Abstract
Minor capsid protein L2 of papillomaviruses plays an essential role in virus assembly by recruiting viral components to PML bodies, the proposed sites of virus morphogenesis. We demonstrate here that the function of L2 in virus assembly requires the chaperone Hsc70. Hsc70 was found dispersed in naturally infected keratinocytes and cultured cells. A dramatic relocation of Hsc70 from the cytoplasm to PML bodies was induced in these cells by L2 expression. Hsc70-L2 complex formation was confirmed by coimmunoprecipitation. The complex was modulated by the cochaperones Hip and Bag-1, which stabilize and destabilize Hsc70-substrate complexes, respectively. Cytoplasmic depletion of Hsc70 caused retention of wild-type and N-terminally truncated L2, but not of C-terminally truncated L2, in the cytoplasm. This retention was partially reversed by overexpression of Hsc70 fused to green fluorescent protein but not by ATPase-negative Hsc70. Hsc70 associated with L1-L2 virus-like particles (VLPs) but not with VLPs composed either of L1 alone or of L1 and C-terminally truncated L2. Moreover, displacement of Hsc70 from L1-L2 VLPs by encapsidation of DNA, generating pseudovirions, was found. These data indicate that Hsc70 transiently associates with viral capsids during the integration of L2, possibly via the L2 C terminus. Completion of virus assembly results in displacement of Hsc70 from virions.
Molecular chaperones, or heat shock proteins (Hsp), are a large family of cellular proteins found in prokaryotes and eukaryotes. They are implicated in preventing protein aggregation after cellular stress and assisting the correct folding of newly synthesized polypeptides as well as fulfilling important functions in cellular transport and assembly processes (3, 15, 27). Not only cells but also viruses benefit from the chaperone system. Some viruses encode chaperones of their own, while others are dependent on host cell chaperones, which have been shown often to be upregulated in response to viral infection (43).
Heat shock cognate protein 70 (Hsc70) is a constitutively expressed member of the Hsp70 family. In eukaryotic cells, it is involved in a large variety of processes, including folding of nascent polypeptide chains (15), translocation of proteins across membranes, intracellular vesicle trafficking and sorting, uncoating of clathrin-coated vesicles, signal transduction, and nuclear transport (27). The mechanism of Hsc70 function often involves delaying or promoting the folding of the protein substrate. Its activity is facilitated through interaction with a number of proteins containing J-like domains, such as Hsp40, which act as cochaperones (19, 29). Positive and negative modulators such as Hip and Bag-1 regulate Hsc70 function by stabilizing and destabilizing the interaction with the protein substrate, respectively (33, 34, 45).
Cellular members of the Hsp70 family have been shown to be involved in the assembly of hepadnaviruses and polyomaviruses (43) as well as enteroviruses (25) and poxviruses (16). Hsc70 regulates the posttranslational translocation of the large envelope protein of human hepatitis B virus into the endoplasmic reticulum (18, 24). Hsc70 also transiently binds to polyomavirus capsid proteins, escorting them to the nucleus. Since Hsc70 is not incorporated into polyoma virions, it has been proposed to inhibit premature assembly of capsids in the cytosol and/or to facilitate the nuclear transport of capsid protein complexes (6, 37). In addition, Hsc70 was recently demonstrated to facilitate the assembly of polyomavirus-like particles in vitro (5). Other viruses, such as influenza virus A, rabies virus, vesicular stomatitis virus (36), and human immunodeficiency virus (14), encapsidate this chaperone into viral particles for yet unknown reasons. Adenoviruses require Hsc70 for delivery of the viral genome to the nucleus (13, 38).
We have been interested in the morphogenesis of human papillomaviruses (HPV). HPV are epitheliotropic viruses, which replicate only in terminally differentiating cells of the skin and mucosa. They induce benign, self-limiting tumors, which sometimes progress to malignancy, e.g., cervical carcinoma (28). The nonenveloped papillomavirus consists of only three components; it has a circularly closed, double-stranded DNA genome in the form of chromatin and two capsid proteins, L1 and L2. The major capsid protein, L1, is the main structural component of the viral protein shell. Three hundred sixty L1 copies assemble to form 72 pentamers, called capsomeres (4, 30), whereas each virion probably contains only 12 copies of the minor capsid protein, L2 (2, 40, 46). Virus-like particles (VLPs), composed of either L1 alone or L1 and L2, as well as pseudovirions, which harbor a heterologous reporter plasmid, have been used for the study of virus morphogenesis and infection. These studies have demonstrated that L2 serves important nonstructural functions during virus assembly and the infection process (8, 10, 11, 17, 47, 50). L2 accumulates at PML bodies (also known as PODs, or ND10) and recruits L1 to these nuclear substructures, where replication of papillomavirus DNA, and probably virus assembly, takes place (44).
It has been demonstrated in a previous study that L1 and L2 are separately translocated to the nucleus (10). Although L1 assembles in the cytoplasm to form capsomeres, and mutants of L1 lacking a nuclear localization signal can even form VLPs in the cytoplasm (35), incorporation of L2 into VLPs requires nuclear localization (2). This raises the question of how the interactions of L2 and L1 are regulated. Given the multiple functions of chaperones in folding and macromolecular assembly, we hypothesized that L2 activity may be controlled by one or several chaperones. To address this question, we have analyzed the interaction of L2 with the chaperone Hsc70. We report here that Hsc70-L2 association is an obligatory intermediate step in papillomavirus assembly.
MATERIALS AND METHODS
Cell lines and antibodies.
The osteosarcoma cell line HuTK−143B, the recombinant vaccinia virus VTF7-3, and the transfer vector pTM1 were generously provided by B. Moss (31). All cells were grown at 37°C in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and antibiotics. L1- and L2-specific mouse monoclonal antibodies (MAbs) and rabbit polyclonal antibodies have been described previously (39, 48, 49). The rat anti-Hsc70 MAb SPA-815 and the rabbit anti-Hsp40 polyclonal antiserum SPA-400 were purchased from Stressgen. The hemagglutinin epitope (HA) tag-specific mouse MAb (clone 16B12) was obtained from Berkeley Antibody Company. MG132, phenylbutyrate, and nocodazole were purchased from Sigma-Aldrich.
Mutagenesis and generation of recombinant vaccinia viruses.
The generation of recombinant vaccinia viruses expressing HPV16, -18, and -33 wild-type L2 (12, 47) and the N- and C-terminal L2 deletion mutants of HPV33 has been described previously (1).
Generation of green fluorescent protein (GFP) fusions.
L2 fragment 399/467 of HPV33 was generated by PCR using oligonucleotides 5′-TCT GAA TTC AAT GGG ATT TGA TAC TCC TGT T-3′ and 5′-GCG GGA TCC CTA GGC CGC CAC ACG GAC ATC-3′ as forward and reverse primers, respectively (boldfacing indicates newly introduced restriction sites). Fragments were purified by agarose gel electrophoresis, cut with EcoRI and BamHI, and cloned into pEGFP-C1 (Clontech) to yield pGFP-33L2-399/467. Bovine cDNAs encoding wild-type Hsc70 and its ATPase-deficient mutant Hsc70K71M were generously supplied by S. Schmid (32) and subcloned into the pEGFP-C1 expression vector. Expression plasmids pCDNA/HA.Hip and pCDNA/HA.Bag-1, which carry the Hip or Bag-1 gene, respectively, fused at the N terminus in frame with an HA tag under the control of the cytomegalovirus promoter, were generously provided by H. H. Kampinga (34).
Protein expression.
Confluent cells were split 1:4 and grown for 24 h. They were washed once with phosphate-buffered saline (PBS) and infected at a multiplicity of infection of 2 with the helper vaccinia virus vTF7-3 alone or with vTF7-3 and a recombinant vaccinia virus expressing wild-type or mutant L2 diluted in serum-free DMEM. After incubation for 1 h at room temperature, the virus-containing medium was replaced with supplemented DMEM. Where indicated, MG132 (final concentration, 10 μM) was added to the cells 3 h later and incubation was continued for an additional 6 h. Cells were processed for immunoprecipitation, sucrose gradient analysis, or immunofluorescence staining after the indicated times at 37°C.
Immunofluorescence staining.
Cells were grown on coverslips and infected with vaccinia viruses as described above, fixed with methanol-0.02 M EGTA (−20°C) for at least 20 min, washed twice with PBS, and blocked in 5% goat serum in PBS. Coverslips were then incubated for 1 h at 37°C with the antibodies indicated. After extensive washing with PBS, coverslips were again blocked for 30 min with 5% goat serum and subsequently incubated for 1 h at 37°C with Cy3-conjugated Affinipure goat anti-rabbit immunoglobulin G (IgG) and Cy2-conjugated Affinipure goat anti-mouse IgG (Jackson Immunoresearch Products), respectively. Coverslips were again washed with PBS and then mounted on slides by using Fluoprep mounting medium (bioMérieux). Pictures were taken by using a ZEISS Axiovert 200M microscope and a ZEISS Axiocam digital camera. Axiovision software (version 3.0) was used for merging images. Some images were deconvoluted by using the software supplied by ZEISS.
Immunoprecipitation.
COS-7 cells were transfected with pCDNA/HA.Hip or pCDNA/HA.Bag-1, respectively, by using a GIBCO BRL CELL-PORATOR (Life Technologies Inc., Gaithersburg, Md.). After 24 h, cells were coinfected with the helper virus vTF7-3 and vac33L2. Alternatively, cells were directly infected with vTF7-3 and vac33L2. Cells were harvested after 20 h, washed once with PBS, and suspended in 1 ml of PBS supplemented with 0.5% NP-40 and a protease inhibitor cocktail. Cell extracts were prepared by sonication for 30 s at 20% output and a 20% interval by using a Branson Sonifier 250. The whole-cell lysate was added to goat anti-mouse IgG-coated Dynabeads (Dynal), to which MAb 16B12, specific for the HA tag, or MAb 33L2-1 had previously been coupled. After constant agitation for 4 h at 4°C, the beads were collected and washed four times with 1 ml of ice-cold PBS. Bound proteins were eluted with 1% sodium dodecyl sulfate (SDS) at 100°C. Lysates and immunoprecipitates were subsequently analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to a nitrocellulose membrane. Hip and Bag-1 were detected by using the HA tag-specific MAb 16B12. Hsc70 and L2 were detected by using the rat MAb SPA-815 and MAb 33L2-1, respectively, and a horseradish peroxidase-coupled secondary antibody (Jackson Immunoresearch Products). The signal was visualized by enhanced chemiluminescence (Amersham Pharmacia).
Preparation of HPV33 pseudovirions.
Pseudovirions were prepared essentially as described previously (12, 47), with slight modifications. Briefly, COS-7 cells were transfected with the marker plasmid pEGFPGFP-NLS by electroporation using a CELL-PORATOR. Transfected cells were grown for 48 h and subsequently infected with recombinant vaccinia viruses for expression of L1 and L2. Pseudovirions were extracted from nuclei after 40 h by sonication in a hypotonic buffer supplemented with 0.5% NP-40 and were purified by cesium chloride buoyant density gradients. Individual fractions were dialyzed and subjected to immunoblot and infectivity assays.
Sucrose gradient analysis.
Pseudovirion- and VLP-containing fractions of the cesium chloride buoyant density gradients were dialyzed against PBS for 2 h and then loaded onto a 10-to-60% sucrose gradient in PBS supplemented with 5 μg of bovine serum albumin/ml. After 150 min at 36,000 rpm and 4°C (in a Beckman SW40 rotor), the gradients were fractionated from the top (750 μl/fraction). Aliquots of each fraction were precipitated by using trichloroacetic acid. Proteins were resuspended in sample buffer at 100°C for 5 min and analyzed by SDS-PAGE and immunoblotting using MAbs 33L1-7 and 33L2-1, the Hsc70-specific rat MAb SPA-815, and horseradish peroxidase-conjugated goat anti-mouse and goat anti-rat IgG, respectively.
RESULTS
L2 expression induces relocation of Hsc70.
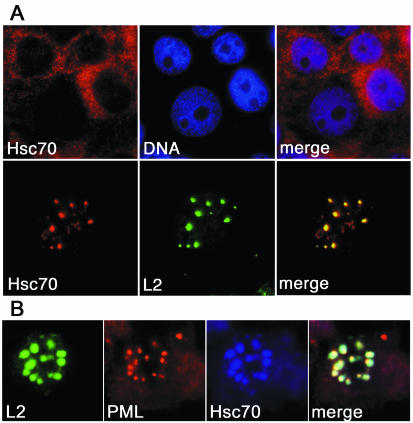
Initially we investigated the interaction of Hsc70 and L2 by using cell culture systems. As shown in Fig. 1 for HuTK−143B cells, Hsc70 was virtually absent from nuclei as well as nucleoli and was localized predominantly in the cytoplasm. The cellular distribution of Hsc70 changed dramatically when minor capsid protein L2 of HPV33 was expressed in these cells. Hsc70 localized mainly to nuclei, exhibited a speckled distribution, and colocalized with L2 in PML bodies as demonstrated by costaining with PML (Fig. 1). Colocalization of Hsc70 and L2 in PML bodies was independent of the mode of L2 expression, the type of HPV, and the cell line used. By use of (i) infection with a recombinant vaccinia virus or transfection with a plasmid, (ii) L2 of HPV16, HPV18, or HPV33, and (iii) COS-7 or HuTK−143B cells, colocalization of L2 and Hsc70 in PML bodies was observed in all cells expressing L2 (data not shown). This dramatic change indicates that L2 and Hsc70 may form a complex, possibly in the cytoplasm, which is then translocated to the nucleus.
FIG. 1.
Colocalization of Hsc70 and L2 at PML bodies. (A) HuTK−143B cells were either control infected with vTF7-3 (upper panels) or coinfected with vTF7-3 and vac33L2 (lower panels) for 12 h. Following infection, cells were stained for DNA, Hsc70, and L2 as indicated. (B) Cells were additionally stained for PML to demonstrate localization of L2 and Hsc70 at PML bodies.
Hsc70 and L2 form an active complex.
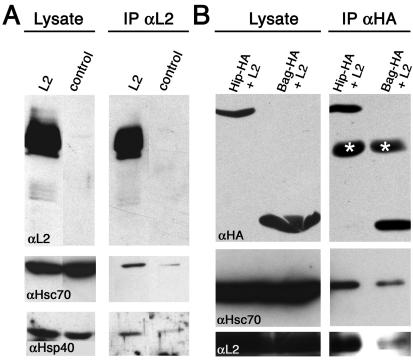
To analyze if this cotransport was due to complex formation between Hsc70 and L2, we coimmunoprecipitated the two proteins. By use of an antibody against L2, Hsc70 was coimmunoprecipitated from lysates of cells expressing L2, but only marginally from control cells (Fig. 2A). Since Hsc70 requires cochaperones such as Hsp40 for full activity, the detection of Hsp40 in immunoprecipitates from lysates of L2-expressing cells indicated that the Hsc70-L2 complex was functional (Fig. 2A). By use of an antibody against Hsc70, Hsp40 and L2 were also coimmunoprecipitated from lysates of cells expressing L2 (data not shown).
FIG. 2.
Coimmunoprecipitation of L2 and chaperones. (A) Lysates from vac33L2- and control-infected cells were subjected to SDS-PAGE followed by L2-specific immunoblotting. For immunoprecipitation (IP), lysates were incubated with L2-specific MAbs before immunoblotting with the L2-specific MAb 33L2-1. The same blots were reprobed with Hsc70- and Hsp40-specific antibodies after removal of the L2-specific antibodies from the membrane. (B) COS-7 cells were transfected with plasmids encoding HA-tagged Hip or Bag-1, respectively, grown for 24 h, and subsequently infected with vaccinia viruses for expression of L2. Extracts were subjected to HA-specific immunoprecipitation prior to HA-specific immunoblotting. In addition to Hip and Bag-1, the IgG heavy chain (marked by asterisks) was detected in the immunoprecipitates. The same blots were repeatedly reprobed with Hsc70- and L2-specific antibodies after removal of the antibodies from the membrane.
Two other cochaperones, Hip and Bag-1, have recently been shown to modify the affinity of Hsc70 for its substrates by stabilizing the ADP-bound form or by favoring nucleotide exchange, respectively (33, 34). Overexpression of these cochaperones should therefore affect the Hsc70-L2 complex. By use of HA-tagged Hip and Bag-1, respectively, for coexpression with L2, both Hip and Bag-1 colocalized with L2 at PML bodies (data not shown). When Hip and Bag-1 were precipitated by using an HA-specific antibody, both Hsc70 and L2 were found in the immunoprecipitates (Fig. 2B). Coimmunoprecipitates of Hip contained significantly larger amounts of Hsc70 and L2 than Bag-1 coimmunoprecipitates (Fig. 2B), a finding consistent with the known functions of Hip and Bag-1. These data indicate that L2 and Hsc70 form an active complex that can be modified by overexpression of cochaperones.
Cytoplasmic depletion of Hsc70 blocks nuclear translocation of L2.
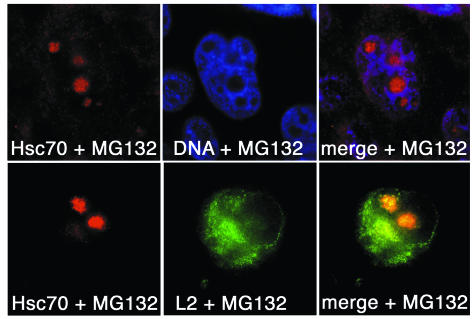
We next investigated if complex formation between L2 and Hsc70 is required for L2 function in virus assembly. Because Hsc70 knockout cells are nonviable, and dominant-negative mutants of Hsc70 produce unwanted pleiotropic effects (S. Schmid, personal communication), we used various treatments to remove Hsc70 from the cytoplasm of L2-expressing cells. Heat shock triggers relocation of Hsc70 from the cytoplasm to nucleoli (21). Similarly, Hsc70 was relocated to nucleoli in cells treated with proteasome inhibitors such as MG132 (Fig. 3) or with phenylbutyrate (data not shown). Under these conditions, L2 protein was retained in the cytoplasm, where it accumulated in perinuclear aggregates, as shown in Fig. 3 for MG132 treatment.
FIG. 3.
Effect of MG132 on localization of Hsc70 and L2. HuTK−143B cells were either control infected with vTF7-3 (upper panels) or coinfected with vTF7-3 and vac33L2 (lower panels). Three hours after infection, cells were grown for an additional 6 h in the presence of 10 μM MG132; they were subsequently stained for DNA, Hsc70, and L2, as indicated. Nucleoli are not stained by Hoechst 33342.
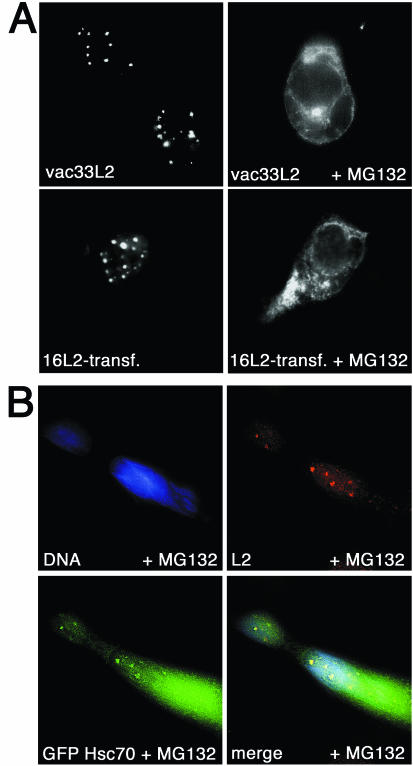
To exclude the possibility that this observation was specific for HPV33 or the vaccinia virus expression system used, we also analyzed the effect of Hsc70 relocation on HPV16 and HPV18 L2 expressed in the vaccinia virus system and on HPV16 L2 synthesized after transfection with a plasmid carrying an L2 expression cassette. In all cases studied, MG132 blocked L2 nuclear translocation, as shown for HPV16 in Fig. 4A. L1 expression also induced nuclear relocation of Hsc70. However, cytoplasmic depletion of Hsc70 affected neither the cellular localization nor the nuclear translocation of any of the L1 proteins analyzed, irrespective of the expression system used (data not shown). This result is in agreement with previous data demonstrating the absence of an inhibitory effect of MG132 on L1 nuclear translocation (10). Taken together, these data strongly suggest that Hsc70 is required for nuclear translocation of L2 but not of L1 and that cytoplasmic retention of L2 under heat shock conditions might be due to a lack of sufficient quantities of Hsc70.
FIG. 4.
(A) MG132-induced L2 retention is independent of the HPV type or expression system used. HPV33 L2 and HPV16 L2 were expressed by using recombinant vaccinia viruses and transfection of codon-optimized HPV16 L2, respectively. Three hours after infection and 24 h after transfection, cells were grown for an additional 6 h in the presence of 10 μM MG132; they were subsequently stained for L2. (B) Rescue of L2 nuclear transport by overexpression of the GFP-Hsc70 fusion. COS-7 cells were transfected with pGFP.Hsc70 and grown for 24 h prior to coinfection with recombinant vaccinia virus vTF7-3 or vac33L2. Three hours after infection, cells were grown for an additional 6 h in the presence of 10 μM MG132. Cells were processed for immunofluorescence analysis.
To confirm this notion, we overexpressed Hsc70 fused to GFP (GFP-Hsc70) in cells treated with MG132. As shown in Fig. 4B, MG132-induced cytoplasmic retention of L2 was partially abolished by GFP-Hsc70, resulting in nuclear translocation of a significant fraction of L2. Under these conditions, GFP-Hsc70 was also found to partially relocate to nucleoli, as expected (data not shown). Overexpression of an ATPase-deficient mutant of GFP-Hsc70 did not lead to reconstitution of L2 nuclear translocation (data not shown). These experiments provide strong additional evidence that Hsc70 and its ATPase function are essential for nuclear translocation of L2.
Hsc70 and L2 colocalize in PML bodies of HPV-infected cells.
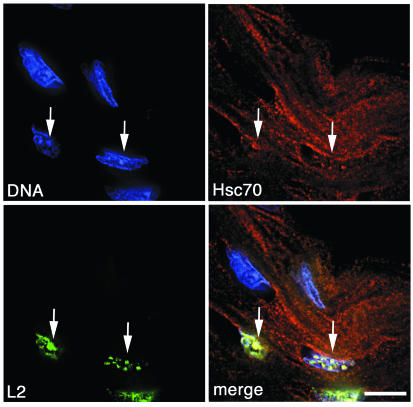
We next analyzed the association of L2 and Hsc70 during natural infection. When we stained HPV33-infected tissue sections obtained from a cervical intraepithelial neoplasia (CIN) for L2 and Hsc70, L2 was detected in distinct nuclear dots (Fig. 5). These dots have previously been identified as PML bodies by demonstrating colocalization of L2 with Sp100, a major component of PML bodies (11). As observed before, L2 was present only in a subset of fully differentiated cells of the keratinized upper layers of this tissue. Hsc70 was detected as dispersed cytoplasmic staining throughout the section, with the exception of L2-expressing cells. Here, Hsc70 was found in the nucleus, colocalizing with L2 at PML bodies (Fig. 5). This finding suggests that interaction and cotransport of L2 and Hsc70 also occur during natural infection and papillomavirus replication.
FIG. 5.
Colocalization of L2 and Hsc70 in cells naturally infected with HPV. Paraffin-embedded tissue sections obtained from a biopsy specimen containing HPV-33 were deparaffinized and stained for DNA, Hsc70, and L2. Arrows highlight colocalization of Hsc70 and L2.
C-terminal L2 truncation abolishes cytoplasmic retention by Hsc70 relocation.
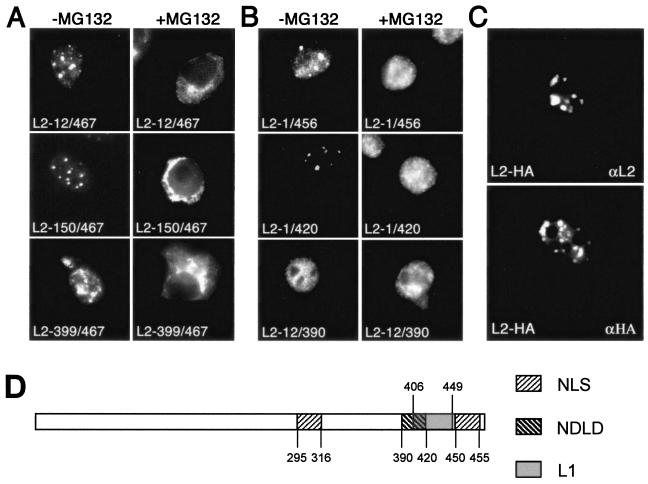
When we analyzed HPV33 L2 mutants carrying deletions at the N or C terminus, we found that N-terminal truncation of 11 (L2-12/467), 149 (L2-150/467), or even 398 (L2-399/467) amino acids did not prevent cytoplasmic retention of L2 through cytoplasmic Hsc70 depletion by use of MG132 (Fig. 6A). In contrast, deletion of only 11 amino acids from the C terminus (L2-1/456) resulted in L2 nuclear translocation in the presence of MG132 (Fig. 6B). Other mutants carrying larger C-terminal deletions (e.g., L2-1/420) or both N- and C-terminal truncations (e.g., L2-12/390) were not cytoplasmically retained by MG132 treatment, either (Fig. 6B). A possible explanation for this observation is a requirement of proteolytic processing at the L2 C terminus for nuclear translocation, which may be blocked by proteasome inhibitors. To test this assumption, an HA tag was fused to the C terminus of codon-optimized HPV16 L2 (20). After transfection of human cells, L2 protein and the HA tag were detected almost exclusively at PML bodies (Fig. 6C). This result excludes the possibility that L2 is proteolytically processed at the C terminus prior to nuclear translocation and accumulation in PML bodies. In addition, immunoblot analysis of L2 synthesized either in the presence or in the absence of MG132 showed no differences in the protein pattern of L2 (data not shown). Our data indicate, therefore, that only L2 molecules carrying an intact C terminus are retained in the cytoplasm after cytoplasmic Hsc70 depletion.
FIG. 6.
Effect of MG132 on cellular localization of mutant L2 proteins. N-terminal (A) or C-terminal (B) deletion mutants were expressed in HuTK−143B cells in the absence or presence of MG132 by use of recombinant vaccinia viruses or as a GFP fusion after transfection (L2-399/467). L2 was detected by immunofluorescence. With the exception of L2-399/467 and L2-12/390, all mutants analyzed accumulated at PML bodies (1). (C) HPV16 L2 was epitope tagged at the C terminus and expressed in COS-7 cells after transfection. Cells were stained for L2 or the HA tag. (D) Schematic diagram of some L2 protein domains (1, 9). NLS, nuclear localization signal; NDLD, ND10 localization domain; L1, L1 interaction domain (9) adopted for HPV33.
Interestingly, the C-terminally truncated L2 mutants were also associated with Hsc70 in nuclear translocation and colocalized with Hsc70 at PML bodies (data not shown). This finding clearly indicates that there are other Hsc70 binding sites in the L2 protein in addition to the C terminus, as expected. However, as shown above by nuclear translocation from Hsc70-depleted cytoplasm, Hsc70 binding to sequences outside the C-terminal region was not needed for translocation to the nucleus.
Hsc70 is associated with VLPs but not with pseudovirions.
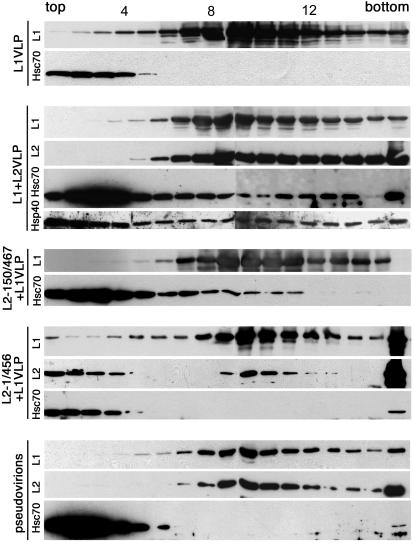
Since L2 is associated with Hsc70 in nuclear translocation, it was interesting to analyze if Hsc70 is integrated into VLPs or virions together with L2. To address this question, DNA-free VLPs and pseudovirions, harboring a marker plasmid, were produced in COS-7 cells and purified by buoyant density gradient centrifugation. This method allows efficient separation of pseudovirions and VLPs (41). Sedimentation velocity analysis through linear sucrose gradients revealed that Hsc70 was not associated with VLPs composed of L1 alone (Fig. 7), although Hsc70 has been shown to associate with L1 in nuclear translocation. Hsc70 was found exclusively at the top of the gradient (fractions 1 to 4), whereas VLPs were detected mainly in fractions 9 to 12. In contrast, small amounts of Hsc70 cosedimented with L1-L2 VLPs (Fig. 7), suggesting an L2-dependent association of Hsc70 with these particles. The cochaperone Hsp40 was also present in L1-L2 VLP-containing fractions (Fig. 7) but not in L1 VLPs (data not shown). Cosedimentation of Hsc70 with VLPs composed of L1 and N-terminally truncated L2 was also observed, as shown for L1-L2-150/467 VLPs (Fig. 7). In contrast, no association with Hsc70 was detected for VLPs containing C-terminally truncated L2, as shown for L2-1/456 (Fig. 7). These findings suggest a particular function for Hsc70, interacting with the L2 C terminus, in the integration of L2 into the viral capsid. To our surprise, Hsc70 was not detectable in L1-L2 pseudovirions (Fig. 7). These data indicate that Hsc70 is only transiently associated with papillomavirus capsids and dissociates during the assembly of virions.
FIG. 7.
Association of Hsc70 with VLPs. Pseudovirions and VLPs were extracted from cells expressing L1 either alone or together with wild-type or mutant L2 and were subsequently purified by cesium chloride density gradient centrifugation. Fractions containing pseudovirions or VLPs were subjected to centrifugation through linear sucrose gradients. Fractions were analyzed by SDS-PAGE and immunoblotting for L1, L2, Hsc70, and Hsp40 as indicated. For detection of L2, Hsc70, and Hsp40, a fourfold-greater amount of protein was used than for L1, as well as longer exposure to film. Sedimentation was from left to right. Fraction numbers are given above the top gel.
DISCUSSION
Our experiments demonstrate a requirement for Hsc70 in nuclear translocation of the papillomavirus minor capsid protein L2. L2 seems to bind to Hsc70 in the cytoplasm, and the Hsc70-L2 complex is translocated to PML bodies. Depletion of Hsc70 from the cytoplasm arrests L2 in the cytoplasm, indicating that Hsc70 has an essential function in viral morphogenesis.
We have shown that Hsc70 is concentrated at PML bodies in cells expressing L2, whereas it is dispersed, mainly in the cytoplasm, in cells without L2 synthesis. This pattern was observed for L2 from three HPV types tested, with two different expression systems used, in several cell lines, and in HPV-infected keratinocytes obtained from a CIN lesion. Taken together, the findings suggest that association of L2 with Hsc70 is an obligatory intermediate step in papillomavirus replication.
Given the many possible functions of Hsc70 within the cell, demonstrating the importance of Hsc70 for L2 nuclear translocation was not straightforward. Since overexpression of dominant-negative Hsc70 mutants might have an adverse effect on cell viability (32), we chose to use stress-induced relocation of Hsc70 to nucleoli as the method for removal from the cytoplasm. Relocating chaperones to nucleoli is part of the cellular heat shock response to physiological and environmental stress, which is mediated by heat shock transcription factors and activated by diverse signals. One such signal is inhibition of the proteasome, and proteasome inhibitors, such as MG132, coactivate heat shock factors HSF1 and HSF2 (26). We have visualized a dramatic change in the subcellular distribution of Hsc70 and L2 under these conditions, i.e., relocation of Hsc70 to nucleoli and retention of L2 in the cytoplasm. Moreover, using overexpression of Hsc70 fused to GFP in MG132-treated cells, we have demonstrated that cytoplasmic retention of L2 was most probably due to specific depletion of Hsc70 and not to one of the other possible pleiotropic effects of inhibition of the proteasome.
In order to further characterize the interaction of L2 with Hsc70, we have shown that binding requires an active ATPase domain of Hsc70. In addition, the cochaperone Hsp40, a member of the family of J-proteins, which enhance the Hsc70 chaperone activity by stimulating the ATPase activity, was found associated with the Hsc70-L2 complex. Moreover, the Hsc70 activity of L2 binding was modified by coexpression with the cochaperones Hip and Bag-1. Hip stabilized the Hsc70-L2 complex, whereas Bag-1 reduced the extent of L2 binding. Thus, cochaperones that regulate the chaperone activity of Hsc70 potentially represent new targets for interference with papillomavirus assembly.
We have also shown by coexpression of L2 with L1 that Hsc70 is associated with VLPs but not with pseudovirions. No association was found with VLPs consisting of L1 alone. One attractive model suggested by these experiments is that Hsc70 participates in the integration of L2 into the viral capsid. Hsc70 may be required to generate or to maintain a specific state of folding of L2 or of L2 domains necessary for assembly with capsomeres. This model is consistent with the inability of purified L1 and L2 to form complexes in vitro without coexpression (9). Alternatively, Hsc70 may play a role in the encapsidation of the viral minichromosome.
Although Hsc70 was indispensable for the nuclear translocation of L2, C-terminally truncated L2 was not retained in the cytoplasm after Hsc70 depletion. In contrast, N-terminally truncated L2 exhibited the wild-type phenotype. This finding suggests a special role of the L2 C-terminal region in the association with Hsc70. It is tempting to speculate that L2 may interact with other proteins in the cytoplasm via its C terminus. In the absence of Hsc70, this interaction might be the reason for cytoplasmic retention of L2. Alternatively, L2 may form aggregates due to incorrect folding induced by the C-terminal region. The function of Hsc70 would then be to mask the C terminus in order to prevent such interactions, which otherwise would cause a premature block of papillomavirus morphogenesis.
Hsc70 is known to bind to short hydrophobic sequences exposed on the surface of a protein. Clearly, the C terminus is not the only Hsc70 binding site in L2. C-terminally truncated mutants of L2 were also associated with Hsc70 in nuclear translocation. In contrast to L2, however, nuclear translocation of these mutants occurred both in the presence and in the absence of Hsc70, as was also observed for L1. Moreover, no association of Hsc70 with VLPs consisting of L1 alone or containing C-terminally truncated L2 could be detected, in contrast to full-length or N-terminally truncated L2. This seemingly paradoxical observation may be due to functional differences between Hsc70 binding to the C terminus and that to more N-terminal sites. This supports our idea that an unprotected C terminus of L2 is detrimental to virion morphogenesis. Hsc70 is therefore released from the L2 C terminus when the L2 C terminus is completely buried inside the virion. Indeed, we have evidence for a strong cytotoxic activity located at the very C terminus of L2 (N. Kaemper, T. Nowak, and M. Sapp, unpublished data). In contrast, Hsc70 binding to more N-terminal sites may simply assist protein folding, in which case Hsc70 can be released once folding is completed, independently of the actual state of virus assembly.
Hsp70 has previously been shown to facilitate HPV DNA replication by promoting E1 complex formation at the origin of replication (23). Subsequently, Hsp70 displaces E2 from E1-ori complexes, thereby reactivating E1 helicase activity (22). Hsc70 is also involved in the disassembly of clathrin-coated vesicles, which are probably used by papillomaviruses for cell entry by endocytosis (7, 42). We have shown here that Hsc70 is indispensable for the integration of L2 into the viral capsid. Thus, transient interactions with chaperones of the Hsp70 family are required for several important steps of the papillomavirus life cycle.
Acknowledgments
We are indebted to B. Moss for the vaccinia virus expression system and to H. H. Kampinga for generously providing expression plasmids for Hip and Bag-1. We thank A. Funk for critical reading of the manuscript.
This work was supported by grants to M.S. and R.E.S. from the Deutsche Forschungsgemeinschaft (DFG Str 157/4-3, 157/4-4, and SFB 490-B5).
REFERENCES
- 1.Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domain (ND) 10 homing and reorganization. Virology 314:161-167. [DOI] [PubMed] [Google Scholar]
- 2.Becker, K. A., C. Sapp, L. Florin, G. G. Maul, and M. Sapp. 2004. Nuclear localization but not PML protein is required for incorporation of the papillomavirus minor capsid protein L2 into virus-like particles. J. Virol. 78:1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchner, J. 1999. Hsp90 & Co.—a holding for folding. Trends Biochem. Sci. 24:136-141. [DOI] [PubMed] [Google Scholar]
- 4.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 5.Chromy, L. R., J. M. Pipas, and R. L. Garcea. 2003. Chaperone-mediated in vitro assembly of Polyomavirus capsids. Proc. Natl. Acad. Sci. USA 100:10477-10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cripe, T. P., S. E. Delos, P. A. Estes, and R. L. Garcea. 1995. In vivo and in vitro association of hsc70 with polyomavirus capsid proteins. J. Virol. 69:7807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, P. M., D. R. Lowy, and J. T. Schiller. 2003. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology 307:1-11. [DOI] [PubMed] [Google Scholar]
- 8.Day, P. M., R. B. Roden, D. R. Lowy, and J. T. Schiller. 1998. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J. Virol. 72:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnen, R. L., K. D. Erickson, X. S. Chen, and R. L. Garcea. 2003. Interactions between papillomavirus L1 and L2 capsid proteins. J. Virol. 77:4818-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florin, L., C. Sapp, R. E. Streeck, and M. Sapp. 2002. Assembly and translocation of papillomavirus capsid proteins. J. Virol. 76:10009-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florin, L., F. Schäfer, K. Sotlar, R. E. Streeck, and M. Sapp. 2002. Reorganization of nuclear domain 10 induced by papillomavirus capsid protein L2. Virology 295:97-107. [DOI] [PubMed] [Google Scholar]
- 12.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 13.Glotzer, J. B., M. Saltik, S. Chiocca, A. I. Michou, P. Moseley, and M. Cotten. 2000. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature 407:207-211. [DOI] [PubMed] [Google Scholar]
- 14.Gurer, C., A. Cimarelli, and J. Luban. 2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol. 76:4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartl, F. U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852-1858. [DOI] [PubMed] [Google Scholar]
- 16.Jindal, S., and R. A. Young. 1992. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J. Virol. 66:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawana, K., H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1998. In vitro construction of pseudovirions of human papillomavirus type 16: incorporation of plasmid DNA into reassembled L1/L2 capsids. J. Virol. 72:10298-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert, C., and R. Prange. 2003. Chaperone action in the posttranslational topological reorientation of the hepatitis B virus large envelope protein: implications for translocational regulation. Proc. Natl. Acad. Sci. USA 100:5199-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufen, T., M. P. Mayer, C. Beisel, D. Klostermeier, A. Mogk, J. Reinstein, and B. Bukau. 1999. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc. Natl. Acad. Sci. USA 96:5452-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leder, C., J. A. Kleinschmidt, C. Wiethe, and M. Müller. 2001. Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 75:9201-9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis, M. J., and H. R. Pelham. 1985. Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 4:3137-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, B. Y., A. M. Makhov, J. D. Griffith, T. R. Broker, and L. T. Chow. 2002. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 22:6592-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, J. S., S. R. Kuo, A. M. Makhov, D. M. Cyr, J. D. Griffith, T. R. Broker, and L. T. Chow. 1998. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 273:30704-30712. [DOI] [PubMed] [Google Scholar]
- 24.Löffler-Mary, H., M. Werr, and R. Prange. 1997. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology 235:144-152. [DOI] [PubMed] [Google Scholar]
- 25.Macejak, D. G., and P. Sarnow. 1992. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J. Virol. 66:1520-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew, A., S. K. Mathur, C. Jolly, S. G. Fox, S. Kim, and R. I. Morimoto. 2001. Stress-specific activation and repression of heat shock factors 1 and 2. Mol. Cell. Biol. 21:7163-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer, M. P., and B. Bukau. 1998. Hsp70 chaperone systems: diversity of cellular functions and mechanism of action. Biol. Chem. 379:261-268. [PubMed] [Google Scholar]
- 28.McMurray, H. R., D. Nguyen, T. F. Westbrook, and D. J. McCance. 2001. Biology of human papillomaviruses. Int. J. Exp. Pathol. 82:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minami, Y., J. Hohfeld, K. Ohtsuka, and F. U. Hartl. 1996. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J. Biol. Chem. 271:19617-19624. [DOI] [PubMed] [Google Scholar]
- 30.Modis, Y., B. L. Trus, and S. C. Harrison. 2002. Atomic model of the papillomavirus capsid. EMBO J. 21:4754-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss, B., O. Elroy-Stein, T. Mizukami, W. A. Alexander, and T. R. Fuerst. 1990. New mammalian expression vectors. Nature 348:91-92. [DOI] [PubMed] [Google Scholar]
- 32.Newmyer, S. L., and S. L. Schmid. 2001. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 152:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nollen, E. A., J. F. Brunsting, J. Song, H. H. Kampinga, and R. I. Morimoto. 2000. Bag-1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol. Cell. Biol. 20:1083-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nollen, E. A., A. E. Kabakov, J. F. Brunsting, B. Kanon, J. Hohfeld, and H. H. Kampinga. 2001. Modulation of in vivo HSP70 chaperone activity by Hip and Bag-1. J. Biol. Chem. 276:4677-4682. [DOI] [PubMed] [Google Scholar]
- 35.Paintsil, J., M. Müller, M. Picken, L. Gissmann, and J. Zhou. 1996. Carboxyl terminus of bovine papillomavirus type-1 L1 protein is not required for capsid formation. Virology 223:238-244. [DOI] [PubMed] [Google Scholar]
- 36.Sagara, J., and A. Kawai. 1992. Identification of heat shock protein 70 in the rabies virion. Virology 190:845-848. [DOI] [PubMed] [Google Scholar]
- 37.Sainis, L., C. Angelidis, G. N. Pagoulatos, and L. Lazaridis. 2000. HSC70 interactions with SV40 viral proteins differ between permissive and nonpermissive mammalian cells. Cell Stress Chaperones 5:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saphire, A. C., T. Guan, E. C. Schirmer, G. R. Nemerow, and L. Gerace. 2000. Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J. Biol. Chem. 275:4298-4304. [DOI] [PubMed] [Google Scholar]
- 39.Sapp, M., U. Kraus, C. Volpers, P. J. Snijders, J. M. Walboomers, and R. E. Streeck. 1994. Analysis of type-restricted and cross-reactive epitopes on virus-like particles of human papillomavirus type 33 and in infected tissues using monoclonal antibodies to the major capsid protein. J. Gen. Virol. 75:3375-3383. [DOI] [PubMed] [Google Scholar]
- 40.Sapp, M., C. Volpers, M. Müller, and R. E. Streeck. 1995. Organization of the major and minor capsid proteins in human papillomavirus type 33 virus-like particles. J. Gen. Virol. 76:2407-2412. [DOI] [PubMed] [Google Scholar]
- 41.Selinka, H. C., T. Giroglou, T. Nowak, N. D. Christensen, and M. Sapp. 2003. Further evidence that papillomavirus particles exist in two distinct conformations. J. Virol. 77:12961-12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selinka, H. C., T. Giroglou, and M. Sapp. 2002. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology 299:279-287. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan, C. S., and J. M. Pipas. 2001. The virus-chaperone connection. Virology 287:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takayama, S., D. N. Bimston, S. Matsuzawa, B. C. Freeman, C. Aime-Sempe, Z. Xie, R. I. Morimoto, and J. C. Reed. 1997. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 16:4887-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trus, B. L., R. B. Roden, H. L. Greenstone, M. Vrhel, J. T. Schiller, and F. P. Booy. 1997. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat. Struct. Biol. 4:413-420. [DOI] [PubMed] [Google Scholar]
- 47.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volpers, C., M. Sapp, C. A. Komly, P. Richalet-Secordel, and R. E. Streeck. 1993. Development of type-specific and cross-reactive serological probes for the minor capsid protein of human papillomavirus type 33. J. Virol. 67:1927-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpers, C., M. Sapp, P. J. Snijders, J. M. Walboomers, and R. E. Streeck. 1995. Conformational and linear epitopes on virus-like particles of human papillomavirus type 33 identified by monoclonal antibodies to the minor capsid protein L2. J. Gen. Virol. 76:2661-2667. [DOI] [PubMed] [Google Scholar]
- 50.Yang, R., P. M. Day, W. H. Yutzy IV, K. Y. Lin, C. F. Hung, and R. B. Roden. 2003. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J. Virol. 77:3531-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]