Abstract
Exposure therapy builds on the mechanism of fear extinction leading to decreased fear responses. How the stress hormone cortisol affects brain regions involved in fear extinction in humans is unknown. For this reason, we tested 32 men randomly assigned to receive either 30 mg hydrocortisone or placebo 45 min before fear extinction. In fear acquisition, a picture of a geometrical figure was either partially paired (conditioned stimulus; CS+) or not paired (CS−) with an electrical stimulation (unconditioned stimulus; UCS). In fear extinction, each CS was presented again, but no UCS occurred. Cortisol increased conditioned skin conductance responses in early and late extinction. In early extinction, higher activation towards the CS− than to the CS+ was found in the amygdala, hippocampus and posterior parahippocampal gyrus. This pattern might be associated with the establishment of a new memory trace. In late extinction, the placebo compared with the cortisol group displayed enhanced CS+/CS− differentiation in the amygdala, medial frontal cortex and nucleus accumbens. A change from early deactivation to late activation of the extinction circuit as seen in the placebo group seems to be needed to enhance extinction and to reduce fear. Cortisol appears to interfere with this process thereby impairing extinction of recently acquired conditioned fear.
Keywords: amygdala, fMRI, fear conditioning, prefrontal cortex, stress hormones
INTRODUCTION
Stress and the accompanying release of stress hormones such as cortisol have a critical impact on a variety of learning and memory processes including simple associative learning such as conditioning (Shors, 2004; Schwabe et al., 2010). Classical fear conditioning and extinction constitute the most widely studied models to comprehend the neuronal mechanisms of fear and anxiety disorders (Graham and Milad, 2011). In fear acquisition, exposure to an aversive unconditioned stimulus (UCS) is coupled with the presentation of a conditioned stimulus (CS+). Subsequently, conditioned fear emerges after the presentation of the CS+. As a reference, a further stimulus (CS−) is introduced, which is not paired with the UCS and controls for habituation processes. Fear conditioned responses (CRs) typically occur in differential skin conductance responses (SCRs) and are conveyed by activation of the amygdala, anterior cingulate cortex, hippocampus, nucleus accumbens (NAcc) and orbitofrontal cortex (Knight et al., 2004a,b; Klucken et al., 2009; Sehlmeyer et al., 2009; Mechias et al., 2010).
When the CS+ is no longer paired with the UCS, the amplitude of conditioned fear is reduced until it is completely vanished. This process is termed extinction learning, meanwhile a new inhibitory association between CS+ and no UCS is established (Milad and Quirk, 2012). The fear extinction circuit encompasses the amygdala, hippocampus and medial prefrontal cortex (mPFC), in particular its ventral part, which inhibits fear responses in the amygdala (Sotres-Bayon and Quirk, 2010; Milad and Quirk, 2012). Distinct neuronal populations in the amygdala have been identified to either convey fear or extinction learning (Herry et al., 2008, 2010). In humans, neuroimaging studies revealed a mixed pattern of results concerning differential fear extinction learning: either CS+/CS− differentiation or larger CS− compared with CS+ responses have been found e.g. in the amygdala (LaBar et al., 1998; Phelps et al., 2004; Milad et al., 2007; Lang et al., 2009). This raises the question of factors contributing to this divergence. Critical variables seem to be the reinforcement schedule (partial vs continuous reinforcement), the exact timing of phases (acquisition, extinction learning) relative to each other, total number of extinction trials and the time window of analysis (early and late vs entire extinction learning).
A manifold literature has repeatedly shown that stress hormones modulate various learning and memory processes depending on the particular timing of cortisol increases relative to encoding, consolidation and retrieval (for reviews, see Wolf, 2009; Schwabe et al., 2010). Glucocorticoids such as cortisol bind to mineralocorticoid and glucocorticoid receptors (Reul and de Kloet, 1985) mediating slow genomic as well as rapid non-genomic signalling. Both receptor types are situated in the fear and the extinction circuit (e.g. in the amygdala or mPFC) and are activated by cortisol administration or stress (Groeneweg et al., 2012). It has already been shown that stress hormones modulate neuronal correlates of fear acquisition depending on sex hormone status (Stark et al., 2006; Merz et al., 2010; Tabbert et al., 2010; Merz et al., 2012b). Thus, sex and sex hormones outline important variables to consider when investigating the neuronal correlates of fear conditioning.
There are only few studies investigating the effect of cortisol or stress on extinction, which strongly vary in time of cortisol/stress intervention and further methodological issues. Stress or cortisol administration before acquisition led to heightened fear responses during extinction in male mice (Izquierdo et al., 2006) and enhanced conditioned SCRs in men (Jackson et al., 2006). A stressor applied between acquisition and extinction in operant conditioning increased behavioural resistance to extinction (Schwabe and Wolf, 2011). Furthermore, stress after extinction of contextual fear increased extinction recall on a separate day in rats (Akirav and Maroun, 2007). But cortisol administration before extinction lowered CRs in male rats during fear extinction (Ninomiya et al., 2010) and fear recall (Yang et al., 2006, 2007). Stress before extinction learning also reduced UCS expectancy at the first trial of fear extinction and recall in men (Bentz et al., 2013).
Similarly, beneficial effects of cortisol administration prior to exposure therapy on the reduction of pathological fear have been reported (Soravia et al., 2006; de Quervain et al., 2011). Since extinction is supposed to be the main mechanism underlying exposure-based treatment in anxiety disorders, cortisol might act by enhancing extinction-related processes in exposure therapy. On the one hand, cortisol is thought to inhibit fear memory retrieval during exposure. On the other hand, cortisol assumedly increases the consolidation of extinction memories after exposure (cf. de Quervain and Margraf, 2008; de Quervain et al., 2009; Bentz et al., 2010). First results indicate that the beneficial effects of cortisol during exposure therapy might primarily be driven by the impact of cortisol on fear retrieval, but not on extinction learning per se (Bentz et al., 2013).
All in all, different designs, samples and timing of stress or cortisol administration relative to acquisition, extinction or extinction recall complicate the picture of cortisol effects on extinction. These phases depict different memory stages (encoding, consolidation, retrieval), on which stress hormones can exert opposing effects (Wolf, 2009; Schwabe et al., 2010). In particular, neuroimaging studies on the direct impact of cortisol on extinction are lacking so far.
The objective of the present functional magnetic resonance imaging (fMRI) study was to examine the effects of cortisol administration on electrodermal and neuronal correlates of fear extinction in healthy men. Men were chosen as a starting point, because the investigation of women is complicated by different stages of the menstrual cycle and the intake of oral contraceptives. On one side, previous studies showed that cortisol reduced neuronal activation during fear acquisition in men (Stark et al., 2006; Merz et al., 2010, 2012b). If the same effect holds true for new learning of an inhibitory association during extinction, an attenuating effect of cortisol on fear extinction and its neuronal correlates could be proposed. On the other side, a beneficial effect of cortisol on the extinction of conditioned fear responses has been demonstrated (Soravia et al., 2006; Ninomiya et al., 2010; de Quervain et al., 2011). Altogether, these findings do not directly lead to a specific hypothesis in the current design, in particular concerning the direction of neuronal correlates of cortisol effects during fear extinction. Nonetheless, the neuronal correlates should include the fear extinction network comprising the amygdala, hippocampus, mPFC (Sotres-Bayon and Quirk, 2010; Milad and Quirk, 2012) and the NAcc as an important structure for changing contingencies (Klucken et al., 2009).
MATERIALS AND METHODS
Participants
In total, 32 healthy men completed the study. All of them were university students except one man, who had already graduated. Exclusion criteria covered standard fMRI exclusion criteria, somatic diseases, in particular endocrine diseases, history of psychiatric or neurological treatment and regular medication. Inclusion criteria comprised an age between 18 and 35 and a body mass index (BMI) between 18 and 28 kg/m2. All participants had to be right-handed as assessed by the Edinburgh Inventory of Handedness (Oldfield, 1971) and had normal or corrected vision. They gave written informed consent and received 20€ for their attendance. All procedures were in accordance with the Declaration of Helsinki and approved by the local ethics committee of the Justus Liebig University Giessen.
Fear conditioning design
A picture of a rhomb and of a square served as CS+ and CS− (counterbalanced stimulus allocation); they were grey in colour, had identical luminance and were presented for 8 s against a black background. Both stimuli were projected onto a screen at the end of the scanner (visual field = 18°) using an LCD projector (EPSON EMP-7250) and were viewed through a mirror mounted on the head coil. The UCS was a 100 ms transcutaneous electrical stimulation (sent by a custom-made impulse-generator, 833 Hz) delivered through two Ag/AgCl electrodes (1 mm2 surface each) attached to the middle of the left shin. UCS intensity was set individually using a gradually increasing procedure to achieve an ‘unpleasant but not painful’ level of sensation.
The conditioning experiment consisted of an acquisition and an extinction phase (Figure 1A). The conditioning procedure was adapted from prior studies in our laboratory (Stark et al., 2006; Merz et al., 2013) and included an additional extinction phase. A total of 16 CS+ and 16 CS− trials were presented in the acquisition and in the extinction phase (total time for each session: ∼10 min). During acquisition, the onset of the UCS presentation started 7.9 s after CS+ onset, but only in 10 out of 16 trials (delay conditioning; 62.5% reinforcement). The CS− was never paired with the UCS, the UCS omission 7.9 s after CS− onset was defined as non-UCS. No electrical stimulation was given during the extinction phase.
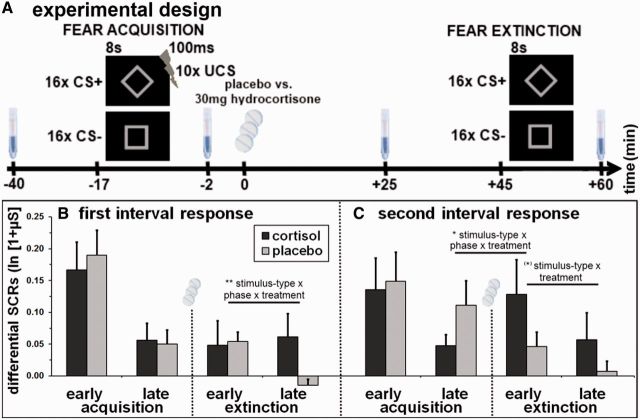
Fig. 1.
(A) Experimental design. After differential fear conditioning with a partial reinforcement schedule, participants either received 30 mg hydrocortisone or placebo, fear extinction was conducted 45 min later. Over the course of the experiment, four saliva samples were obtained to verify the effectiveness of the experimental treatment. Mean differential (CS+ minus CS−) SCRs are separately shown for the first interval response (B) and the second interval response (C) in early and late fear acquisition and extinction, respectively. Error bars indicate standard errors of the mean. **P < 0.01, *P < 0.05, (*) P < 0.10.
Between the CS, a black screen was shown lasting between 9.5 and 12 s (randomly jittered inter-trial interval). For both sessions, a pseudo-randomized stimulus order was used comprising the following restrictions: no more than two consecutive presentations of the same CS as well as within the first and the second half of the experiment an equal quantity of CS+ (for acquisition: five CS+ reinforced and three CS+ unreinforced trials; for extinction: eight CS+ unreinforced trials) and CS− trials (eight trials).
A partial reinforcement schedule was used to slow acquisition and extinction and to make learning non-trivial. Thus, early and late phases in each stage (each consisting of the mean of eight trials per stimulus-type) could be investigated reflecting the gradual development of fear learning and its extinction (cf. LaBar et al., 1998; Schiller et al., 2008). Early and late phases were defined as the first and the second halves of both phases, respectively.
Experimental procedure and cortisol
Individual sessions were scheduled between 2 and 5 p.m. to guarantee low and relatively stable endogenous cortisol concentrations. All participants were instructed to refrain from smoking, food intake and drinking anything but water for at least 2 h before the experimental session. At the beginning, they received a detailed explanation of the procedure in general. All participants were informed about a possible relationship between CS and UCS in advance of fear acquisition, which they have to detect, but received no details about the absence of the UCS during extinction.
Saliva samples for the analyses of free cortisol were collected from the participants by means of Salivette collection devices (Sarstedt, Nümbrecht, Germany). Samples were taken directly before (first sample, baseline) as well as directly after fear acquisition (second sample; see Figure 1A). Immediately after the second sample, 16 participants received three 10 mg tablets of cortisol (30 mg hydrocortisone; Hoechst) in a double-blind, randomized design. Visually identical placebos (tablettose and magnesium) were given to the other 16 participants. After that, participants had to rate the percentage occurrence (0–100%) of the UCS after presentation of the CS+ and CS−. To further confirm contingency awareness, we also handed a forced choice questionnaire, in which one of the two CS had to be chosen as the stimulus preceding the electrical stimulation.
Twenty-five minutes after tablet intake, participants provided a third saliva sample right before the second scan session started with a structural scan. Functional scans for fear extinction took place 45 min after tablet administration. After extinction, a fourth saliva sample was taken and participants had to fill out a treatment guess with the possible answers: ‘placebo’, ‘cortisol’ or ‘I do not know’. Directly after sampling, the saliva was stored at −20°C until assayed. A commercial available enzyme immunoassay (IBL International, Hamburg, Germany) was used to measure free cortisol concentrations. Intra-assay coefficients of variations were below 5% with an inter-assay coefficient of variation below 8%. Parallel to each saliva sample, participants rated their affect (see Supplementary Data for further details).
Statistical analyses were all performed in IBM SPSS Statistics for Windows 21.0 with Greenhouse–Geisser correction where appropriate and the statistical significance level was set to α = 0.05. We conducted analyses of variance (ANOVA) for cortisol including the repeated measurement factor time (first, second, third vs fourth measurement) and the between subjects factor treatment (placebo vs cortisol). For significant effects, post hoc analyses included two sample t-tests between the placebo and the cortisol group. The treatment guess was analysed using Fisher’s exact test with the directed answers ‘placebo’ and cortisol’ only (excluding ‘I do not know’).
Skin conductance responses
SCRs were sampled with an in-house built optical fibre SCR coupler especially designed for measuring SCRs concurrently to fMRI. Ag/AgCl electrodes were used filled with isotonic (0.05 M NaCl) electrolyte medium placed hypothenar at the non-dominant hand. Raw SCR data were low pass filtered with a cut-off frequency of 10 Hz. As previously (Stark et al., 2006; Merz et al., 2010, Tabbert et al., 2010, 2011; Merz et al., 2012a,b, 2013) SCRs were defined in three analysis windows (cf. Prokasy and Ebel, 1967): the maximum amplitude within a window of 1–5 s after the CS onset was counted as the first interval response (FIR), within 5–8.5 s as the second interval response (SIR), and within 8.5–13 s as the unconditioned response. The FIR reflects an orienting response, whereas the SIR reflects the anticipation of the UCS. So, they depict distinct processes, which might be differentially responsive for modulating effects to occur.
The baseline was the skin conductance level immediately preceding the inflexion point. Data were transformed with the natural logarithm to attain a normal distribution. Electrodermal data of two participants (one in each group) had to be discarded because of malfunction of the SCR coupler and a random noise in the dataset.
Statistical comparisons of SCRs were performed with the within-subject factors stimulus-type (CS+ and CS−) and phase (early vs late; cf. LaBar et al., 1998; Schiller et al., 2008) as well as the between-subjects factor treatment (placebo vs cortisol) separately for the acquisition and extinction phase in repeated measures ANOVA. Since we were also interested in the transition from acquisition to extinction, we compared late acquisition and early extinction as well. In addition, we calculated a percentage change index for decreases in extinction learning indicating to what extent the electrodermal responding of the CS+ declined from early to late extinction [1 − (SCR to the CS+ in late extinction) / (SCR to the CS+ in early extinction) ×100] and compared this index between groups using two-sample t-tests.
fMRI
Brain images were acquired using a 1.5 T whole-body tomograph (Siemens Symphony with a quantum gradient system) with a standard head coil. Structural image acquisition encompassed 160 T1-weighted sagittal images (MPRAGE; 1-mm slice thickness). For functional imaging, 245 volumes for fear acquisition as well as for extinction were registered using a T2*-weighted gradient echoplanar imaging sequence with 25 slices covering the whole brain (slice thickness = 5 mm; 1 mm gap; descending slice order; TA = 100 ms; TE = 55 ms; TR = 2.5 s; flip angle = 90°; field of view = 192 × 192 mm2; matrix size = 64 pixel × 64 pixel). The first three volumes of each session were discarded because of an incomplete steady state of magnetization. The axial slices were oriented parallel to the orbitofrontal cortex–bone transition to minimize susceptibility artifacts in prefrontal areas. A gradient echo field map sequence was measured before both functional runs to get information for unwarping B0 distortions.
All imaging data were analysed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK, 2009) implemented in MatLab R2012a (Mathworks Inc., Sherborn, MA, USA). We included the following pre-processing steps for both sessions separately: unwarping and realignment, slice time correction, co-registration of functional data to each participant’s anatomical image, segmentation into grey and white matter, normalization to the standard space of the Montreal Neurological Institute (MNI) brain, and spatial smoothing (isotropic 3D Gaussian filter; FWHM: 9 mm).
Fear acquisition and extinction were integrated as separate sessions in one first-level model in SPM8 including the following 12 experimental conditions: CS+ (early and late), CS− (early and late), UCS and non-UCS for acquisition as well as CS+ (early and late), CS− (early and late), UCS omission after CS+ and CS− for extinction. All regressors were modelled by a stick function convolved with the canonical hemodynamic response function in the general linear model, without specifically modelling the durations of the different events (i.e. event-related design). According to the event-related approach, the fMRI analyses closely match the SCR analyses regarding the FIR component, both depicting initial responding to the CS. Covariates in the model comprised the six movement parameters from the realignment step. Furthermore, a high-pass filter (time constant = 128 s) was implemented.
Random effects group analyses were conducted in SPM8 and focused on the contrast CS+ minus CS−. Time-dependent changes in neuronal activation between early and late extinction were compared between and within groups using the flexible factorial design. Furthermore, two sample t-tests were performed in SPM8 to test differences between the placebo and cortisol group in early as well as late extinction separately. Within the same two sample t-tests, overall effects of stimulus-type (contrast CS+ vs CS−) were tested for extinction as well as fear acquisition.
For all statistical analyses, we used exploratory whole brain as well as region of interest (ROI) analyses including the following ROI, which were identified in previous experiments examining cortisol effects on fear conditioning (for a review, see Rodrigues et al., 2009; e.g. Merz et al., 2010, 2012a,b,) and fear extinction in general (Phelps et al., 2004; Sotres-Bayon and Quirk, 2010; Milad and Quirk, 2012): amygdala, anterior cingulate gyrus, hippocampus, medial frontal cortex (MFC), NAcc and orbitofrontal cortex. We tested all ROI separately for the left and the right hemisphere except the anterior cingulate gyrus and the MFC. The required masks for these analyses were maximum probability masks with the probability threshold set to 0.50 taken from the Harvard–Oxford Cortical and Subcortical Structural Atlases provided by the Harvard Centre for Morphometric Analysis (http://www.cma.mgh.harvard.edu/fsl_atlas.html). Regarding the exploratory whole-brain analyses, the intensity threshold was set to P ≤ 0.05 corrected for multiple testing [family-wise error (FWE) correction], the minimal cluster size (k) was 10 voxels, and the significance threshold was set to P ≤ 0.05 on voxel-level, FWE-corrected. For the ROI analyses, the intensity threshold was set to P ≤ 0.05 uncorrected, k = 0, and the significance threshold was set to P ≤ 0.05 on voxel-level, FWE-corrected (using the small volume correction option of SPM8).
RESULTS
Sample characteristics, cortisol concentrations and treatment guess
There were no significant differences between the placebo and the cortisol group in mean age [placebo: M = 24.9, SD = 4.3; cortisol: M = 24.3, SD = 3.6; t(29.1) = 0.45; P > 0.65] or BMI [placebo: M = 24.2, SD = 2.1; cortisol: M = 23.5, SD = 1.8; t(29.4) = 1.07; P > 0.29]. Contingency awareness after fear acquisition was confirmed in all participants by a higher percentage of perceived UCS presentations after the CS+ (40–100% of the cases) compared with the CS− (0–10% of the cases). Furthermore, all participants marked the correct geometrical figure as CS+.
Five men displayed extremely high cortisol concentrations (>800 nmol/l) 25 min after hydrocortisone intake (third sample). These high levels most likely reflect micro hydrocortisone residues of the uncoated tablet in the mouth of the participants, thus, they were excluded from cortisol analyses. ANOVA revealed a significant main effect of time [F(1.2,29.5) = 43.8; P < 0.001], treatment [F(1,25) = 102.1; P < 0.001] and a time × treatment interaction [F(1.2,29.5) = 46.7; P < 0.001]. In the cortisol group, cortisol concentrations were elevated in the third and fourth sample compared with the placebo group (both P < 0.001; first and second sample: P > 0.40; Table 1), pointing to a successful pharmacological treatment during extinction.
Table 1.
Mean (SE) cortisol concentrations (in nmol/l) before fear acquisition, after fear acquisition, before (25 min after tablet intake) and after fear extinction
| Cortisol (nmol/l) | Before fear acquisition | After fear acquisition | Before fear extinction | After fear extinction |
|---|---|---|---|---|
| Cortisol | 9.70 (2.01) | 9.62 (2.00) | 335.29 (55.59) | 194.55 (17.24) |
| Placebo | 11.74 (1.48) | 10.80 (2.48) | 6.99 (1.16) | 5.18 (0.70) |
Results of the treatment guess showed that participants were not able to correctly identify, which substance they had been administered (Fisher’s exact test: P > 0.30). In the placebo group, seven participants correctly supposed the intake of placebo, but two were mistaken in assuming cortisol. Only one man in the cortisol group correctly indicated to have received cortisol, the remaining 22 participants had no treatment guess at all.
Skin conductance responses
Higher SCRs towards the CS+ than towards the CS− were established during fear acquisition as indicated by a significant main effect of stimulus-type [FIR: F(1,28) = 30.4; P < 0.001; SIR: F(1,28) = 20.6; P < 0.001]. Furthermore, this CS+/CS− differentiation declined over time [main effect phase; FIR: F(1,28) = 31.0; P < 0.001; SIR: F(1,28) = 15.2; P = 0.001; interaction stimulus-type × phase; FIR: F(1,28) = 27.6; P < 0.001; SIR: F(1,28) = 5.2; P = 0.030; Figure 1B and C]. As expected, no main effect or interaction with the factor treatment was found in early and late fear acquisition.
In extinction, the CS+/CS− differentiation still remained, but to a lesser degree [main effect stimulus-type; FIR: F(1,28) = 4.1; P = 0.053; SIR: F(1,28) = 10.6; P = 0.003], also declining over time [main effect phase; FIR: F(1,28) = 13.0; P = 0.001; SIR: F(1,28) = 5.0; P = 0.033; interaction stimulus-type × phase; FIR: F(1,28) = 4.7; P = 0.040; SIR: n.s.]. As indicated by a significant stimulus-type × phase× treatment interaction in the FIR [F(1,28) = 10.0; P = 0.004; Figure 1B] as well as a trend in the interaction stimulus-type × treatment in the SIR [F(1,28) = 3.2; P = .084; Figure 1C], participants in the cortisol group displayed heightened conditioned SCRs, whereas participants in the placebo group showed attenuated CRs over time.
The comparison between late acquisition and early extinction revealed a significant interaction between stimulus-type, phase, and treatment in the SIR [F(1,28) = 6.9; P = 0.014], demonstrating higher conditioned SCRs in the cortisol compared with the placebo group during early extinction relative to late acquisition.
Comparisons of the percentage change index for decreases in extinction learning revealed a trend to enhanced reduction of SCRs from early to late extinction to the CS+ in the placebo group (67.1%) compared with the cortisol group (39.0%) in the FIR [T(24.9) = 1.9; P = 0.075]. The same direction was found in the SIR (placebo: 46.4%; cortisol: 26.9%), however, this effect was not significant.
Hemodynamic responses
In early fear acquisition, we detected significant CRs (contrast CS+ minus CS−) in the anterior cingulate cortex, right hippocampus, left orbitofrontal cortex and bilaterally in the NAcc (Table 2). In late fear acquisition, a significant CS+/CS− differentiation was found in the left NAcc and left orbitofrontal cortex (Table 2).
Table 2.
Localization and statistics of the peak voxels for the contrast CS+ minus CS−, separately for early and late fear acquisition
| Early acquisition | Brain structure | x | y | z | Tmax | Pcorr |
|---|---|---|---|---|---|---|
| CS+ – CS− | Anterior cingulate | −3 | 17 | 34 | 4.60 | 0.009 |
| R hippocampus | 33 | −22 | −11 | 4.42 | 0.006 | |
| L nucleus accumbens | −12 | 11 | −11 | 3.60 | 0.008 | |
| R nucleus accumbens | 12 | 11 | −11 | 3.29 | 0.014 | |
| L orbitofrontal cortex | −15 | 14 | −17 | 3.82 | 0.036 |
| Late acquisition | Brain structure | x | Y | Z | Tmax | Pcorr |
|---|---|---|---|---|---|---|
| CS+ – CS− | L nucleus accumbens | −6 | 14 | −5 | 2.93 | 0.030 |
| L orbitofrontal cortex | −12 | 17 | −20 | 3.87 | 0.030 |
The significance threshold was Pcorr≤ 0.05 (FWE-corrected; small volume correction in SPM8).
All coordinates (x, y, z) are given in MNI space. L = left, R = right.
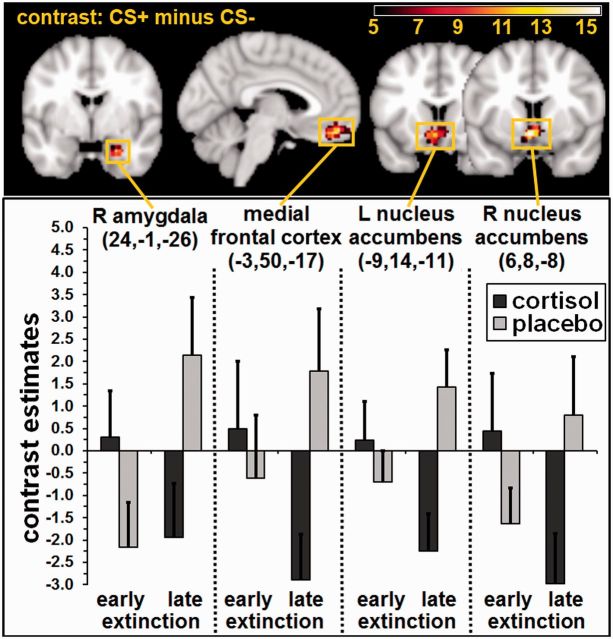
Differential BOLD responses changed over the course of extinction (early vs late) between groups in the MFC, left NAcc, and right amygdala, whereas a further trend emerged in the right NAcc (phase × treatment interaction; Table 3 and Figure 2). This undirected interaction effect was subsequently further analysed. First, early was compared with late extinction in the placebo and cortisol group separately. In the placebo group, neuronal activation increased from early to late extinction in the MFC, a further trend emerged in the right amygdala. In the cortisol group, neuronal activation decreased from early to late extinction as a trend in the left NAcc (Table 3).
Table 3.
Localization and statistics of the peak voxels for the contrast CS+ vs CS− for the comparison between early and late fear extinction as well as separately for early and late extinction
| Early vs late ext. | Brain structure | x | Y | Z | Fmax | Pcorr |
|---|---|---|---|---|---|---|
| Phase × treatment | R amygdala | 24 | −1 | −26 | 14.82 | 0.025 |
| Medial frontal gyrus | −3 | 50 | −17 | 15.12 | 0.042 | |
| L nucleus accumbens | -9 | 14 | −11 | 10.48 | 0.035 | |
| R nucleus accumbens | 6 | 8 | −8 | 8.23 | 0.068 | |
| Groups separated | Brain structure | x | y | z | Tmax | pcorr |
| Placebo (late–early) | R amygdala | 24 | −1 | −26 | 3.30 | 0.080 |
| Medial frontal gyrus | −3 | 44 | −23 | 4.61 | 0.020 | |
| Cortisol (early–late) | L nucleus accumbens | −9 | 14 | 11 | 2.58 | 0.088 |
| Early extinction | Brain structure | x | y | z | Tmax | pcorr |
| CS+ – CS− | No significant differences | |||||
| CS− – CS+ | L amygdala | −21 | −13 | −14 | 2.89 | 0.066 |
| R amygdala | 21 | −13 | −14 | 3.42 | 0.027 | |
| L hippocampus | −33 | −28 | −14 | 5.34 | 0.001 | |
| R hippocampus | 27 | −19 | −14 | 4.69 | 0.003 | |
| L posterior parahippocampal gyrus (WB) | −36 | −31 | −11 | 5.92 | 0.038 | |
| Placebo–cortisol | No significant differences | |||||
| Cortisol–placebo | No significant differences | |||||
| Late extinction | Brain structure | x | Y | Z | Tmax | Pcorr |
| CS+ – CS− | No significant differences | |||||
| CS− – CS+ | No significant differences | |||||
| Placebo–cortisol | L amygdala | −24 | −10 | −14 | 3.14 | 0.044 |
| R amygdala | 24 | −4 | −23 | 2.94 | 0.075 | |
| Medial frontal gyrus | −3 | 41 | −23 | 3.92 | 0.018 | |
| L nucleus accumbens | −9 | 14 | −11 | 3.11 | 0.021 | |
| R nucleus accumbens | 12 | 17 | −8 | 2.41 | 0.077 | |
| Cortisol–placebo | No significant differences | |||||
| Groups separated | Brain structure | x | Y | Z | Tmax | Pcorr |
| Placebo (CS+ – CS−) | L nucleus accumbens | −12 | 11 | −8 | 3.04 | 0.044 |
| Cortisol (CS− – CS+) | L amygdala | −24 | −10 | −14 | 3.08 | 0.084 |
| L nucleus accumbens | −9 | 14 | −11 | 2.70 | 0.071 | |
| R nucleus accumbens | 9 | 14 | −8 | 2.99 | 0.041 | |
The significance threshold was Pcorr≤ 0.05 [FWE-corrected; for the small volume correction as well as for the whole-brain (WB) correction]. Trends up to a threshold of Pcorr≤ 0.10 are written in italics. The peak voxel from the WB analysis was labelled based on the Harvard–Oxford Subcortical Structural Atlas. All coordinates (x, y, z) are given in MNI space. L = left, R = right.
Fig. 2.
Neuronal activation during early vs late extinction for the contrast CS+ minus CS−. Data are masked with the respective ROI and illustrated with F ≥ 5.0 (see colour bar for exact F-values). The depicted slices were selected according to the reported activations in the right amygdala (y = −1), the medial frontal cortex (x = −3), as well as in the left (y = 14) and right nucleus accumbens (y = 8). Additionally, mean contrast estimates as well as the respective standard errors in the respective peak voxels are displayed in the bar graphs separately for the cortisol and the placebo group. Cortisol application reduced differential neuronal activation in fear extinction-related structures during late extinction. L = left, R = right.
Second, cortisol effects were tested separately for the early and late extinction phase. In early fear extinction, no significant group differences emerged for the contrast CS+ minus CS−. The analysis of the contrast CS+ minus CS− across both groups yielded no significant results. However, the reversed contrast (CS− minus CS+) revealed significant BOLD responses in the right amygdala, left posterior parahippocampal gyrus, bilaterally in the hippocampus, and as a trend in the left amygdala across both groups (Table 3). In late fear extinction, analyses across both groups did not result in any significant effect. However, significantly higher CRs emerged in the placebo compared with the cortisol group during late extinction in the left amygdala, MFC and left NAcc. Further trends were detected in the right amygdala and right NAcc (Table 3). Thus, cortisol exerted its effects on late, but not on early extinction.
Third, the CS+/CS− differentiation was tested separately for both groups during late extinction. In the placebo group, higher CS+ compared with CS− responses were found in the left NAcc. In the cortisol group, the opposite contrast (CS− minus CS+) revealed a significant result in the right NAcc and further trends in the left amygdala and left NAcc (Table 3).
DISCUSSION
In this fMRI study, cortisol given prior to fear extinction changed electrodermal as well as neuronal correlates of extinction learning. Higher differential SCRs were observed in the cortisol compared with the placebo group pointing to still remaining CRs during extinction after cortisol administration, in particular regarding late extinction. On the neuronal level, these higher fear-associated SCRs during late extinction were reflected in diminished activation of the amygdala, MFC and NAcc in the cortisol compared with the placebo group.
Fear extinction in general
In early extinction, higher BOLD responses towards the CS− compared with the CS+ were found in the amygdala, hippocampus and posterior parahippocampal gyrus across both groups replicating previous extinction studies (Phelps et al., 2004; Klucken et al., 2013). This initial shift in neuronal activation might be connected to extinction learning and coding the predictive CS+ property being reversed when new information is available. In contrast, higher responses to the CS+ than to the CS− have also been reported in particular in the amygdala (LaBar et al., 1998). However, LaBar and colleagues used a 100% reinforcement schedule during fear acquisition, after which rapid extinction occurs as opposed to a partial reinforcement schedule implemented in the current and previous designs (Phelps et al., 2004) leading to slowed extinction learning. Extinction in a continuous reinforcement design might be more comparable to late extinction in a partial reinforcement schedule.
Within the amygdala, a rodent model proposes that two classes of neurons are activated during fear conditioning (Herry et al., 2008, 2010): whereas fear neurons convey fear learning receiving inputs from the hippocampus, extinction neurons indicate fear extinction while being reciprocally connected to the mPFC. Furthermore, fear neurons in the amygdala display reduced activation towards the CS+ during extinction leading to overall higher responses towards the CS− (Herry et al., 2008), thus being in line with the current fMRI results of both groups in early extinction. This activation pattern however changed from early to late extinction towards higher responses to the CS+ compared with the CS− in the amygdala and MFC in the placebo group. Both the CS+/CS− differentiation in the amygdala and mPFC have already been found particularly during late extinction (Milad et al., 2007), supporting the idea that interactions between amygdala and mPFC serve encoding of long-term extinction memories. Besides, activation of the MFC also increased in the placebo group from early to late extinction, which might also foster adequate fear extinction learning. Of note in this context, a lesser vmPFC activation was found during late extinction in patients with post-traumatic stress disorder (PTSD) compared with healthy controls, which might contribute to a failure of adequate extinction consolidation (Milad et al., 2009). Reduced extinction learning (i.e. higher CS+/CS− differentiation) has also been implicated in the development of PTSD (Guthrie and Bryant, 2006; Lommen et al., 2013).
Additionally, the NAcc was recruited during late extinction. The NAcc as part of the ventral striatum has been implicated in the formation of contingency awareness during fear acquisition (Pezze and Feldon, 2004; Carter et al., 2006; Klucken et al., 2009). Our current data in early and late acquisition not only confirm these prior results, but also extend them by showing an additional involvement of the NAcc in late fear extinction. Similarly to fear acquisition, in which an association between CS+ and UCS establishes, a new relationship (no UCS occurring after the CS+) has to be learned in extinction as well.
All in all, the current results together with rodent data (Herry et al., 2008, 2010) suggest a model as follows: during early extinction, higher CS− compared with CS+ responses could mirror deactivation of fear neurons in the amygdala towards the CS+. These fear neurons are closely connected to the hippocampal complex, which encodes the relevant contextual information (cf. Ji and Maren, 2007). During late extinction, extinction neurons in the amygdala take over leading again to a larger BOLD signal to the CS+ than to the CS−, possibly conveyed by the additional activation of the MFC. At the same time, conditioned SCRs diminish indicating successful fear extinction. This interpretation is consistent with the idea that the mPFC plays a critical role in the inhibition of CRs by suppressing the output of the amygdala (Milad and Quirk, 2002; Maren and Quirk, 2004). Furthermore, the NAcc might be involved in relearning and coding the new contingencies during late extinction.
Cortisol effects on fear extinction
The observed changes in CS+/CS− differentiation in the amygdala, MFC and NAcc over the course of fear extinction in the placebo group seem to be necessary for adequate extinction learning. Cortisol interrupted this shift, thereby attenuating extinction learning and promoting prolonged conditioned fear reflected in still increased differential SCRs and slightly reduced extinction of electrodermal responding to the CS+ in late relative to early extinction. In addition to reduced extinction learning after cortisol administration, an enhanced recall of the acquired fear memory in the cortisol group might be assumed, which can be seen in the differential SIR (Figure 1C). Moreover, cortisol might have delayed extinction learning due to an initially enhanced fear recall. The proposed switch in neuronal processing as seen in the placebo group might simply occur later in the cortisol group. However, this interpretation (increased fear retrieval) would contradict the literature on declarative memory supporting reduced memory retrieval (particularly of emotional information; for reviews, see Wolf, 2009; Schwabe et al., 2012) or attenuated fear retrieval (Bentz et al., 2013) after stress or cortisol administration. Further research is needed to disentangle the effects of cortisol on fear retrieval vs extinction learning processes in designs accounting for a prolonged extinction session as well as investigating consolidated fear.
During late extinction, cortisol reduced differential BOLD responses in the amygdala, MFC and NAcc. Previous studies have already shown that glucocorticoids attenuate prefrontal activation in general (Diamond et al., 2007) or during memory retrieval (Oei et al., 2007). Furthermore, cortisol also impairs working memory (Oei et al., 2006; Schoofs et al., 2009), which relies on intact prefrontal functioning (Fuster, 2000). As already mentioned, the NAcc might be concerned with encoding of the new contingencies during late extinction. This relearning seems to be successful in the placebo group, in which a higher CS+/CS− differentiation in the NAcc was detected. But in the cortisol group, the reversed pattern was observed not only in the NAcc, but also in the amygdala. This opposite pattern that also emerged during early extinction (in both groups) might explain the still increased SCRs in the cortisol group. Furthermore, cortisol has already been implicated in decreasing amygdala activation during psychosocial stress (Prüssner et al., 2008) or during fear acquisition (Merz et al., 2010).
In accordance with the current results, an operant conditioning study in humans showed that stress after initial learning weakened subsequent extinction (Schwabe and Wolf, 2011). The same picture emerged in men exposed to psychosocial stress before fear acquisition and subsequent extinction (Jackson et al., 2006). These human studies confirm effects of acute stress exposure before conditioning attenuating fear extinction in male mice (Izquierdo et al., 2006). Besides, stress also impaired extinction recall in rodents (Akirav and Maroun, 2007).
However, studies in male rats showed facilitated extinction and recall after prior glucocorticoid application, when different phases of fear conditioning are divided into several days (Yang et al., 2006, 2007; Ninomiya et al., 2010). Similarly, clinical studies showed that cortisol administration after trauma reduced the incidence of PTSD (Schelling et al., 2001, 2004), alleviated existing PTSD symptoms (Aerni et al., 2004) and enhanced exposure therapy in specific and social phobia (Soravia et al., 2006; de Quervain et al., 2011). At first sight, this framework would predict opposite results in the current study, i.e. lowered conditioned SCRs during extinction (cf. de Quervain and Margraf, 2008; de Quervain et al., 2009; Bentz et al., 2010, 2013). However, the proposed mechanism does not necessarily rely on cortisol directly influencing fear extinction per se (as in the present data). In these studies, rather fear retrieval and extinction consolidation might be affected by cortisol administration, but not fear extinction learning.
Further important differences between these previous experiments and the present data exist: clinical studies investigated patients with anxiety disorders (Soravia et al., 2006; de Quervain et al., 2011), in whom pathological fear acquisition supposedly happened long time ago and therefore is well consolidated. Besides, the mentioned studies in rodents (Yang et al., 2006, 2007; Ninomiya et al., 2010) were also conducted in paradigms involving several days. In contrast, the present experiment established a fear memory and conducted fear extinction immediately afterwards, thus interfering with the consolidation of fear acquisition. Once the fear memory is consolidated (e.g. on the next day), the effect of cortisol on fear retrieval (Bentz et al., 2013) might emerge and attenuate CRs. This picture is obviously reversed when extinction takes place immediately after fear acquisition, when consolidation has not been finished yet. So, our results in healthy men cannot be directly compared with patients with long existing anxiety disorders and remote acquired fear. All together, the exact timing between cortisol administration, fear acquisition and extinction plays a critical role for further research.
Limitations
At least, four limitations should be mentioned: first, only men participated in this study, so the results cannot be generalized to women and might even be opposing as suggested by some of our previous work on cortisol effects on fear acquisition (Stark et al., 2006; Merz et al., 2010; Tabbert et al., 2010; Merz et al., 2012b). However, cortisol administration before fear acquisition led to a reduction of neuronal activation during extinction learning in women taking oral contraceptives (Tabbert et al., 2010), which is consistent with the present results in men. Besides, cortisol given prior to fear acquisition reduced CRs in several brain regions in men (Stark et al., 2006; Merz et al., 2010, 2012b), which supports the notion of comparable effects of cortisol on fear acquisition and extinction at least in male participants.
Second, we have to acknowledge that the current results reflect the effects of supraphysiological cortisol concentrations, which cannot be directly translated to stress-induced cortisol concentrations. As far as fear acquisition is concerned, we could recently show that both supraphysiologcial levels obtained after administration of 30 mg hydrocortisone (as in the present study) as well as physiological cortisol increases after psychosocial stress exert the same effects on neuronal activation in men and women taking oral contraceptives (Merz et al., 2013). This effect might also apply to our current data on fear extinction, but this hypothesis has to be proven in future studies. Accordingly, the effect of a physiological cortisol dose on fear extinction should be tested to complement the picture.
Third, we neither conducted extinction on a separate day nor tested extinction recall on an additional day. These additional tests would have shed light on direct effects and long-lasting consequences of cortisol application before extinction learning. The exact timing of different phases of fear conditioning by itself has to be considered in future studies along with the time when cortisol is given.
Fourth, the FIR concerns orienting responses to the CS and closely matches the event-related approach of the fMRI results in terms of timing. The SIR reflects anticipatory responses to the UCS, but this component is not adequately reflected in the fMRI data. It is quite difficult to determine when exactly anticipation processes start on the neuronal level, so, an adequate modelling of this component needs further attention.
Conclusion
To summarize, cortisol administration impaired fear extinction learning in healthy men. Higher differential SCRs in the cortisol group indicate still remaining, inappropriate fear during extinction. This result pattern was accompanied with lowered neuronal activation in the amygdala, MFC and NAcc. A shift from deactivation during early extinction to activation during late extinction seems to promote adequate fear extinction as seen in the placebo group. Cortisol administration however suppressed this shift thereby attenuating fear extinction. These results critically emphasize the importance of timing of cortisol application relative to fear acquisition and extinction. Future studies should systematically examine the impact of different time points of cortisol administration on neuronal and electrodermal correlates of fear extinction.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of interest
None declared.
Supplementary Material
Acknowledgments
This study was carried out at the Bender Institute of Neuroimaging, Justus Liebig University Giessen. We thank Dr. Carlo Blecker (Bender Institute of Neuroimaging) for technical assistance and Dr. Bertram Walter (Bender Institute of Neuroimaging) for statistical support as well as Prof. Dr. Clemens Kirschbaum (Technical University of Dresden) for analysing the saliva samples. Furthermore, we thank Naomi de Haas, Sonja Reichert and Liliane Weis for subject recruitment and data collection. In addition, we acknowledge the helpful comments raised by the reviewers.
Funding for this study was provided by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) to O.T.W. (WO 733/13-1; part of the DFG Research Unit FOR 1581, Extinction Learning). The DFG had no role in study design, collection, analysis and interpretation of data, writing of the manuscript or in the decision to submit the paper for publication.
REFERENCES
- Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. The American Journal of Psychiatry. 2004;161:1488–90. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ-F, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders. 2010;24:223–30. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bentz D, Michael T, Wilhelm FH, et al. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013;38:1186–97. doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;29:1007–12. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30:358–70. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-F, Bentz D, Michael T, et al. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6621–25. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ-F, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. European Journal of Pharmacology. 2008;583:365–71. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Experimental Brain Research. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. The American Journal of Psychiatry. 2011;168:1255–65. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, Kloet ER, de Joels M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology. 2012;350:299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosomatic Medicine. 2006;68:307–11. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Mueller C, Luethi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–U28. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luethi A. Neuronal circuits of fear extinction. The European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. The Journal of Neuroscience. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–22. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Klucken T, Tabbert K, Schweckendiek J, et al. Contingency learning in human fear conditioning involves the ventral striatum. Human Brain Mapping. 2009;30:3636–44. doi: 10.1002/hbm.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Vaitl D, Stark R. Dissociation of neuronal electrodermal, and evaluative responses in disgust extinction. Behavioral Neuroscience. 2013;127:380–6. doi: 10.1037/a0032331. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of Neuroscience. 2004a;24:218–28. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive, Affective & Behavioral Neuroscience. 2004b;4:317–25. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, et al. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. The European Journal of Neuroscience. 2009;29:823–32. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen MJJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behaviour Research and Therapy. 2013;51:63–7. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Stark R, Vaitl D, Tabbert K, Wolf OT. Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biological Psychology. 2013;92:82–9. doi: 10.1016/j.biopsycho.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive and Affective Neuroscience. 2012a;7:819–30. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Hormones and Behavior. 2012b;62:531–8. doi: 10.1016/j.yhbeh.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.05.015. doi: 10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ninomiya EM, Martynhak BJ, Zanoveli JM, Correia D, da Cunha C, Andreatini R. Spironolactone and low-dose dexamethasone enhance extinction of contextual fear conditioning. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1229–35. doi: 10.1016/j.pnpbp.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Elzinga BM, Wolf OT, et al. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging and Behavior. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, Well S, van Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress - The International Journal on the Biology of Stress. 2006;9:133–41. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Prokasy WF, Ebel HC. Three components of the classically conditioned GSR in human subjects. Journal of Experimental Psychology. 1967;73:247–56. [Google Scholar]
- Prüssner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63:234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annual Review of Neuroscience. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biological Psychiatry. 2001;50:978–85. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, de Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Annals of the New York Academy of Sciences. 2004;1032:158–66. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. The Journal of Neuroscience. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–53. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neuroscience and Biobehavioral Reviews. 2012;36:1740–9. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress increases behavioral resistance to extinction. Psychoneuroendocrinology. 2011;36:1287–93. doi: 10.1016/j.psyneuen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: quantity and quality. Neuroscience and Biobehavioral Reviews. 2010;34:584–91. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Learning during stressful times. Learning & Memory. 2004;11:137–44. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5585–90. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:1–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, et al. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32:1290–8. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, et al. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiology of Learning and Memory. 2010;94:392–401. doi: 10.1016/j.nlm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: twelve years of progress? Brain Research. 2009;1293:142–54. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Yang Y-L, Chao P-K, Lu K-T. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropharmacology. 2006;31:912–24. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yang Y-L, Chao P-K, Ro L-S, Wo Y-YP, Lu K-T. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropharmacology. 2007;32:1042–51. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.