Abstract
Background.
To evaluate the longitudinal associations between menopausal status, related hormonal changes, and level of self-reported physical functioning.
Methods.
Study included 2,495 women (age: 45–57 between 2000 and 2001) from the Study of Women’s Health Across the Nation. Physical functioning scale of the Medical Outcomes Study Short-Form (SF-36; score 0–100) was categorized as: no limitation (86–100), moderate limitation (51–85), and substantial limitation (0–50). Study variables were collected between 2000 (visit-04) and 2011 (visit-12) at five timepoints. Statistical models were adjusted for age at visit-04, time since visit-04, ethnicity, site, economic status, level and change in body mass index, level and change in physical activity, and presence of comorbid conditions.
Results.
In final models, natural and surgical postmenopausal women had significantly higher odds of functional limitation, compared with premenopausal women. Less reduction in estradiol and testosterone since visit-04 were significantly associated with lower odds of functional limitation, while greater increase in sex hormone-binding globulin was associated with higher odds of functional limitation.
Conclusions.
Our findings suggest the menopause-related changes in endogenous sex hormones as a possible mechanism of action to explain the greater limitation in physical functioning reported in women at midlife.
Key Words: Physical function, Epidemiology, Functional performance, Geriatric endocrinology, Menopause.
Functional limitations, defined as the degree of difficulty in performing daily living activities (1), have been used as a surrogate marker of the overall impact of disease and environment (2). Self-reported functional limitations have been observed more frequently in women compared with men (3–5), even at relatively young ages of 40–55 years (6). This period of women’s lives coincides with the menopausal transition; a time when women are subjected to several physiological, physical, and psychological changes. The physiological changes accompanying the menopausal transition, including changes in endogenous sex hormone levels, may predispose women to the development of functional limitations. Earlier research from the Study of Women’s Health Across the Nation (SWAN) documented self-reported functional limitations among 20% of women aged 40–55 (7). Interestingly, postmenopausal women have been found to report greater functional limitations (both self-reported and objectively assessed) than premenopausal women (8–10). Additionally, women with surgical menopause (8,11) and earlier age at menopause (11) reported worse physical functioning. These findings were limited by the cross-sectional design (8,10,11) and/or the inability to adjust for over-time change in factors that may vary during the menopausal transition such as changes in body mass index (BMI) and level of physical activity (8–11).
Whether or not the changes in endogenous sex hormones that accompany the menopausal transition contribute to the increase in functional limitations has yet to be evaluated. The aim of the current study was to assess the longitudinal relationships between endogenous sex hormones, menopausal status, and self-reported functional limitations in women transitioning through the menopause while controlling for factors known to change over the menopausal transition that could potentially impact physical functioning.
Methods
Study Participants
SWAN is an ongoing, longitudinal, multiethnic study of the menopausal transition. The study design has been previously reported (12). In brief, 3,302 participants aged 42–52 years (1996–1997), were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were (i) an intact uterus and at least one ovary, (ii) at least one menstrual period within the past 3 months, (iii) no hormone therapy use within the past 3 months, (iv) self-identified race as African American, Caucasian, Chinese, Hispanic, or Japanese.
Of the 3,302 women enrolled in SWAN, 2,868 women were available to complete the physical functioning subscale of the Medical Outcomes Study SF-36 as part of the following SWAN follow-up visits: 04, 06, 08, 10, or 12 (in 2011). Due to administrative issues at the New Jersey site (the only site that recruited Hispanic participants), data collection was halted during visit-06 through visit-10. Therefore, data from this site was excluded (n = 284). Women (n = 89) were also excluded due to missing data for physical functioning, menopausal status, and sex hormones at all visits. This resulted in a final sample of 2,495 women for longitudinal analysis (n = 10,651 observations).
For the sex hormones analysis, observations at which women were using hormone therapy, missing data on hormone therapy use, or missing data on sex hormones (179 women use/missing hormone therapy or sex hormones at all timepoints) were dropped, resulting in a sample of 2,316 women (n = 8,995 observations) for longitudinal sex hormones and functional limitations analysis. The research protocols were approved by the institutional review board at each site and all participants provided written informed consent before enrollment.
Study Measures
Physical functioning limitations.
Self-reported physical functioning was assessed by the 10-item physical functioning subscale of the SF-36 (13), which has been extensively evaluated for validity, internal consistency, and test–retest reliability in diverse ethnic groups and age ranges. It includes a three-item response of “limited a lot,” “limited a little,” or “not limited at all” to the following items: vigorous and moderate activities; lifting or carrying groceries; climbing several flights of stairs; climbing one flight of stairs; bending, kneeling, or stooping; walking more than 1 mile; walking several blocks; walking one block; and bathing or dressing. Participant responses were calculated using the original algorithm that produces a physical functioning score between 0 and 100 with higher scores representing better physical functioning (14). Scores on the SF-36 are highly skewed with many respondents scoring 100 (15). Therefore, SF-36 physical functioning scores were categorized into a three-level functional limitations variable using cutoffs as recommended by Rose and colleagues (15): no limitation (86–100), moderate limitation (51–85), and substantial limitation (0–50).
Menopausal status.
Menopausal status was based on bleeding patterns, current hormone use, hysterectomy/oophorectomy as follow: (i) premenopausal: menses in the last 3 months with no change in regularity in the last 12 months; (ii) early perimenopausal: menses in the last 3 months with some change in regularity during the prior 12 months; (iii) late perimenopausal: no menses within the last 3 months, but some menstrual bleeding over the prior 12 months; (iv) postmenopausal: no menses within the last 12 months; (v) surgical postmenopausal: history of hysterectomy or bilateral oophorectomy; (vi) hormone users/unknown: postmenopausal women using hormone therapy and women with undetermined menopausal status due to hormone therapy use.
Endogenous sex hormones.
A fasting blood sample was drawn at each visit during the early follicular phase (days 2–5) if women were still menstruating. If a timed sample could not be obtained, a random fasting sample was taken within the 90-day period of recruitment. Sex hormones were measured using the Automated Chemiluminescence System (ACS)-180 (Bayer Diagnostics Corp., Norwood, MA). Estradiol (E2) was measured using a modified, off-line ACS-180 (E2-6; lower limit of detection [LLD]: 1–7 pg/mL; averaged interassay coefficient of variation [CV]: 10.6%; averaged intra-assay CV: 6.4%). Serum testosterone (T) concentration was evaluated with the ACS-180 total T assay modified to increase precision in the low ranges (LLD: 2–2.2ng/dL; interassay CV: 10.5%; intra-assay CV: 8.5%). Sex hormone-binding globulin (SHBG) was measured with a two-site chemiluminescent immunoassay (LLD: 1.9–3.2 nM; interassay CV: 9.9%; intra-assay CV: 6.1%). Only E2 assays were conducted in duplicate. The arithmetic mean for the duplicate measures was calculated and reported (CV: 3%–12%). Hormone values below the LLD were replaced with a random value between zero and the LLD. Cycle day of blood draw was reported as days 2–5 (for regularly menstruating women) or outside of that period (for irregularly and nonmenstruating women).
Study covariates.
In addition to race/ethnicity as a study covariate, annual BMI was calculated using measured weight (kg)/height (m)2. Age, current smoking status, and difficulty paying for basics (degree of difficulty in paying for basics such as food, housing, and health care on a three-item scale “very hard, somewhat hard, or not very hard at all”) were derived from questionnaires administered annually. Annual reporting of comorbid medical conditions known to be associated with poor physical functioning including depression, osteoarthritis, diabetes, and hypertension were also considered. Depression was assessed using the Center for Epidemiologic Studies Depression scale with a cut point ≥16 indicating current presence of depressive symptoms (16). Osteoarthritis was self-reported as present or absent. Participants who self-reported diabetes, had fasting glucose levels ≥126mg/dL, or reported any use of insulin or antidiabetic agents on at least 70% of the visits and/or for at least three consecutive visits were classified as diabetic. Participants with systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or self-reported using antihypertensive medication were classified as having hypertension. Physical activity was assessed longitudinally via a modified Baecke scores of habitual physical activity; with higher scores indicating more physical activity (17).
Statistical analysis.
For longitudinal analysis, time variant menopausal status, log-transformed E2, SHBG, and T (to account for skewed distributions) were modeled separately as a function of functional limitations categories using ordinal generalized estimating equation since the proportional odds assumptions were met. Time was modeled as age at visit-04 and time since visit-04 to account for chronological aging. Sex hormones, BMI, and physical activity score were modeled as visit-04 (cross-sectional effect) and change from visit-04 (longitudinal effect) to distinguish between the cross-sectional effect and the longitudinal effect (18). Interactions between the menopausal status, sex hormones (in separate models), ethnicity, and BMI were evaluated and none were significant. Smoking status was initially included in multivariable analyses and then excluded as adding it to models did not improve the models or change the main results. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). All models were two-sided at alpha = 0.05.
Results
Participants were followed for a maximum of 11.2 years (median follow-up time = 9.7 years) and contributed an average of 4.3 observations per woman. Table 1 presents characteristics of the study participants at visit-04. About 52% of the study population were pre- or early perimenopausal and about 17% were natural or surgical postmenopausal. By visit-12, 92% were natural or surgical postmenopausal and only 2.6% were still menstruating (Supplementary Table 1).
Table 1.
Characteristics of the Study Population at SWAN Visit-04
| Study Variables | N = 2,495* |
|---|---|
| Age (y), mean ± SD | 50.5±2.7 |
| Ethnicity, n (%) | |
| Caucasian | 1,225 (49.1) |
| African American | 769 (30.8) |
| Japanese | 265 (10.6) |
| Chinese | 236 (9.5) |
| Status, n (%) | |
| Premenopause | 175 (7.5) |
| Early perimenopause | 1,043 (44.7) |
| Late perimenopause | 246 (10.5) |
| Natural postmenopause | 355 (15.2) |
| Surgical postmenopause | 45 (1.9) |
| Hormone users/unknown | 472 (20.2) |
| Body mass index, kg/m2, mean ± SD | 28.7±7.5 |
| Smoker, n (%) | 306 (13.4) |
| Physical activity†, mean ± SD | 7.7±1.7 |
| How hard to pay for basics, n (%) | |
| Very hard | 183 (7.4) |
| Somewhat | 674 (27.1) |
| Not very | 1,630 (65.5) |
| Hypertension, n (%) | 778 (34.8) |
| Diabetes, n (%) | 167 (7.1) |
| Osteoarthritis, n (%) | 311 (13.3) |
| Depression, n (%) | 416 (18.0) |
| Endogenous sex hormones, median (Q1, Q3) | |
| E2, pg/mL | 31.5 (18.8, 72.2) |
| SHBG, nM | 38.2 (25.5, 53.7) |
| T, ng/dL | 33.4 (24.1, 47.0) |
| Self-reported physical functioning‡, n (%) | |
| No limitation | 1,319 (58.4) |
| Some limitation | 700 (31.0) |
| Substantial limitation | 238 (10.5) |
Notes: E2 = estradiol; SHBG = sex hormone-binding globulin; SWAN = Study of Women’s Health Across the Nation; T = testosterone.
*Denominator for presented proportions for some variables may not be the same due to missing related information at visit-04 for different variables.
†Range of physical activity score 3–13.2. Higher score indicates higher level of physical activity.
‡No limitation: physical functioning score 86–100; some limitation: physical functioning score 51–85; substantial limitation: physical functioning score: ≤50.
Overall, 58.4% reported no functional limitations, 31.0% reported some limitation, and 10.5% reported substantial limitation at follow-up visit-04. A slight increase in substantial limitation was observed from visit-04 (11.0%) to visit-12 (15.0%). Additionally, classification as having no limitation decreased over time (58.4% at visit-04 vs 52.1% at visit-12), Figure 1. The odds of functional limitation per 7-year increase was found to be 1.24 (95% confidence interval: 1.16, 1.32) adjusting for age at visit-04, study site, and race (data not shown).
Figure 1.
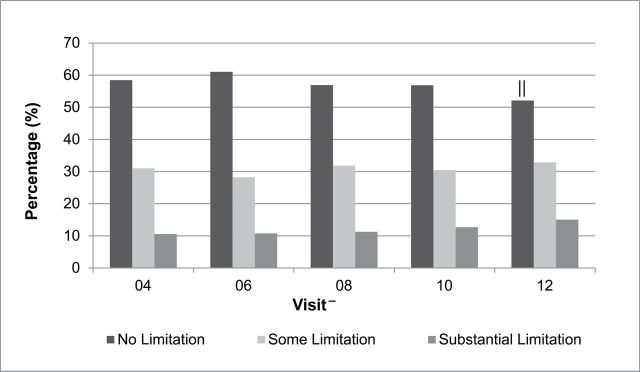
Percentages of physical function limitation categories over time. *
No limitation: physical functioning score 86–100; some limitation: physical functioning score 51–85; substantial limitation: physical functioning score: ≤50.
-Cross-sectional sample size at each visit: visit-04 = 2,257, visit-06 = 2,142, visit-08 = 2,126, visit-10 = 2,091, visit-12 = 2,035. Common reasons for loss to follow-up: refusing to complete the form, missing the study visit and study deactivation.
|| p < .0001 for greater odds of substantial limitation versus no limitation and some limitation at visit-12 compared with visit-04.
In longitudinal analysis (Table 2), the odds of substantial limitation versus no limitation and some limitation were higher in late perimenopausal, natural postmenopausal, surgical postmenopausal, and in hormone user/unknown menopausal status women (All p ≤ .05) compared with premenopausal women adjusting for age, site, race, difficulty paying for basics, level at visit-04, and change since visit-04 of BMI and physical activity. Further adjustment for comorbid conditions did not change the results for late peri-, surgical, and natural postmenopausal women.
Table 2.
Longitudinal Associations Between Menopausal Status and Higher Levels of Physical Functioning Limitation
| Menopausal Status | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| Proportional Odds Ratio (95% CI) | p Value | Proportional Odds Ratio (95% CI) | p Value | Proportional Odds Ratio (95% CI) | p Value | |
| Premenopause | 1 (Reference) | — | 1 (Reference) | — | 1 (Reference) | — |
| Early perimenopause | 1.55 (1.08, 2.24) | .02 | 1.46 (1.00, 2.14) | .05 | 1.42 (0.96, 2.10) | .10 |
| Late perimenopause | 1.73 (1.19, 2.53) | .004 | 1.62 (1.09, 2.41) | .02 | 1.51 (1.00, 2.27) | .049 |
| Natural postmenopause | 1.89 (1.29, 2.77) | .001 | 1.72 (1.16, 2.56) | .01 | 1.61 (1.07, 2.41) | .02 |
| Surgical postmenopause | 2.00 (1.25, 3.19) | .004 | 1.88 (1.17, 3.04) | .01 | 1.73 (1.06, 2.83) | .03 |
| Hormone users/unknown | 1.61 (1.09, 2.37) | .02 | 1.64 (1.09, 2.47) | .02 | 1.46 (0.97, 2.22) | .10 |
Notes: Proportional odds ratios present the odds of substantial limitation versus no limitation and some limitation in each status category compared with premenopausal status. BMI = body mass index; CI = confidence interval.
*Model 1: adjusted for age at visit-04, time since visit-04 and site.
†Model 2: adjusted for age at visit-04, time since visit-04, site, race, ability to pay for basics, BMI at visit-04, change in BMI since visit-04, physical activity at visit-04, and change in physical activity since visit-04.
‡Model 3: additionally adjusted for hypertension, diabetes, osteoarthritis, and depression.
In Table 3, higher levels of E2 at visit-04 and less reduction in E2 and T since visit-04 were significantly associated with lower odds of reporting functional limitations. On the other hand, a greater increase in SHBG since visit-04 was associated with greater odds of functional limitations. Except for E2 level at visit-04, results did not change in final models (model 3, Table 3).
Table 3.
Longitudinal Associations Between Level and Changes of Endogenous Sex Hormones and Higher Levels of Physical Functioning Limitation
| Endogenous Sex Hormones, Separate Models (log transformed) | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| Proportional Odds Ratio (95% CI) | p Value | Proportional Odds Ratio (95% CI) | p Value | Proportional Odds Ratio (95% CI) | p Value | |
| E2 level at visit04 | 0.87 (0.79, 0.96) | .01 | 0.87 (0.79, 0.97) | .01 | 0.91 (0.82, 1.00) | .06 |
| E2 change since visit04 | 0.91 (0.85, 0.98) | .01 | 0.91 (0.85, 0.97) | .01 | 0.93 (0.87, 0.99) | .03 |
| SHBG level at visit04 | 1.08 (0.93, 1.25) | .30 | 1.08 (0.93, 1.25) | .30 | 1.08 (0.94, 1.26) | .30 |
| SHBG change since visit04 | 1.17 (1.03, 1.34) | .02 | 1.19 (1.04, 1.36) | .01 | 1.23 (1.08, 1.41) | .002 |
| T level at visit04 | 0.92 (0.77, 1.10) | .40 | 0.94 (0.79, 1.12) | .50 | 0.93 (0.79, 1.10) | .40 |
| T change since visit04 | 0.84 (0.73, 0.97) | .02 | 0.87 (0.75, 0.99) | .047 | 0.84 (0.73, 0.97) | .02 |
Notes: Proportional odds ratios present the odds of substantial limitation versus no limitation and some limitation per 1 unit increase (log transformed) in sex hormones (level or change). BMI = body mass index; CI = confidence interval; E2 = estradiol; SHBG = sex hormone-binding globulin; T = testosterone.
*Model 1: adjusted for age at visit-04, time since visit-04, BMI at visit-04, change in BMI since visit-04, and site.
†Model 2: adjusted for age at visit-04, time since visit-04, BMI at visit-04, change in BMI since visit-04, site, race, cycle day of blood draw, ability to pay for basics, physical activity at visit-04, and change in physical activity since visit-04.
‡Model 3: additionally adjusted for hypertension, diabetes, osteoarthritis, and depression.
Discussion
The results from the current study suggest menopause-related changes in sex hormones as possible triggering factors for the significant limitation reported in surgical and postmenopausal women.
Our findings that both surgical and natural postmenopausal women are more likely to report higher levels of limitation were in agreement with previous cross-sectional analyses from SWAN (7,8). We expanded these cross- sectional results to the longitudinal setting while controlling for changes in factors that potentially could impact physical functioning level. Using longitudinal data from the Michigan Bone Health and Metabolism study, Sowers and colleagues (19) reported significant reduction in levels of self-reported and performance-based physical functioning in midlife women. The study was limited by only including Caucasian women. We reported similar results, however, using a larger sample with multiethnic groups of midlife women. Additionally, we evaluated the potential contributions of changes in sex hormones on functional limitations, which was not evaluated in Sowers and colleagues (19) or any previous study.
Several changes accompanying the menopausal transition could potentially predispose women to be more vulnerable to a greater functional limitation. According to the Nagi model (3) functional limitation and disability could be consequences of an active pathological situation. This pathological situation can lead to an impairment status. We hypothesized that the hormonal changes either due to natural or surgical menopause could easily create a pathological situation and the consequences of the hormonal changes (discussed below) could lead to an impairment status that results in functional limitations (8). Our results support our hypothesis (8) that hormonal changes could play a primary role in the etiology for the disablement process as proposed by the Nagi model (3).
Functional limitations in performing daily living activities are mainly affected by muscle strength, mass, and performance (20). The reduction in muscle strength can play a substantial role in the degree of difficulty in performing daily living activities (21,22). The loss of muscle mass may contribute to the loss of skeletal mass by reducing mechanical stress on the skeleton (23). It has been reported that an accelerated loss of muscle strength occurs earlier in women than men (24). Women tend to lose muscle strength (24) and mass (23) around the 5th decade of age which is the time at which most women go through menopause.
We reported significant positive associations between the changes in E2 and T levels, and functional limitations during the menopausal transition. Previous studies showed significant associations between the estrogen and testosterone levels and muscle strength (25–27), a potential determinant of physical functioning. A recent meta-analysis showed that muscle strength for women on hormone therapy were ~5% significantly greater than those not on hormone therapy (28). Combined estrogen/testosterone treatment was associated with an increase in lower body mass, reduction in body fat, and an increase in lower muscle strength in postmenopausal women (29).
The menopause-related changes in sex hormones could potentially affect the physical functioning level via several mechanisms. Estrogen may have a direct effect on muscle via estrogen receptors at the messenger RNA level (30). The skeletal muscles have a higher proportion of alpha estrogen receptor on type II fibers, the twitch muscle fibers that produce fast and strong contractions (20). Estrogen may also act on muscle indirectly via the somatotropic axis of growth hormone and insulin growth factor 1 (31). Both growth hormone and insulin growth factor 1 have anabolic effects on muscle and bone tissues (20,32).
Androgens are also important for women as they act on several tissues including the central nervous system, cardiovascular system, bone, breast, muscle, adipose, and genital tissues (33). Androgen acts either directly by binding to androgen receptors or indirectly by local aromatization to estrogens (33). T increases muscle protein synthesis through binding to the androgen receptors in muscle tissue, which in turn stimulates protein synthesis in both type I and II muscle fibers (25). Adding T to estrogen therapy was associated with an increase in lower muscle strength in postmenopausal women (29).
In the current study, the odds of reporting higher levels of functional limitations were the highest among surgical menopause women. After menopause, the production of estrogen falls rapidly, while levels of androgen slightly decrease over time. Oophorectomy reduces serum T by half (29) and this may explain the greatest functional limitations in surgical menopause reported in the current study. Another possible explanation is that the greater functional limitation among surgical menopause women could be a consequence of the conditions that lead to surgical menopause and impact women’s health status. More than 20% of surgical menopause women described their health status as fair or poor compared with less than 7% of pre- and perimenopausal women (19).
We found a significant positive effect between increase of SHBG and greater functional limitations. SHBG is a glycoprotein synthesized in the liver and binds with higher affinity to androgens more than estrogens (34). An increase in SHBG is associated with a decreased androgenic status. Therefore, the positive association that we reported may be explained by the direct relationship between SHBG and androgen. The use of hormone therapy increases SHBG production and therefore leads to a reduction in active T levels (33).
This study has a number of limitations. Self-reported physical functioning from the SF-36 was used as longitudinal physical functioning performance measures were not available at time of this study. Although we utilized self-reported physical functioning rather than an objective measure, the SF-36 physical functioning scale is a widely used scale with high reproducibility and validity measures (13,14). We excluded Hispanic women due to their shorter follow-up time. Therefore, the current results may not be generalizable to Hispanic women. It is unlikely that excluding Hispanics introduced bias to the main results as a sensitivity analysis including them showed similar main results. Additionally, women excluded from the current analysis probably biased results toward the null, as those who were excluded were less healthy (women excluded were slightly older, less physically active, more likely to be surgical post or natural postmenopausal, on hormone therapy, hypertensive, self-reported osteoarthritis, and reported more depressive symptoms than women included). Including those women would strengthen our results. Finally, because women with prior hysterectomy were ineligible for SWAN enrollment, women who had a hysterectomy after enrollment may not be representative of all women with hysterectomy.
Using a well-characterized cohort of multiethnic women at midlife, we reported higher levels of functional limitations in both surgical and natural postmenopausal women that were not explained by potential confounders. The greater limitations observed among surgical and natural postmenopausal women are most likely to be resulted from the changes in endogenous estrogen and androgens accompanying the menopausal transition. Future studies should assess if similar associations exist with objective physical functioning measures. Additionally, it is important to test whether the effect of type and timing of menopause are still seen later in life once all women transition completely to the postmenopausal stage.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgment
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
References
- 1. Applegate WB, Blass JP, Williams TF. Instruments for the functional assessment of older patients. N Engl J Med. 1990;322:1207–1214 [DOI] [PubMed] [Google Scholar]
- 2. Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989;79:703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467 [PubMed] [Google Scholar]
- 4. Ross CE, Bird CE. Sex stratification and health lifestyle: consequences for men’s and women’s perceived health. J Health Soc Behav. 1994;35:161–178 [PubMed] [Google Scholar]
- 5. Murray ET, Hardy R, Strand BH, Cooper R, Guralnik JM, Kuh D. Gender and life course occupational social class differences in trajectories of functional limitations in midlife: findings from the 1946 British birth cohort. J Gerontol A Biol Sci Med Sci. 2011;66:1350–1359. 10.1093/gerona/glr139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions—United States, 1991–1992. MMWR Morb Mortal Wkly Rep. 1994;43:730–731, 737––739 [PubMed] [Google Scholar]
- 7. Sowers M, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–1492 [DOI] [PubMed] [Google Scholar]
- 8. Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause. 2012;19:1186–1192. 10.1097/gme.0b013e3182565740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurina LM, Gulati M, Everson-Rose SA, et al. The effect of menopause on grip and pinch strength: results from the Chicago, Illinois, site of the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160:484–491 [DOI] [PubMed] [Google Scholar]
- 10. Cheng MH, Wang SJ, Yang FY, Wang PH, Fuh JL. Menopause and physical performance–a community-based cross-sectional study. Menopause. 2009;16:892–896. 10.1097/gme.0b013e3181a0e091 [DOI] [PubMed] [Google Scholar]
- 11. Tom SE, Cooper R, Patel KV, Guralnik JM. Menopausal characteristics and physical functioning in older adulthood in the National Health and Nutrition Examination Survey III. Menopause. 2012;19:283–289. 10.1097/gme.0b013e3182292b06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sowers M, Crawford S, Sternfed B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000:175–188 [Google Scholar]
- 13. Ware J. Measures for a new era of health assessment. In: Stewart AL, Ware JE, eds. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992:3–11 [Google Scholar]
- 14. Ware J. SF-36 Health Survey. Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993 [Google Scholar]
- 15. Rose MS, Koshman ML, Spreng S, Sheldon R. Statistical issues encountered in the comparison of health-related quality of life in diseased patients to published general population norms: problems and solutions. J Clin Epidemiol. 1999;52:405–412 [DOI] [PubMed] [Google Scholar]
- 16. Randloff LS. The CESD scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401 [Google Scholar]
- 17. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323 [DOI] [PubMed] [Google Scholar]
- 18. Hedeker D, Gibbons RD. Longitudinal Data Analysis. NJ: A John Wiley & Sons. INC.; 2006:69–76 [Google Scholar]
- 19. Sowers M, Tomey K, Jannausch M, Eyvazzadeh A, Nan B, Randolph J., Jr Physical functioning and menopause states. Obstet Gynecol. 2007;110:1290–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9:186–197 [PubMed] [Google Scholar]
- 21. Alexander NB, Schultz AB, Ashton-Miller JA, Gross MM, Giordani B. Muscle strength and rising from a chair in older adults. Muscle Nerve Suppl. 1997;5:S56–S59 [PubMed] [Google Scholar]
- 22. Bassey EJ, Fiatarone MA, O’, Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82:321–327 [DOI] [PubMed] [Google Scholar]
- 23. Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH. Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr. 1991;53:1378–1383 [DOI] [PubMed] [Google Scholar]
- 24. Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84:95–98 [DOI] [PubMed] [Google Scholar]
- 25. van Geel TA, Geusens PP, Winkens B, Sels JP, Dinant GJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol. 2009;160:681–687. 10.1530/EJE-08-0702 [DOI] [PubMed] [Google Scholar]
- 26. Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120–1131 [DOI] [PubMed] [Google Scholar]
- 27. Häkkinen K, Pakarinen A. Muscle strength and serum testosterone, cortisol and SHBG concentrations in middle-aged and elderly men and women. Acta Physiol Scand. 1993;148:199–207 [DOI] [PubMed] [Google Scholar]
- 28. Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. 10.1093/gerona/glp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dobs AS, Nguyen T, Pace C, Roberts CP. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1509–1516 [DOI] [PubMed] [Google Scholar]
- 30. Lemoine S, Granier P, Tiffoche C, Rannou-Bekono F, Thieulant ML, Delamarche P. Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc. 2003;35:439–443 [DOI] [PubMed] [Google Scholar]
- 31. Friend KE, Hartman ML, Pezzoli SS, Clasey JL, Thorner MO. Both oral and transdermal estrogen increase growth hormone release in postmenopausal women–a clinical research center study. J Clin Endocrinol Metab. 1996;81:2250–2256 [DOI] [PubMed] [Google Scholar]
- 32. Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693–721 [DOI] [PubMed] [Google Scholar]
- 33. Cooper AL, Nelson DF, Doran S, et al. Testosterone treatment in women—an overview. Curr Women’s Health Rev. 2009;5:29–43. 10.2174/157340409787721267 [Google Scholar]
- 34. Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. ; SWAN Investigators. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111:1242–1249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


