In all, 150 image volumes aligned to AC-PC were acquired.
Before the resting-state data acquisition started, participants were in the scanner for about 10 min during which a localizer and the anatomical images were aquired so that subjects could get used to the scanner noise.
Procedure
Before the resting-state measurement subjects were instructed to keep their eyes closed, relax and not think of anything in particular. Immediately after the resting-state measurement, participants were asked about unwanted thoughts during the past measurement. Unwanted thoughts were conceptualized as repeatedly occurring thoughts that are unwanted or negative. They were measured by means of three items that have been used previously (Brose et al., 2011; Kühn et al., 2013), of which two were adapted from the Stress Coping Inventory (Janke and Erdmann, 2002). Participants were asked to rate how much the statements ‘During the past five minutes, I could not get certain thoughts out of my mind.’ and ‘During the past five minutes, I kept thinking about something over and over again.’ matched their thoughts and feelings. A third item was developed to capture self-related unwanted thoughts, ‘During the past five minutes, I had difficulties suppressing thoughts about myself.’ Previously, these items were used with a an eight-point answering scale [ranging from 0 (does not apply at all) to 7 (does apply very well)]. Due to the constaints of the scanner environment we were only able to use a 4-point answering scale, ranging from 0 (does not apply at all) to 3 (does apply very well). The average score across all three items was used for analysis, indicating the presence of unwanted thoughts during the resting-state measurement.
Questionnaires
In order to assess trait-based tendencies to ruminate we administered the Rumination Response Scale (RRS, Treynor et al., 2003, in the Dutch translation used by Schoofs et al., 2010) with the subscales reflection and brooding. The brooding subscale is of most interest because it has been related to vulnerability to depression (Nolan et al., 1998; Roberts et al., 1998; Nolen-Hoeksema, 2000). Moreover, we measured depression by means of the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). Trait and state anxiety was determined by means of the State Trait Anxiety Inventory (STAI, Spielberger, 1970, in its Dutch translation by van der Ploeg, 1982). For all questionnaires, higher scores are indicative of respectively, more rumination, depressive symptoms and more state or trait anxiety.
Resting-state analysis
The first five volumes were discarded to allow the magnetization to approach a dynamic equilibrium. Part of the data pre-processing, including slice timing, head motion correction (a least squares approach and a six-parameter spatial transformation) and spatial normalization to the Montreal Neurological Institute (MNI) template (resampling voxel size of 3 mm × 3 mm × 3 mm), were conducted using the SPM5 and Data Processing Assistant for Resting-State fMRI (DPARSF, Chao-Gan and Yu-Feng, 2010). A spatial filter of 4 mm FWHM (full-width at half maximum) was used. Participants showing head motion above 3 mm of maximal translation (in any direction of x, y or z) and 1.0° of maximal rotation throughout the course of scanning would have been excluded.
After pre-processing, linear trends were removed. Then the fMRI data were temporally band-pass filtered (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency respiratory and cardiac noise (Biswal et al., 1995). ReHo analysis (Zang et al., 2004; Liu et al., 2006; Wu et al., 2009, 2011) was performed using DPARSF. ReHo is based on previous reports that fMRI activity is more likely to occur in clusters of several spatially contiguous voxels than in a single voxel (Tononi et al., 1998; Kantanoda et al., 2002). Therefore, ReHo assumes that a given voxel is temporally similar to that of its neighbors. For each participant, ReHo analysis was performed on a voxel-wise basis by calculating Kendall’s coefficient of concordance (KCC, Kendall and Gibbons, 1990) of the time series of a given voxel with those of its nearest neighbors (26 voxels). Then, the KCC value was given to this center voxel and individual KCC maps were obtained. ReHo was calculated within a brain-mask, which was obtained by removing the tissues outside the pial surface using the software MRIcro (by Chris Rorden, http://www.psychology.nottingham.ac.uk/staff/cr1/mricro.html). On the resulting ReHo maps, a whole brain correlation with the mean unwanted thought score was computed. A height threshold of P < 0.001 and cluster-size corrected by means of Monte Carlo simulation. Significant effects were reported when the volume of the cluster was greater than the Monte Carlo simulation determined minimum cluster size on the whole brain volume (>25 voxels), above which the probability of type I error was below 0.05 (AlphaSim, Ward, 2000).
To explore whether the unwanted thought-related resting-state ReHo activation was related to habitual rumination tendencies, the beta values extracted from the significant clusters of the whole brain ReHo analysis were correlated by means of Pearson product–moment correlation coefficient with the brooding subscore oft the RRS.
Additionally, we conducted an exploratory analysis computing functional connectivity maps with a seed region consisting of the cluster of ReHo activation found to be positively correlated with unwanted thoughts (left striatum) by means of DPARSF. The resulting whole brain functional connectivity maps were correlated with the mean unwanted thought score to identify brain regions that were jointly activated with the left striatum in particular in participants who were experiencing unwanted thoughts. Coordinates are reported in MNI space.
RESULTS
Mean and standard deviations of the subscales of the questionnaires are listed in Table 1. The three unwanted thought state questions asked after the resting-state acquisition were highly correlated with one another [question 1 and 2: r(41) = 0.586, P < 0.001; 2 and 3: r(41) = 0.376, P < 0.05, 1 and 3: r(41) = 0.625, P < 0.001), and therefore we used the average of them in the following. The average unwanted thought score was positively correlated with the brooding subscale of the RRS trait questionnaire [r(41) = 0.372, P < 0.05].
Table 1.
Mean and standard deviation of the questionnaires used
| Intrusive thoughts | RRS brooding | RRS reflection | RRS total | STAI state | STAI trait | BDI | |
|---|---|---|---|---|---|---|---|
| Mean | 0.76 | 10.2 | 10.1 | 50.7 | 33.2 | 36.7 | 5.31 |
| s.d. | 0.66 | 3.1 | 3.9 | 13.4 | 7.7 | 8.5 | 4.4 |
A whole brain analysis on the ReHo maps revealed the typical activation in the default mode network, in particular in the posterior cingulate cortex (Long et al., 2008). To test our hypothesis of an association between unwanted thoughts and prefrontal cortex resting-state activation, we correlated the whole brain ReHo maps reflecting the temporal homogeneity of regional spontaneous brain activity with the total score of unwanted thoughts during resting state. As a result, we found a cluster of significant negative correlation in right DLPFC (54, 42, 39; BA 46) and a significant cluster of positive correlation in left striatum (putamen, −36, −3, 3) (Figure 1). To exclude that the results were driven in part by depressiveness or state anxiety of the participants, we included BDI and STAI state as nuisance regressors. Since these additional covariates do not change the results we conclude that the findings are specific for unwanted thoughts.
Fig. 1.
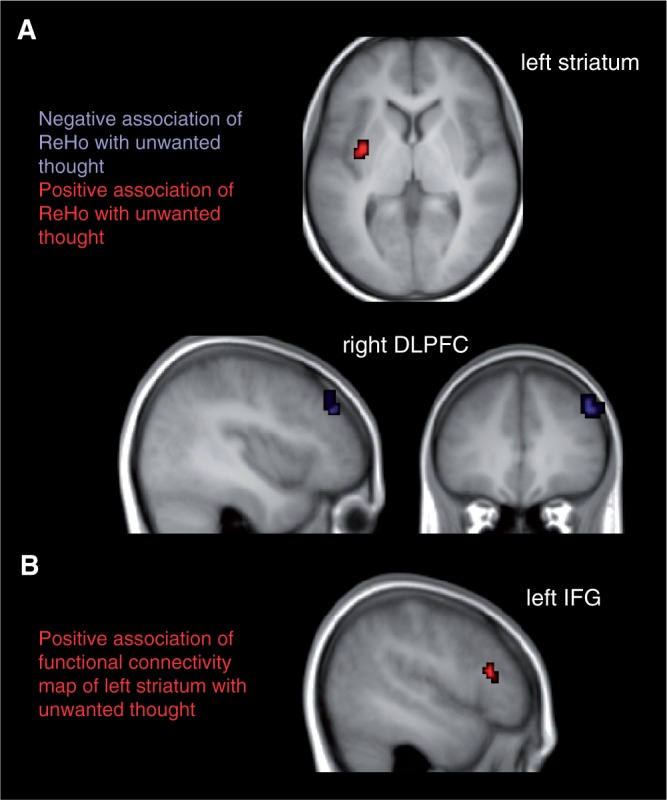
(A) Results of whole brain correlation of ReHo and mean unwanted thoughts during the resting-state measurement (P < 0.001, cluster-size corrected by means of Monte Carlo simulation). Red indicates regions of positive, blue of negative correlation. (B) Results of whole brain correlation of functional connectivity maps with left striatum (putamen) and mean unwanted thoughts during resting-state measurement (P < 0.001, cluster-size corrected by means of Monte Carlo simulation). Results are overlayed onto the mean T1 image of all participants.
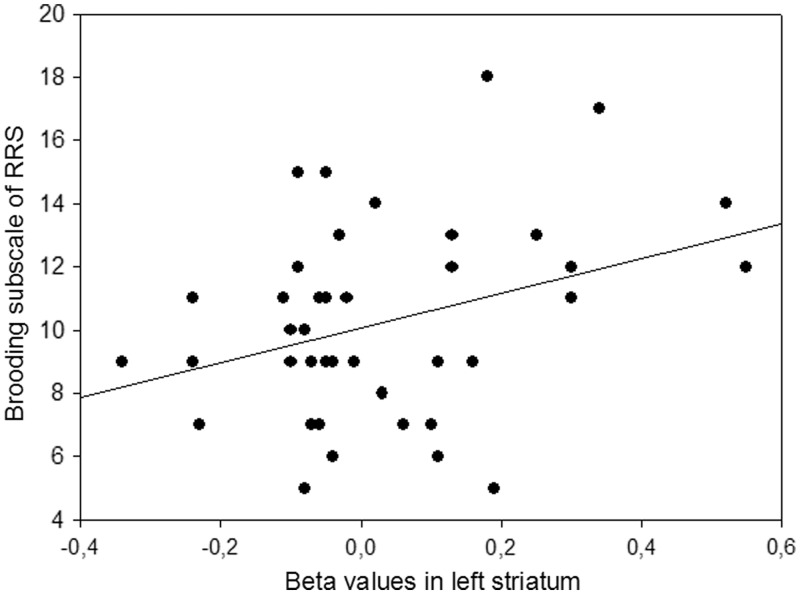
In order to explore whether the unwanted thought-related resting-state activation in healthy participants was related to the brooding subscore of the RRS, that has been related to vulnerability to depression, we correlated the extracted beta values from the left striatum and the right DLPFC with this subscore. ReHo resting-state brain activity in left striatum was positively correlated with brooding (Figure 2), indicating that higher local connectivity in left striatum leads to higher habitual tendency of maladaptive rumination [Pearson product–moment correlation coefficient, r(41) = 0.334, P < 0.05]. The DLPFC activity was not correlated with brooding (P > 0.77). Neither DLPFC nor left striatum were correlated with reflection (P > 0.59).
Fig. 2.
Scatter plot of the correlation between beta values in left striatum and brooding subscore of the Ruminative Response Style questionnaire; r(41) = 0.334, P < 0.05.
Within the scope of an exploratory analysis, we aimed at investigating other brain regions that are functionally related to the significant cluster in left striatum during unwanted thoughts. We computed functional connectivity maps of a mask comprising the cluster in left striatum and correlated these maps with the mean unwanted thought score. One significant cluster was found in left IFG, Broca’s area, −57, 24, 15).
DISCUSSION
Within the scope of the present study, correlates of self-reported unwanted thoughts during brain resting state were investigated. Lower ReHo in right DLPFC and higher ReHo in left striatum (putamen) was associated with more self-reported unwanted thoughts. Moreover, we explored brain regions functionally connected to the overactive striatum. We found higher functional connectivity of the left striatum with left IFG in individuals experiencing more unwanted thoughts. Furthermore, the state-dependent hightened resting-state activity in left striatum was positively associated with trait-level rumination, a measure known to be a vulnerability factor of depression.
The lower ReHo in the right DLPFC associated with more unwanted thoughts could reflect deficiencies in control over the stream of thoughts. Previous studies reported the suppression of unwanted memories to be associated with increased activity in DLPFC (Anderson et al., 2004). Likewise, a study in which participants were instructed not to think about a certain topic (e.g. a white polar bear) has associated thought suppression with sustained activity in DLPFC and transient activity in anterior cingulate cortex (Mitchell et al., 2007). In accordance with these findings, we observed a negative correlation of ReHo in DLPFC with unwanted thoughts which fits to the notion that DLPFC shows tonic activity during thought suppression. Similarly, studies on emotion regulation have associated DLPFC activity with successful control of unwanted emotions (Beauregard et al., 2001; Ochsner et al., 2004; Goldin et al., 2008). Accordingly, the negative correlation between local connectivity in DLPFC with the amount of self-reported unwanted thought could be the neural reflection of a deficit in mechanisms that usually achieve the suppression of undesirable content of thought. Moreover, it has been argued that DLPFC related cognitive control deficiencies may not only explain the inability to interrupt negative thinking, but would also be a vulnerability factor for depression (De Raedt and Koster, 2010).
The observed positive association between ReHo in the left striatum and the trait-level assessment of brooding in RRS likewise supports a certain degree of consent between trait and state-level assessment of unwanted thoughts. Because this is, to our best knowledge, the first time that brooding scores have been linked with the striatum, caution is warranted. Nevertheless, it implicates a certain degree of clinical relevance because, although individuals scoring high on the brooding subscale of the RRS are not necessarily currently depressed, brooding has been described as a trait characteristic that increases the vulnerability to depression (Nolan et al., 1998; Roberts et al., 1998; Nolen-Hoeksema, 2000). An alternative interpretation may be that the resting-state activity in the striatum could be indicative of an increased ability to block incoming external information, providing more cognitive capacity for inner thought.
In line with our exploratory finding of functional connectivity between left striatum and IFG, the frontal cortex has been shown to be strongly connected with the striatum (Cummings, 1993). A fronto-striatal circuitry has been implicated in the gating and inhibition of actions (Frank, 2006) and representations in decision making (Frank and Claus, 2006). The striatum has been proposed to implement a generic selection mechanism that facilitates or suppresses representations in the frontal cortex. Particularly in OCD, the fronto-striatal neural circuit has been suggested to underpin impulsive and compulsive acts as well as intrusive thoughts (Fineberg et al., 2010). In a previous study, we have shown an association between the trait to ruminate and a reduction of resting-state activity in the right IFG.
In philosophy, it has been suggested that thought involves language production and inner speech (Wittgenstein, 1953; Davidson, 1975). The present data might be interpreted as a link between unwanted thoughts and language-related processes based on the functional connectivity analysis in which we found higher connectivity of the left striatum with left IFG, also called Broca’s area, in individuals that experienced more unwanted thoughts during the measurement. Left IFG activation has previously been reported in neuroimaging studies investigating inner speech and verbalization (McGuire et al., 1996; Shergill et al., 2002; Morin and Michaud, 2007). Therefore, our findings suggest that unwanted thoughts are represented in a language format. But the present data are silent as to whether this association holds true for unwanted thoughts only, or applies to thoughts in general as proposed by certain streams of philosophy (Wittgenstein, 1953; Davidson, 1975).
The observed connectivity of the striatum with left IFG is in line with our previous results of a correlation between the amount of unwanted thoughts assessed over 100 days and task-related deactivation in left IFG (Kühn et al., 2013). We found that participants with a stronger tendency to ruminate activated the left IFG stronger during fixation periodes. But the longitudinal assessment of unwanted thoughts in this previous study will most likely reflect rumination on a trait-level, whereas our present assessment was tailored on the period of the resting-state acquisition itself and represents a more state-like measurement of unwanted thoughts.
A possible limitation of the present study is the lack of male participants. Moreover, ‘wanted’ thoughts such as an exciting upcoming event, could likewise have resulted in high scores on the three state questions assessed after resting state. However, the significant positive correlation with the brooding subscale of the RRS does support the interpretation that most of the thoughts that occurred were ruminations and therefore ‘unwanted’. Future research is needed to explore the relationship between state- and trait-aspects of rumination and unwanted thoughts in more detail. Furthermore, it would be of interest to focus onto the content of unwanted thoughts in a non-clinical population and in essence compare the neural correlates of obsessive intrusive thoughts that have been associated with OCD and worries that have been associated with depression and anxiety. In the literature, worries have been described as more egosyntonic, meaning that their content is in closer agreement with the belief system than obsessive intrusive thoughts that are typically described as egodystonic (Turner et al., 1992; Langlois et al., 2000). Moreover, the content of worries is usually not perceived as unacceptable, whereas the content of intrusive thoughts is.
To summarize, we have shown an association between self-reported unwanted thoughts and activity within the fronto-striatal network. Lower ReHo in resting-state activity within right DLPFC and higher ReHo in left striatum were related to more self-reported unwanted thoughts during rest. The reduction in DLPFC could result from deficiencies in thought suppression, whereas the hightened resting-state activity in striatum could reflect disturbances within the gate-keeping function of the basal ganglia. Stronger functional connectivity of the left striatum with left IFG was observed in individuals experiencing more unwanted thoughts. The involvement of the so-called Broca’s are could reflect a heightened involvement speech generation processes during unwanted thoughts.
Conflict of interest
S.K., M.-A.V., R.D.R. report no financial relationships with commercial interests. J.G. has received research funding from the German Federal Ministry of Education and Research (BMBF 01GS08159), research funding from AstraZeneca, Eli Lilly & Co, Janssen-Cilag, Bristol-Myers Squibb and speaker fees from AstraZeneca, Janssen-Cilag and Bristol-Myers Squibb. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the article.
Acknowledgments
S.K. and M.-A.V. are Postdoctoral Fellows of the Research Foundation Flanders (FWO).
REFERENCES
- Anderson MC, Ochsner KN, Kuhl B, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21(RC165):1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berman MG, Nee DE, Casement M, et al. Neural and behavioural effects of interference resolution in depression and rumination. Cognitive Affective Behavioural Neuroscience. 2011;11:85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance Medicine. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brose A, Schmiedek F, Lövden M, Lindenberger U. Normal aging dampens the link between intrusive thoughts and negative affect in reaction to daily stressors. Psychology of Aging. 2011;26:488–502. doi: 10.1037/a0022287. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function and relevance to disease. Annual New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for ‘pipeline’ data analysis of resting-state fMRI. Frontiers in System Neuroscience. 2010;4:1–7. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive Affective Behavioral Neuroscience. 2010;10:470–8. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives in Neurology. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Davidson D. Thought and talk. In: Guttenplan S, editor. Mind and Language. Oxford: Oxford University Press; 1975. [Google Scholar]
- De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cognitive Affective Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, et al. Probing compulsive and impuslive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19:1120–36. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychology Review. 2006;113:300–26. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reapprasial and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a basline: functional imaging and the resting human brain. Nature Review Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biology Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke W, Erdmann G. SVF 78. Eine Kurzform des Stressverarbeitungsfragebogens SVF 120. Göttingen, Germany: Hogrefe; 2002. [Google Scholar]
- Julien D, O’Connor KP, Aarema F. Intrusive thoughts, obsessions, and appraisals in obsessive-compulsive disorder: a critical review. Clinical Psychology Review. 2007;27:366–83. doi: 10.1016/j.cpr.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Matsuda Y, Sugishita M. A spatial–temporal regression model for the analysis of functional MRI data. NeuroImage. 2002;17:1415–28. doi: 10.1006/nimg.2002.1209. [DOI] [PubMed] [Google Scholar]
- Kendall M, Gibbons JD. Rank Correlation Methods. Oxford: Oxford University Press; 1990. [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–45. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kühn S, Schmiedek F, Brose A, Schott B, Lindenberger U, Lövden M. The neural representation of intrusive thoughts. Social Cognitive Affective Neuroscience. 2013;8:688–93. doi: 10.1093/scan/nss047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Vanderhasselt MA, De Raedt R, Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and ist relation to depression. Journal of Affective Disorders. 2012;141(2–3):352–60. doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Langlois F, Freeston MH, Ladouceur R. Differences and similarities between obsessive intrusive thoughts and worry in a non-clinical population: study 1. Behavioral Research Therapy. 2000;38:157–73. doi: 10.1016/s0005-7967(99)00027-3. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan D, Weiller E, et al. The Mini International Neuropsychiatric Interview. (MINI), a short diagnostic interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–31. [Google Scholar]
- Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. NeuroReport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Long XY, Zuo XN, Kiviniemi V, et al. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. Journal of Neuroscience Methods. 2008;171:349–55. doi: 10.1016/j.jneumeth.2008.03.021. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, et al. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory verbal hallucinations. British Journal of Psychiatry. 1996;169:148–59. doi: 10.1192/bjp.169.2.148. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Macrae CN. Separating sustained from transient aspects of cognitive control during thought suppression. Psychological Science. 2007;18:292–7. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. NeuroImage. 2007;37:1073–982. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morin A, Michaud J. Self-awareness and the left inferior frontal gyrus: inner speech use during self-related processing. Brain Research Bulletin. 2007;74:387–96. doi: 10.1016/j.brainresbull.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Nolan SA, Roberts JE, Gotlib IH. Neuroticism and ruminative response style as predictorsof change in depressive symptomatology. Cognitive Therapy Research. 1998;22:445–55. [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109:504–11. [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Neill ML, Nenzel ME, Cadwell W. Intrusive thoughts and psychopathy in a student and incarcerated sample. Journal of Behavioral Therapy Experimental Psychiatry. 2009;40:147–57. doi: 10.1016/j.jbtep.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Putnam KM, McSweeney LB. Depressive symptoms and baseline prefrontal EEG alpha activity: a study utilizing Ecological Momentary Assessment. Biological Psychology. 2008;77:237–40. doi: 10.1016/j.biopsycho.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive Affective Behavioral Neuroscience. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Gilboa E, Gotlib IH. Ruminative response style and vulnerability to episodes of dysphoria: gender, neuroticism, and episode duration. Cognitive Therapy Research. 1998;22:401–23. [Google Scholar]
- Schoofs H, Hermans D, Raes F. Brooding and reflection as subtypes of rumination: evidence from confirmatory factor analysis in nonclinical samples using the Dutch Ruminative Response Scale. Journal of Psychopathology and Behavioral Assessment. 2010 Advance online publication. doi: 10.1007/s10862-010-9182-9. [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, et al. Modulation of activity in temporal cortex during generation of inner speech. Human Brain Mapping. 2002;16:219–27. doi: 10.1002/hbm.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckens AE, Ehlers A, Hackmann A, Ruths FA, Clark DM. Intrusive memories and rumination in patients with post-traumatic stress disorder: a phenomenological comparison. Memory. 2007;15:249–57. doi: 10.1080/09658210701256449. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety Inventory, Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Tononi G, McIntosh AR, Russell DP, Edelman GM. Functional clustering: identifying strongly interactive brain regions in neuroimaging data. NeuroImage. 1998;7:133–49. doi: 10.1006/nimg.1997.0313. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognitive Therapy Reseach. 2003;27:247–59. [Google Scholar]
- Turner SM, Beidel DC, Stanley MA. Are obsessional thoughts and worry different cognitive phenomena? Clinical Psychology Review. 1992;12:257–70. [Google Scholar]
- Van der Ploeg HM. DeZelf-BeoordelingsVragenlijst (STAI-DY). De ontwikkeling envalidatievaneenNederlandstaligevragenlijstvoorhetmeten van angst. Tijdschrift voor Psychiatrie. 1982;24:576–88. [Google Scholar]
- Ward BD. AFNI AlphaSim Documentation. Medical College of Wisconsin; 2000. Simultaneous inference for fMRI data. [Google Scholar]
- Wittgenstein L. Philosophical Investigations. Oxford: Blackwell; 1953. [Google Scholar]
- Wu QZ, Li DM, Kuang WH, et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Human Brain Mapping. 2011;32:1290–9. doi: 10.1002/hbm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, et al. Regional homogeneity changes in patients with parkinson’s disease. Human Brain Mapping. 2009;30:1502–10. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook K, Him KH, Suh SY, Lee KS. Intolerance of uncertainty, worry and rumination in major depressive disorder and generalized anxiety disorder. Journal of Anxiety Disorders. 2010;24:623–8. doi: 10.1016/j.janxdis.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:515–24. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]