Abstract
Dopamine beta-hydroxylase (DBH), an enzyme that converts dopamine to norepinephrine, has broad influences on social functions. In this study, we examined to what extent two polymorphisms (−1021C/T and a 19 bp insertion/deletion) in DBH gene modulate individuals’ empathic perception and response, which were measured, respectively, by reading the mind in the eyes test and the empathic concern subscale of interpersonal reactivity index. Results showed that polymorphism at −1021C/T, but not the 19 bp insertion/deletion, accounts for 2.3% variance of empathic perception and 1.4% variance of empathic response. Individuals with the CC genotype, which is associated with higher DBH activity, manifested greater empathic ability than those with CT/TT genotypes. These findings demonstrate the importance of DBH −1021C/T as a genetic basis of empathy and in predicting individual differences in social and affective processing.
Keywords: dopamine beta-hydroxylase, DBH, −1021C/T, polymorphism, empathy
INTRODUCTION
Empathy, the ability to understand and experience the mental state of another person, is fundamental for living in social groups and caring for others. It is composed of two major components, cognitive empathy and affective empathy, both of which can be further divided into a variety of subskills and systems, such as empathic perception (the ability to perceive and identify another person’s internal state) and empathic response (the ability to share other persons’ feelings) (Baron-Cohen and Wheelwright, 2004; Batson, 2008; Shamay-Tsoory, 2008). These abilities allow us to predict and understand others’ motives, intentions, thoughts and emotions, so as to promote altruistic behavior and inhibit aggressive behavior (Mehrabian et al., 1988). Impaired empathic ability is a central characteristic of social behavioral abnormalities such as autism spectrum disorders (Dziobek et al., 2008) and schizophrenia (Shamay-Tsoory et al., 2007).
Empathic abilities vary widely between individuals. A twin study suggested that the heritability of empathy is 0.34–0.47 (Knafo et al., 2008). However, the existing evidence is insufficient for us to clearly understand the molecular basis of empathy. The main purpose of this study was to investigate to what extent dopamine beta-hydroxylase (DBH) gene modulates empathic perception and response.
Animal and human studies concerning the biochemical foundation of empathy suggest that the dopaminergic system and noradrenergic system are crucial for empathy-related behaviors. Human studies demonstrated that lower dopamine levels are associated with higher donation of money to a poor child in a developing country (Reuter et al., 2011) and with better performance in a theory of mind task measuring the ability to predict the behavior or thoughts of others in a simple social context (Bassett et al., 2007). Human studies also showed that higher norepinephrine levels are associated with better recognition and recall of positive emotional stimuli (Harmer et al., 2009) and with increased interpersonal cooperation in daily interaction (Tse and Bond, 2003). Given the positive relationship between norepinephrine levels and empathy-related behaviors and the negative relationship between dopamine levels and social behaviors, it is plausible that an enzyme with the ability to modulate the dopamine and norepinephrine levels, would in turn modulate individuals’ empathic ability and empathy-related behaviors.
DBH is an enzyme that converts dopamine to norepinephrine. Inhibiting DBH activity increases dopamine levels and decreases norepinephrine levels (Robertson et al., 1986). Previous studies confirmed the important role of DBH in social functions: Dbh knockout mice exhibit deficits in discriminating familiar and unfamiliar mice (Marino et al., 2005) and in retrieving neonates scattered in the home cage (Thomas and Palmiter, 1997). Humans evidencing social dysfunctions such as autistic patients (and their mothers) have lower plasma DBH activity than controls (Lake et al., 1977; Robinson et al., 2001).
DBH is coded by a single gene DBH which is located on chromosome 9q34 (Craig et al., 1988; Kobayashi et al., 1989). In humans, the genetic variations of DBH account for 98% of variance in plasma DBH activity (Oxenstierna et al., 1986). Two polymorphisms (−1021C/T, a 19 bp insertion/deletion) are tightly linked to the plasma DBH activity. −1021C/T (also labeled as rs1611115), a genetic variant located in the 5′ upstream region of DBH, accounts for 35–52% of variance in plasma DBH activity (Zabetian et al., 2001). Homozygosity for the T allele of −1021C/T is associated with lower plasma DBH activity. The 19 bp insertion/deletion (GeneBank: X63418), a polymorphism located in the 4.5 kb upstream of the transcriptional start site, also plays a role in the plasma DBH activity. The deletion (D) allele of this polymorphism indicates lower plasma DBH activity, whereas the insertion (I) allele indicates higher plasma DBH activity (Cubells et al., 2000).
Given the link between the DBH enzyme and empathic ability and behaviors and the link between the DBH activity and DBH genetic variations, we hypothesize that genotypes of −1021C/T and the 19 bp insertion/deletion are associated with empathy. Specifically, we predict that individuals with the genotypes leading to higher DBH activity (CC of −1021C/T, II of the 19 bp insertion/deletion), would have higher empathic abilities or tendency than individuals with the genotypes leading to lower DBH activity (CT or TT of −1021C/T, ID or DD of the 19 bp insertion/deletion). Moreover, as −1021C/T accounts for a majority of variation in DBH activity (Zabetian et al., 2001), it is possible that the genetic variations in −1021C/T could account for more individual differences in empathic perception and response than the variations in the 19 bp insertion/deletion. To measure participants’ empathic perception, we used the reading the mind in the eyes test (RMET; Baron-Cohen et al., 2001) in which participants recognized or inferred others’ emotional states by using visual cues from eye regions. This task has been shown to have high validity in measuring the individual’s ability of inferring others’ internal emotional state (Baron-Cohen et al., 2001; Vellante et al., 2012) and it has been widely used in previous studies to link empathic perception with individuals’ genetic polymorphisms or hormone levels (Domes et al., 2007; Rodrigues et al., 2009; van Honk et al., 2011). To measure participants’ empathic response, we used the empathic concern subscale in interpersonal reactivity index (IRI; Davis, 1983). This subscale has been shown to be sensitive to individuals’ empathic response to others’ misfortune (Davis, 1983; Rankin et al., 2006; Rodrigues et al., 2009). Previous studies showed that patients with abnormality in the dopaminergic system, including patients with Parkinson’s disease or schizophrenia, have deficits both in tasks measuring empathic perception (Tsuruya et al., 2011; Kucharska-Pietura et al., 2012) and in tasks measuring empathic response (Smith et al., 2012; Narme et al., 2013). The DBH polymorphisms, thus, might modulate individuals’ empathic perception and response in similar manners. On the other hand, previous neuroimaging studies also showed that empathic perception and response have both the same (e.g. inferior frontal gyrus) and differential neural substrates (e.g. posterior superior temporal sulcus for empathic perception, anterior insular for empathic response) (for reviews, see Adams et al., 2010; Bernhardt and Singer, 2012). It is thus also plausible that the DBH polymorphisms modulate individuals’ empathic perception and response in different ways.
METHODS
Participants
Three hundred and twenty-nine unrelated, unselected Chinese Han senior students (202 female, mean age = 22.3 ± 1.0 years) were recruited from Henan University of Science and Technology, China. The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Department of Psychology, Peking University. Informed written consents were obtained from each participant.
Genotyping
Genomic DNA was extracted from hair follicle cells using Chelex-100 method (de Lamballerie et al., 1994). −1021C/T (rs1611115) in DBH gene was amplified by polymerase chain reaction (PCR). The upstream primer, 5′-GGAGGGACAGCTTCTAGTCC-3′, and the downstream primer, 5′-TCAGTCTCACCACGGCAC-3′, were recruited. A 149 bp product was amplified with an initial 3 min denaturation at 94°C, followed by 35 cycles of 94°C for 30 s, 63°C for 45 s, 72°C for 1 min and a final extension period at 72°C for 10 min. Genotyping was performed by single strand conformation polymorphism method. On genotyping, six samples selected randomly were sequenced to determinate the alleles of genotyping results. The distribution of genotypes (CC = 225, CT = 96, TT = 8) showed no deviation from Hardy–Weinberg equilibrium (χ2 = 0.36, P = 0.55).
The 19 bp insertion/deletion polymorphism (GeneBank: X63418) in DBH gene was amplified using the upstream primer, 5′-GCA AAAGTCAGGCACATGCACC-3′ and the downstream primer, 5′-GTCAGCGAGATGGGGAGGTGGA-3′. Cycling conditions consisted of an initial denaturation at 94°C lasting for 5 min, followed by 35 cycles with denaturation at 94°C for 30 s, an annealing at 60°C for 30 s and an extension at 72°C for 1 min. Finally, an extension period at 72°C was conducted for 5 min, and then the PCR products were genotyped by 8% polyacrylamide gel electrophoresis for 3 h. On genotyping, six of the samples randomly selected from each of genotype groups were sequenced to further determinate the allele of the genotyping results. The distribution of genotypes (II = 109, ID = 148, DD = 59) also showed no deviation from Hardy–Weinberg equilibrium (χ2 = 0.49, P = 0.49).
Reading the mind in the eyes test
RMET is a paper-and-pencil test that consists of 36 items; each item consists of a photograph displaying eye regions of a Caucasian individual and four possible adjectives describing the current emotional or mental state of the pictured individual. These adjectives were presented in both the original English and in Chinese to keep it as close as possible to the original RMET. Participants made a forced choice from the four alternatives without time constraints. The internal consistency (Cronbach's α) in this study was 0.670, which is comparable to what was reported in the previous study (Vellante et al., 2012; α = 0.605).
Empathic concern
The participants completed the 28-item IRI (Davis, 1983). It consists of four 7-item subscales, two affective subscales (empathic concern, personal distress) and two cognitive subscales (perspective taking, fantasy). Empathic concern measures the feeling of affection and concern in response to the misfortune of others (e.g. ‘I often have tender, concerned feelings for people less fortunate than me’). Personal distress taps into ‘self-oriented’ feelings of personal anxiety and unease when observing the anguish and pain endured by others. Perspective taking evaluates the individuals' cognitive propensity to spontaneously adopt the psychological point of view of others. Fantasy assesses the extent to which people immerse themselves into the feelings and actions of fictitious characters. For each item, the participant judged on a five-point Likert scale to what extent the description applied to himself/herself, with 0 indicating ‘does not describe me well’ and 4 indicating ‘describes me very well’. The internal consistencies for empathic concern, personal distress, perspective taking and fantasy, as measured with Cronbach’s α, were 0.630, 0.728, 0.614 and 0.507, respectively. They were slightly lower than the scores reported in the original work (Davis, 1980; 0.68 ≤ α ≤ 0.79).
RESULTS
Empathic perception
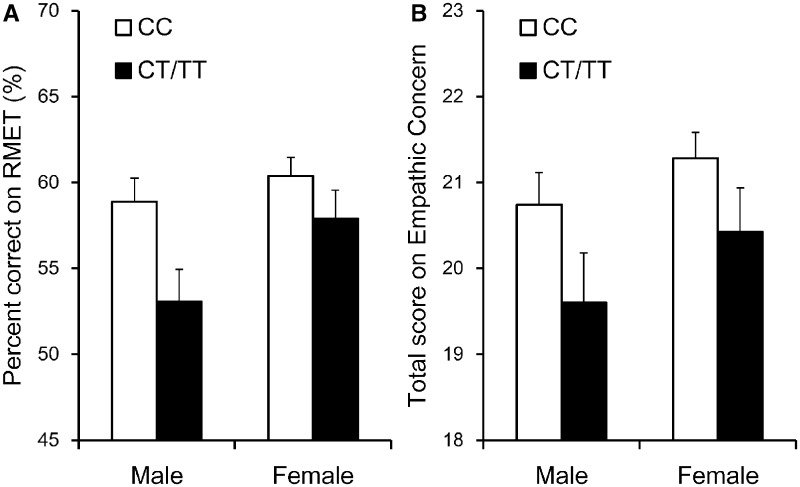
To assess the individuals’ ability in emotion recognition and empathic perception, we analyzed the percentage of correct responses on RMET. Seven participants (2.1%, five females) were excluded from analysis because their scores were at chance level (25%). The mean response accuracy for the remaining 322 participants was 59% (s.d. = 11%), which was lower than the 78% (s.d. = 10%) accuracy originally reported in Baron-Cohen et al. (2001). However, this difference was consistent with Adams et al. (2010) who demonstrated a cultural difference in RMET. Given that there is gender difference in empathic perception (Baron-Cohen et al., 2001) and empathic response (O’Brien et al., 2013), we include gender as a between-participant factor in the following analyses (Figure 1).
Fig. 1.
Effects of −1021C/T polymorphism on the response accuracy in RMET and on the total score on the IRI empathic concern subscale in males and females. (A) Individuals with the CC (N = 220) genotype performed better in RMET than individuals with the CT/TT (N = 102) genotypes. Seven participants were excluded due to their random guessing in the task. (B) Individuals with the CC (N = 225) genotype showed stronger empathic responses than individuals with the CT/TT (N = 104) genotypes. A five-point Likert scale (0 = ‘does not describe me well’ and 4 = ‘describes me very well’) was used for each item. Standard errors of the means are shown as error bars.
For −1021C/T, a 2 (gender: male vs female) × 2 (genotype: CC vs CT/TT) ANOVA revealed a main effect of gender, F(1, 318) = 5.242, P = 0.023, partial η2 = 0.016, with females performed better than males (60% ± 11% vs 57% ± 12%). Importantly, the main effect of genotype was also significant, F(1, 318) = 8.975, P = 0.003 and partial η2 = 0.027. This effect of genotype remained to be significant when the seven excluded participants were included, F(1, 325) = 5.824, P = 0.016 and partial η2 = 0.018. Individuals with CC genotype (60% ± 11%) performed significantly better than individuals with CT/TT genotypes (56% ± 12%). The interaction between gender and genotype was not significant, F(1, 318) = 1.445, P = 0.230 and partial η2 = 0.005. Regression analysis with −1021C/T polymorphism (0 = CT/TT, 1 = CC) as a single predictor of RMET indicated that this polymorphism accounted for a significant proportion of the variance in RMET, F(1, 320) = 7.460, P = 0.007, β = 0.151, R2 = 0.023 and adjusted R2 = 0.020. This result continues to hold after controlling for gender (step 1, entering gender with 0 = male and 1 = female; step 2, entering both gender and −1021C/T polymorphism), F(1, 319) change = 7.765, P = 0.006, β = 0.153 and R2 change = 0.023. For the 19 bp insertion/deletion, however, a 2 (gender: male vs female) ×3 (genotype: II vs ID vs DD) ANOVA found no significant RMET score difference between individuals with II (58% ± 12%), ID (59% ± 11%) and DD (59% ± 10%) genotypes, F(2, 303) = 0.078, P = 0.925, partial η2 = 0.001, nor the interaction between gender and genotype, F(2, 303) = 0.752, P = 0.472 and partial η2 = 0.005.
Empathic response
We used the total score on the IRI empathic concern subscale to measure participants’ empathic responses. For −1021C/T, a 2 (gender: male vs female) × 2 (genotype: CC vs CT/TT) ANOVA showed no main effect of gender, F(1, 325) = 2.275, P = 0.132, partial η2 = 0.007, but a main effect of genotype, F(1, 325) = 4.895, P = 0.028 and partial η2 = 0.015. Individuals with CC genotype (21.1 ± 3.5) showed greater empathic response to others’ misfortune than those with CT/TT genotypes (20.1 ± 3.9). The interaction between gender and genotype was not significant, F(1, 325) = 0.098, P = 0.754 and partial η2 < 0.001. Regression analysis with −1021C/T polymorphism as the only predictor indicated that this polymorphism accounted for a significant proportion of the variance in empathic concern, F(1, 327) = 4.669, P = 0.031, β = 0.119, R2 = 0.014 and adjusted R2 = 0.011. This finding continues to hold after controlling for gender (step 1, entering gender; step 2, entering both gender and −1021C/T polymorphism), F(1, 326) change = 4.872, P = 0.028, β = 0.121 and R2 change = 0.015. For the 19 bp insertion/deletion, a 2 (gender: male vs female) ×3 (genotype: II vs ID vs DD) ANOVA found no significant difference in empathic concern between individuals with II (20.9 ± 3.7), ID (20.5 ± 3.6) and DD (21.3 ± 3.9) genotypes, F(2, 310) = 0.672, P = 0.512, partial η2 = 0.004, nor interaction between gender and genotype, F(2, 310) = 0.017, P = 0.983 and partial η2 < 0.001.
When testing participants, we also included the other three subscales of IRI (fantasy, perspective taking, personal distress). For −1021C/T, when we submitted the scores in these subscales to 2 (gender: male vs female) × 2 (genotype: CC vs CT/TT) ANOVAs respectively, we observed neither a main effect of genotype nor an interaction between genotype and gender, all Ps > 0.10. For the 19 bp insertion/deletion, when we submitted the scores in these subscales to 2 (gender: male vs female) ×3 (genotype: II vs ID vs DD) ANOVAs, respectively, we found only a significant main effect of genotype for perspective taking, with individuals of the DD type (19.0 ± 3.3) showed greater tendency in perspective taking than individuals of II (17.7 ± 3.5) or ID (17.6 ± 3.7) type, both Ps < 0.05 (post hoc LSD). However, given that the number of participants of the DD genotype was relatively small compared with the number of participants of the other types, this finding needs to be verified in further studies.
We also examined the possible associations between DBH polymorphisms and the overall scores on the cognitive subscales (perspective taking + fantasy) and the affective subscales (empathic concern + personal distress) in IRI (Davis, 1983). For DBH −1021C/T, 2 (gender: male vs female) × 2 (genotype: CC vs CT/TT) ANOVAs on affective and cognitive subscales revealed neither main effects of genotype nor interactions between genotype and gender (Ps > 0.10) but a significant main effect of genotype on affective subscales, F(1, 325) = 4.121, P = 0.043 and partial η2 = 0.013. Individuals with CC genotype (36.0 ± 6.1) showed a greater tendency of affective empathy than those with CT/TT genotypes (34.6 ± 6.9), consistent with the effect of −1021C/T on empathic concern. However, it should be noted that there was no genotype effect on personal distress alone. This is not surprising given that items in personal distress (e.g. ‘In emergency situations, I feel apprehensive and ill at ease’) assess emotional self-control rather than the tendency to share others’ feelings (Baron-Cohen and Wheelwright, 2004). The null effect on perspective taking and fantasy was inconsistent with the significant effect on RMET, possibly because these tasks measure different aspects of cognitive empathy, as outlined previously. For the 19 bp insertion/deletion, no effect of genotype was found on the combined affective or cognitive subscales.
DISCUSSION
In this population-based study, we found that −1021C/T, but not the19 bp insertion/deletion, of DBH gene modulates individuals’ empathic perception and response. As we predicted, individuals with the CC genotype of −1021C/T manifested greater empathic ability than those with one or two copies of the T allele.
The functional dissociations between −1021C/T and the19 bp insertion/deletion are not entirely surprising given that variations in DBH activity are mainly accounted for by −1021C/T polymorphism (Zabetian et al., 2001). Importantly, the present findings concerning −1021C/T polymorphism are consistent with previous observations regarding the positive association between DBH activity and affiliative behavior and social memory (Thomas and Palmiter, 1997; Marino et al., 2005). Given that DBH is the unique synthetic enzyme that converts dopamine to norepinephrine (Thomas et al., 1998), our findings are also consistent with studies that demonstrated the roles of dopaminergic and noradrenergic systems in empathic abilities and empathy-related behaviors (Tse and Bond, 2003; Bassett et al., 2007; Harmer et al., 2009; Reuter et al., 2011). The important advance made by this study is that we directly demonstrated the link between −1021C/T polymorphism and individuals’ empathic abilities.
Our demonstration concerning the importance of DBH gene in empathic perception and response may have clinical implications for individuals with severe impairment in empathy-related behaviors. Clinical studies have found that individuals with autism spectrum disorders have lower DBH activity (Lake et al., 1977) and perform worse in empathy-related tasks (Baron-Cohen et al., 2001; Dapretto et al., 2006; Dziobek et al., 2008) than controls. Although these studies as a whole evidenced the impaired empathic abilities and lower DBH activity in autistic patients, they failed to directly test the link between DBH activity and autistic symptoms. This study went further by demonstrating that the DBH gene, the main determiner of DBH activity, is associated with empathic abilities in healthy population. It would be a fruitful endeavor for further studies to investigate in detail the genotyping of DBH −1021C/T and the diagnosis, treatment and prognosis of autism spectrum disorders (and other psychiatric disorders).
Several limitations of this study should be noted. First, the tasks we used to measure participants’ empathic abilities may not be optimal in revealing the underlying constructs of empathy. Here, Chinese participants were tested with a Caucasian version of RMET and the cultural differences in expressing emotional cues around the eye regions or in perceiving these cues could have added noises to our measurement (Duchenne and Cuthbertson, 1990; Jack et al., 2012). These noises could lead to either false positive in statistical analysis or underestimation of the contribution of DBH gene polymorphism to individuals’ empathic abilities. Second, we focused on the modulatory role of a single gene in empathic abilities while these abilities are likely to be influenced by multiple genes and by the interaction between genetic variations and environment. More systematic studies are needed to take into consideration a variety of genetic, neurophysiological and social factors in revealing the underlying mechanisms for individual differences in empathic and affective processing. Finally, all the participants in this study were Chinese. As some studies showed that the relations between genes and social behaviors can be modulated by culture (Kim et al., 2010, 2011), it would be interesting to investigate the potential cultural differences in the association between DBH polymorphisms and empathic abilities.
Acknowledgments
This study was supported by grants from National Basic Research Program of China (973 Program: 2010CB833904), China Postdoctoral Science Foundation (2013M530002), Natural Science Foundation of China (30110972, 91232708), and China Postdoctoral Science Foundation (2013M530002). We thank Mr Peizhe Zhang, Mr Guochang Cao, Miss Hua Ma and Miss Lin Lei for their assistances in data collection, and Mr Philip Blue and two anonymous reviewers for their comments on an earlier version of the manuscript.
REFERENCES
- Adams RB, Rule NO, Franklin RG, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience. 2010;22:97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:241–51. [PubMed] [Google Scholar]
- Batson CD. These things called empathy: eight related but distinct phenomena. In: Decety J, Lckes W, editors. The Social Neuroscience of Empathy. Cambridge, MA: The MIT Press; 2008. pp. 3–15. [Google Scholar]
- Bassett AS, Caluseriu O, Weksberg R, Young DA, Chow EWC. Catechol-O-methyl transferase and expression of schizophrenia in 73 adults with 22q11 deletion syndrome. Biological Psychiatry. 2007;61:1135–40. doi: 10.1016/j.biopsych.2006.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual Review of Neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Craig SP, Buckle VJ, Lamouroux A, Mallet J, Craig IW. Localization of the human dopamine beta hydroxylase (DBH) gene to chromosome 9q34. Cytogenetics and Cell Genetics. 1988;48:48–50. doi: 10.1159/000132584. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, et al. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS: Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–26. [Google Scholar]
- de Lamballerie X, Chapel F, Vignoli C, Zandotti C. Improved current methods for amplification of DNA from routinely processed liver tissue by PCR. Journal of Clinical Pathology. 1994;47:466–7. doi: 10.1136/jcp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Duchenne GB, Cuthbertson RA, editors. The Mechanisms of Human Facial Expression or an Electrophysiological Analysis of the Expression of the Emotions. New York: Cambridge University Press; 1990. [Google Scholar]
- Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) Journal of Autism and Developmental Disorders. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. The American Journal of Psychiatry. 2009;166:1178–84. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Jack RE, Garrod OGB, Yu H, Caldara R, Schyns PG. Facial expressions of emotion are not culturally universal. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7241–4. doi: 10.1073/pnas.1200155109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Mojaverian T, et al. Gene-culture interaction: oxytocin receptor polymorphism (OXTR) and emotion regulation. Social Psychological and Personality Science. 2011;2:665–72. [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15717–21. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Zahn-Waxler C, Van Hulle C, Robinson JL, Rhee SH. The developmental origins of a disposition toward empathy: genetic and environmental contributions. Emotion. 2008;8:737–52. doi: 10.1037/a0014179. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kurosawa Y, Fujita K, Nagatsu T. Human dopamine beta-hydroxylase gene: two mRNA types having different 3’-terminal regions are produced through alternative polyadenylation. Nucleic Acids Research. 1989;17:1089–102. doi: 10.1093/nar/17.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska-Pietura K, Tylec A, Czernikiewicz A, Mortimer A. Attentional and emotional functioning in schizophrenia patients treated with conventional and atypical antipsychotic drugs. Medical Science Monitor. 2012;18:CR44–49. doi: 10.12659/MSM.882202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Archives of General Psychiatry. 1977;34:553–6. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdélat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behavioural Brain Research. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mehrabian A, Young AL, Sato S. Emotional empathy and associated individual differences. Current Psychology. 1988;7:221–240. [Google Scholar]
- Narme P, Mouras H, Roussel M, Duru C, Krystkowiak P, Godefroy O. Emotional and cognitive social processes are impaired in Parkinson’s disease and are related to behavioral disorders. Neuropsychology. 2013;27:182–92. doi: 10.1037/a0031522. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Konrath SH, Grühn D, Hagen AL. Empathic concern and perspective taking: linear and quadratic effects of age across the adult life span. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2013;68:168–75. doi: 10.1093/geronb/gbs055. [DOI] [PubMed] [Google Scholar]
- Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals—a genetic study. Journal of Psychiatric Research. 1986;20:19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Frenzel C, Walter NT, Markett S, Montag C. Investigating the genetic basis of altruism: the role of the COMT Val158Met polymorphism. Social Cognitive and Affective Neuroscience. 2011;6:662–8. doi: 10.1093/scan/nsq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Goldberg MR, Onrot J, et al. Isolated failure of autonomic noradrenergic neurotransmission. Evidence for impaired beta-hydroxylation of dopamine. The New England Journal of Medicine. 1986;314:1494–7. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- Robinson PD, Schutz CK, Macciardi F, White BN, Holden JJ. Genetically determined low maternal serum dopamine beta-hydroxylase levels and the etiology of autism spectrum disorders. American Journal of Medical Genetics. 2001;100:30–6. doi: 10.1002/ajmg.1187. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. Empathic processing: its cognitive and affective dimensions and neuroanatomical basis. In: Decety J, Lckes W, editors. The Social Neuroscience of Empathy. Cambridge, MA: The MIT Press; 2008. pp. 215–32. [Google Scholar]
- Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology. 2007;21:431–8. doi: 10.1037/0894-4105.21.4.431. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophrenia Research. 2012;137:196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–92. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. Journal of Neurochemistry. 1998;70:2468–76. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- Tse WS, Bond AJ. Reboxetine promotes social bonding in healthy volunteers. Journal of Psychopharmacology. 2003;17:189–95. doi: 10.1177/0269881103017002007. [DOI] [PubMed] [Google Scholar]
- Tsuruya N, Kobayakawa M, Kawamura M. Is “reading mind in the eyes” impaired in Parkinson’s disease? Parkinsonism and Related Disorders. 2011;17:246–8. doi: 10.1016/j.parkreldis.2010.09.001. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Bos PA, Kruijt A-W, Lentjes EG, Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3448–52. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellante M, Baron-Cohen S, Melis M, et al. The “Reading the Mind in the Eyes” test: systematic review of psychometric properties and a validation study in Italy. Cognitive Neuropsychiatry. 2012;2012:1–29. doi: 10.1080/13546805.2012.721728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. American Journal of Human Genetics. 2001;68:515–22. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]