Abstract
In major depressive disorder (MDD), as a network-level disease, the pathophysiology would be displayed to a wide extent over the brain. Moreover, the network-wide changes could be dependent on the context of affective processing. In this study, we sought affective state-dependent changes of the brain functional network by applying a graph-theoretical approach to functional magnetic resonance imaging data acquired in 13 patients with MDD and 12 healthy controls who were exposed to video clips inducing the negative, neutral or positive affective state. For each affective condition, a group-wise brain functional network was constructed based on partial correlation of mean activity across subjects between brain areas. Network parameters, global and local efficiencies, were measured from the brain functional network. Compared with controls’, patients’ brain functional network shifted to the regular network in the topological architecture, showing decreased global efficiency and increased local efficiency, during negative and neutral affective processing. Further, the shift to the regular network in patients was most evident during negative affective processing. MDD is proposed to provoke widespread changes across the whole brain in an affective state-dependent manner, specifically in the negative affective state.
Keywords: major depressive disorder, fMRI, graph-theoretical analysis, affective processing
INTRODUCTION
The pathophysiology of major depressive disorder (MDD) includes affective dysfunction, which is supposed to be rooted in the cortico-limbic neural circuit. In MDD, changes in the cortico-limbic connectivity have been exhibited at rest (Anand et al., 2005, 2009; Horn et al., 2010; Zhou et al., 2010; Cao et al., 2012; Zhu et al., 2012) and during affective processing (Anand et al., 2005; Almeida et al., 2009; Friedel et al., 2009; Carballedo et al., 2011; Lisiecka et al., 2011; Perlman et al., 2012).
Considering widespread dysfunction among a variety of brain areas, MDD is conceptualized as a network-level disease. The pathophysiology of MDD may therefore be seen over the whole brain network. Notably, topological configuration of the whole brain network can be described in terms of network parameters provided by graph-theoretical analysis, which has successfully identified abnormalities of network configuration in patients with various diseases (for reviews, see Bassett and Bullmore, 2009; Guye et al., 2010; Wen et al., 2011).
Graph-theoretical analysis indeed showed alterations in topological architecture of the brain functional network in MDD (Leistedt et al., 2009; Jin et al., 2011; Zhang et al., 2011). Those studies employed neuroimaging data at rest (Jin et al., 2011; Zhang et al., 2011) or during sleeping (Leistedt et al., 2009). Even though the task-independent changes may reflect intrinsic disruptions, topological characteristics of the brain functional network could depend on the context of processing.
In task-related neuroimaging studies, patients with MDD have repeatedly demonstrated alterations of activity and connectivity specifically to negative affective stimuli. The hypothesis that cortical regulation of limbic brain areas is impaired during negative affective processing in MDD could be extended to task context-dependent topological characteristics of the brain functional network. For the aim, in this study, we applied graph-theoretical analysis to functional magnetic resonance imaging (fMRI) data acquired during affective processing to stimuli with different valence. Disruption of topological architecture in MDD was assessed by comparing network parameters between controls and patients. Context-dependence of the network disruption was searched for by exploring topological characteristics to different stimuli.
Here, we hypothesized that disruption of topological architecture in MDD would be found in the task-related brain functional network as well. Furthermore, task context-dependent changes in connectivity in previous studies could be represented in terms of topological characteristics.
METHODS
Subjects
Thirteen female patients with MDD (age: 35.3 ± 9.8 years) were recruited for this study. Twelve age- and sex-matched healthy subjects (age: 36.5 ± 4.9) served as controls. The shared exclusion criteria for the patients and controls were meeting DSM-IV criteria for any other psychiatric disorders other than MDD, history of alcohol or substance abuse or electroconvulsive therapy within the prior 6 months, chronic neurological disorders, severe or acute medical conditions, breast-feeding or any contraindications to MRI scanning.
The patients with MDD were diagnosed according to MINI International Neuropsychiatric Interview (Sheehan et al., 1998) by a board-certified psychiatrist (K.U.L.). Depression and anxiety were measured by the 17-item Hamilton (1960) Rating Scale for Depression (HAMD), Hamilton (1959) Rating Scale for Anxiety (HAMA), Beck Depression Inventory (BDI) (Beck and Steer, 1987) and State and Trait Anxiety Inventory (S/TAI) (Spielberger, 1983).
The study was conducted with the understanding and full written consent of each subject and was approved by the Institutional Review Board at the Uijeongbu St. Mary’s Hospital, the Catholic University of Korea.
Experimental paradigm
During fMRI scanning, the subjects attempted to solve geometric puzzles that had no solution while they were shown pre-recorded video clips of facial expressions depicting negative, neutral and positive feedback regarding their performance. Each puzzle was presented for 4 s followed by 4 s of feedback of facial expressions, and then a 4 s period in which subjects rated their subjective reaction to the feedback they received. Each trial lasted a total of 28 s and the whole experiment included 60 trials for each type of affective stimuli.
Acquisition of imaging data
Magnetic resonance (MR) images were acquired using a 1.5 T Avanto system (Siemens AG, Erlangen, Germany). In each subject, T2*-weighted echo planar functional MR images were acquired under three consecutive sessions. At each session, a total of 307, 341 and 312 whole brain images were collected, respectively, with the blood-oxygen-level-dependent contrast (repetition time = 2000 ms, echo time = 24 ms, number of slices = 29, slice thickness = 4 mm, matrix size = 64 × 64, in-plane resolution = 3.59 mm × 3.59 mm). A T1-weighted structural MR image was also acquired for coregistration to functional images in each subject (number of slices = 160, slice thickness = 1 mm, matrix size = 512 × 512, in-plane resolution = 0.45 mm × 0.45 mm).
Analysis of functional images
Preprocessing and statistical analysis of functional images were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing steps included spatial realignment of a series of volumes, normalization into the same coordinate frame as the MNI template brain with transformation parameters derived from segmentation of the high resolution structural image coregistered to the mean functional image and smoothing using a Gaussian filter of 8 mm FWHM (full width at half maximum).
In statistical analysis, the general linear model included five regressors: three affective conditions (positive, neutral and negative), fixation and a mean over scans. Voxel-wise parameter estimates were calculated in the model and contrasts of the parameter estimates of each affective condition relative to fixation were collected.
Correlation of mean activity
Seventy-three brain areas were parcellated from the whole brain grey matter, based on the automated anatomical labelling brain atlas (Tzourio-Mazoyer et al., 2002). The list of the 73 brain areas is provided in Table 1.
Table 1.
Brain areas used for defining nodes of the brain functional network. Except the cerebellar vermis, all brain areas exist as a pair in the left and right hemispheres
| No. | Brain area | Sub-brain region |
|---|---|---|
| 1/2 | Precentral gyrus | Sensorimotor |
| 3/4 | Supplementary motor area | |
| 5/6 | Postcentral gyrus | |
| 7/8 | Paracentral lobule | |
| 9/10 | Superior frontal gyrus | Frontal |
| 11/12 | Middle frontal gyrus | |
| 13/14 | Inferior frontal gyrus | |
| 15/16 | Olfactory cortex | |
| 17/18 | Straight gyrus | |
| 19/20 | Superior parietal gyrus | Parietal |
| 21/22 | Inferior parietal gyrus | |
| 23/24 | Supramarginal gyrus | |
| 25/26 | Angular gyrus | |
| 27/28 | Precuneus | |
| 29/30 | Calcarine fissure and surrounding cortex | Occipital |
| 31/32 | Cuneus | |
| 33/34 | Lingual gyrus | |
| 35/36 | Superior occipital gyrus | |
| 37/38 | Middle occipital gyrus | |
| 39/40 | Inferior occipital gyrus | |
| 41/42 | Fusiform gyrus | |
| 43/44 | Heschl gyrus | Temporal |
| 45/46 | Superior temporal gyrus | |
| 47/48 | Middle temporal gyrus | |
| 49/50 | Inferior temporal gyrus | |
| 51/52 | Insula | Limbic/paralimbic |
| 53/54 | Anterior cingulate gyrus | |
| 55/56 | Median cingulate gyrus | |
| 57/58 | Posterior cingulate gyrus | |
| 59/60 | Hippocampus | |
| 61/62 | Parahippocampal gyrus | |
| 63/64 | Amygdala | |
| 65/66 | Caudate nucleus | Subcortical |
| 67/68 | Putamen | |
| 69/70 | Thalamus | |
| 71/72 | Cerebellar hemisphere | Cerebellar |
| 73 | Cerebellar vermis | |
Mean activity of each brain area for individual affective condition was measured by reading contrast values acquired in subject-level analysis. Under the condition that there were more than 10 suprathreshold voxels which were activated for each affective condition at an uncorrected P-value ≤ 0.05, the mean activity was calculated from those voxels; otherwise, from all voxels within a brain area. Thus, from each brain area, a series of mean activity values across subjects (12 values for the control group and 13 values for the patient group) were collected. The distribution of mean activity values of each group for individual affective conditions is displayed using a box plot in Supplementary Figure S1. We intended to exclude any subjects who were marked as outliers (red crosses in Supplementary Figure S1) consistently across the three affective conditions for each brain area or consistently across the 73 brain areas for each affective condition, but no subjects were excluded under the criteria.
Group-wise functional connectivity was estimated by correlating the mean activity series between every pair of brain areas. Partial correlation was used to correct for dependencies between brain areas. Finally, a 73 × 73 correlation matrix, each element of which being a correlation coefficient between two brain areas, was acquired for each group.
Network construction
A network can be constructed by defining nodes and estimating edges. Here, 73 brain areas constituted nodes of the whole brain functional network and edges between the nodes were estimated from the correlation matrix of mean activity. A specific selection of a threshold would convert the correlation matrix to a binary matrix consisting of 1s (presence of edges) and 0s (absence of edges) corresponding to correlation coefficients above and below the threshold, respectively. That is, each threshold determines the sparsity of a network, which can be described by a number of edges or connection density (ratio of the number of existing edges to the number of maximally possible edges).
Network parameters
We employed global efficiency and local efficiency to assess the efficiency in information transport across a brain functional network. Shortest path length is the minimum number of edges between two nodes, and efficiency between them is the inverse of shortest path length. Global efficiency of a network is the mean efficiency for every pair of nodes in the network, and local efficiency of a network is the mean efficiency for local subnetworks centring every node in the network, where a local subnetwork consists of the neighbours of a vertex (Latora and Marchiori, 2001). The physical meaning of global and local efficiencies is the efficiency in transporting information in global and local scales, respectively.
Small-world properties
We explored a range of connection densities to find consistent topological characteristics across the changing network sparsity. The range of connection densities was decided to satisfy small-world properties, which suggest a balance between functional segregation and integration in a brain functional network.
In terms of global and local efficiencies, a small-world network has higher global efficiency than the regular network and higher local efficiency than the random network (Latora and Marchiori, 2001). We found the range of connection densities, so-called small-world regime, across which the brain functional network acquired from the control group satisfied small-world properties by comparing global and local efficiencies of it with those of the two extreme networks with the same number of nodes and edges. From randomly generated 100 representations of the random and regular networks, the mean and standard deviation of global and local efficiencies were measured.
Comparison of network parameters between groups
For each affective condition, differences in global and local efficiencies between the two groups were calculated at each connection density. The statistical significance of the differences was estimated by non-parametric permutation tests. Given the null hypothesis that there were no differences in global and local efficiencies between the two groups, the group label of each subject was randomly reassigned. The statistical significance of an observed difference was decided in comparison with the distribution of differences obtained from the repetitive random reassignment of the group label (10 000 times). The significance level was determined at a P-value of 0.05, with false discovery rate correction for multiple comparisons for the three affective conditions.
Comparison of network parameters between conditions
In each group, differences in global and local efficiencies between two affective conditions (negative vs positive, positive vs neutral and negative vs neutral) were calculated at each connection density. The statistical significance of the differences was still estimated by non-parametric permutation tests. In this comparison, the condition label was randomly reassigned and an observed difference was compared with the distribution of differences acquired from the repetitive random reassignment of the condition label (10 000 times). The significance level was determined at a P-value of 0.05, with false discovery rate correction for multiple comparisons for the three pairs of affective conditions in each group.
As we were specifically interested in differences in global and local efficiencies between negative and positive affective conditions in the patient group, we further searched for the differences at the sub-brain level. Considering eight sub-brain regions, including sensorimotor, frontal, parietal, occipital, temporal, limbic/paralimbic, subcortical and cerebellar regions, as in Table 1, global and local efficiencies of each sub-brain region were measured by averaging the contributions of brain areas constituting the sub-brain region. Between negative and positive affective conditions, regional global efficiency was compared for each pair of sub-brain regions, and regional local efficiency was compared for each sub-brain region. Here, the statistical significance of the differences was estimated by non-parametric permutation tests. The distribution of maximum differences among the eight sub-brain regions was found to circumvent correction for multiple comparisons. The significance level was determined at a P-value of 0.05.
RESULTS
Behavioural results
Demographic and clinical characteristics of the patients and controls are summarized in Table 2. The patients were more depressed than the controls as measured by HAMD and BDI. Also, the patients were more anxious than the controls as measured by HAMA and TAI. Twelve of the patients were taking medications: 4 paroxetine CR, 3 escitalopram, 2 mirtazapine, 1 venlafaxine, 1 fluoxetine and 1 bupropion.
Table 2.
Demographic and clinical characteristics of patients with major depressive disorder and controls who participated in this study
| Patients | Controls | Statistics | P-value | ||
|---|---|---|---|---|---|
| Age (years) | 35.3 ± 9.8 | 36.5 ± 4.9 | −0.38 | NS | |
| HAMD | 17.1 ± 4.7 | 0.7 ± 1.1 | 12.208 | <0.001 | |
| HAMA | 14.3 ± 5.6 | 2.1 ± 2.4 | 7.202 | <0.001 | |
| BDI | 25.3 ± 8.8 | 4.4 ± 3.9 | 7.760 | <0.001 | |
| SAI | 22.9 ± 6.4 | 24.5 ± 4.6 | −0.699 | NS | |
| TAI | 33.4 ± 7.5 | 24.7 ± 4.6 | 3.459 | <0.005 | |
| Subjective emotional rating | Negative | 2.3 ± 0.7 | 3.2 ± 0.5 | −3.916 | <0.005 |
| Neutral | 4.2 ± 0.6 | 4.7 ± 0.3 | −2.770 | <0.05 | |
| Positive | 5.2 ± 0.9 | 5.4 ± 0.4 | −0.758 | NS | |
HAMD, Hamilton Rating Scale for Depression; HAMA, Hamilton Rating Scale for Anxiety; BDI, Beck Depression Inventory; SAI, State Anxiety Inventory; TAI, Trait Anxiety Inventory; NS, not significant.
The participants’ subjective emotional ratings varied based on each emotional feedback type. As expected, the subjects rated their subjective emotional responses as negative when receiving negative facial expressions feedback, as positive when receiving positive facial expressions feedback and as neutral when receiving neutral facial expressions feedback. The patients felt more negatively when viewing negative and neutral facial expressions feedback as compared with the controls.
Correlation of mean activity
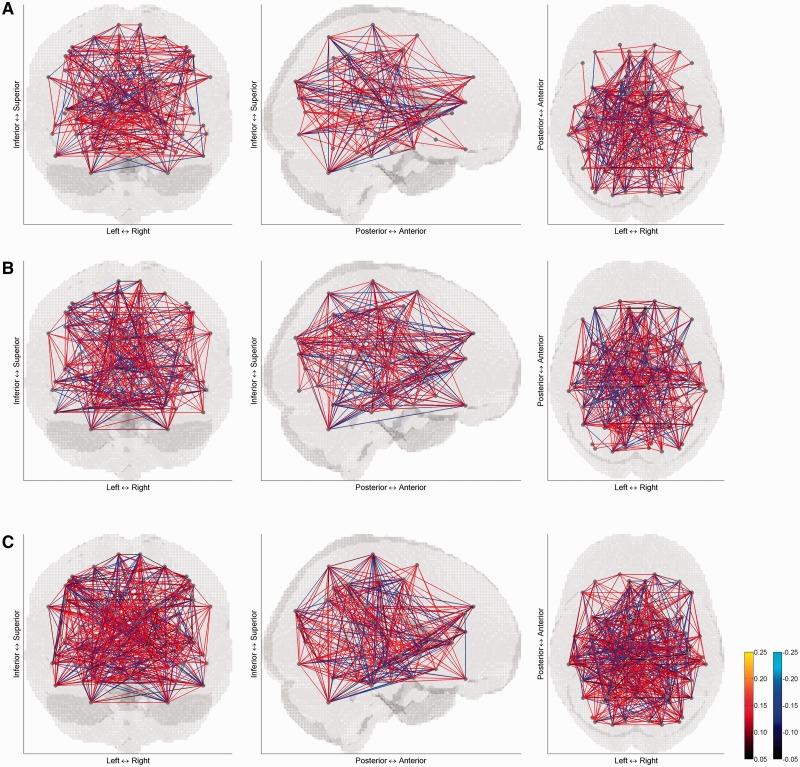
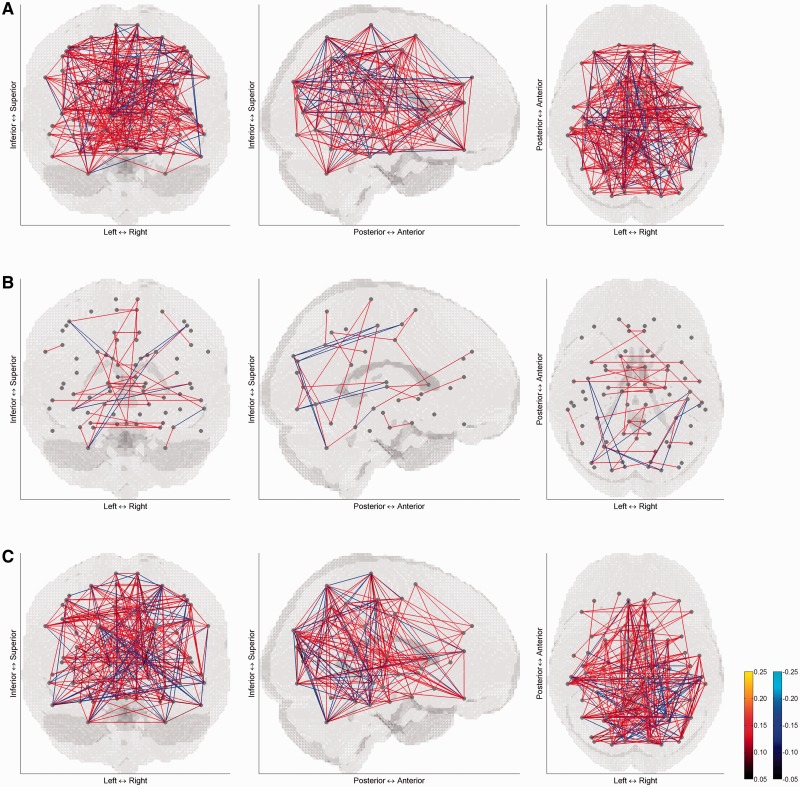
The correlation matrices of mean activity are displayed on the brain in Figures 1 and 2 for the control group and patient group, respectively. The pattern of correlation looks different between the two groups depending on the condition type. Especially for the negative affective condition, correlation in the patient group is much sparser, compared with the control group and compared with other affective conditions.
Fig. 1.
Correlation matrices of mean activity in (A) positive, (B) negative and (C) neutral affective conditions of controls. For sparse views on the brain, correlation coefficients were thresholded at ±0.05. The left panel is a coronal view from posterior to anterior, the middle panel is a sagittal view from right to left, and the right panel is a transverse view form superior to inferior. Grey dots correspond to 73 brain areas and lines indicate correlation between pairs of the brain areas.
Fig. 2.
Correlation matrices of mean activity in (A) positive, (B) negative and (C) neutral affective conditions of patients with major depressive disorder. For sparse views on the brain, correlation coefficients were thresholded at ±0.05. The left panel is a coronal view from posterior to anterior, the middle panel is a sagittal view from right to left, and the right panel is a transverse view form superior to inferior. Grey dots correspond to 73 brain areas and lines indicate correlation between pairs of the brain areas.
Small-world regime
For the three affective conditions, the brain functional network of the control group consistently showed higher global efficiency than the regular network and lower local efficiency than the random network across a range of connection densities from 0.10 to 0.35 (Supplementary Figure S2). Therefore, in this small-world regime, global and local efficiencies were compared between the two groups and between the affective conditions.
Comparison of network parameters between groups
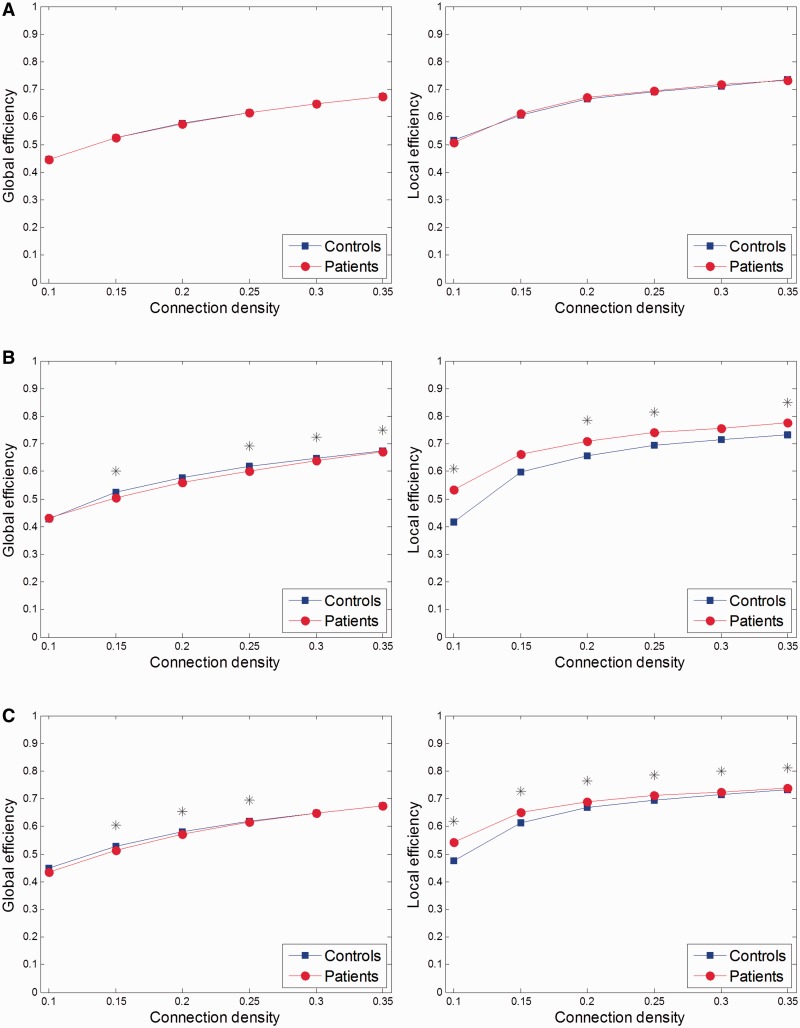
When comparing global and local efficiencies of the patient group with the control group, the differences were dependent on the condition type. As compared with the control group, the patient group exhibited no differences in the positive affective condition (Figure 3A), but they showed decreased global efficiency and increased local efficiency in the negative and neutral affective conditions (Figure 3B and C).
Fig. 3.
Comparisons of global and local efficiencies in (A) positive, (B) negative and (C) neutral affective conditions between patients with major depressive disorder and controls across a range of connection densities from 0.10 to 0.35. Stars indicate statistical significance.
Comparison of network parameters between conditions
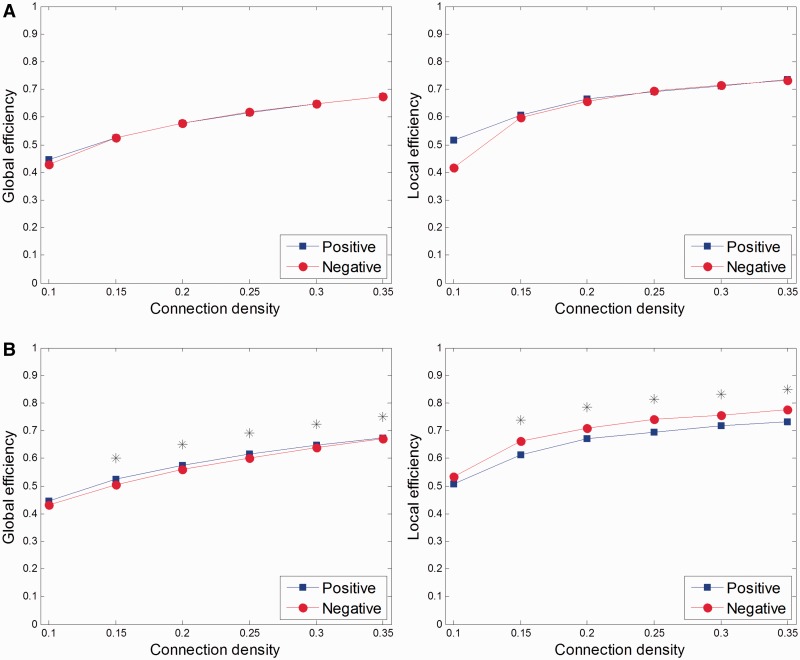
When comparing global and local efficiencies between two conditions in each group, the patient group showed differences between negative and positive affective conditions (Figure 4B) and between negative and neutral affective conditions (Supplementary Figure S4B). The control group displayed no differences between any two conditions (Figure 4A and Supplementary Figures S3A and S4A).
Fig. 4.
Comparisons of global and local efficiencies between negative and positive affective conditions in (A) controls and (B) patients with major depressive disorder across a range of connection densities from 0.10 to 0.35. Stars indicate statistical significance.
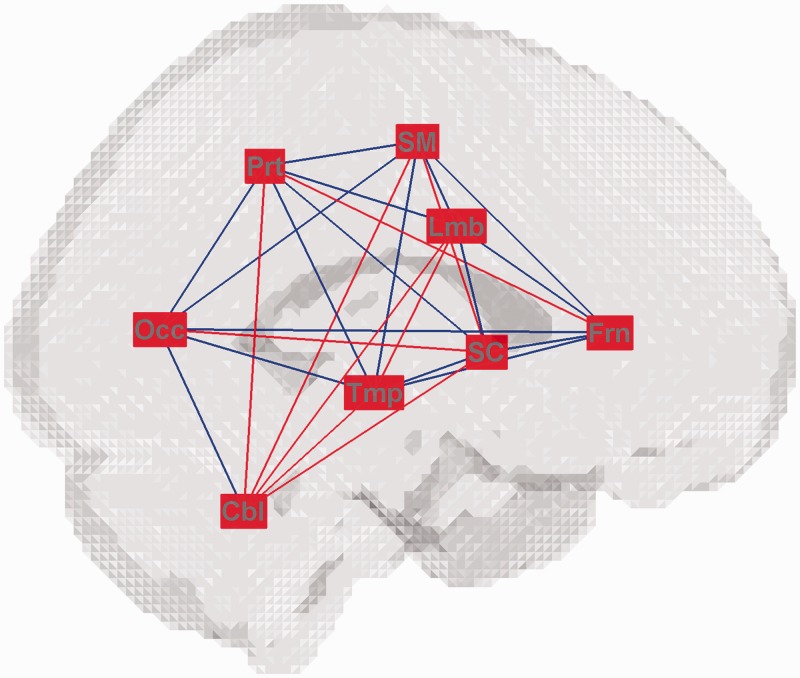
At the sub-brain level, between negative and positive affective conditions, regional global efficiency between limbic and cerebellar sub-brain regions was different at connection density 0.25. Regional local efficiency was different over the sensorimotor, frontal, parietal and temporal sub-brain regions at connection densities 0.30 and 0.35 (Table 3). Compared with the positive affective condition, regional global efficiency among widespread sub-brain regions consistently decreased or increased, and regional local efficiency in all sub-brain regions dominantly increased in the negative affective condition (Figure 5 and Supplementary Figure S5).
Table 3.
Differences in regional global and local efficiencies at a sub-brain level between positive and negative affective conditions in patients with major depressive disorder
| Connection density | Regional efficiency | Sub-brain region | Difference (negative − positive) | P-value |
|---|---|---|---|---|
| 0.25 | Global efficiency | Lmb–Cbl | 0.1905 | <0.0001 |
| 0.30 | Local efficiency | SM | 0.0588 | <0.0001 |
| 0.35 | Local efficiency | Frn | 0.0526 | <0.0001 |
| Prt | 0.0633 | <0.0001 | ||
| Tmp | 0.0552 | <0.0001 |
Lmb, limbic/paralimbic; Cbl, cerebellar; SM, sensorimotor; Frn, frontal; Prt, parietal; Tmp, temporal.
Fig. 5.
Consistent changes in global and local efficiencies between negative and positive affective conditions in patients with major depressive disorder at a sub-brain level across above half of a range of connection densities from 0.10 to 0.35. The red colour of each sub-brain region indicates an increase in regional local efficiency, and the red and blue colours of each pair of sub-brain regions indicate an increase and a decrease, respectively, in regional global efficiency in the negative affective condition compared with the positive affective condition. The acronyms of sub-brain regions conform to Table 1.
DISCUSSION
We showed group-wise network-wide changes in responses to different kinds of affective stimuli in MDD. We constructed a brain functional network based on correlation of mean activity of brain areas across subjects and found task context-dependent changes in topological characteristics. MDD patients relative to controls exhibited decreased global efficiency and increased local efficiency in negative and neutral affective conditions. The topological changes in MDD patients were most evident in the negative affective condition when compared with other affective conditions.
Topological changes of the brain functional network in MDD from this study can be characterized compared with previous connectivity studies. Most of all, the topological changes were task context-specific; MDD patients’ correlation of mean activity was much weakened in the negative affective condition (Figure 2B), and corresponding topological characteristics were different from controls’ (Figure 3). In previous task-related studies using affective stimuli, there have been changes specific to the negative affective condition shown (Anand et al., 2005; Friedel et al., 2009; Carballedo et al., 2011; Perlman et al., 2012). Subtle abnormalities of neural structures in MDD could be shown as changes in interregional functional connections and furthermore changes in topological characteristics at the whole brain level under a specific task context.
MDD patients displayed topological changes in the neutral affective condition as well, towards the same direction (decreased global efficiency and increased local efficiency) as in the negative affective condition (Figure 3C). Although neutral affective stimuli provoked less profound changes than negative affective stimuli (Supplementary Figure S3), MDD patients appear to respond abnormally even in response to neutral affective stimuli. On the other hand, in spite of no differences compared with controls in terms of topological characteristics (Figure 3A), connectivity changes to positive affective stimuli, which were employed to differentiate bipolar disorder from MDD (Almeida et al., 2009), could be still related to a pathophysiological mechanism of MDD.
The main pattern of topological changes in MDD patients involved decreased global efficiency and increased local efficiency compared with controls. Even though the brain functional network of patients with MDD seems to keep small-world properties, it shifted to the regular network from that of controls in topological architecture. This would indicate alterations of the brain functional network in a less optimized way under the pathological condition of MDD.
Although graph-theoretical analysis has been widely applied to characterize the brain functional network in pathological conditions thus far, not all of the studies appear to provide completely consistent results. For various neuropsychiatric diseases, some studies showed shifts to the random network (Rubinov et al., 2009; Stam et al., 2009; Zhang et al., 2011) and some other studies showed shifts to the regular network (Fallani et al., 2010). Indeed, with respect to MDD, previous graph-theoretical approaches showed a shift to the random network (Zhang et al., 2011) as well as a shift to the regular network (Leistedt et al., 2009). Although the direction of the shift may be differently represented depending on various factors such as neuroimaging modality, brain state (task-performing state vs resting state) and network modelling method even for the same disease, topological deviation from the healthy state could be generally shown.
With respect to network modelling, we specifically constructed the group-wise brain functional network based on mean activity-based functional connectivity. This type of network modelling would reveal different aspects of the brain functional network in comparison with usual approaches based on time series-based functional or effective connectivity. A high correlation between brain areas in our approach is attributable to a similar pattern of changes in mean activity across subjects, so that the efficiency in transporting information described by global and local efficiencies would correspond to the group-wise consistency in responses to specific affective stimuli among brain areas. Thus, decreased global efficiency and increased local efficiency during negative affective processing in MDD patients could reflect that activity changes in response to negative affective stimuli became less consistent between remote brain areas, but more consistent between close brain areas. This may be considered a general notion of impaired long range connections, including alterations of frontal-limbic couplings (Anand et al., 2005; Friedel et al., 2009), to negative affective stimuli in MDD patients.
At the sub-brain level, between negative and positive affective conditions, differences in regional global and local efficiencies were shown among various sub-brain regions. Increased local efficiency of the brain functional network was attributable to increased regional local efficiency in the sensorimotor, frontal, parietal and temporal regions (Table 3). Especially, all sub-brain regions showed consistent increases in regional local efficiency across a wide range of connection density (Figure 5 and Supplementary Figure S5). Local increases in information transport in other sub-brain regions than the limbic region in affective processing may show a failure to segregate affective processing from other processing, such as cognitive and sensorimotor processing, in MDD (Epstein et al., 2011).
Compared with positive affective processing, regional global efficiency consistently increased as well as decreased among widespread sub-brain regions during negative affective processing (Figure 5). It may be notable that the limbic region exhibited decreases in regional global efficiency in connections with cortical regions such as the sensorimotor, frontal and parietal regions. It is proposed that extensive changes over the brain functional network, beyond the cortico-limbic circuitry, may be provoked depending on the context of affective processing.
Considering that MDD is associated with alterations of affective processing, abnormality in functional connections would be shown more clearly in the context of affective stimuli. Given task-related neuroimaging data, although network modelling based on functional (Anand et al., 2005; Vasic et al., 2009; Epstein et al., 2011; Lee et al., 2011) or effective connectivity (Almeida et al., 2009; Carballedo et al., 2011; Desseilles et al., 2011; Hamilton et al., 2011; Perlman et al., 2012) between brain areas provides more specific views on the network structure and strength of connections, graph-theoretical approaches to the modelled network offer alternative views on the network topology at a higher level. Network parameters as network-wise summarized values may have to be considered cautiously in that those values abstract a network to a high degree (Smith, 2012). Nonetheless, complementary information on functional connections available at different levels would be still useful in connectivity studies of MDD.
CONCLUSION
This study revealed that task context-dependent changes in functional connections could be represented at the whole brain level in a topological fashion in MDD patients. Most evidently to negative affective stimuli, MDD patients exhibited altered topological characteristics deviated from the healthy state. Although dysfunction of cortical and limbic areas has been mainly shown in MDD in terms of activity and connectivity, MDD is actually supposed to involve widespread dysfunction among various brain areas. In this sense, graph-theoretical approaches would be a promising tool for searching for the pathophysiological mechanism of MDD as a network-level disease.
FUNDING
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2044932).
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
REFERENCES
- Almeida JRCD, Versace A, Mechelli A, et al. Abnormal Amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66(5):451–9. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research—Neuroimaging. 2009;171(3):189–98. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57(10):1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Human brain networks in health and disease. Current Opinion in Neurology. 2009;22(4):340–7. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory. San Antonio: HarcourtBrace Jovanovich; 1987. [Google Scholar]
- Cao X, Liu Z, Xu C, et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naïve patients with major depressive disorder. Journal of Affective Disorders. 2012;141(2-3):194–203. doi: 10.1016/j.jad.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Scheuerecker J, Meisenzahl E, et al. Functional connectivity of emotional processing in depression. Journal of Affective Disorders. 2011;134(1–3):272–9. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Schwartz S, Dang-Vu TT, et al. Depression alters “top-down” visual attention: a dynamic causal modeling comparison between depressed and healthy subjects. Neuroimage. 2011;54(2):1662–8. doi: 10.1016/j.neuroimage.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Epstein J, Perez DL, Ervin K, et al. Failure to segregate emotional processing from cognitive and sensorimotor processing in major depression. Psychiatry Research—Neuroimaging. 2011;193(3):144–50. doi: 10.1016/j.pscychresns.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Fallani FDV, Costa LDF, Rodriguez FA, et al. A graph-theoretical approach in brain functional networks. Possible implications in EEG studies. Nonlinear Biomedical Physics. 2010;4(Suppl. 1):S8. doi: 10.1186/1753-4631-4-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel E, Schlagenhauf F, Sterzer P, et al. 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology. 2009;205(2):261–71. doi: 10.1007/s00213-009-1536-1. [DOI] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magnetic Resonance Materials in Physics, Biology and Medicine. 2010;23(5–6):409–21. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16(7):763–72. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British Journal of Medical Psychology. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4:33. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Gao C, Chen C, et al. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neuroscience Letters. 2011;503(2):105–9. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Physical Review Letters. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lee TW, Wu YT, Yu YWY, Chen MC, Chen TJ. The implication of functional connectivity strength in predicting treatment response of major depressive disorder: a resting EEG study. Psychiatry Research—Neuroimaging. 2011;194(3):372–7. doi: 10.1016/j.pscychresns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Leistedt SJJ, Coumans N, Dumont M, Lanquart JP, Stam CJ, Linkowski P. Altered sleep brain functional connectivity in acutely depressed patients. Human Brain Mapping. 2009;30(7):2207–19. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisiecka D, Meisenzahl E, Scheuerecker J, et al. Neural correlates of treatment outcome in major depression. International Journal of Neuropsychopharmacology. 2011;14(4):521–34. doi: 10.1017/S1461145710001513. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, et al. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. Journal of Affective Disorders. 2012;139(1):75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, et al. Small-world properties of nonlinear brain activity in schizophrenia. Human Brain Mapping. 2009;30(2):403–16. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. quiz: 34–57. [PubMed] [Google Scholar]
- Smith SM. The future of FMRI connectivity. NeuroImage. 2012;62(2):1257–66. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stam CJ, De Haan W, Daffertshofer A, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain. 2009;132(1):213–24. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychological Medicine. 2009;39(6):977–87. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Wen W, He Y, Sachdev P. Structural brain networks and neuropsychiatric disorders. Current Opinion in Psychiatry. 2011;24(3):219–25. doi: 10.1097/YCO.0b013e32834591f8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological Psychiatry. 2011;70(4):334–42. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Zheng H, et al. Increased neural resources recruitment in the intrinsic organization in major depression. Journal of Affective Disorders. 2010;121(3):220–30. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry. 2012;71(7):611–7. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.