Abstract
Werner syndrome is a progeric syndrome characterized by premature atherosclerosis, diabetes, cancer, and death in humans. The knockout mouse model created by deletion of the RecQ helicase domain of the mouse Wrn homologue gene (Wrn∆hel/∆hel) is of great interest because it develops atherosclerosis and hypertriglyceridemia, conditions associated with aging liver and sinusoidal changes. Here, we show that Wrn∆hel/∆hel mice exhibit increased extracellular matrix, defenestration, decreased fenestration diameter, and changes in markers of liver sinusoidal endothelial cell inflammation, consistent with age-related pseudocapilliarization. In addition, hepatocytes are larger, have increased lipofuscin deposition, more frequent nuclear morphological anomalies, decreased mitochondria number, and increased mitochondrial diameter compared to wild-type mice. The Wrn∆hel/∆hel mice also have altered mitochondrial function and altered nuclei. Microarray data revealed that the Wrn∆hel/∆hel genotype does not affect the expression of many genes within the isolated hepatocytes or liver sinusoidal endothelial cells. This study reveals that Wrn∆hel/∆hel mice have accelerated typical age-related liver changes including pseudocapillarization. This confirms that pseudocapillarization of the liver sinusoid is a consistent feature of various aging models. Moreover, it implies that DNA repair may be implicated in normal aging changes in the liver.
Key Words: Sinusoid, Endothelium, Mitochondria, Electron microscopy, Seahorse analyzer.
Old age is associated with increased vulnerability, susceptibility to disease, and disability and is the major independent risk factor for most diseases of the Western world including atherosclerosis, cancer, and arthritis as well as prototypical aging diseases such as dementia and osteoporosis (1). Although the aging process is the main determinant of disease beyond the third decade of life (2), our understanding of how old age predisposes to disease remains rudimentary (3). It has been recognized that the liver may be central to the aging process because it plays a pivotal role in many metabolic and detoxification processes that impact on aging and disease susceptibility in the rest of the body.
The liver had long been thought to be preserved from any significant age-related changes. A growing body of evidence, however, shows that older age is associated with marked changes in all cells of the liver (4,5) and particularly the liver sinusoidal endothelial cells (LSECs) (6). These age-related changes to the LSEC, called pseudocapillarization, are now known to affect directly hepatic metabolism through inhibiting transfer of substances such as lipids and drugs between the blood and liver parenchyma (7,8). In particular, fenestrations, which are transcellular pores that facilitate transfer of substrates across the LSEC, are reduced in both number and diameter in old age in all species yet reported including humans.
Werner Syndrome (WS) is a human autosomal recessive progeric condition that causes the premature onset of clinical signs of aging, such as cardiovascular disease, cancer, and osteoporosis. It is caused by a mutation of the WRN (RECQL2) gene, which encodes a RecQ family 3′–5′ DNA helicase protein that also has a 3′–5′ exonuclease domain at its N-terminus region (9,10). The protein is involved in DNA recombination, transcription, repair, and telomere maintenance (11–13). The life expectancy of a person with WS has climbed from 46 years in the 1990s (14) to 55 years due to better medical care (15) and the introduction of antidiabetic drugs, underlining again the abnormal metabolic profile of these patients (16).
A number of mouse models of WS have been developed (17–19). In this report, we investigate the Wrn helicase mutant mice, Wrn∆hel/∆hel, which lack the helicase unit of the Wrn gene. Although these mice do not develop a severe aging phenotype, they do exhibit many of the WS phenotypes including hypertriglyceridemia, insulin resistance, an increased incidence of cancer, and a decreased life expectancy (20,21). Previously, we have shown that vitamin C supplementation and resveratrol treatment have beneficial effects on the health and/or life span of these Wrn∆hel/∆hel mice. Many of these effects were mediated in part by the liver, and we believe that the liver sinusoidal endothelium played an integral role (22,23). Hence, the aim of this study was to systematically investigate the morphology, gene expression, and function of the liver sinusoidal endothelium as well as the hepatocytes in Wrn∆hel/∆hel mice and to further evaluate these mice for use in aging liver studies.
Methods
Homozygous WRN helicase transgenic (Wrn∆hel/∆hel) on a C57Bl6 background and C57Bl6 wild-type (WT) control male mice aged 4–7 months and 14–16 months were used for all parameters in this study, with the exception of the microarray analysis where 3- to 4-month-old and 13- to 14-month-old animals were used. Control C57Bl6 mice were bred independently. All animals were housed in the Molecular Physiology unit at the ANZAC Research Institute and allowed free access to water and commercial pellets. This study was approved by the Sydney South Western Area Health Service Animal welfare committee.
Biochemical Parameters
Basic liver function tests (alanine transaminase and aspartate transaminase) were measured in plasma blood with the Roche Hitachi cobas 8000 Modular Analyzer (Roche Diagnostics, Indianapolis, IN) in the Biochemical Department of Concord Hospital (n = 4 mice per age group and genotype). Plasma insulin levels were measured using the Ultrasensitive Mouse Insulin ELISA Kit (Alpco Diagnostics, Salem, New Hampshire) (n = 4 mice per age group and genotype).
Morphological and Ultrastructural Studies
For liver morphology studies, liver perfusion was performed as described previously (24). In addition, one lobe was ligated and placed in 4% phosphate-buffered paraformaldehyde for histology and immunohistochemistry prior to introduction of fixative for electron microscopy analysis.
Following fixation, liver samples for transmission electron microscopy were embedded in Spurrs resin, sectioned, and examined using a Philips CM10 transmission electron microscope. Liver morphology was assessed for mitochondrial density, lipid deposition, nuclei morphology, and fibrotic changes. Liver pieces for scanning electron microscopy were prepared as previously described (7), and fenestration density and size were examined using a JEOL 6380 scanning electron microscope. Five mice per age group and genotype were analyzed by electron microscopy techniques. Not less than 10 hepatocytes were analyzed per animal, and at least 350 mitochondria per animal were analyzed to calculate mitochondria size and number. Nuclei area was excluded from hepatocyte area for calculation of mitochondrial density.
For histology, liver samples were embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin for gross morphology or Sirius red, a stain for liver collagen deposition, a marker of liver fibrosis (25).
Immunohistochemistry was used to determine the expression of LYVE1, perlecan, and ICAM 1 (antigens expressed on LSECs and extracellular matrix) on frozen sections, and the expression of F4/80 (Kupffer cell antigen) was analyzed on paraffin sections.
Endogenous peroxidase was blocked with 0.3% hydrogen peroxide. The primary antibodies, which were applied for 1 hour were LYVE1 (ALY7 eBioscience, 1:200), perlecan (A7L6 -NeoMArkers,1:200), ICAM-1, and F4/80 (kindly provided by P.Bertolino, 1:10). Sections were then treated with anti-rat biotinylated secondary antibody and ExtrAvidin peroxidase (Sigma). 3,3′-Diaminobenzidine was used to reveal peroxidase activity. The immunohistochemistry staining was rated blindly and independently. Staining was rated between zero and three for the intensity of staining in the periportal sinusoids, pericentral sinusoids, portal vein, and central veins (n = 5 mice per age group and genotype were analyzed using these histological and immunohistochemical techniques).
Microarray Studies
For gene expression study, total RNA was isolated from hepatocytes and LSEC isolated from 3- to 4-month-old and 13- to 14-month-old Wrn∆hel/∆hel and WT mice (3 mice per age and genotype) using RNAeasy (Qiagen). Cells were obtained after liver perfusion with liberase (Roche), Percoll gradient separation of nonparenchymal cells and subsequent removal of Kupffer cells by selective adherence. Amplified cRNA was hybridized onto Illumina.SingleColor.MouseWG-6 v2.0 Whole-Genome Expression BeadChip containing 45,280 probes for 30,853 genes. Raw fluorescence intensity values were quantile normalized with The BeadStudio software (Illumina Inc.) and log-transformed prior to two-way ANOVA analysis with Benjamini–Hochberg false discovery rate correction.
Seahorse Mitochondrial Studies
Mitochondrial respiration and oxidation in the livers were assessed using a Seahorse Extracellular Flux Analyzer. Substrates studied were glutamate/malate and succinate, and responses to ADP, Oligomycin, FCCP, and Antimycin A were quantified (n = 4 per genotype, age 4–7 months). Data were extracted from Excel spreadsheets using “R” and respiration states calculated using the point-to-point calculations.
Mitochondrial DNA Studies
For quantification of mitochondrial DNA, total DNA was extracted from isolated hepatocytes from four groups of mice using DNeasy kit (Qiagen) (n = 3 mice per age and genotype).
Two mitochondria-encoded genes (NADH dehydrogenase subunit 5 and ATP synthase F0 subunit 6 ) and one nuclear-encoded gene (transferrin receptor, which is a single copy nuclear gene) were quantified by RealTime PCR using TaqMan chemistry. Amplification efficiency was calculated from standard curves for each gene generated by RealTime PCR of serial dilutions of most concentrated DNA sample and appeared to be 94.7% for NADH dehydrogenase subunit 5, 94.5% for ATP synthase F0 subunit 6, and 81.2% for transferrin receptor. Quantities of target genes in the samples were estimated from RealTime PCR–generated C t (cycle threshold) values corrected for amplification efficiency.
Statistical Analysis
Statistical analysis was performed using SigmaPlot 11.0 (SyStat Software, Chicago, IL). All results are expressed as mean ± SEM. Comparisons between groups were performed with t test, ANOVA, or Kruskal–Wallis where appropriate. Differences were considered significant at a p value <.05.
Results
At 4–7 months, Wrn∆hel/∆hel mice appeared healthy and in good condition; however at 14–16 months, the Wrn∆hel/∆hel mice had some grey fur and were beginning to develop bald patches. Wrn∆hel/∆hel mice were significantly heavier at 14–16 months than at 4–7 months (Figure 1); there were no differences in weight between the other groups.
Figure 1.
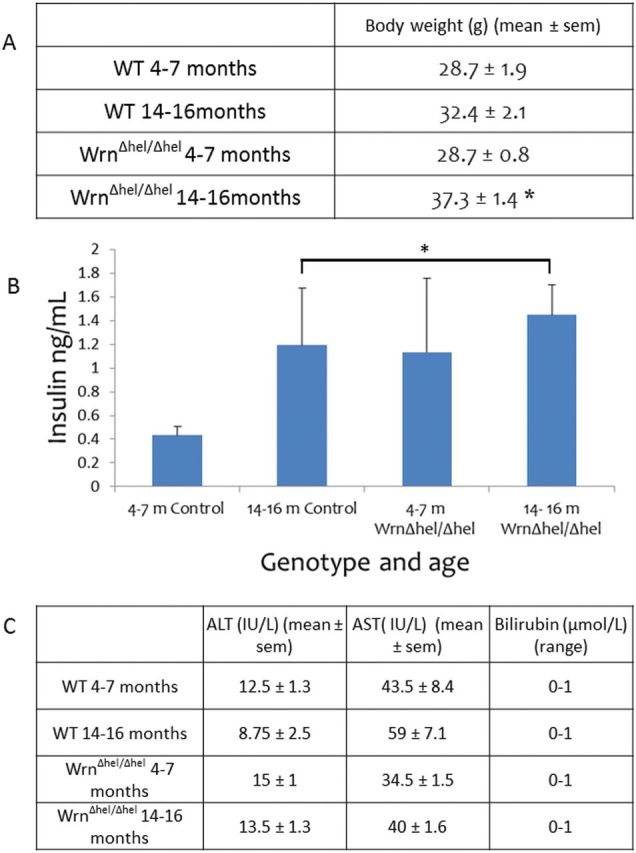
(A) Animal weight was significantly increased in the 14- to 16-month-old Wrn∆hel/∆hel mice and WT mice compared with the 4- to 7-month-old animals (p = .001). (B) Hyperinsulinemia was seen in the progeroid 4- to 7-month-old Wrn∆hel/∆hel mice and in both the WT and Wrn∆hel/∆hel mice at 14–16 months (*p = .014). (C) No difference in liver function was detected between genotype and age.
Biochemical Parameters
Plasma insulin levels were increased in the Wrn∆hel/∆hel mice at both 4–7 months and 14–16 months of age compared with 4- to 7-month-old WT. There was also an age-related increase in plasma insulin levels in the WT mice (p = .014, Figure 1B);
alanine transaminase, aspartate transaminase, and bilirubin levels in plasma were within the normal range in all age groups and genotypes (Figure 1C).
Morphological Studies
All livers from the 4- to 7-month-old and 14- to 16-month-old Wrn∆hel/∆hel and WT mice were free from pathological changes on hematoxylin and eosin staining (Figure 2A–D). However, a dramatic increase in inflammatory infiltrate was observed both in the 4- to 7-month-old and 14- to 16-month-old Wrn∆hel/∆hel mice compared with their age-matched controls (Figure 2A–D). Sirius red staining also revealed a significant increase in fibrotic changes in the parenchyma of the 4- to 7-month-old and 14- to 16-month-old Wrn∆hel/∆hel mice and parenchyma and vessels of the 14- to 16-month-old WT (Figure 2E–H).
Figure 2.
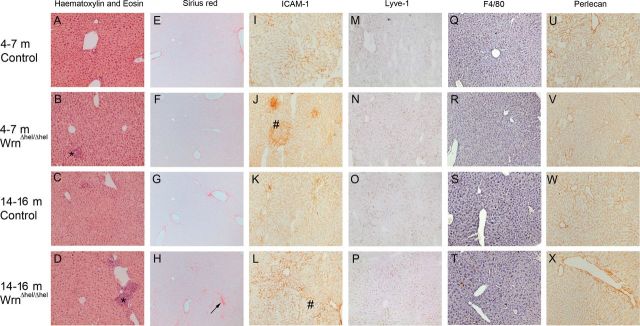
Histology and Immunohistochemistry of the liver. Hematoxylin and eosin staining of the liver (A–D) confirmed that although the livers of all mice were free of pathology, there is an increase in inflammatory cell aggregation (*) with age and Wrn∆hel/∆hel genotype. Sirius red staining (E–H) revealed an increase in collagen deposition along the sinusoids with age (→) and Wrn∆hel/∆hel genotype. Upregulation of ICAM-1 expression (#) (I–L) confirmed the hematoxylin and eosin finding of increased inflammation in the livers of Wrn∆hel/∆hel and 14- to 16-month-old WT mice. No age- or genotype-dependent changes in expression of Lyve-1 (M-P), F4/80 (Q-T), or Perlecan (U–X) were detected. (Original magnification ×400).
Immunohistochemical Studies
ICAM 1 staining increased in both Wrn∆hel/∆hel age groups and in the 14- to 16-month-old WT group, reflecting the increased inflammation seen on histology (Figure 2I–L). In contrast, expression of LYVE-1, the marker for LSECs (Figure 2M–P), Perlecan, the marker for vascular extracellular matrix (Figure 2Q–T), and the Kupffer cell marker, F4/80 (Figure 2U–X) did not change with age or genotype.
Electron Microscopic Studies
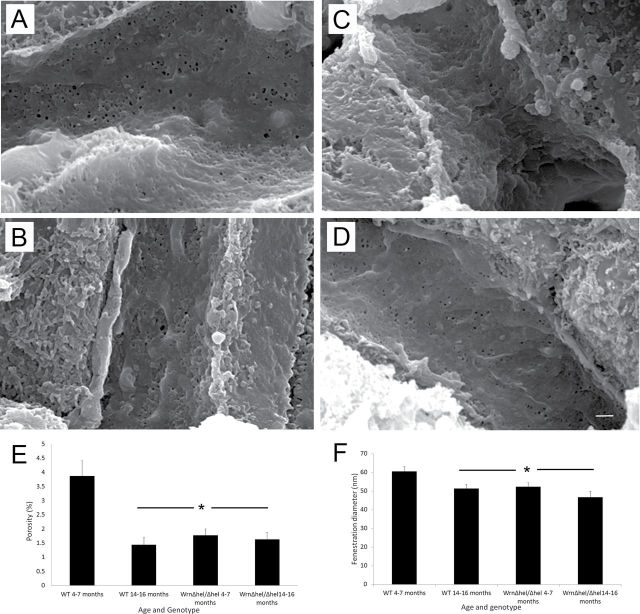
Scanning electron microscopy revealed a significantly decreased number of fenestrations in 14- to 16-month-old WT mice and in both the 4- to 7-month-old and 14- to 16-month-old Wrn∆hel/∆hel mice (p < .001, Figure 3E). In addition, fenestration diameter was significantly decreased with age and genotype (p = .004, Figure 3F).
Figure 3.
Scanning electron microscopy of the liver sinusoidal endothelium. (A–D) Typical scanning electron micrographs from 4- to 7-month-old WT, 4- to 7-month-old Wrn∆hel/∆hel, 14- to 16-month-old WT, and 14- to 16-month-old Wrn∆hel/∆hel mice, respectively. Reduced porosity and fenestration diameter in both age groups of the Wrn∆hel/∆hel mice and 14- to 16-month-old WT mice. Figure 3E is quantification of the genotype- and age-related defenestration of the sinusoidal endothelium (*p < .001), and Figure 6F shows the decreased fenestration diameter (*p = .004). Original magnification ×15000, Scale bar = 1 µm).
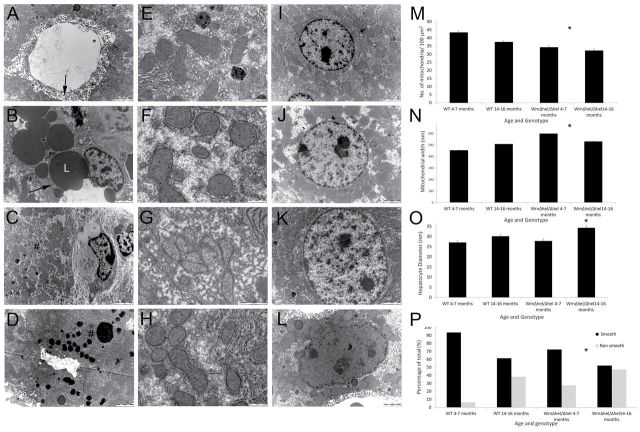
Transmission electron microscopy supported the histological findings of increased collagen deposition along the sinusoids in the space of Disse in the mice and more inflammatory cells in the sinusoids seen on light microscopy (Figure 4A–D). In addition, the hepatocytes were larger with age and genotype (p = .001, Figure 4A–D), and there were significantly increased levels of lipofuscin deposition in the 4- to 7-month-old Wrn∆hel/∆hel mice. Examination of the nucleus revealed an increase in irregularly shaped nuclei with Wrn∆hel/∆hel genotype (chi squared, 20.879, p = .0001, Figure 4I–L). There was no change in nuclei diameter (Wrn∆hel/∆hel: 4–7 months, 8.269 ± 0.312 µm; 14–16 months, 9.365 ± 0.35 µm; WT: 4–7 months, 8.067±0.287 µm; 14–16 months, 19.091 ± 0.601 µm, p = .052) or ploidy (data not shown) with age or genotype. Localized fatty changes were found in the Wrn∆hel/∆hel mice in both age groups (Figure 4I–L).
Figure 4.
Transmission electron microscopy of the liver in Wrn∆hel/∆hel and WT mice. (A) It depicts the liver of a 4- to 7-month-old WT mouse. The endothelium is fenestrated (*) and the space of Disse (→) is free from extracellular matrix deposition. (B) Micrograph taken from a 4- to 7-month-old Wrn∆hel/∆hel mouse; there is significant lipid deposition (L) in the hepatocyte, and the space of Disse (→) has significant extracellular matrix deposition. Micrographs (C) and (D) are taken from 14- to 16-month-old WT and 14- to 16-month-old Wrn∆hel/∆hel mice, respectively. The increased lipofuscin deposition seen in Wrn∆hel/∆hel is clearly apparent (#). (E–H) They display the changes seen in mitochondrial number and size with age and genotype: in E, 4- to 7-month-old WT; F, 4- to 7-month-old Wrn∆hel/∆hel; G, 14- to 16-month-old WT; and H, 14- to 16-month-old Wrn∆hel/∆hel mice. Changes in mitochondrial number per 100 µm2 are quantified in M (*p = .036), and changes in mitochondrial diameter are shown in N (*p = .004). Changes in nuclear shape with age and genotype are seen in micrographs: I, 4- to 7-month-old WT; J, 4- to 7-month-old Wrn∆hel/∆hel; K, 14- to 16-month-old WT; and L, 14- to 16-month-old Wrn∆hel/∆hel mice. Increases in maximal hepatocyte diameter with genotype and age are shown in O (*p = .001), and P displays the proportional increase in ruffled nuclei with genotype and age (*p = .0001). Scale bars as marked on the micrographs.
As assessed by maximal diameter measurements, there was an increase in hepatocyte mitochondrial size in the Wrn∆hel/∆hel mice in both age groups compared with WT controls (p = .004, Figure 4E–H). In addition, there was a reduction in hepatocyte mitochondrial numbers per 100 µm2 in both age groups of the Wrn∆hel/∆hel mice and also in 14- to 16-month-old the WT control mice (p = .036, Figure 4N). The mitochondria appeared to be ultrastructurally normal in all groups, with cristae and membranes intact and uncompromised.
Mitochondrial Function Studies
Mitochondria extracted from the livers of 4- to 7-month-old WT and Wrn∆hel/∆hel mice and analyzed using the Seahorse analyzer showed significant differences in: ADP-dependent state III respiration (p = .002); ADP-independent state IV respiration (p = .028); and FCCP uncoupled respiration rates (p = .001). There were no differences in ADP:O ratio, a measure of overall ETC efficiency or hydrogen peroxide production (Figure 5A–F).
Figure 5.
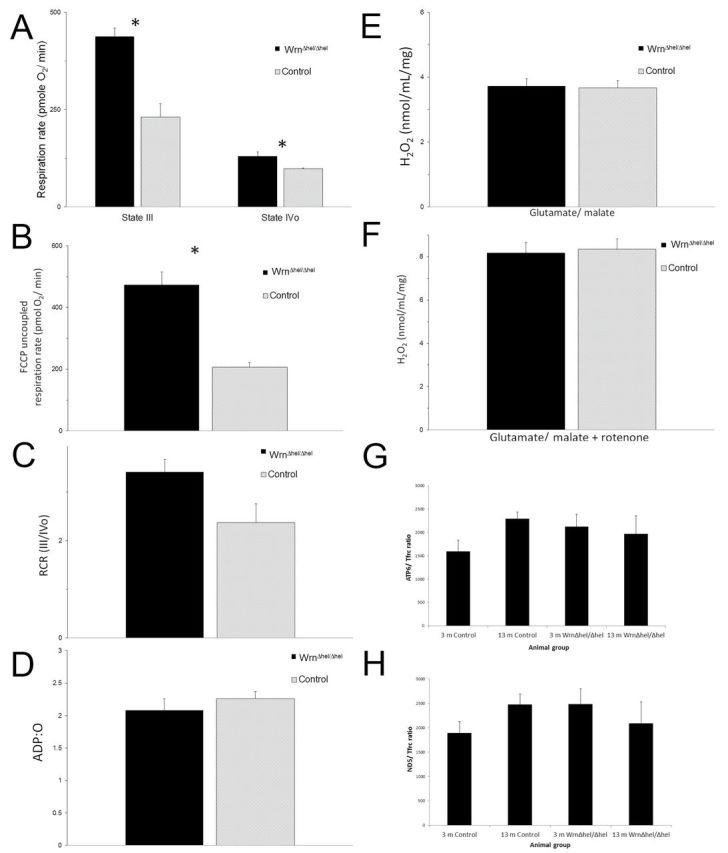
Mitochondrial function in Wrn∆hel/∆hel and control mice. Mitochondrial function as determined by Seahorse technology revealed significant loss of mitochondrial function in liver mitochondrial function in Wrn∆hel/∆hel mice. (A) It shows decreased State III and IV respiration (*State III: p = .002, State IV: p = .028) and (B) FCCP uncoupled respiration rate (p = .001) can be seen. (C) The ratio of respiration rates was not significant but shows a trend to reduction (p = .067). No changes in (D) ADP production or (E and F) H2O2 synthesis were detected. Quantification of mitochondrial DNA (G and H) was also performed, and no age- or genotype-dependent changes were found.
Mitochondrial DNA was quantified by measuring mtDNA copy numbers as measured by NADH dehydrogenase subunit 5 and ATP synthase F0 subunit 6 levels normalized against nuclear DNA copy numbers, measured by transferrin receptor; no significant difference was found in this parameter using this method of measurement (Figure 5G and H).
Microarray Studies
Gene regulation by both genotype and age was assessed using microarray in both hepatocytes and LSECs.
In LSECs, the scavenger receptor class A (Msr1) and transmembrane protein 66 (Tmem66) were both upregulated in the mutant mice, whereas the RNA-binding motif protein 13 (Rbm13) was significantly downregulated in Wrn∆hel/∆hel LSECs (Figure 6A).
Figure 6.
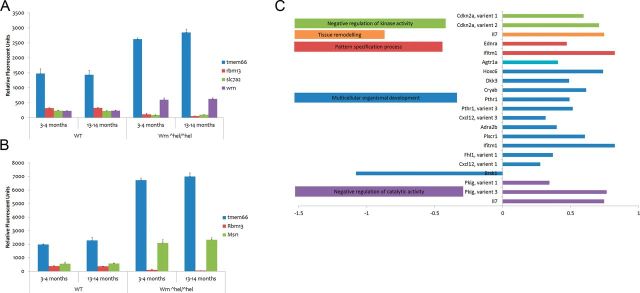
Significant LSEC and hepatocyte microarray results in Wrn∆hel/∆hel and WT mice. Differential expression of genes in LSECs are displayed in (A), whereas (B) displays those differentially expressed in Wrn∆hel/∆hel in hepatocytes. The classes of genes differentially regulated by age in LSECs are shown in (C).
In hepatocytes, the WS homolog (human) (Wrn) and transmembrane protein 66 (Tmem66) were significantly upregulated, whereas RNA-binding motif protein 13 (Rbm13) and solute carrier family 7 (cationic amino acid transporter) member 2 (Slc-7a2) (Figure 6B) were significantly downregulated in the mutant mice. Of note, expression patterns of Tmem66g and Rbm13 were very similar in LSECs and hepatocytes.
Age-dependent gene regulation.
Sixty-six genes were differentially regulated with age in LSECs independently of genotype. Figure 6C shows Gene Ontology terms statistically overrepresented within this list (Supplementary File). Aging was associated with significant upregulation of, for example, chemokine (C-X-C motif) ligand 12 (Cxcl12); arginine-rich domain–containing protein (Aard); collectin subfamily member 11 (Colec11); angiotensin II receptor, type 1a (Agtr1a); transmembrane protein 56 (Tmem56); and musculoskeletal, embryonic nuclear protein 1 (Mustn1) in both Wrn∆hel/∆hel and WT animals although always to a lesser extent in the former.
No transcripts reached statistical significance for the age-related changes in hepatocytes when Benjamini–Hochberg false discovery rate correction was applied. However, when less strict statistical criteria were used, 513 transcripts were identified (p < .005). The Supplementary file shows the genes statistically overrepresented within this list. For example, aging was associated with significant upregulation of cell death–inducing DNA fragmentation factor; alpha subunit–like effector A (Cidea); CCR4 carbon catabolite repression 4-like (Saccharomyces cerevisiae) (Ccrn4l); glypican 1 (Gpc1); leucine-rich repeats and transmembrane domains 1 (Lrtm1); and WAP four-disulfide core domain 2 (Wfdc2) in both Wrn∆hel/∆hel and WT hepatocytes. In contrast to LSECs, age-related upregulation of transcription was less pronounced in WT animals.
Discussion
Ultrastructural changes during aging contribute to impaired liver metabolism and detoxification. Many of these changes occur in the hepatic blood vessels or sinusoids and occur independently of any disease pathology. In young disease-free liver, the hepatic sinusoids have a unique fenestrated endothelium that allows bidirectional transfer of substances between the blood and the hepatocytes (26). However, during aging, a loss of fenestrations, an increase in extracellular matrix deposition, and changes in cell differentiation patterns impede transfer across the endothelium leading to impaired liver function and increased risk factors for adverse drug reactions and dyslipidemic conditions (7,24). This pattern of age-related changes in the liver sinusoid has been termed “pseudocapillarization.”
We have now demonstrated that the Wrn∆hel/∆hel progeria model replicates age-related pseudocapillarization from an early age. All animals included in this study are free from exogenously induced pathological changes as evidenced by the histological findings, liver enzyme levels, and gross morphological observations. The Wrn∆hel/∆hel mice exhibit defenestration and decreased fenestration diameter (Figure 3) together with increased extracellular matrix (Figure 2) and changes in markers of LSEC inflammation, such as increased ICAM-1 expression and increased cell aggregates (Figure 2) from as early as 4–7 months. These changes are all hallmarks of the normal aging liver sinusoid and have been studied by numerous groups (27–32) since our original reporting in 2000 (33).
In addition to changes in the LSEC, there are changes in the hepatocytes of the Wrn∆hel/∆hel mice. Hepatocytes are larger in the Wrn∆hel/∆hel mice compared with WT control mice. In addition, the hepatocytes of Wrn∆hel/∆hel mice have increased lipofuscin deposition, more frequent nuclear morphological anomalies, decreased mitochondria number, and increased mitochondrial diameter compared with WT hepatocytes as evidenced by our electron microscopy analyses (34–36) (Figure 4). Such findings are typical of aging hepatocytes in many species (37).
The Wrn∆hel/∆hel mice also have altered mitochondrial function as measured by the Seahorse technology (Figure 5A–F). The decreased number of mitochondrial organelles and the presence of larger mitochondria measured by electron microscopy in the Wrn∆hel/∆hel mice reflect altered cell energy metabolism and are typical of aging mitochondria in many tissues. However, mtDNA copy number measurements (Figure 5G and H) were unchanged in the Wrn∆hel/∆hel mice. The most likely reason for this is that a single mitochondrion can contain 2–10 copies of its DNA (38), and the decreased number of mitochondria in Wrn∆hel/∆hel mice and in older 13- to 14-month-old WT mice could be compensated for by increased copies of DNA in the remaining mitochondria. In addition, Seahorse measurements of mitochondrial function showed increased respiration rate and uncoupling. Together the data indicate that mitochondrial function is altered in the Wrn∆hel/∆hel mice, leading to increased mitochondrial volume as a compensatory mechanism or as part of the pathological progeroid process.
Nuclei morphology was changed with Wrn∆hel/∆hel genotype and age, consisting of a shift from regular round-shaped nuclei in the 4- to 7-month-old control mice to irregularly shaped, invaginated nuclei in the progeric Wrn∆hel/∆hel mice and the 14- to 16-month-old controls. No change in nuclear ploidy or nuclei size was found, which agrees with a previous report in WS (39).
The increased insulin levels in the serum of Wrn∆hel/∆hel mice may be reflective of impaired insulin uptake in the liver of these animals. We speculate that this may be due to changes in their sinusoidal endothelium. Endothelial integrity affects insulin transfer into the liver as shown in the PDGF-α knockout mouse. Such mice exhibit an 80% increased insulin sensitivity that correlate with the substantially increased number of fenestrations (40).
The microarray data (Figure 6) revealed that the Wrn∆hel/∆hel genotype does not affect the expression of many genes within the isolated hepatocytes, with only four genes that are differentially expressed in the Wrn∆hel/∆hel mice. TMEM66 gene expression, which encodes SARAF, a regulator of calcium entry into cells (41), is upregulated in Wrn∆hel/∆hel mice compared with WT mice (Figure 6B). RBM13, also known as MAK 16, is a RNA-binding protein involved in the stability and translation of RNA following stress and cell damage (42) and is downregulated (Figure 6C) in Wrn∆hel/∆hel mice compared with WT mice. In addition, MSR-1, which encodes the macrophage scavenger receptor-1, is upregulated in the LSECs of Wrn∆hel/∆hel mice. This protein is implicated in many physiological and pathological conditions such as Alzheimer’s disease, atherosclerosis, and cancers (43). It is believed to be implicated in NASH and several dyslipidemic liver conditions (44). Finally, SLC-7a2 is downregulated in the hepatocytes of Wrn∆hel/∆hel mice. SLC-7a2 encodes the solute carrier family 7 (cationic amino acid transporter) member 2, which is responsible for uptake of arginine, lysine, and ornithine. A dysfunction of this receptor has been shown to have implications in hyperinsulinemia (45) as seen in the Wrn∆hel/∆hel mice.
Microarray date also reveal that the LSEC is very susceptible to age-related changes in gene expression. No gene was differentially expressed across the age groups examined in the hepatocytes, whereas 66 genes were changed with age in the LSECs (Figure 6C).
Other progeroid mouse models have been created that recapitulate premature aging phenotypes (31,46–49). All models show abnormal nuclear morphology. The liver has been studied in the Ercc− /∆ mouse model, which shows pseudocapillarization, increased nuclear invagination, and lipofuscin deposits. The hepatocyte mitochondria were not studied in this model, but the observation of increased oxidative damage suggests mitochondrial dysfunctions (31). Although there are no comprehensive studies of the liver in the other progeroid syndrome models, lamin A/C gene mutations are associated with hyperlipidemia, hyperglycemia, and atherosclerosis (50), also suggestive of possible liver changes.
In summary, pseudocapillarization and other hallmarks of aging such as mitochondrial dysfunction are apparent from as early as 4 months of age in the Wrn∆hel/∆hel mice and resemble the phenotypic changes seen in the liver tissue of much older WT animals. Progeria and aging changes were more remarkable in the LSEC than in the hepatocytes, confirming the importance of the sinusoidal cells in liver aging. As such, the Wrn∆hel/∆hel model provides a valuable model for studying the important role the liver plays in aging.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
We acknowledge the financial support of the National Health and Medical Research Council (570937) and Ramaciotti Foundation (RN 23/08).
Supplementary Material
Acknowledgment
We gratefully acknowledge the contribution of Mamdouh Khalil of the Molecular Physiology unit, ANZAC Research Institute, Dorothy Kouzios of the Diagnostic Pathology unit, Concord Hospital and all members of the Electron Microscopy Unit at Concord Hospital. We thank Dr. Patrick Bertolino for his kind gift of the ICAM-1 and F4/80 antibodies. We acknowledge the financial support of the National Health and Medical Research Council and Ramaciotti Foundation.
References
- 1. Le Couteur DG, Cogger VC, Hilmer SN, et al. Aging, atherosclerosis and the liver sieve. In: Randall LO, ed. Aging and the Elderly: Psychology, Sociology and Health. New York: NOVA Science Publishers; 2006:153–176 [Google Scholar]
- 2. Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21 [DOI] [PubMed] [Google Scholar]
- 3. Andrews GR, Khalil Z, Le Couteur DG. Experimental gerontological research in Australia. Exp Gerontol. 2002;37:1303–1310 [DOI] [PubMed] [Google Scholar]
- 4. Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62:973–978 [DOI] [PubMed] [Google Scholar]
- 5. Popper H. Aging and the liver. Prog Liver Dis. 1986;8:659–683 [PubMed] [Google Scholar]
- 6. Le Couteur DG, Warren A, Cogger VC, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken). 2008;291:672–683 [DOI] [PubMed] [Google Scholar]
- 7. Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell SJ, Huizer-Pajkos A, Cogger VC, et al. Age-related pseudocapillarization of the liver sinusoidal endothelium impairs the hepatic clearance of acetaminophen in rats. J Gerontol A Biol Sci Med Sci. 2011;66:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamath-Loeb AS, Shen JC, Loeb LA, Fry M. Werner syndrome protein. II. Characterization of the integral 3’ –> 5’ DNA exonuclease. J Biol Chem. 1998;273:34145–34150 [DOI] [PubMed] [Google Scholar]
- 10. Shen JC, Gray MD, Oshima J, Kamath-Loeb AS, Fry M, Loeb LA. Werner syndrome protein. I. DNA helicase and dna exonuclease reside on the same polypeptide. J Biol Chem. 1998;273:34139–34144 [DOI] [PubMed] [Google Scholar]
- 11. Constantinou A, Tarsounas M, Karow JK, et al. Werner’s syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 2000;1:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Opresko PL, Otterlei M, Graakjaer J, et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell. 2004;14:763–774 [DOI] [PubMed] [Google Scholar]
- 13. Turaga RV, Paquet ER, Sild M, et al. The Werner syndrome protein affects the expression of genes involved in adipogenesis and inflammation in addition to cell cycle and DNA damage responses. Cell Cycle. 2009;8:2080–2092 [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto K, Imakiire A, Miyagawa N, Kasahara T. A report of two cases of Werner’s syndrome and review of the literature. J Orthop Surg (Hong Kong). 2003;11:224–233 [DOI] [PubMed] [Google Scholar]
- 15. Goto M, Ishikawa Y, Sugimoto M, Furuichi Y. Werner syndrome: a changing pattern of clinical manifestations in Japan (1917~2008). Biosci Trends. 2013;7(1):13–22 [PubMed] [Google Scholar]
- 16. Kitamoto T, Takemoto M, Fujimoto M, et al. Sitagliptin successfully ameliorates glycemic control in Werner syndrome with diabetes. Diabetes Care. 2012;35:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lebel M, Leder P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc Natl Acad Sci USA. 1998;95:13097–13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Ogburn CE, Ware CB, et al. Cellular Werner phenotypes in mice expressing a putative dominant-negative human WRN gene. Genetics. 2000;154:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lombard DB, Beard C, Johnson B, et al. Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol Cell Biol. 2000;20:3286–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lebel M, Lavoie J, Gaudreault I, Bronsard M, Drouin R. Genetic cooperation between the Werner syndrome protein and poly(ADP-ribose) polymerase-1 in preventing chromatid breaks, complex chromosomal rearrangements, and cancer in mice. Am J Pathol. 2003;162:1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massip L, Garand C, Turaga RV, Deschênes F, Thorin E, Lebel M. Increased insulin, triglycerides, reactive oxygen species, and cardiac fibrosis in mice with a mutation in the helicase domain of the Werner syndrome gene homologue. Exp Gerontol. 2006;41:157–168 [DOI] [PubMed] [Google Scholar]
- 22. Labbé A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278 [DOI] [PubMed] [Google Scholar]
- 23. Massip L, Garand C, Paquet ER, et al. Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J. 2010;24:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cogger VC, Hilmer SN, Sullivan D, Muller M, Fraser R, Le Couteur DG. Hyperlipidemia and surfactants: the liver sieve is a link. Atherosclerosis. 2006;189:273–281 [DOI] [PubMed] [Google Scholar]
- 25. Huang Y, de Boer WB, Adams LA, et al. Image analysis of liver collagen using sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int. 2013;33:1249–1256 [DOI] [PubMed] [Google Scholar]
- 26. Cogger VC, Le Couteur DG. Fenestrations in the liver sinusoidal endothelial cell. In: Arias IM, Boyer JL, eds. The Liver: Biology and Pathobiology. Hoboken, NJ: John Wiley & Sons, Ltd; 2009, 387–404 [Google Scholar]
- 27. Simon-Santamaria J, Malovic I, Warren A, et al. Age-related changes in scavenger receptor-mediated endocytosis in rat liver sinusoidal endothelial cells. J Gerontol A Biol Sci Med Sci. 2010;65:951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanaka T, Kono T, Terasaki F, et al. Thiamine prevents obesity and obesity-associated metabolic disorders in OLETF rats. J Nutr Sci Vitaminol (Tokyo). 2010;56:335–346 [DOI] [PubMed] [Google Scholar]
- 29. Stacchiotti A, Lavazza A, Ferroni M, et al. Effects of aluminium sulphate in the mouse liver: similarities to the aging process. Exp Gerontol. 2008;43:330–338 [DOI] [PubMed] [Google Scholar]
- 30. Furrer K, Rickenbacher A, Tian Y, et al. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc Natl Acad Sci USA. 2011;108:2945–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gregg SQ, Gutiérrez V, Robinson AR, et al. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito Y, Sørensen KK, Bethea NW, et al. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Couteur DG, Cogger VC, Markus AM, et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537–543 [DOI] [PubMed] [Google Scholar]
- 34. Miquel J, Economos AC, Fleming J, Johnson JE, Jr. Mitochondrial role in cell aging. Exp Gerontol. 1980;15:575–591 [DOI] [PubMed] [Google Scholar]
- 35. Schmucker DL, Sachs H. Quantifying dense bodies and lipofuscin during aging: a morphologist’s perspective. Arch Gerontol Geriatr. 2002;34:249–261 [DOI] [PubMed] [Google Scholar]
- 36. Andrew W. An electron microscope study of age changes in the liver of the mouse. Am J Anat. 1962;110:1–18 [DOI] [PubMed] [Google Scholar]
- 37. Le Couteur DG, Sinclair DA, Cogger VC, et al. The aging liver and longterm caloric restriction. In: Everitt AV, Rattan SIS, Couteur DG, Cabo R, eds. Calorie Restriction, Aging and Longevity. Dordrecht, Netherlands; Springer Press; 2010:191–216 [Google Scholar]
- 38. Wiesner RJ, Rüegg JC, Morano I. Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun. 1992;183:553–559 [DOI] [PubMed] [Google Scholar]
- 39. Gahan PB, Middleton J. Euploidization of human hepatocytes from donors of different ages and both sexes compared with those from cases of Werner’s syndrome and progeria. Exp Gerontol. 1984;19:355–358 [DOI] [PubMed] [Google Scholar]
- 40. Raines SM, Richards OC, Schneider LR, et al. Loss of PDGF-B activity increases hepatic vascular permeability and enhances insulin sensitivity. Am J Physiol Endocrinol Metab. 2011;301:E517–E526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438 [DOI] [PubMed] [Google Scholar]
- 42. Abdelmohsen K, Srikantan S, Yang X, et al. Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J. 2009;28:1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowdish DM, Gordon S. Conserved domains of the class A scavenger receptors: evolution and function. Immunol Rev. 2009;227:19–31 [DOI] [PubMed] [Google Scholar]
- 44. Bieghs V, Verheyen F, van Gorp PJ, et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS One. 2012;7:e34378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. González M, Flores C, Pearson JD, Casanello P, Sobrevia L. Cell signalling-mediating insulin increase of mRNA expression for cationic amino acid transporters-1 and -2 and membrane hyperpolarization in human umbilical vein endothelial cells. Pflugers Arch. 2004;448:383–394 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Herron AJ, Worman HJ. Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery-Dreifuss muscular dystrophy. Hum Mol Genet. 2006;15:2479–2489 [DOI] [PubMed] [Google Scholar]
- 47. Mounkes LC, Kozlov SV, Rottman JN, Stewart CL. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum Mol Genet. 2005;14:2167–2180 [DOI] [PubMed] [Google Scholar]
- 48. Sullivan T, Escalante-Alcalde D, Bhatt H, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arimura T, Helbling-Leclerc A, Massart C, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169 [DOI] [PubMed] [Google Scholar]
- 50. Csoka AB, Cao H, Sammak PJ, Constantinescu D, Schatten GP, Hegele RA. Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J Med Genet. 2004;41:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.