Abstract
Exaggerated anticipatory anxiety during expectation of performance-related situations is an important feature of the psychopathology of social anxiety disorder (SAD). The neural basis of anticipatory anxiety in SAD has not been investigated in controlled studies. The current study used functional magnetic resonance imaging (fMRI) to investigate the neural correlates during the anticipation of public and evaluated speaking vs a control condition in 17 SAD patients and 17 healthy control subjects. FMRI results show increased activation of the insula and decreased activation of the ventral striatum in SAD patients, compared to control subjects during anticipation of a speech vs the control condition. In addition, an activation of the amygdala in SAD patients during the first half of the anticipation phase in the speech condition was observed. Finally, the amount of anticipatory anxiety of SAD patients was negatively correlated to the activation of the ventral striatum. This suggests an association between incentive function, motivation and anticipatory anxiety when SAD patients expect a performance situation.
Keywords: social phobia, anticipatory anxiety, fMRI, ventral striatum, insula, amygdala
INTRODUCTION
Individuals suffering from social anxiety disorder (SAD), classified as ‘social phobia’ in DSM-IV-TR show exaggerated fear responses when confronted with social situations, especially concerning performance situations such as giving a speech. Even when such social situations are anticipated, pronounced anxiety symptoms emerge.
In search of the neural basis of SAD, brain circuits have been identified that are involved in the pathophysiology of this disorder. By means of functional brain imaging, increased amygdala activation was shown in response to disorder-related stimuli as well as during symptom provocation (for an overview, see Miskovic and Schmidt, 2012). These findings support influential models suggesting a significant role of the amygdala in the processing of threat-related stimuli, especially in the rapid detection of threat and the initiation of defense behaviors (LeDoux, 1998; Öhman and Mineka, 2001). An involvement of other brain areas such as the insula (e.g. Straube et al., 2004; Yoon et al., 2007) and medial prefrontal cortex (mPFC; e.g. Stein et al., 2002; Blair et al., 2011) has also been reported in SAD. For example, activation of the insula, a brain region strongly involved in interoception and representation of bodily states, might support aversive feelings by the perception of bodily states of arousal (e.g. Straube et al., 2004), whereas activation of the mPFC was suggested to reflect changed self-referential attention in SAD (e.g. Blair et al., 2011).
Furthermore, several studies reported decreased responses of the striatum during a cognitive task and a reduced function of the mesolimbic dopamine system in general in SAD patients (e.g. Tiihonen et al., 1997; Sareen et al., 2007; Schneier et al., 2009). It has been proposed that dysfunctions of the striatum, especially of the ventral section, are associated with avoidance behaviors and impaired motivation. Thus, this dysfunction might impair successful coping with threat of potential negative evaluation (Schneier et al., 2009). However, the role of the striatum in SAD patients has not been investigated in the context of symptom provocation or during the processing of disorder-related stimuli.
In contrast to several studies on brain activation during the presence of disorder-related stimuli and performance situations, there are no sufficiently controlled functional imaging studies examining anticipatory anxiety in SAD. A positron emission tomography study by Tillfors et al. (2002) reported increased amygdala activation in SAD patients during private speaking when it was known that public speaking followed, as compared to private speaking that followed after public speaking. This differential amygdala response was interpreted to be related to the anticipatory component. However, the absence of a control group, the small sample size (n = 9), the unbalanced order of experimental conditions and the presence of actual speech during anticipation of public speaking all might affect the findings of this study. Lorberbaum et al. (2004) compared neural correlates of anticipating a public speech vs a rest condition in SAD patients during functional magnetic resonance imaging (fMRI). Findings showed increased activation in temporal lobe and limbic regions, including amygdala and insula, and decreased activation in prefrontal areas in SAD patients as compared to healthy controls (HC). However, a small sample size (n = 8) and the absence of an appropriate control condition limit the conclusions which can be drawn from this study.
Several studies in healthy subjects and individuals with other anxiety disorders have investigated the functional neuroanatomy of anticipatory anxiety to specific threat stimuli (e.g. Boshuisen et al., 2002; Nitschke et al., 2006; Simmons et al., 2006; Straube et al., 2007, 2008, 2009; Somerville et al., 2010; Carlson et al., 2011). These studies reported, for example, activation in the insula (Boshuisen et al., 2002; Nitschke et al., 2006; Simmons et al., 2006; Straube et al., 2007, 2008; Carlson et al., 2011) and the bed nucleus of the stria terminalis (BNST; Straube et al., 2007; Somerville et al., 2010), which belongs to the so-called extended amygdala and has been proposed to be involved in sustained and unpredictable threat (Davis et al., 2009). Furthermore, depending on several factors, activations and deactivations in different prefrontal areas (Boshuisen et al., 2002; Ploghaus et al., 2003; Kalisch et al., 2006; Nitschke et al., 2006; Simmons et al., 2006; Straube et al., 2007, 2008, 2009) have been reported during anticipatory anxiety.
The neural basis of anticipatory anxiety in SAD patients remains to be investigated with appropriate paradigms. In the present study, we used fMRI to examine brain activation in the amygdala, insula, mPFC, BNST, dorsal and ventral striatum during the anticipation of public speaking vs the anticipation of a control condition in both SAD patients and HC subjects. In addition to the factorial approach, we also investigated the association between the magnitude of experienced anticipatory anxiety and brain activation.
MATERIALS AND METHODS
Subjects
Seventeen patients with a primary diagnose of SAD and 17 HC subjects participated in the study. All participants were right-handed, with normal or corrected-to-normal vision. They were recruited via public announcement. All participants provided written, informed consent for the study. The study was approved by the ethics committee of the University of Jena. Diagnoses were confirmed by clinical psychologists administering the Structured Clinical Interview for DSM-IV Axis I and II disorders (SCID I and II; Fydrich et al., 1997; Wittchen et al., 1997). Exclusion criteria were any of the following: (i) a diagnosis of obsessive–compulsive disorder, current alcohol or substance abuse, any psychotic disorder or dementia and current primary or secondary major depression; (ii) a history of seizures or head injury with loss of consciousness; (iii) a severe uncontrollable medical condition; or (iv) the use of any psychotropic medication within the preceding 6 months. HC were free of any psychopathology. In the SAD sample under study, two patients met the criteria of another anxiety disorder (generalized anxiety disorder and agoraphobia with history of panic disorder), nine patients were diagnosed with affective disorder in their past (dysthymia or past major depressive disorder, recurrent, in full remission), and 10 patients fit criteria of an Axis II personality disorder [anxious (avoidant) personality disorder, obsessive–compulsive personality disorder, dependent personality disorder, paranoid personality disorder]. SAD and HC subjects were matched for age, education (Table 1) and gender (SAD: seven females; HC: six females; χ2[1] = 0.13; P > 0.05). Before scanning, all participants completed the LSAS (Liebowitz Social Anxiety Scale, German version; Stangier and Heidenreich, 2005) and BDI (Beck Depression Inventory, German version; Hautzinger et al., 1995) questionnaire. SAD patients scored significantly higher on both LSAS and BDI questionnaires than the control subjects (Table 1).
Table 1.
Demographic and questionnaire characteristics for patients with SAD and HC concerning age, education, symptom severity (LSAS) and depression (BDI)
| SAD | HC | t-value | |
|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | ||
| Age | 31.12 | 30.82 | 0.09 |
| (10.52) | (8.63) | ||
| Education (in years) | 11.29 | 11.50 | 0.63 |
| (0.99) | (0.89) | ||
| LSAS | 75.06 | 19.65 | 10.23* |
| (19.71) | (10.49) | ||
| BDI | 13.56 | 4.76 | 3.64* |
| (9.08) | (4.09) |
*P < 0.05.
Paradigm
Subjects were told that the experiment consists of several speech and computer test blocks presented in random order. Furthermore, participants were advised that during the computer test blocks the complicated and interference prone equipment had to be tested by the experimenter to avoid problems during the experiment. Both anticipatory conditions started with a 20 s baseline fixation cross which was followed by a 3 s cue signaling either the speech (announced by the word ‘speech’) or the control condition (‘computer test’). After this cue, a 40 s anticipatory phase started. During this phase, a fixation cross was shown. After the speech anticipatory phase, a very common topic (e.g. ‘Tell me something about your favorite film’ or ‘Tell me something about your last vacation’) was visually presented and a 2 min speech time followed. Task performance was analyzed by means of the number of words produced during the speech conditions. After the anticipatory phase in the control condition a word was displayed and subjects simply had to say the word aloud. The speech topic and the control word were presented for 3 s. A dummy video camera was attached to the MRI to heighten the social character of the situation. Subjects were told that their speeches, but not the reading of the test word, would be recorded by this camera. Subjects were also told that the tape will be evaluated by two independent experts concerning word fluency and eloquence. Participants were debriefed after the experiment.
During fMRI scanning, there were three speech and three control sessions presented in counterbalanced order across participants. Scanning did not include the speech or the reading of the test word. After MRI scanning, participants rated the unpleasantness, arousal and anxiety they felt during anticipatory phases in the speech as well as in the test condition using a nine-point Likert scale (unpleasantness: 1 = very pleasant to 9 = very unpleasant, whereas 5 = neutral; arousal: 1 = not arousing/sedate to 9 = very arousing; anxiety: 1 = not anxious, 9 = very anxious). Behavioral data were analyzed by repeated measures analyses of variance (ANOVA) and t-tests using the SPSS software (Version 19.0.0.1, SPSS, Inc.). For ANOVA, a probability level of P < 0.05 was considered statistically significant.
Functional MRI
The neural data were recorded in a 3 tesla magnetic resonance scanner (‘Magnetom TIM TRIO’, Siemens, Medical Solutions, Erlangen, Germany). After a T1-weighted anatomical scan, six runs with 26 volumes (3 × speeches and 3 × control conditions) were conducted using a T2*-weighted echo-planar sequence (echo time = 30 ms, flip angle = 90°, matrix = 64 × 64, field of view = 192 mm, repetition time = 3 s). Each volume consisted of 40 axial slices (thickness = 3 mm, gap = 0 mm, in plane resolution = 3 × 3 mm). The first four volumes of each run were discarded from analysis to ensure that steady-state tissue magnetization was reached.
FMRI data preprocessing and analyzing were conducted by using the BrainVoyager QX software package (Version 1.10.4; Brain Innovation, Maastricht, The Netherlands). To begin, all volumes were realigned to the first volume in order to minimize artifacts due to head movements. Data were controlled for movement artifacts (>3 mm in any direction). No participant showed movement artifacts and thus no participant had to be excluded from analysis. Further data preprocessing comprised spatial (8 mm full-width half-maximum isotropic Gaussian kernel) and temporal smoothing (low pass filter: 2.8 s). The anatomical and functional images were co-registered and normalized to the Talairach space (Talairach and Tournoux, 1988).
Statistical analyses were performed by multiple linear regression of the signal time course at each voxel. The expected blood oxygen-level-dependent (BOLD) signal change for each event type (predictor) was modeled by a canonical hemodynamic response function. The anticipatory phases of speech and control test were defined as events of interest. Statistical comparisons were conducted using a mixed effect analysis, which considers inter-subject variance and permits population-level inferences. First, voxel-wise statistical maps were generated and the relevant, planned contrasts of predictor estimates (beta-weights) were computed for each individual. Second, a random effect group analysis of these individual contrasts was performed.
Analyses were conducted for specific regions of interest [ROIs; defined by using the Talairach daemon software (http://www.ric.uthscsa.edu/projects/talairachdaemon.html), BrainMap (Fox and Lancaster, 2002); the ROI definition of the BNST based on the atlas of (Mai et al., 1997) and our previous studies (e.g. Straube et al., 2004; Straube et al., 2007)]. ROIs were the amygdala, insula, mPFC, BNST, dorsal and ventral striatum.
Statistical parametric maps resulting from voxel-wise analyses were considered statistically significant for clusters that survived a correction for multiple comparisons. For this purpose, we used the approach as implemented in Brain Voyager [based on a 3D extension of the randomization procedure described by Forman et al. (1995)]. First, voxel-level threshold was set at P < 0.005 (uncorrected). Threshold maps were then submitted to a ROI-based correction for multiple comparisons. The correction is based on the estimation of the cluster threshold that is the minimal number of voxels, which is necessary to control for multiple comparisons. The cluster threshold criterion was based on the estimate of map’s spatial smoothness (Forman et al., 1995) and on an iterative procedure (Monte Carlo simulation). The Monte Carlo simulation used 1000 iterations in order to estimate the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5%. This cluster size threshold was applied to the statistical maps. Our search space comprised all ROIs. The cluster threshold for the comparison of SAD vs HC subjects and speech > control anticipation was 120 mm3 in this combined ROI map. Finally, correlation analyses were conducted between brain activation within the ROIs and anxiety ratings in SAD patients. Clusters of voxels with P < 0.005 (uncorrected) were considered as significant when size >134 mm3 (estimated via Monte Carlo simulation, see above).
RESULTS
Behavioral data
Ratings
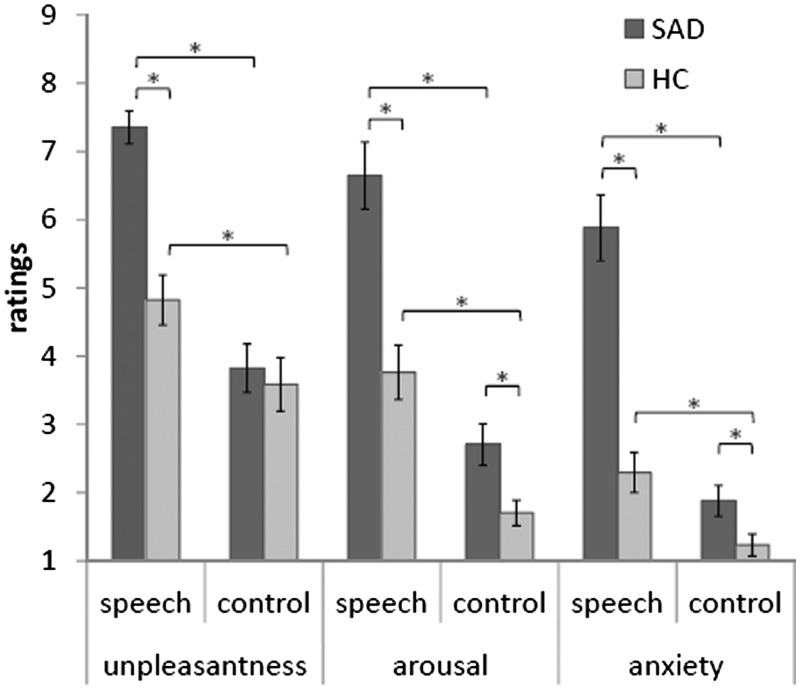
Analyzing rating data revealed that both SAD and HC subjects rated speech anticipation, in comparison to the control condition, as more negative (F[1, 32] = 55.48, P < 0.05), more arousing (F[1, 32] = 94.26, P < 0.05) and more anxiety-inducing (F[1, 32] = 83.93, P < 0.05). There was also a Group × Anticipatory condition effect (unpleasantness: F[1, 32] = 12.86, P < 0.05; arousal: F[1, 32] = 9.28, P < 0.05; anxiety: F[1, 32] = 28.37, P < 0.05) based on increased ratings in SAD patients as compared to HC subjects in the speech vs the control condition. Figure 1 summarizes results of the rating data.
Fig. 1.
Unpleasantness, arousal and anxiety ratings for the speech and control anticipatory phases in patients with SAD and HC. Asterisks mark significant differences.
Task performance
Analyses of produced number of words during the speech condition revealed that SAD patients spoke significantly fewer words than HC subjects (SAD: 96.68 words; HC: 153.14 words; t[32] = −3.52, P < 0.05).
FMRI data
Interaction group by anticipatory condition
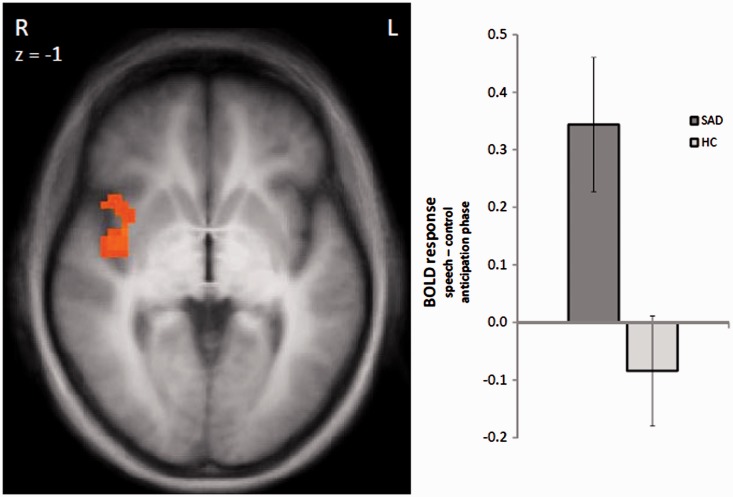
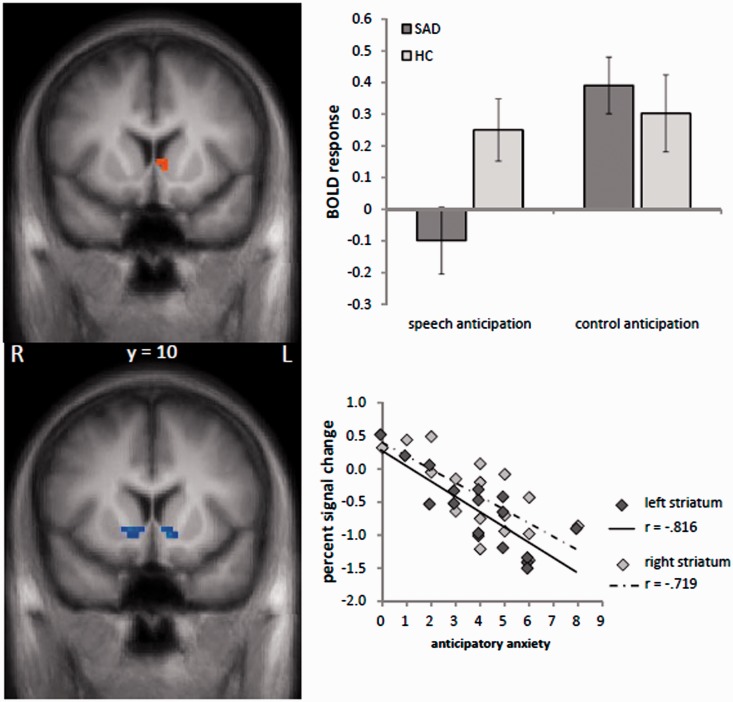
Increased brain activation in SAD patients compared to HC subjects in response to the speech vs control anticipation was found in the right insula (peak voxel Talairach coordinates: x = 47; y = −3; z = 5; size = 1328 mm3; t-value = 4.20; Figure 2). HC subjects showed stronger activation than SAD patients in the ventral striatum in response to the speech vs control anticipation (peak voxel Talairach coordinates: x = −4; y = 10; z = 6; size = 243 mm3; t-value = 3.18). The bar chart in Figure 3 indicates that this effect is mainly due to a relative deactivation in SAD patients in response to the speech vs the control condition. Plotted against baseline (Figure 3), this effect seems to be due to the speech condition, even though effects against the fixation baseline are only interpretable with great caution. There were no significant effects in the other ROIs.
Fig. 2.
Differential brain activation during speech vs control anticipation. Patients with SAD display an enhanced activation in the right insula as compared to HC subjects (speech > control anticipatory phase). Statistical parametric maps are overlaid on a T1 scan (radiologicalconvention: left = right). The plots on the bottom display contrasts of parameter estimates (speech > control anticipatory phase; mean ± s.e. for maximally activated voxel).
Fig. 3.
Upper column: Differential brain activation during speech vs control anticipation. Patients with SAD display a decreased activation in the left ventral striatum as compared to HC subjects (speech > control anticipatory phase). Bottom column: Brain activation in the left and right ventral striatum correlated significantly to anticipatory anxiety (self rated via nine-point Likert scale) in patients with SAD. The scatter plot on the right side displays the relationship between contrasts of parameter estimates (speech–control anticipatory phase) and means anticipatory anxiety in SAD patients. Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right).
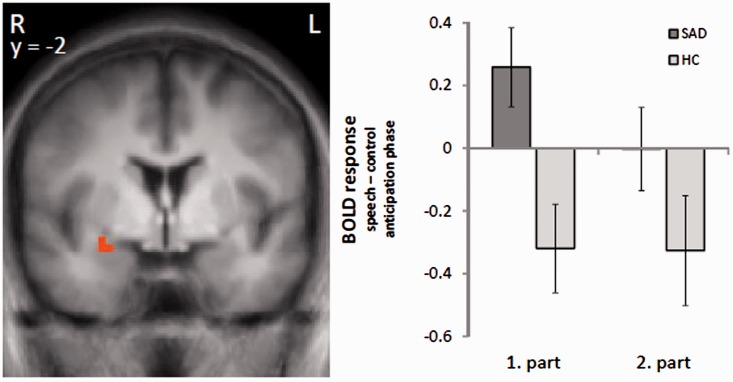
In the amygdala, we found a cluster of increased brain activation in SAD patients compared to HC subjects in response to the speech vs control anticipation that did not exceed the required cluster size of activated voxels (sub-threshold cluster size: 65 mm3). As initial amygdala responses to threat conditions have been suggested (e.g. LeDoux, 1998; Öhman and Mineka, 2001; Wright et al., 2001; Straube et al., 2007; Wendt et al., 2008), we analyzed the time course specifically of the amygdala response in more detail by dividing the 40 s anticipatory phase into two 20 s sections (the new required cluster size is 81 mm3; estimation via Monte Carlo simulation; see Materials and Methods section). The analysis showed a significant differential activation in the right amygdala (peak voxel Talairach coordinates: x = 26; y = −2; z = −8; size = 83 mm3; t-value = 3.14) in speech vs control anticipation, in SAD patients vs HC subjects, in the first 20 s but not in the last 20 s of the anticipatory phase (Figure 4).
Fig. 4.
Differential brain activation during speech vs control anticipation in either the first or second part of the anticipatory phase (each 20 s). Patients with SAD, as compared to HC, display an enhanced activation in the right amygdala in the first half of the anticipatory phase (speech > control anticipatory phase). Statistical parametric maps are overlaid on a T1 scan (radiological convention: left = right). The plots on right side display contrasts of parameter estimates (speech − control anticipatory phase; mean ± s.e. for maximally activated voxel).
Correlation analysis
Correlation of anxiety ratings and brain activation in SAD patients revealed a negative correlation between perceived anxiety during the anticipatory phases and the activation in the ventral striatum (right: peak voxel Talairach coordinates: x = 8; y = 7; z = 0; size = 212 mm3; r-value = 0.72; left: peak voxel Talairach coordinates: x = −7; y = 6; z = 2; size = 387 mm3; r-value = 0.82; Figure 3). There were no significant correlations in the other ROIs.
DISCUSSION
The study’s objective was to investigate the neural correlates of anticipatory anxiety in patients with SAD. Results show a hyperactivation of the insula in SAD patients during speech anticipation. Furthermore, the amygdala was significantly activated at the beginning of the speech anticipatory phase. The ventral striatum was deactivated in SAD patients during the speech anticipation. Moreover, this deactivation was greater in patients who rated the speech anticipation more anxiety-inducing.
Several studies have shown an insular involvement in the processing of aversive emotional cues in SAD patients (e.g. Lorberbaum et al., 2004; Straube et al., 2004; Yoon et al., 2007; but see Tillfors et al., 2001; Stein et al., 2002), and in patients with anxiety disorder, in general (e.g. Etkin and Wager, 2007). The insular region was shown to play an important role in the representation of visceral and autonomic responses to emotional stimuli (e.g. Damasio et al., 2000; Critchley, 2004) and the integration of perceived feelings and other conditions of the specific situation (Craig, 2009). The right anterior insula might be particularly involved in the re-representation of sympathicus activity (Critchley, 2004). Therefore, the present insular hyperactivation may indicate an increased processing of (negative) bodily sensations in SAD patients even when a threatening event is merely anticipated. Given that, it seems that anticipation of an anxiety-provoking event is accompanied by an attentional shift toward detailed monitoring of internal information (anxious feelings and physical fear responses). Such processes were proposed to lead to a vicious circle of amplifying anxiety reactions and to contribute to the maintenance of social anxiety (Clark and Wells, 1995).
The amygdala hyperactivation during anticipatory anxiety in SAD was restricted to the first part of the anticipatory phase. This suggests a temporally restricted role of the amygdala in anticipatory anxiety and is in accordance with findings in specific phobia (Straube et al., 2007) or anticipatory anxiety in healthy subjects (Straube et al., 2009). The initial stronger amygdala response concurs with theoretic assumptions (LeDoux, 1998; Whalen, 1998; Öhman and Mineka, 2001) and allocates the amygdala a central role within a transient threat detection system. This is also in accord with previous results that showed an involvement of the amygdala in threat processing in SAD patients (e.g. Stein et al., 2002; Tillfors et al., 2002; Lorberbaum et al., 2004; Straube et al., 2004; Phan et al., 2006; Yoon et al., 2007; Schmidt et al., 2010).
The most important finding of our study relates to the differential activation in the ventral striatum between SAD and HC subjects. During speech vs control anticipation, patients with SAD as compared to HC subjects showed a deactivation. Furthermore, the activation in this area was negatively associated with the perceived anticipatory anxiety in SAD patients, suggesting a link between incentive function, motivation and anticipatory anxiety when SAD patients expect performance situations. This could be related to increased aversion of expected rejection (Tom et al., 2007) or an increased fear of negative evaluation by others that is often accompanied by avoidance behaviors. The striatal pattern might also reflect differences in reward anticipation. SAD participants may consider the control condition while not being evaluated, as rewarding in comparison to the evaluated speech condition. A lack of motivation to face the threat due to the overestimation of negative consequences (Clark and Ehlers, 2002) may also be related to the described activation patterns in the striatum. All this can trigger safety behaviors that was identified as an important maintenance factor of social anxiety, especially when avoidance behaviors take place merely in anticipation of socially threatening situations (Clark and Wells, 1995; Clark and Ehlers, 2002). Task performance data indicated that SAD patients produced less words than HC subjects during the speech condition, which might be at least partly also a consequence of reduced striatum activity during the anticipation period. However, due to the correlational nature of our study, this possible link remains speculative.
Future studies should also investigate anticipatory anxiety during variable anticipatory intervals and different kinds of anticipated threat to clarify the role of other brain areas. It has been suggested that the BNST is associated with anxiety during unpredictable threat and sustained anxiety periods in anxiety disorders (e.g. Straube et al., 2007; Davis et al., 2009). The current study found no effect in the BNST. However, the BNST might be more relevant for vigilant anxiety states associated with unpredictably occurring external threat signals (Straube et al., 2007; Somerville et al., 2010). In our paradigm, there was rather a predictable threat situation and SAD patients seem to be strongly engaged in monitoring internal states. Future paradigms might investigate anticipatory anxiety during unpredictable and suddenly occurring external threat in SAD. Furthermore, PFC activation seems to be related to different cognitive strategies (e.g. Miller and Cohen, 2001; Ochsner and Gross, 2005) and an involvement of this region might occur when measures of coping behavior during the anticipatory phase will be included.
In summary, the present study revealed neural correlates of anticipatory anxiety in patients with SAD. The hyperactivated right insula suggests a link between bodily responses and anticipatory anxiety in SAD. The initial amygdala response revealed a time-restricted role in anticipatory anxiety in SAD patients. Furthermore, decreased activation of the ventral striatum suggests an association between incentive function, motivation and anticipatory anxiety when expecting performance situations in SAD individuals. This hypothesis is also supported by the negative correlation between activation in the ventral striatum and perceived anticipatory anxiety in patients suffering from SAD.
REFERENCES
- Blair KS, Geraci M, Otero M, et al. Atypical modulation of medial prefrontal cortex to self-referential comments in generalized social phobia. Psychiatry Research: Neuroimaging. 2011;193(1):38–45. doi: 10.1016/j.pscychresns.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AMJ, Reinders AATS, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biological Psychiatry. 2002;52(2):126–35. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience. 2011;6(1):74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, Ehlers A. Soziale Phobie: Eine kognitive Perspektive. In: Stangier U, Fydrich T, editors. Soziale Phobie und soziale Angststörung: Psychologishce Grundlagen - Diagnostik - Therapie. Göttingen: Hogrefe; 2002. pp. 157–80. [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment. New York: Guilford Press; 1995. [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6333–4. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2009;35(1):105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3(4):319–21. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Fydrich T, Renneberg B, Schmitz B, Wittchen HU. Strukturiertes Klinisches Interview für DSM-IV, Achse II (Persönlichkeitsstörungen) Göttingen: Hogrefe; 1997. [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck-Depressions-Inventar (BDI). Testhandbuch der deutschen Ausgabe. Bern: Huber; 1995. [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30(4):1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biological Psychiatry. 1998;44(12):1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701–5. [PubMed] [Google Scholar]
- Mai J, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diago: Academic Press; 1997. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neuroscience and Biobehavioral Reviews. 2012;36(1):459–78. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29(1):106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends in Cognitive Science. 2003;7(5):197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Sareen J, Campbell DW, Leslie WD, et al. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biological Psychiatry. 2007;61(3):396–404. doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mohr A, Miltner WHR, Straube T. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biological Psychology. 2010;84(2):304–12. doi: 10.1016/j.biopsycho.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Abi-Dargham A, Martinez D, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depression and Anxiety. 2009;26(5):411–8. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60(4):402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–24. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangier U, Heidenreich T. Liebowitz Social Anxiety Scale. In: Scalarum CIP, editor. Internationale Skalen für Psychiatrie (Internatioal Scales for Psychiatry) Weinheim: Beltz; 2005. pp. 299–306. [Google Scholar]
- Stein M, Goldin P, Sareen J, Zorrilla L, Brown G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives General Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel H, Miltner W. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56(12):921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H, Miltner W. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Human Brain Mapping. 2008;30(2):689–98. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel H-J, Miltner WHR. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44(3):975–81. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- Tiihonen J, Kuikka J, Bergström K, Lepola U, Koponen H, Leinonen E. Dopamine reuptake site densities in patients with social phobia. American Journal of Psychiatry. 1997;154(2):239–42. doi: 10.1176/ajp.154.2.239. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. American Journal of Psychiatry. 2001;158(8):1220–6. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M. Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biological Psychiatry. 2002;52(11):1113–9. doi: 10.1016/s0006-3223(02)01396-3. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Wendt J, Lotze M, Weike A, Hosten N, Hamm A. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45(2):205–15. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7(6):177–88. [Google Scholar]
- Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV, Achse-I (SKID-I) Göttingen: Hogrefe; 1997. [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-tesla functional MRI study. Psychiatry Research. 2007;154(1):93–8. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]