Abstract
The effect of social rejection on cardiac and brain responses was examined in a study in which participants had to decide on the basis of pictures of virtual peers whether these peers would like them or not. Physiological and behavioral responses to expected and unexpected acceptance and rejection were compared. It was found that participants expected that about 50% of the virtual judges gave them a positive judgment. Cardiac deceleration was strongest for unexpected social rejection. In contrast, the brain response was strongest to expected acceptance and was characterized by a positive deflection peaking around 325 ms following stimulus onset and the observed difference was maximal at fronto-central positions. The cardiac and electro-cortical responses were not related. It is hypothesized that these differential response patterns might be related to earlier described differential involvement of the dorsal and ventral portion of the anterior cingulate cortex.
Keywords: anterior cingulate, ERP, HR, P3, social rejection
INTRODUCTION
Human beings have strong social needs and one of these primary needs is the need to belong (Baumeister and Leary, 1995). People feel comfortable in the presence of close others and feel distressed when they are rejected by them. Social rejection and social exclusion have been studied with various paradigms and considerable knowledge has been acquired about consequences for well-being and the possible role in the development of psychiatric diseases, like for instance, major depression (Davey et al., 2008). More recently, research has focused on the neural basis of social rejection and exclusion by using functional magnetic resonance imaging (fMRI; e.g. Eisenberger et al., 2003; Crowley et al., 2009; Gutz et al., 2011).
In a recent fMRI study (Somerville et al., 2006), a new paradigm was introduced to measure the brain mechanisms involved in social rejection. In this task participants were shown pictures of unfamiliar faces and were told that these people had previously seen a picture of the participant and had formed a positive or negative first impression of the participant. In the task, the participants had to determine whether they thought that this unknown person had formed a positive or negative first impression of them. After this prediction, the participants were confronted with the computer-generated ‘real’ answer, which could be either positive or negative. In this way, four stimulus categories were created, representing either expected or unexpected acceptance (positive first impression) or rejection (negative first impression). In this study, a clear dissociation was found between activation in the ventral and the dorsal part of the anterior cingulate cortex (vACC vs dACC). The vACC appeared to be more sensitive to the valence of the actual judgment (positive vs negative first impression), whereas the dACC was more sensitive to congruency (expected vs unexpected first impression). In a cardiac study that used the same paradigm (Gunther Moor et al., 2010), it was found that unexpected rejection induced a transient cardiac deceleration. This cardiac deceleration is similar to the cardiac deceleration found after negative feedback and after violations of social rules, which has been related to motivationally relevant violations of expectancy (e.g. van der Veen and Sahibdin, 2011). Gunther Moor et al. (2010) speculated that this cardiac response might be related to activation in the dACC, which is also thought to be involved in the representation and control of cardiac responses (Critchley et al., 2003; Gianaros, Van Der Veen, and Jennings, 2004). However, as far as we know, no event-related brain potential (ERP) studies have been performed addressing the electrophysiological correlates of social rejection.
In contrast to social rejection, social exclusion, a related but different construct, has been studied using ERPs by employing the Cyberball paradigm. The Cyberball paradigm is a virtual ball-toss game in which participants can either be included or excluded from the game. A first ERP study using the Cyberball paradigm found that the distress caused by social exclusion was related to frontal slow wave activity (580–900 ms post-stimulus) and it was found that this slow wave was more negative going for participants experiencing more distress and more positive going for participants experiencing less distress (Crowley et al., 2009). A second study (Gutz et al., 2011) using the Cyberball paradigm found that exclusion was associated with an increase in P3 amplitude. The P3 is a positive going ERP component that is maximal between 300 and 800 ms after stimulus onset. In their study, Gutz et al. distinguished between a fronto-central P3a and a more parietal P3b component. The P3a is thought to reflect a ‘stimulus-driven frontal attention mechanism during task processing’ and the P3b is thought to reflect ‘attention and appears related to subsequent memory processing’ (Polich, 2007). In paradigms that have used feedback stimuli, which might be related to the exclusion events in the Cyberball paradigm, it has been found that the P3 amplitude is larger to feedback associated with larger, more important monetary incentives and is larger for positive feedback (e.g. Van den Berg et al., 2012). Gutz et al. found that the P3a amplitude was related to the affective processing of the exclusion and P3b amplitude was related to its perceived intensity.
The main goal of this study was to examine the ERP associated with social rejection in the paradigm developed by Somerville et al. (2006). As no study examined social rejection using ERPs before, we hypothesized that the effects of rejection on various ERP components are comparable with those found in the ERP studies using the Cyberball paradigm. Gutz et al. (2011) reported a larger P3 to acceptance trials in a rejection block. Therefore, we expected larger P3 amplitudes for acceptance trials, and especially unexpected acceptance trials, which are more or less similar to the infrequent, unexpected acceptance trials in the exclusion block of the Cyberball paradigm. A second goal was to replicate the finding of a cardiac deceleration to unexpected rejection, as was found in the study of Gunther Moor et al. (2010).
METHODS
Participants
Participants were 19 healthy, right-handed volunteers (mean age 21.6 years, s.d. = 2.0, 3 males) who had normal or corrected-to-normal vision. The study was approved by the local medical ethics committee. Participants provided written informed consent and received a small fixed monetary reward (10 Euros) at the end of the study. Participants were screened with a general health questionnaire. Exclusion criteria were serious general health problems and neurological or psychiatric disorders in past or present.
Stimuli and procedure
The design of the experiment was based on the task developed by Somerville et al. (2006) who used this task in an fMRI study, and Gunther Moor et al. (2010) who used this task to examine cardiac responses. A cover story was used in which participants were told that they would participate in a larger ongoing study in which different universities were involved and in which first impressions of people were evaluated. In the first session, the participants were asked whether their picture could be taken and could be sent to the collaborating research group for judgment. Participants were told that their picture was evaluated by a panel and that the evaluation was categorized in terms of ‘like’ or ‘do not like’. The participants were, furthermore, told that this evaluation combined with the picture of the panel members would be sent back to the research laboratory in which the study was performed and that these pictures and evaluations would be used in the second session. The second session, which was held ∼1 week later, consisted of the social rejection task combined with two unrelated tasks, which will not be discussed in the present article. Participants were told that they would see pictures of the panel members and that they would have to say whether they thought that the panel members would like them or not. In the task, first the face of the panel member was presented with the question ‘Do you think this person likes you?’. This screen was presented for 3 s and the participants had to push either the left or right button of a response panel to indicate whether they thought the panel member liked them (right button) or not (left button). After this the same face was presented again, but now for 1 s and with the given answer printed left of the picture of the panel member. Finally, the same face was presented one more time, but now for 2 s and with the expectation on the left side and the actual evaluation printed on the right side of the screen. The faces of the participants were not evaluated by existing persons but instead, a fixed, computer-generated randomized sequence with 50% YES answers and 50% NO answers was used. Faces of the evaluators were taken from the AR face database (Martinez and Benavente, 1998). Neutral facial expressions were used, separate faces were used only once and an equal amount of male and female faces were used. A total of 120 faces were presented in a single block, which lasted about 10 min. Participants did not receive information about the percentage ‘like’ and ‘do not like’ evaluations they could expect.
Data acquisition
Electro-encephalography (EEG), electro-oculography (EOG) and electro-cardiography (ECG) signals were amplified, sampled and stored on a portable amplifier (Vitaport System, Temec Instruments B.V., Kerkrade). EEG was derived from F3, Fz, F4, C3, Cz, C4 and Pz according to the international 10–20 system (Sharbrough et al., 1991) and signals were referred to physically connected mastoids. Vertical EOG was derived from electrodes placed on the infraorbital and supraorbital regions on the left eye. EOG and EEG were sampled at 256 Hz, low-pass filtered at 30 Hz and high-pass filtered with a time constant of 0.5 Hz. Electrode impedance was kept <8 kOhm. The EEG signal was locked to the onset of the stimulus showing both the expected evaluation and the given evaluation and epochs were extracted between 100 ms preceding and 700 ms following the onset of this stimulus. The epochs were corrected for vertical EOG artifacts by using an often used correction method (Gratton et al., 1983). As a final check, epochs were visually inspected and checked for artifacts and epochs were excluded from analysis when necessary. ECG was recorded from pre-cordial leads and sampled at 512 Hz. R-peaks were detected offline and the R-peak occurrence times were visually inspected for artifacts and corrected when necessary. We followed the logic of Gunther Moor et al. (2010) and selected seven inter-beat intervals (IBIs) surrounding the evaluation stimulus were selected for further analysis; i.e. two preceding IBIs (IBIs −2 and −1), the concurrent IBI (i.e. IBI 0) and three subsequent IBIs (i.e. IBIs 1, 2, 3 and 4). Like in the Gunther Moor study, IBI 0 to IBI 4 were referenced to the second IBI preceding stimulus onset (IBI −2). Based on visual inspection of the data that showed responses already returned to baseline after IBI 3 and preliminary statistical tests that failed to show a main effect of sequential IBI, we decided to restrict the analysis to IBI 0 to IBI 3.
Statistical analysis
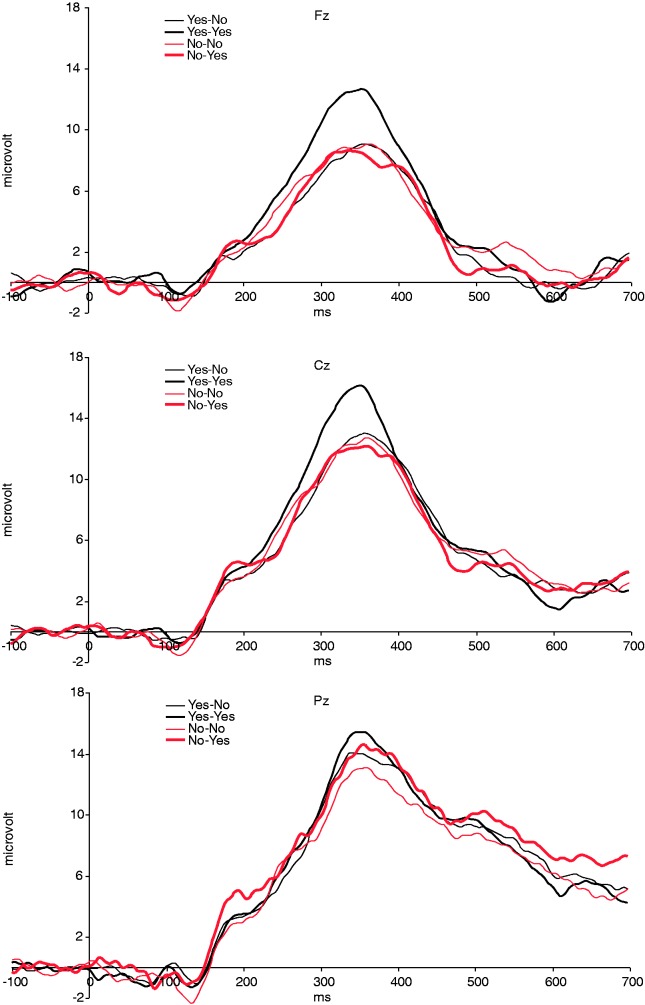
Behavioral, cardiac and electro-cortical measures were statistically evaluated using SPSS 16 (SPSS Inc., Chicago, IL, USA). Analysis of variance was performed using a general linear model (GLM) repeated-measures design. After visual inspection of the grand average wave forms (Figure 1), we could not detect a clear negative wave, which was maximal for unexpected rejections (expected answer YES/given answer NO; YN). We did, however, detect a broad positive wave, which peaked around 325 ms after stimulus onset and clearly differentiated between the four stimulus categories and electrodes. Therefore, we decided to compute an area measure separately for all stimulus categories in the area between 275 and 375 ms after evaluation onset was chosen. The area measure was tested in a design with electrode (three levels; Fz, Cz and Pz), expectation (two levels; Yes vs No) and evaluation (two levels; Yes vs No). Stimulus-locked cardiac responses were tested in a design with sequential IBI (four levels; IBI0, IBI1, IBI2 and IBI3), expectation and evaluation as within-subjects factors. Huynh-Feldt corrections of degrees of freedom were applied whenever appropriate, but uncorrected degrees of freedom are reported. Effects size is reported as partial eta squared (η2).
Fig. 1.
Grand average ERPs for expected (No–No) and unexpected (Yes–No) rejections and expected (Yes–Yes) and unexpected (No–Yes) acceptance at mid-line electrodes (n = 17).
RESULTS
Performance
A first global analysis showed that two participants performed the task very differently as compared to the other participants and were labeled as outliers in a box-plot analysis. Both participants expected that only a very small percentage of people would like them (5% and 13%, respectively), whereas the average percentage for the remaining 17 participants was 48%. We therefore decided to exclude these participants from further analysis. For the remaining participants we computed a bias measure (number of expected to like ratings divided by the total number of ratings), which can be seen as a measure of positive (>0.5) or negative (<0.5) expectations. The average bias was 0.48 ± 0.026 (minimum 0.32, maximum 0.67), which means that on average expected about the same amount positive and negative ratings (bias did not significantly differ from 0.5, P > 0.5).
Event-related brain potentials
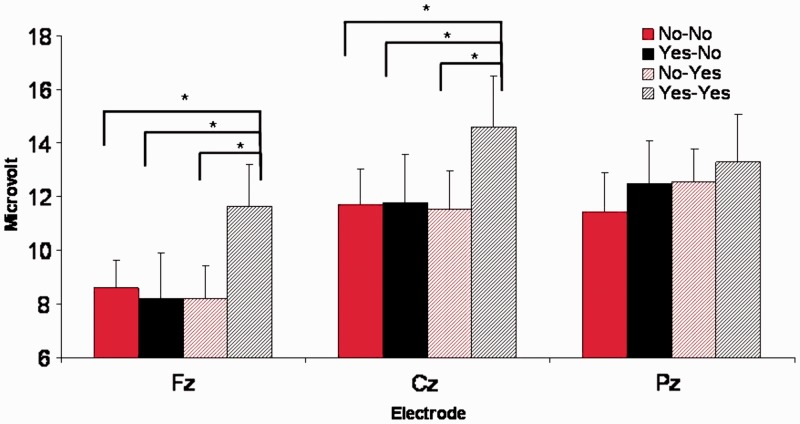
Based on the performance analysis in the previous section we decided to only analyze the 17 participants with sufficient negative and positive expectations. For both the cardiac and the ERP analysis the average number of trials in the various categories was 33 (YY), 30 (NN), 28 (YN) and 27 (NY). Average wave-forms for all four stimulus categories and for the three central electrode positions are shown in Figure 1. The average amplitude in an interval between 275 and 375 ms post-judgment onset was computed for the four stimulus categories and three central electrodes separately (Figure 2) and was tested in a design with expectation, evaluation and electrode as within-subjects factors. In this analysis we found a main effect of evaluation, F1,16 = 6.7, P < 0.05, η2 = 0.295, electrode, F1,16 = 15.7, P < 0.0005, η2 = 0.494 and a three-way interaction between expectation, evaluation and electrode, F2,32 = 6.9, P < 0.05, η2 = 0.300. Follow-up analyses showed that YY stimuli elicited a more positive-going wave on Fz (mean ± s.e.m. = 11.6 ± 1.6 µVolt) and Cz (14.6 ± 1.9) as compared with YN (Fz: 8.1 ± 1.7, t = 3.7, P < 0.005; Cz: 11.8 ± 1.8, t = 2.5, P < 0.05), NY (Fz: 8.2 ± 1.2, t = 2.6, P < 0.05; Cz: 11.5 ± 1.4, t = 2.1, P < 0.05) and NN (Fz: 8.5 ± 1.0, t = 2.9, P < 0.05; Cz: 11.7 ± 1.3, t = 2.5, P < 0.05) stimuli. This difference was not significant on Pz (P-values >0.1) and differences between other stimulus categories were also not significant (P-values >0.3).
Fig. 2.
Average ERP amplitude between 275 and 375 ms after evaluation onset at midline electrodes (n = 17).
Cardiac responses
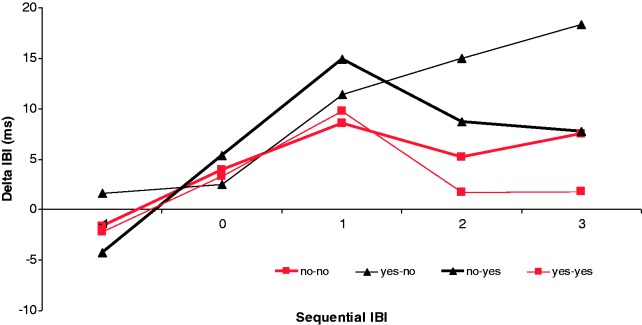
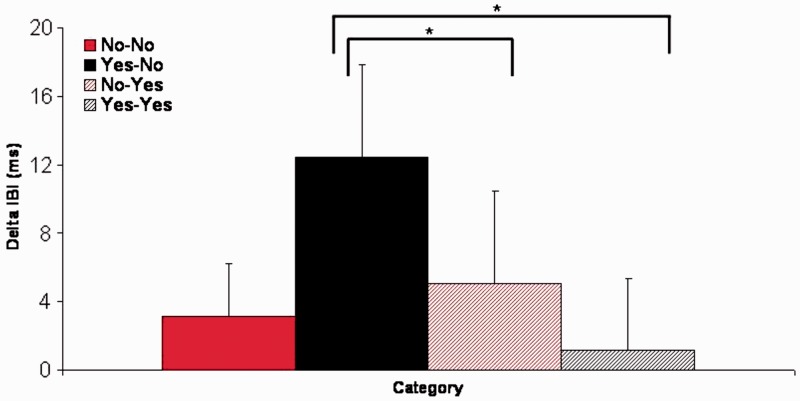
Cardiac responses to the four different stimuli are shown in Figure 3 and were analyzed in a design with expectation, evaluation and sequential IBI as within-subjects factors. In this analysis we found an interaction between evaluation and sequential IBI, F3,48 = 3.9, P < 0.05, η2 = 0.196. Follow-up analyses showed that for IBI2, deceleration was marginally larger for unexpected rejection (YN) as compared with expected acceptance (YY; P = 0.05) and for IBI3 deceleration this difference was significantly larger (P < 0.05). All other comparisons did not result in significant differences. For illustrative purposes, we computed a deceleration measure for all stimulus categories separately by computing the average deceleration on IBI1, IBI2 and IBI3 and subtracting IBI0 from this average. This measure is shown in Figure 4.
Fig. 3.
Cardiac response in terms of delta IBI for expected (No–No) and unexpected (Yes–No) rejections and expected (Yes–Yes) and unexpected (No–Yes) acceptance (n = 17).
Fig. 4.
Average cardiac deceleration computed by summation of IBI1, IBI2 and IBI3 and subtracting IBI0.
Association cardiac and brain responses
The association between brain and cardiac responses was explored by computing correlations between both measures. We computed the correlation between average cardiac deceleration as shown in Figure 4 (see ‘Cardiac responses’ section) and the amplitude in the earlier mentioned interval on Fz for all categories separately. A total of four correlations was computed and these analyses did not yield a significant result (all P-values >0.2).
DISCUSSION
This study aimed at examining the ERP associated with social rejection and replicating the finding of additional cardiac slowing to unexpected social rejection. The present findings show that, as compared with the other conditions, expected acceptance evoked a stronger positive wave around 325 ms post-stimulus onset. With respect to the cardiac results, we found the expected stronger cardiac slowing following unexpected rejection.
The finding that expected acceptance elicited a larger fronto-central positive wave was not in line with our main hypothesis. We hypothesized that especially ‘unexpected’ acceptance would elicit a larger P3 because these events could possibly elicit some kind of orienting response due to the mismatch between expectancy and judgment. Possibly, the social context and present form of the task prevented the build-up of strong expectancies with respect to the negative events. At first sight, the findings seem to be at odds with most theories about impression formation which stress the importance of negative information (e.g. Anderson, 1965). According to these theories, negative information is weighted more heavily than positive information, and in this way it could be expected that unexpected negative events would elicit a larger P3. However, this study did not directly examine impression formation, but examined how participants judged the impression formation process by virtual judges. In this process positive information could be more salient, because it seems more important that a complete stranger likes us than the other way around. In this way the enhanced P3 effect for expected acceptance (YY trials) could be explained by the fact that expected acceptance is linked with some kind of social reward. Several studies using monetary rewards show that the P3 is larger in response to positive outcomes (Hajcak et al., 2007; Bellebaum et al., 2010; van den Berg et al., 2012). It can be hypothesized that it is particularly rewarding for humans to learn that people who you expect to like you indeed confirm that they like you. In this way the P3 amplitude can be linked to the motivational relevance of the stimulus as proposed by Nieuwenhuis et al. (2005a). The idea that participants might have a bias toward wanting to see their predictions for being ‘liked’ confirmed, could potentially have broad significance for their lay theories of social interaction and who is/not likely to be friendly to them. In this way the expected acceptance is the most relevant stimulus. In the study of van den Berg et al. (2012), it was shown for feedback stimuli in a time-estimation paradigm that more relevant stimuli, that is, positive feedback associated with a monetary reward, evoke a larger P3.
The enhanced P3 for expected acceptance is somewhat difficult to relate to the findings with respect to social exclusion and inclusion in the Cyberball paradigm. In this way the current findings point in the direction of a clear distinction between social exclusion as measured in the Cyberball paradigm and social rejection measured in this study. Crowley et al. found that more distress during exclusion events was associated with more negative going late waves on frontal electrodes. They did, however, not directly compare expected and unexpected events. Moreover, they did also not directly compare inclusion and exclusion events. Gutz et al., on the other hand, directly examined expectation and compared inclusion and exclusion events. They found the largest P3a amplitude on events that can be seen as somewhat comparable with our unexpected acceptance (NY) trials. They compared two conditions which differed in terms of the frequency of ball possession. The condition in which the ball possession was lowest was defined as the exclusion condition, and in this condition the largest P3a amplitude was found on ball possession events that can be seen as unexpected and inclusive. The P3a in their study was defined as an area measure between 240 and 320 ms after stimulus onset and in this way overlaps with our area measure. Two important differences between their study and our study make a direct comparison difficult. First, inclusion in the Cyberball paradigm can be seen as the default social rule, whereas the ‘like’ response in the social rejection (SR) paradigm is not a standard social convention. Most people are used to other people liking or disliking them and do not expect that all people will like them on the basis of a first impression. Second, expectation differs between both paradigms. In the Cyberball game, expectation is based on the social rule and how the game is played by others. In the SR task expectation results from individual bias and in this way the participants can regulate the amount of unexpected rejections, whereas in the Cyberball paradigm participants completely depend on external factors. Unexpected acceptance in the Cyberball paradigm is also less frequent and therefore attracts more attention which is reflected in the oddball effect (enhanced P3). New studies should examine the role of exact role of probability, control over expectancy and different social contexts defining social exclusion or rejection. Besides these differences in basic task characteristics, we would like to stress that the most important difference between the Cyberball paradigm and the task used in this study is, of course the studied process itself. In the Cyberball paradigm participants are confronted with exclusion events that violate implicit social rules. In this study, participants are confronted with ‘do not like’ judgments by virtual judges which can be labeled as a mild form of social rejection. Most likely, there is some overlap in the experienced emotions in both paradigms, but the underlying processes leading to these emotions clearly differ. As is the case with many other social laboratory experiments, it is doubtful whether the present ‘do not like’ judgments come close to social rejection experienced in real life situations. For experiencing real social rejection it is necessary that someone has had the opportunity to form a more thorough impression and learn about another person and then receive the negative ‘do not like’ judgment. For both practical and ethical reasons, experiencing real social rejection is not possible in a laboratory situation. Therefore, we think that the present form of rejection is the closest we can get in an ethical experiment and in a controlled laboratory environment.
The cardiac responses were in line with study of Gunther Moor et al. (2010). Like in their study, we found a stronger deceleration to unexpected rejection. As can be seen in Figure 3, the response to unexpected rejections differed from the responses to other stimuli, which shows that only the combination of the negative outcome and the unexpected outcome leads to this cardiac deceleration. Gunther Moor et al. argued on the basis of their findings that the cardiac response to unexpected rejection might be linked to activation of the dACC and is possibly related to the role of the dACC as a neural alarm system that was proposed by Eisenberger and Lieberman (2004).
The differential cardiac and electro-cortical response to the different events in this task and the lack of correlation show that these responses might reflect different processes involved in the evaluation of these complex social stimuli. We speculate that this dissociation between the ERP and HR results might be explained by the differential involvement of the vACC and dACC in this task, as was shown by Somerville et al. (2006). They showed that the dACC was especially active during a violation of expectancy and the vACC was more active during positive vs negative events. The role of the dACC in the detection of violation of expectancy suggests a relation with the cardiac response, which was also sensitive to such a violation of expectancy. A possible relation with dACC activation is also in line with the suggestion of Gunther Moor et al. (2010) who linked the cardiac response to the dACC. We speculate that the enhanced electro-cortical response to expected acceptance might be more related to the vACC and reflects the positive valence of this stimulus. Although we have no direct evidence to support this link, there appears to be some indirect evidence in support of this hypothesis. First, Gutz et al. (2011) found that P3a amplitude in their study was related to the negative mood evoked by exclusion and hypothesized that their P3a amplitudes reflected affective processing and P3b amplitudes reflected the more cognitive aspects of the stimulus. As was argued before, the positive wave found in the present study might be similar to their P3a and, therefore, it could be argued that the amplitude of the positive wave in this study might also reflect the affective aspects of the stimulus. A well-known theory about the differential role of the vACC and dACC states that the vACC is more strongly involved in emotional aspects, whereas the dACC is more involved in cognitive aspects of information processing (Bush et al., 2000). It should be noted, however, that this strict dichotomy has been challenged by more recent reviews (e.g. Eisenberger and Lieberman, 2004; Shackman et al., 2011). Eisenberger and Lieberman noted that ‘Intuitively, pain studies should cluster within the rostral, affective division of the ACC, but instead typically activate dACC’. Shackman et al. proposed the ‘adaptive control hypothesis’ which suggests that the dACC processes affect properties, especially information with a negative value (punishment, pain) to bias responding when the most adaptive course of action is uncertain and therefore integrates emotion, pain and cognitive control. Second, additional indirect evidence comes from an earlier study that showed that the positive going waves to positive feedback in the same latency range in a time estimation paradigm might reflect vACC activation (Nieuwenhuis et al., 2005b). They used the time estimation task in an fMRI experiment and an EEG experiment and found that positive feedback in the fMRI experiment was associated with additional activation in the vACC. Moreover, they found in the EEG experiment that the more positive going wave to positive feedback around 300 ms post-stimulus could be modeled with dipoles located in the vACC and the posterior cingulate cortex. Due to conceptual similarities between positive feedback in their study and expected acceptance in this study, it could be argued that vACC could also be one of the underlying structures involved in evoking the positive going wave in this study. Third, a final piece of indirect evidence comes from a source localization study, which showed that the low gamma-band part of the P3a response can be localized in the vACC (Lee et al., 2007). We would like to stress once more that the hypothesized relation between our positive wave and the activation of the vACC and the relation between the cardiac response and dACC activation needs to be supported by more direct evidence. Furthermore, if the positive wave is related to valence and vACC activation, one would also expect a larger response in the unexpected acceptance condition, which was clearly not the case. It is possible that only in the case of predicting that a person would like them the participants get really involved in the SR task. Only in this special instance the acceptance gives the pleasurable experience evoking the ERP response.
A somewhat unexpected finding was that participants expected about the same percentage rejection as acceptance. This seems to be at odds with previous research showing a person positivity effect or Polyanna effect (Matlin and Stang, 1978). It might be the case that participants in the present experiment changed their expectation during the course of the experiment due to the given feedback. We have tested this hypothesis by comparing the first 20 expectations with the last 20 expectations, but these did not differ significantly (data not shown). There was, however, a large amount of individual variation in the change of expectation over the course of the experiment. This variation might be related to different personality characteristics. As was argued in the discussion with respect to the Cyberball paradigm, people who expect to be rejected might feel rejected much earlier in the task and adjust behavior accordingly. This leads to the prediction that individuals with low self-esteem or high rejection sensitivity may be particularly likely to feel rejected early in the task and might show a different behavioral pattern and therefore a different pattern in ERP responses. More research is clearly needed to further investigate these interesting individual differences.
To summarize, this study replicated the findings of Gunther Moor et al. (2010) by showing that unexpected rejection in the SR task gives rise to additional cardiac slowing. Expected acceptance elicited a stronger fronto-central positive going wave which is possibly related to social reward. It was hypothesized that cardiac and electro-cortical responses were possibly differentially related to activation of the dorsal and the ventral part of the ACC. Future studies have to test this hypothesis by combining imaging methods and relating measures of brain activation and cardiac function to subjective measures and individual differences.
Conflict of Interest
None declared.
REFERENCES
- Anderson NH. Primacy effects in personality impression formation using a generalized order effect paradigm. Journal of Personality and Social Psychology. 1965;2:1–9. doi: 10.1037/h0021966. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Bellebaum C, Polezzi D, Daum I. It is less than you expected: the feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia. 2010;48:3343–50. doi: 10.1016/j.neuropsychologia.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, McCarty ER, David DH, Bailey CA, Mayes LC. Exclusion and micro-rejection: event-related potential response predicts mitigated distress. Neuroreport. 2009;20:1518–22. doi: 10.1097/WNR.0b013e328330377a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–30. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, Crone EA, van der Molen MW. The heartbrake of social rejection: heart rate deceleration in response to unexpected peer rejection. Psychological Science. 2010;21:1326–33. doi: 10.1177/0956797610379236. [DOI] [PubMed] [Google Scholar]
- Gutz L, Kupper C, Renneberg B, Niedeggen M. Processing social participation: an event-related brain potential study. Neuroreport. 2011;22:453–8. doi: 10.1097/WNR.0b013e3283476b67. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It's worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44:905–12. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Park KS, Kang DH, Kang KW, Kim YY, Kwon JS. Generators of the gamma-band activities in response to rare and novel stimuli during the auditory oddball paradigm. Neuroscience Letters. 2007;413:210–5. doi: 10.1016/j.neulet.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. 1998 The AR face database. CVC Tech. Report #24. [Google Scholar]
- Matlin MW, Stang DJ. The Pollyanna Principle: Selectivity in Language, Memory and Thought. Cambridge, MA: Schenkman; 1978. [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005a;131:510–32. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005b;21:3161–8. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Lüders H, Nuwer M, Picton TW. AEEGS guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1991;8:200–2. [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Van den Berg I, Shaul L, Van der Veen FM, Franken IH. The role of monetary incentives in feedback processing: why we should pay our participants. Neuroreport. 2012;23:347–53. doi: 10.1097/WNR.0b013e328351db2f. [DOI] [PubMed] [Google Scholar]
- Van der Veen FM, Sahibdin PP. Dissociation between medial frontal negativity and cardiac responses in the ultimatum game: Effects of offer size and fairness. Cognitive, Affective, & Behavioral Neuroscience. 2011;11:516–25. doi: 10.3758/s13415-011-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]